Abstract
Systematic review (SR) is a rigorous, protocol-driven approach designed to minimise error and bias when summarising the body of research evidence relevant to a specific scientific question. Taking as a comparator the use of SR in synthesising research in healthcare, we argue that SR methods could also pave the way for a “step change” in the transparency, objectivity and communication of chemical risk assessments (CRA) in Europe and elsewhere. We suggest that current controversies around the safety of certain chemicals are partly due to limitations in current CRA procedures which have contributed to ambiguity about the health risks posed by these substances. We present an overview of how SR methods can be applied to the assessment of risks from chemicals, and indicate how challenges in adapting SR methods from healthcare research to the CRA context might be overcome. Regarding the latter, we report the outcomes from a workshop exploring how to increase uptake of SR methods, attended by experts representing a wide range of fields related to chemical toxicology, risk analysis and SR. Priorities which were identified include: the conduct of CRA-focused prototype SRs; the development of a recognised standard of reporting and conduct for SRs in toxicology and CRA; and establishing a network to facilitate research, communication and training in SR methods. We see this paper as a milestone in the creation of a research climate that fosters communication between experts in CRA and SR and facilitates wider uptake of SR methods into CRA.
1. Introduction
Systematic review (SR) is a rigorous, protocol-driven approach to minimising error and bias1 in the aggregation and appraisal of evidence relevant to answering a research question. SR techniques were initially developed in the fields of psychology, social science and health care and have, since the 1980s, provided a valuable tool for evidence-informed decision-making across many domains (Lau et al. 2013). In medicine, SRs have provided a valuable response to the need for consistent, transparent and scientifically-robust interpretations of the results of increasing numbers of often conflicting studies of the efficacy of healthcare interventions. SRs have taken on an increasingly fundamental role both in supporting decision-making in healthcare and, by channelling resources towards questions for which the answers are not yet known, reducing waste in research (Chalmers, Glasziou 2009; Salman et al. 2014). It is now accepted practice in healthcare to use SR methods to assess evidence not only for the efficacy of interventions, but also on diagnostic tests, prognostics and adverse outcomes.
The extension of SR techniques to other fields is based on a mutual need across disciplines to make the best use of existing evidence when making decisions, a move for which momentum has been growing for several decades. For example, the What Works Clearinghouse was established in 2002 to apply SR techniques in support of American educational policy (US Institute of Education Sciences 2015), and in 2000 the international Campbell Collaboration research network was convened to undertake and disseminate systematic reviews on the effects of social interventions in diverse fields such as crime and justice, education, international development and social welfare (Campbell Collaboration 2015). Meta-analysis and SR in ecology have contributed to evidence-based environmental policy since the mid-1990s (Stewart 2010); more recently, the Collaboration for Environmental Evidence (CEE) has been established to encourage conduct of SRs on a wide range of environmental topics (Collaboration for Environmental Evidence 2015).
The potential advantages of adapting SR methodology to the field of chemical risk assessment (CRA) have also been recognised, with multiple research groups and organisations either developing and adopting (Woodruff, Sutton 2014; Birnbaum et al. 2013; European Food Safety Authority 2010; Rooney et al. 2014; Aiassa et al. 2015) or recommending (US National Research Council 2014a, 2014b; US Environmental Protection Agency 2013; Silbergeld, Scherer 2013; Hoffmann, Hartung 2006; Zoeller et al. 2015) the use of SR methods for evaluating the association between health effects and chemical exposures to inform decision-making. There are, however, a number of recognised challenges in extending SR methods to CRA, many of which derive from key differences in the evidence base between the healthcare and toxicological sciences.
SRs in medicine often focus on direct evidence for benefits and adverse effects of healthcare interventions derived from randomised controlled trials (RCTs) in humans. The evidence base for CRA is generally more complex, with a need to extrapolate from investigations in animals, in vitro and in silico, and then to synthesise findings with those from human studies if available. Furthermore, the human data tend to come from observational studies with greater and more varied potential for bias and confounding than RCTs, and the range of outcomes to be considered is usually much wider than in the assessment of healthcare interventions. Thus, when the various types of toxicological research are combined into a single overall conclusion about the health risks posed by a chemical exposure, reviewers are challenged with integrating the results from a broad and heterogeneous evidence base.
In spite of these differences, there is reason for thinking that SR methods can be applied successfully to CRA. For example, techniques for aggregating the results of different study types are already addressed in various frameworks currently in use in toxicology. These include: International Agency of Research on Cancer (IARC) Monographs (International Agency for Research on Cancer 2006); the Navigation Guide (Woodruff, Sutton 2014); and the US Office for Health Assessment and Translation (OHAT) (Rooney et al. 2014; US National Toxicology Panel 2015) – though it should be noted that none of these approaches have yet applied SR methods to the exposure assessment component of CRA. Heterogeneous sources of evidence are a familiar challenge in all domains including clinical medicine (Lau et al. 1998), and SR of observational studies has a crucial role in identifying complications and side-effects of healthcare interventions (Sterne et al. 2014; Higgins, Green 2011). The need for SR of pre-clinical animal trials of healthcare interventions, in order to better anticipate benefits and harms to humans, is another area in which methods being developed and implemented by a number of groups including SYRCLE (Hooijmans et al. 2012; van Luijk et al. 2014) and CAMARADES (Macleod et al. 2005; Sena et al. 2014). (Stewart, Schmid 2015) argue that research synthesis methods (including systematic review) are generic and applicable to any domain if appropriately contextualised.
Given the sometimes controversial outcomes of CRAs and the growing public and media profile of the risks that chemicals may pose to humans and the environment, SR is increasingly viewed as a potentially powerful technique in assessing and communicating how likely it is that a chemical will cause harm. SR methods add transparency, rigour and objectivity to the process of collecting the most relevant scientific evidence with which to inform policy discussions and could provide a critical tool for organising and appraising the evidence on which chemical policy decisions are based.
Consequently, in November 2014 a group of 35 scientists and researchers from the fields of medicine, toxicology, epidemiology, environmental chemistry, ecology, risk assessment, risk management and SR participated in a one-day workshop to consider the application of SR in CRA. The purpose was three-fold:
Identify from expert practitioners in risk assessment and SR the obstacles, in terms of practical challenges and knowledge gaps, to implementing SR methods in CRA;
Develop a “roadmap” for overcoming those obstacles and expediting the implementation of SR methods, where appropriate, by the various stakeholders involved in CRA;
Establish the foundations of a network to co-ordinate research and activities relating to the implementation of SR methods in CRA. The aim would be to support best practice in the application of SR techniques and promote the wider adoption of SR in CRA, both in Europe and elsewhere.
Participants heard seven presentations about recent developments in SR methods, their application to the risk assessment process, and their potential value to policy-makers. There were two break-out sessions in which participants were divided into three facilitated groups, firstly to discuss challenges to implementing SR methods in CRA, and then to suggest ways in which the obstacles could be overcome. These ideas were discussed in plenary before being summarised, circulated for comment, and then published in this paper. The Workshop was conducted under the “Chatham House Rule” such that participants were free to refer to the information presented and discussed, provided they did not attribute it to identifiable individuals or organisations.
The purpose of this overview paper is to present the rationale for exploring the application of SR methods to CRA, the various experts’ views on the challenges to implementing SR methods in CRA, and their suggestions for overcoming them. The remaining goals of the meeting are ongoing work, including the development of the roadmap concept for publication and the establishment of a network for supporting the use of SR in CRA.
2. The appeal of SR methods in CRA
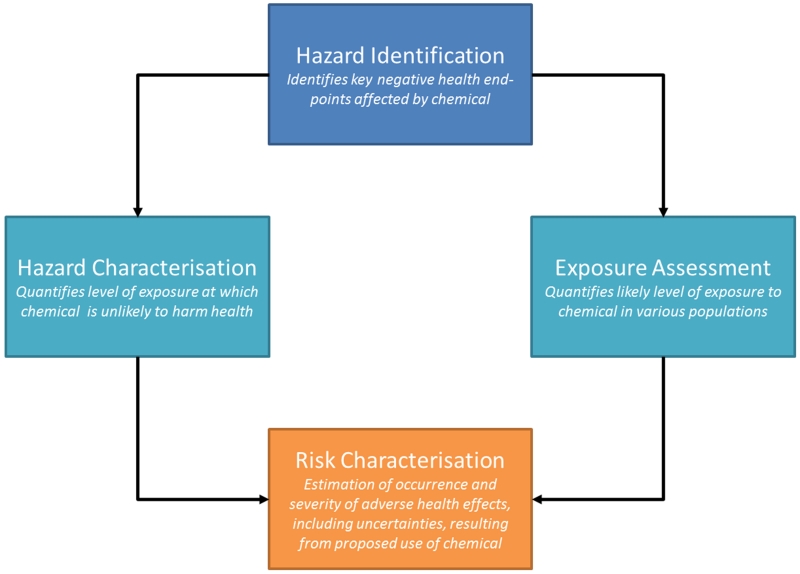
Chemical risk assessment is a multi-step process leading to a quantitative characterisation of risk, which can then be used to inform the management of chemical substances so as to ensure that any risks to human health or the environment are managed optimally. CRAs entail four fundamental steps: hazard identification; hazard characterisation (often a dose-response assessment); exposure assessment; and risk characterisation (see Figure 1). These steps draw on various fields of scientific research including environmental chemistry, toxicology (encompassing in vivo, in vitro, ecotoxicological and in silico methods), ecotoxicology, human epidemiology, and mathematical modelling.
Figure 1. An overview to the chemical risk assessment(CRA) process, whereby risk is a function of hazard and exposure. While SR methods could in principle be applied to all steps of the CRA process, it is the view of the workshop participants that up to this point in time most attention has been focused on the hazard identification and hazard characterisation steps. There are issues around conducting a systematic review for exposure assessment which were not discussed at the workshop, such as the requirement for a very different tool for assessing risk of bias in exposure studies which may necessitate very specialised knowledge of analytical/environmental chemistry.
There are many ways in which errors can occur in the interpretation of evidence from these varied disciplines, including failure to consider all relevant data, failure to allow appropriately for the strengths and limitations of individual studies, and over- or underestimating the relevance of experimental models to real-world scenarios (to name a few). Whether the appraisal of evidence is based on objective processes, or on subjective expert judgement and opinion, may also be an important factor in accurate interpretation of evidence: the assessment process always requires input from technical experts, which inevitably brings an element of subjectivity to the interpretation of the scientific evidence. Different experts may have varying degrees of practical and cognitive access to relevant information, place differing weight on individual studies and/or strands of evidence that they review and, when working in committee, may be more or less influenced by dominant personalities. This can result in misleading conclusions in which the potential for health risks is overlooked, underestimated or overstated. Furthermore, if the factors determining their assessment of evidence are undocumented, when expert opinions are in conflict it can be very challenging to distinguish which opinion is likely to represent the most valid synthesis of the totality of available evidence.
A recent illustrative example (see Box 1) of when expert scientists and reputable organisations have come to apparently contradictory conclusions about the likelihood of a chemical causing harm is the case of bisphenol-A (BPA). BPA is a monomer used in the manufacture of the resinous linings of tin cans and other food contact materials such as polycarbonate drinks bottles. It has been banned from use in infant-feed bottles across the EU (European Commission 1/28/2011) because of “uncertainties concerning the effect of the exposure of infants to Bisphenol A” (European Commission 5/31/2011).
Box 1: Examples of conflicting opinions from scientists and government agencies about the risks to health posed by bisphenol-A at current exposure levels.
Five conflicting opinions about risks to health posed by bisphenol-A at current exposure levels
“no health concern for any age group from dietary exposure and low health concern from aggrkegated exposure” (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) 2015)
“The conclusions of the risk assessment show […] a potential risk to the unborn children of exposed pregnant women. The identified effects relate to a change in the structure of the mammary gland in the unborn child, that could promote subsequent tumour development” (French Agency for Food, Environmental and Occupational Health & Safety 2013)
“DTU evaluates that [EFSA’s TDI for BPA of] 4 μg/kg bw/day is not sufficiently protective with regards to endocrine disrupting effects of BPA. DTU finds that a TDI for BPA has to be 0.7 μg/kg bw/day or lower to be sufficiently protective” (National Food Institute, Denmark 2015)
“BPA is safe at the current levels occurring in foods” (US Food and Drug Administration 2014)
“we are confident that consistent, reproducible, low dose effects have been demonstrated for BPA […] the doses that reliably produce effects in animals are 1–4 magnitudes of order lower than the current LOAEL of 50 mg/kg/day and many should be considered adverse” (Vandenberg et al. 2014)
The European Food Safety Authority (EFSA) considers that current levels of exposure to BPA present a low risk of harm to the public (European Food Safety Authority 2015a). The French food regulator ANSES takes a different stance on the risks to health posed by BPA (French Agency for Food, Environmental and Occupational Health & Safety 4/7/2014), determining there to be a “potential risk to the unborn children of exposed pregnant women”. On this basis, ANSES has proposed classifying BPA as toxic to reproduction in humans (French Agency for Food, Environmental and Occupational Health & Safety 2013), a proposal which has contributed to the French authorities’ decision to implement an outright ban on BPA in all food packaging materials (France 12/24/2012). While the ban has been challenged by some stakeholders as being disproportionate under EU law (Tošenovský 2014, 2015; Plastics Europe 1/15/2015), the Danish National Food Institute has argued that EFSA has overestimated the safe daily exposure to BPA and that some populations are exposed to BPA at levels higher than can be considered safe (National Food Institute, Denmark 2015); a view reflected in the conclusions of some researchers, e.g. (Vandenberg et al. 2014) but not others, e.g. (US Food and Drug Administration 2014).
The example of BPA illustrates the challenges in reaching consensus even when interpreting the same evidence base regarding the potential toxicity of chemical exposures, either in terms of what is known and what is uncertain about the risks to health posed by BPA, and/or what response is appropriate to managing those risks and uncertainties. It also shows how, in the absence of that consensus, there is a danger that policy on BPA may become disconnected from the evidence base, either risking harm to health through continued exposure or incurring unnecessary economic costs through restricting the use of a chemical which is in fact sufficiently safe. It also suggests that if the reasons for disagreement about health risks posed by a chemical are not accessible to various stakeholders in the debate, it then becomes much more difficult for regulators to credibly resolve controversies about chemical safety, potentially undermining their authority in the long term.
This example highlights the potential for differences in the interpretation of evidence when assessing chemical toxicity and the need for a process that is not only scientifically robust but also transparent, so that the reasons for any disagreement can be readily identified – including giving stakeholders greater opportunity to understand when differences in policy stem from divergent assessments of risk, and when they stem from divergent opinions as to how those risks are best managed. It also suggests the importance of the following characteristics in risk assessments that are used to inform risk management decisions:
Transparency, in that the basis for the conclusions of the risk assessment should be clear (otherwise they may not be trusted and errors may go undetected).
Validity, in that CRAs should be sufficiently (though not necessarily maximally) scientifically robust in their methodology and accurate in their estimation of risks and characterisation of attendant uncertainties as to optimise the decisions that must be made in risk management.
Confidence, providing the user with a clear statement as to the overall strength of evidence for the conclusions reached and a characterisation of the utility of the evidence for decision-making (e.g. “appropriate for hazard identification but inappropriate for identification of a reference dose”).
Utility, in that the output of the risk assessment should be in a form that is convenient and intelligible to those who will use it (outputs that are too detailed and complex to validate and readily comprehend lead to inefficiency and possibly erroneous decisions).
Efficiency, providing a clear justification of the choice of research question in the context of efficiently solving a CRA problem. Resources for CRA are often limited and it is wasteful to expend unnecessary effort on aspects of an assessment that will not be critical to decision-making (although for the purposes of transparency and validity, the reasons for focusing on a particular outcome or otherwise restricting the evaluation should be explained).
Reproducibility, in that the conclusions of the SR process when applied to the same question and data should ideally produce the same answer even when undertaken by different individuals (also described as “consistency”). In practice, different experts may reach difference conclusions because they will not all make the same value judgments about the scope, quality and interpretation of evidence. Therefore, the process should be sufficiently rigorous that it is highly likely that scientific judgment would result in the same conclusion independent of the experts involved, and as a minimum the SR process should render transparent the reasons for all conclusions.
It may be perceived that the value of SR methods lies in their provision of unequivocal assessments of whether or not a chemical will induce specific harm to humans and/or wildlife in given circumstances. In practice, however, this will happen only if the evidence base is sufficiently extensive, there is unanimity in identification of the problem and in assessment of the quality of the evidence base, and also how the evidence is to be interpreted in answering the review question (without this, SRs will also produce different results). Often, the consensus and/or information may be relatively limited; in such circumstances, a SR will instead clearly state the limitations of the available data and consequent uncertainties. The value here is in the provision of a comprehensive and transparent assessment of what is not known and insight into the drivers of divergent opinion. From a research perspective, this yields valuable information about how research limitations and knowledge gaps contribute to ongoing uncertainty about environmental and health risks, allowing the subsequent efforts of researchers to be more clearly focused. From a policy perspective, SRs offer a transparent explanation as to why there are differences in opinion which can then be communicated to stakeholders.
Overall, SR contributes to achieving consensus not by eliminating expert judgement, nor by eliminating conflicting opinions about whether a compound should be banned (for example), but by providing a robust, systematic and transparent framework for reviewing evidence of risks, such that when there is disagreement, the reasons for it are clearly visible and the relative merits of differing opinions can be appraised. In this way, it may help to resolve controversies in the interpretation of the science which informs the risk management process.
3. SR and its application to CRA
3.1. Traditional vs. SR methods
SR methods are often contrasted with “traditional”, non-systematic narrative approaches to describing what is and is not already known in relation to a research question. In reality, the distinction between systematic and narrative review is a crude one, with narrative reviews encompassing a number of different approaches to reviewing evidence, from the caricature of one researcher writing about “my field, from my standpoint […] using only my data and my ideas, and citing only my publications” (Caveman 2000), to thorough narrative critiques of comprehensively identified evidence relevant to answering an explicitly articulated question, as conducted by organisations such as IARC (International Agency for Research on Cancer 2006).
Nonetheless, it is worth noting that only relatively recently has it been recognised that traditional narrative reviews are, to varying degrees, vulnerable to a range of methodological shortcomings which are likely to bias their summarisation of the evidence base (Chalmers et al. 2002). These include selective rather than comprehensive retrieval of evidence relevant to the review topic, inconsistent interpretation of the impact of methodological shortcomings on the validity of included studies, and even an absence of clear review objectives or conclusions which are drawn directly from the strengths and limitations of the evidence base (Mulrow 1987; Mignini, Khan 2006).
The presence of these shortcomings seriously challenges the reader’s ability to determine the credibility of a review. When there exist multiple competing reviews, each using opaque methods, it becomes almost impossible to judge their relative merits and therefore to base decisions on current best available evidence. The consequence is a proliferation of conflicting opinions about best practice that fail to take proper account of the body of research evidence. In the healthcare sciences, this was initially shown by Antman and colleagues when they found that, in comparison to recommendations of clinical experts, systematic aggregation of data from existing clinical trials of streptokinase to treat myocardial infarction would have demonstrated benefit some years before recommendations for its use became commonplace (Antman et al. 1992). More recently, cumulative meta-analyses have been shown to be more accurate in summarising current understanding of the size of effect of a wide range of healthcare interventions than researchers planning new clinical trials who have not used these methods (Clarke et al. 2014).
A SR is an approach to reviewing evidence which specifically sets out to avoid these problems, by methodically attempting “to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question,” using “explicit, systematic methods that are selected with a view to minimizing bias” (Higgins, Green 2011).
In detail, this amounts to the pre-specification of the objective and methods of the SR in a written protocol, in which the aim of conducting the review is clearly stated as a structured question (for a SR of the effects of an intervention or exposure, this can establish a testable hypothesis or quantitative parameter that is to be estimated), along with the articulation of appropriate methods. The methods specified should include the techniques for identifying literature of potential relevance to the research question, the criteria for inclusion of the studies of actual relevance to the research question, how the internal validity2 of the included studies will be appraised, and the analytical techniques used for combining the results of the included studies. The purposes of the protocol are to discourage ad-hoc changes to methodology during the review process which may introduce bias, to allow any justifiable methodological changes to be tracked, and also to allow peer-review of the work that it is proposed, to help ensure the utility and validity of its objectives and methods.
The final SR itself consists of a statement of the objective, the search method, the criteria for including relevant studies for analysis, and the results of the appraisal of internal validity of the included studies, e.g. implemented as a “risk of bias” assessment in Cochrane Reviews of randomised trials (Higgins et al. 2011). The evidence is then synthesised using statistical meta-analytical techniques, narrative methods or both (depending on the extent to which meta-analysis is possible) into an overall answer to the research question. An assessment is then made of the strength of the evidence supporting the answer; in Cochrane reviews, this typically follows the GRADE methodology (Atkins et al. 2004), taking into account overall features of the evidence base including risk of bias across the included studies, publication bias in the evidence base, external validity or applicability of the evidence to the population of interest, heterogeneity of the evidence, and the overall precision of the evidence. This is finally followed by a concluding interpretation of what the SR as a whole determines is and is not known in relation to its objective.
In this, we emphasise the distinction between a SR and a meta-analysis. A meta-analysis pools the results of a number of separate studies in a single statistical analysis and may be a component of a SR; however, it does not necessarily incorporate the full set of methodological features which define the SR process (e.g. a meta-analysis may or may not include an assessment of the internal validity of included studies). While we acknowledge that some researchers use the terms “systematic review” and “meta-analysis” interchangeably, we believe the two approaches should be disambiguated. It is also worth noting that many reviews employ a combination of narrative and systematic methods; there were differing opinions among workshop participants as to the extent to which it is reasonable to expect all reviews to fully incorporate SR methods.
3.2. The current status of SR in environmental health, toxicology and CRA
While the use of SR methodologies is well established in healthcare to determine the effect of interventions on health outcomes or the accuracy of a diagnostic test, application of SR is relatively novel in the fields of toxicology and environmental health. Workshop participants heard how methods for SR of medical interventions have in the United States been adapted in both academic and federal contexts to the gathering and appraising of evidence for the effects of chemical exposures on human health: researchers at the University of California have developed the Navigation Guide (Woodruff, Sutton 2014), and the US Office of Health Assessment and Translation (OHAT) at the US National Toxicology Program has developed the OHAT Framework for systematically reviewing environmental health research for hazard identification (Rooney et al. 2014).
The two approaches adapt the key elements of SR methods to questions in environmental health (which is directly relevant to the CRA process but does not include assessment of dose-response). Features that the two approaches have in common include: conducting a SR according to a pre-specified protocol; the development of a specific research question and use of “PECO” statements (see Box 2) in systematising review objectives and the methods that will be used to answer that question; an approach to appraising the internal validity of included studies adapted from the risk of bias appraisal tool developed by the Cochrane Collaboration (Higgins et al. 2011); an adaptation of the GRADE methodology (Atkins et al. 2004) for describing the certainty or strength of a body of evidence, incorporating risk of bias elements with other criteria such as for the assessment of relevance or external validity; and a methodology for combining the results of human and animal research into a statement of confidence about the hazard which a chemical poses to health.
Box 2. The use of PECO statements in the SR process.
“PECO” is an acronym representing: Population (the exposure group of interest, e.g. people of a certain age or rats in laboratory studies); Exposure (the compounds or exposure scenarios of interest, e.g. respiratory exposure to fine particulate matter); Comparator (the group to which the exposure group is being compared, e.g. vehicle-exposed controls in laboratory experiments or less exposed groups in epidemiological studies); Outcome (a deleterious change or marker thereof hypothesised to be brought about by the exposure). The purpose of a PECO statement is to provide a framework for developing the key question which a SR will answer, and also to determine the rationale for the inclusion and exclusion criteria that explicitly define which studies are relevant for the review.
Other tools are being developed to contribute to the systematic assessment of in vivo and ecotoxicity studies which have not been directly derived from Cochrane Collaboration methods. Presented at the Workshop was SciRAP (Science in Risk Assessment and Policy), a system developed to improve the consistency with which the relevance and reliability of studies are appraised in the context of conducting a chemical risk assessment for regulatory purposes. It is also intended to reduce the risk of selection bias in the risk assessment process by providing a mechanism for including non-standardised study methods yielding potentially valuable data (Beronius et al. 2014; SciRAP 2014).
There are a number of other initiatives promoting and developing the use of SR methodologies in environmental and chemical risk assessment. Participants heard about how the European Food Safety Authority is integrating SR methods into its assessments of food and feed safety (European Food Safety Authority 2015b, 2015c), and about the UK Joint Water Evidence Group methods for rapid and systematic assessments of evidence (Collins et al. 2014). Other coordinated initiatives include the Evidence-Based Toxicology Collaboration (Hoffmann, Hartung 2006); the Collaboration for Environmental Evidence (Bilotta et al. 2014a; Land et al. 2015); and the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE).
3.3. Overcoming the challenges in implementing SR methods in CRA
Risk assessment for a chemical or group of chemicals is a multi-faceted process that normally requires consideration of multiple endpoints in relation to a variety of exposure scenarios, integrating evidence from epidemiological studies, bioassays in animals, mechanistic studies and studies on the distribution and determinants of exposure by different pathways and routes. In addition to resolving methodological issues relating to underdeveloped methods (e.g. how SR methods can be used as part of dose-response assessment or how they can be applied to exposure assessment), it is important to consider how SR should fit into the CRA process. One challenge going forward is to explore the circumstances in which applying more rigorous SR methods to assess scientific evidence would be warranted, which would require insight into the practicality and cost-effectiveness of applying such methods in those situations.
In principle, it should be possible to conduct SRs in any aspect of a CRA. Given the success in employing SR methods to support evidence-based practice in healthcare, it is intuitive that SRs could address specific questions arising within toxicology, human epidemiology and environmental health (e.g. hazard assessment within a CRA) and this view appears to be gaining momentum within the environmental health literature. The SR method may also lend itself to answering questions concerning e.g. the accuracy of the reported physical-chemical properties of a substance, doses predicted by quantitative exposure assessment, concentrations of a chemical in the environment and biota, and the derivation of a No Observed Adverse Effect Level (NOAEL) or Benchmark Dose Lower 95% confidence limit (BMDL). European Food Safety Authority (2015c) explores these issues in more detail.
Depending on scope, the resources (time and cost) to undertake an SR can be considerable. Currently there is a lack of empirical evidence relating to the resource-effectiveness of SR approaches in CRA and there was a difference of opinion among workshop participants as to whether the effort required for conducting a SR tends to be under- or overestimated. It was suggested that, where effort is likely to be substantial, efficient use of resources may be achieved by focusing on high-value questions developed through initial scoping exercises. For example, a low-dose adverse effect may be evident in animal models and supported to some extent by human epidemiology and hence a question may be formulated around this initial evidence; there may be little point, however, in pursuing a question related to non-carcinogenic toxicity in wildlife if a substantial part of the literature points towards that substance being a potential human carcinogen. There is also growing interest in rapid reviews, when full SR methods are considered overly onerous (Collins et al. 2014; Schünemann, Moja 2015).
The priorities for expediting the adaptation of SR methods to CRA identified at the Workshop are as follows:
- The development of a number of prototype CRA-focused SRs to explore how readily SR procedures can be integrated into the CRA process, to:
- identify additional methodological challenges in adapting SR methods to the CRA context and develop techniques to address them;
- acquire practical experience in managing resources when conducting SRs in CRA, including the conduct of scoping exercises for identifying high-value review questions, the further development and/or application of novel “rapid evidence review” methods (UK Civil Service 2015), and how SR methods can be integrated into existing regulatory structures such as REACH (see Box 3).
- Technical development of SR methodologies for CRA purposes, in particular the further advancement of techniques for appraising and synthesising mechanistic, toxicological and human epidemiological studies, to include:
- refining tools for more consistent and scientifically robust appraisal of the internal validity of individual studies included in a CRA and the implications for interpretation of their findings; see e.g. Bilotta et al. (2014b). This might include further development and validation of tools such as the SYRCLE methodology for assessing the internal validity of animal studies (Hooijmans et al. 2014); for SR of observational studies see e.g. Sterne et al. (2014), the methods employed in the NTP/OHAT and Navigation Guide protocols, and the applicability of other assessment methods such as SciRAP (Beronius et al. 2014);
- the development of tools for the hazard characterisation and exposure assessment components of the CRA process;
- the further development of software akin to the Cochrane Collaboration’s Review Manager (Nordic Cochrane Centre 2014) and the Systematic Review Data Repository (Ip et al. 2012), and tools such as DRAGON (ICF International 2015) and the Health Assessment Workspace Collaborative (Rusyn, Shapiro 2013) to support extraction, analysis and sharing of data from studies included in reviews;
The development an empirical evidence base for the different types of bias that operate in the CRA domain, including their direction and potential magnitude, and the extent to which any methods being adopted to address them are appropriate and effective.
The development of a recognised “gold standard” for SRs in toxicology and risk assessment equivalent to the Cochrane Collaboration in evidence-based medicine, to address the growing number of purported SRs of unclear validity which are increasingly prevalent in the environmental health literature.
The creation of a climate of constructive discussion that fosters advancement of methods whereby chemical risk practitioners, industry, competent authorities, academic researchers and policy makers can research, discuss and evaluate SR methods and the potential advantages they can bring.
The establishment of a network of scientists and CRA practitioners to pursue research into and discussion of SR methodologies and facilitate their implementation.
The implementation of training programmes for risk assessment practitioners and stakeholders, focusing specifically on application of SR methods to CRA as a complement to current courses which largely cover SR methods in healthcare.
Box 3: The potential utility of SR methods in application to REACH registrations.
Systematic review and REACH regulations
Regulations such as REACH emphasise collating at the point of registration all evidence relevant to evaluating risks to human and environmental health posed by a chemical. As yet, however, there is very little guidance on how registrants should assemble REACH-compliant dossiers, nor is there detailed guidance on how the assembled evidence is to be assessed (Beronius et al. 2014). The subsequent quality of many of the REACH registration dossiers, with 172 out of 283 compliance checks resulting in a request for further information (European Chemicals Agency 2/26/2015), suggests a need for the development of a standardised, scientifically robust approach to dossier assembly which can be consistently followed by registrants.
4. Conclusions
While systematic review methods have proven highly influential in healthcare, they have yet to make widespread impact on the process of chemical risk assessment. While there is much promise in the concept of adapting SR methods to CRA to give definitive answers to specified research questions, or to enable identification of the reasons for failure to resolve debate, a number of challenges to implementing SR methods in CRA have been identified. These include particular concerns about approaches to assessing bias and confounding in observational studies, the effort involved in conducting SRs, and the subsequent benefits of conforming to SR standards. Recent experience from both regulatory agencies and academics already yields some clear recommendations which would expedite the wider implementation of SR methods in CRA, potentially increasing the efficiency, transparency and scientific robustness of the CRA process.
Acknowledgements
Funding for the workshop was provided through the Economic & Social Science Research Council grant “Radical Futures in Social Sciences” (Lancaster University) and Lancaster Environment Centre. CH, PW, AR are grateful to Lancaster University’s Faculty of Science & Technology “Distinguished Visitors” funding programme. The Royal Society of Chemistry is acknowledged for generously providing a meeting room, refreshments and facilitating the workshop proceedings. The PhD studentship of PW is partly funded through Lancaster Environment Centre. The contribution of non-author workshop participants to the development of the manuscript is also greatly appreciated.
Footnotes
It is worth drawing a distinction between three sources of bias in the review process. There is potential for bias in the conduct of a review (e.g. because of inappropriate methods for identifying and selecting evidence for inclusion in the review); bias because the material available for the review is not representative of the evidence base as a whole (due to selective publication); and bias arising from flaws in the design, conduct, analysis and reporting of individual studies included in the review that can cause the effect of an intervention or exposure to be systematically under- or over-estimated. One of the major functions of SRs is to minimise bias in the conduct of a review and, as far as possible, to ensure that potential bias from selective publication and methodological flaws in the evidence are properly taken into account when drawing conclusions in response to a research question.
“Internal validity” is a term used in Cochrane Collaboration guidance on conduct of SRs specifically intended to supersede the use of terms such as “methodological quality” or their equivalents, which are considered ambiguous (Higgins, Green 2011). The internal validity of a piece of research is appraised in a “risk of bias” assessment. The target of the risk of bias assessment is the likelihood, magnitude and direction of systematic error in the size of an observed effect, as caused by flaws in the design, conduct, analysis and reporting of a study. Throughout this document, we follow Cochrane Collaboration conventions in using “internal validity” as a technical term in place of “methodological quality”.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of their employers or otherwise affiliated organisations. EA is employed by the European Food Safety Authority (EFSA); however, the present article is published under her sole responsibility and may not be considered as an EFSA scientific output.
Publication bibliography
- Aiassa E, Higgins JPT, Frampton GK, Greiner M, Afonso A, Amzal B, et al. Applicability and feasibility of systematic review for performing evidence-based risk assessment in food and feed safety. Critical reviews in food science and nutrition. 2015;55(7):1026–1034. doi: 10.1080/10408398.2013.769933. [DOI] [PubMed] [Google Scholar]
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268(2):240–248. [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronius A, Molander L, Rudén C, Hanberg A. Facilitating the use of non-standard in vivo studies in health risk assessment of chemicals: a proposal to improve evaluation criteria and reporting. Journal of applied toxicology : JAT. 2014;34(6):607–617. doi: 10.1002/jat.2991. [DOI] [PubMed] [Google Scholar]
- Bilotta GS, Milner AM, Boyd I. On the use of systematic reviews to inform environmental policies. Environmental Science & Policy. 2014a;42:67–77. doi: 10.1016/j.envsci.2014.05.010. [DOI] [Google Scholar]
- Bilotta GS, Milner AM, Boyd IL. Quality assessment tools for evidence from environmental science. Environ Evid. 2014b;3(1):14. doi: 10.1186/2047-2382-3-14. [DOI] [Google Scholar]
- Birnbaum LS, Thayer KA, Bucher JR, Wolfe MS. Implementing systematic review at the National Toxicology Program: status and next steps. Environ. Health Perspect. 2013;121(4):A108–9. doi: 10.1289/ehp.1306711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Collaboration . The Campbell Collaboration; [accessed 6/13/2015]. 2015. Available online at http://www.campbellcollaboration.org/ [Google Scholar]
- Caveman The invited review - or, my field, from my standpoint, written by me using only my data and my ideas, and citing only my publications. J. Cell. Sci. 2000;113(Pt 18):3125–3126. doi: 10.1242/jcs.113.18.3125. [DOI] [PubMed] [Google Scholar]
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. The Lancet. 2009;374(9683):86–89. doi: 10.1016/S0140-6736(09)60329-9. [DOI] [PubMed] [Google Scholar]
- Chalmers I, Hedges LV, Cooper H. A Brief History of Research Synthesis. Evaluation & the Health Professions. 2002;25(1):12–37. doi: 10.1177/0163278702025001003. [DOI] [PubMed] [Google Scholar]
- Clarke M, Brice A, Chalmers I. Accumulating research: a systematic account of how cumulative meta-analyses would have provided knowledge, improved health, reduced harm and saved resources. PLoS ONE. 2014;9(7):e102670. doi: 10.1371/journal.pone.0102670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration for Environmental Evidence . The Collaboration for Environmental Evidence; [accessed 6/13/2015]. 2015. Available online at http://www.environmentalevidence.org/ [Google Scholar]
- Collins A, Miller J, Coughlin D, Kirk S. The Production of Quick Scoping Reviews and Rapid Evidence Assessments: A How to Guide (Beta Version 2) Joint Water Evidence Group; 2014. Available online at https://sbri.innovateuk.org/documents/3058188/3918930/The+Production+of+QSRs+and+REAs-+A+How+to+guide.pdf/45975020-be7d-4788-b74b-f3b6ed32c73a. [Google Scholar]
- European Commission [accessed 6/15/2015];Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles, Directive 2011/8/EU. Official Journal of the European Union. 2011 Jan 28; Available online at http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:026:0011:0014:EN:PDF.
- European Commission [accessed 2/17/2015];Bisphenol A: EU ban on baby bottles to enter into force tomorrow. Brussels. 2011 May 31; Available online at http://europa.eu/rapid/press-release_IP-11-664_en.htm.
- European Food Safety Authority Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal. 2010;8(6) doi: 10.2903/j.efsa.2010.1637. 2010. [DOI] [Google Scholar]
- European Food Safety Authority [accessed 2/18/2015];No consumer health risk from bisphenol A exposure. 2015a Press Release 21 Jan 2015. Parma. Available online at http://www.efsa.europa.eu/en/press/news/150121.htm.
- European Food Safety Authority [accessed 8/4/2015];Outcome of the targeted consultation of the EFSA Journal editorial on increasing openness, robustness and transparency of scientific assessments. 2015b Available online at http://www.efsa.europa.eu/en/supporting/pub/785e.htm.
- European Food Safety Authority Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal. 2015c;13(5):4121. doi: 10.2903/j.efsa.2015.4121. [DOI] [Google Scholar]
- France [accessed 6/15/2015];LOI n° 2012-1442 du 24 décembre 2012 visant à la suspension de la fabrication, de l’importation, de l’exportation et de la mise sur le marché de tout conditionnement à vocation alimentaire contenant du bisphénol A. 2012 Dec 24; Legifrance.gouv.fr. Available online at http://legifrance.gouv.fr/affichTexte.do;jsessionid=F6553AACC19D178279D8DF154EAC8558.tpdila17v_1?cidTexte=JORFTEXT000026830015.
- French Agency for Food, Environmental and Occupational Health & Safety Bisphenol A: ANSES demonstrates potential health risks and confirms the need to reduce exposure. 2013 Available online at https://www.anses.fr/en/content/bisphenol-anses-demonstrates-potential-health-risks-and-confirms-need-reduce-exposure.
- French Agency for Food, Environmental and Occupational Health & Safety [accessed 2/18/2015];Bisphenol A: ANSES publishes its comments in response to the EFSA draft Opinion for consultation. 2014 Apr 7; Available online at https://www.anses.fr/en/content/bisphenol-anses-publishes-its-comments-response-efsa-draft-opinion-consultation.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins Julian P. T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; [accessed 2/18/2015]. 2011. Available online at http://handbook.cochrane.org/ [Google Scholar]
- Hoffmann S, Hartung T. Toward an evidence-based toxicology. hum exp toxicol. 2006;25(9):497–513. doi: 10.1191/0960327106het648oa. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers M, Vries R.B. de, Leenaars M, Ritskes-Hoitinga M. An initiative to facilitate well-informed decision-making in laboratory animal research: report of the First International Symposium on Systematic Reviews in Laboratory Animal Science. Lab. Anim. 2012;46(4):356–357. doi: 10.1258/la.2012.012052. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, de Vries Rob B M, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC medical research methodology. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICF International [accessed 8/4/2015];DRAGON: An Online Tool for Systematic Review. 2015 Available online at http://www.icfi.com/insights/products-and-tools/dragon-online-tool-systematic-review.
- International Agency for Research on Cancer . Preamble to the IARC Monographs. Lyon, France: [accessed 9/10/2015]. 2006. Available online at http://monographs.iarc.fr/ENG/Preamble/index.php. [Google Scholar]
- Ip S, Hadar N, Keefe S, Parkin C, Iovin R, Balk EM, Lau J. A Web-based archive of systematic review data. Systematic reviews. 2012;1:15. doi: 10.1186/2046-4053-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, Wit C.A. de, Cousins IT, Herzke D, Johansson J, Martin JW. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review protocol. Environ Evid. 2015;4(1):3. doi: 10.1186/2047-2382-4-3. [DOI] [Google Scholar]
- Lau J, Ioannidis JPA, Schmid CH. Summing up evidence. One answer is not always enough. The Lancet. 1998;351(9096):123–127. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- Lau J, Rothstein HR, Stewart GB. History & progress of meta-analysis. In: Koricheva Julia, Gurevitch Jessica, Mengersen Kerrie., editors. Handbook of meta-analysis in ecology and evolution. Princeton University Press; Princeton: 2013. Chapter 25. [Google Scholar]
- Macleod MR, Ebrahim S, Roberts I. Surveying the literature from animal experiments: systematic review and meta-analysis are important contributions. BMJ. 2005;331(7508):110. doi: 10.1136/bmj.331.7508.110-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini LE, Khan KS. Methodological quality of systematic reviews of animal studies: a survey of reviews of basic research. BMC medical research methodology. 2006;6:10. doi: 10.1186/1471-2288-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrow CD. The medical review article: state of the science. Annals of internal medicine. 1987;106(3):485–488. doi: 10.7326/0003-4819-106-3-485. [DOI] [PubMed] [Google Scholar]
- National Food Institute, Denmark Evaluation of EFSA’s new Scientific Opinion on Bisphenol A. Søborg, Denmark (REG-no. DK 30 06 09 46) 2015 Available online at http://www.food.dtu.dk/english/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2015/Evaluation_BisphenolA.ashx?la=da.
- Nordic Cochrane Centre [accessed 6/18/2015];Review Manager (RevMan). Version 5.3: Cochrane Collaboration. 2014 Available online at http://tech.cochrane.org/revman.
- Plastics Europe . French ban on the use of Bisphenol A (BPA) in food contact: In conflict with European law and risk assessment - severe distortion of the market - no safety benefit for consumers. Jasmin Bird; [accessed 6/15/2015]. Jan 15, 2015. Available online at http://www.bisphenol-a-europe.org/uploads/Modules/Mediaroom/stm_re_french-bpa-ban-being-enforced-01-01-2015.pdf. [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environmental health perspectives. 2014;122(7):711–718. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Shapiro A. [accessed 8/4/2015];Health Assessment Workspace Collaborative (HAWC). Version Solid Hammer: UNC-CH Software. 2013 Available online at https://hawcproject.org/
- Salman RA-S, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. The Lancet. 2014;383(9912):176–185. doi: 10.1016/S0140-6736(13)62297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Moja L. Reviews: Rapid! Rapid! Rapid! …and systematic. Systematic reviews. 2015;4:4. doi: 10.1186/2046-4053-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SciRAP . Science in Risk Assessment and Policy. Department of Applied Environmental Science at Stockholm University; Institute of Environmental Medicine at Karolinska Institutet in Stockholm; MistraPharma; [accessed 3/11/2015]. 2014. Available online at http://www.scirap.org/ [Google Scholar]
- Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically. J. Cereb. Blood Flow Metab. 2014;34(5):737–742. doi: 10.1038/jcbfm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E, Scherer RW. Evidence-based toxicology: strait is the gate, but the road is worth taking. ALTEX. 2013;30(1):67–73. doi: 10.14573/altex.2013.1.067. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Higgins JPT, Reeves BC. A Cochrane Risk Of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI) The Cochrane Collaboration; [accessed 9/29/2014]. 2014. Available online at https://sites.google.com/site/riskofbiastool/ [Google Scholar]
- Stewart G. Meta-analysis in applied ecology. Biol. Lett. 2010;6(1):78–81. doi: 10.1098/rsbl.2009.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GB, Schmid CH. Lessons from meta-analysis in ecology and evolution: the need for trans-disciplinary evidence synthesis methodologies. Research synthesis methods. 2015;6(2):109–110. doi: 10.1002/jrsm.1152. [DOI] [PubMed] [Google Scholar]
- Tošenovský E. [accessed 6/15/2015];Question for written answer to the Commission, Rule 130. European Parliament, Parliamentary questions, P-008546/2014, Subject: Possible negative impact on the internal market of measures concerning BPA adopted by the French authorities. 2014 Oct 30; Available online at http://www.europarl.europa.eu/sides/getDoc.do?type=WQ&reference=P-2014-008546&language=EN.
- Tošenovský E. [accessed 6/15/2015];Question for written answer to the Commission, Rule 130. European Parliament, Parliamentary questions, E-004315-15, Subject: Measures concerning Bisphenol A. 2015 Mar 17; Available online at http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+WQ+E-2015-004315+0+DOC+XML+V0//EN&language=en.
- UK Civil Service [accessed 6/13/2015];What is a Rapid Evidence Assessment? 2015 Available online at http://www.civilservice.gov.uk/networks/gsr/resources-and-guidance/rapid-evidence-assessment/what-is.
- US Environmental Protection Agency [accessed 6/16/2015];Process for Developing IRIS Health Assessments. 2013 Available online at http://www.epa.gov/IRIS/process.htm.
- US Food and Drug Administration Bisphenol A (BPA): Use in Food Contact Application. Update on Bisphenol A (BPA) for Use in Food Contact Applications. 2014 Available online at http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm064437.htm.
- US Institute of Education Sciences [accessed 6/13/2015];What Works Clearinghouse. 2015 Available online at http://ies.ed.gov/ncee/wwc/default.aspx.
- US National Research Council . A framework to guide selection of chemical alternatives. The National Academies Press; Washington, D.C.: 2014a. [PubMed] [Google Scholar]
- US National Research Council . Review of EPA’s Integrated Risk Information System (IRIS) Process. The National Academies Press; Washington, D.C.: 2014b. [PubMed] [Google Scholar]
- US National Toxicology Panel [accessed 1/13/2015];Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. 2015 Available online at http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf.
- van Luijk J, Bakker B, Rovers MM, Ritskes-Hoitinga M, de Vries Rob B M, Leenaars M. Systematic reviews of animal studies; missing link in translational research? PLoS ONE. 2014;9(3):e89981. doi: 10.1371/journal.pone.0089981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, et al. Low dose effects of bisphenol A. Endocrine Disruptors. 2014;1(1):e26490. doi: 10.4161/endo.26490. [DOI] [Google Scholar]
- Woodruff TJ, Sutton P. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environmental health perspectives. 2014;122(10):1007–1014. doi: 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Bergman Å, Becher G, Bjerregaard P, Bornman R, Brandt I, et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ Health. 2015;14:118. doi: 10.1186/1476-069X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]