Abstract
Metastatic involvement of the viscera in men with advanced castration-resistant prostate cancer (CRPC) has been poorly characterized to date.
In 359 CRPC patients treated between June 2003 and December 2011, the frequency of radiologically detected visceral metastases before death was 32%. Of the 92 patients with computed tomography performed within 3 months of death, 49% had visceral metastases. Visceral metastases most commonly involved the liver (20%) and lung (13%). Median survival from diagnosis of visceral disease was 7.1 months (95% CI 5.9 - 8.3). Survival was impacted by the degree of bone involvement at detection of visceral disease, varying from 6.1 months in men with >6 bone metastases to 18.2 months in men with no bone metastases (p = 0.001). Heterogeneity was noted in clinical phenotypes and PSA trends at development of visceral metastases. Visceral metastases are now more commonly detected in men with CRPC, likely due to the introduction of novel survival-prolonging treatments.
Visceral disease was previously considered uncommon in men with advanced prostate cancer and has been associated with neuroendocrine phenotypes and poor outcome[1]. In recent Phase III post-docetaxel trials, 23-29% of participants had visceral metastases[2–4]. Autopsy studies on men who died from prostate cancer suggested a higher prevalence of visceral metastases, up to 66% of selected cases[5], but these metastases did not appear clinically relevant. Subset analyses of patients with visceral disease in the post-docetaxel abiraterone and enzalutamide Phase III trials showed hazard ratios of 0.79 (0.60–1.05) and 0.78 (0.56–1.09) respectively, suggesting these patients may derive benefit from targeting of the androgen receptor (AR)[3, 4]. The Phase III clinical trials of sipuleucel-T, radium223 and abiraterone in chemotherapy-naïve CRPC specifically excluded men with visceral disease. With the introduction of several new survival-prolonging treatments (reviewed in[6]) we hypothesized that more patients will develop visceral involvement, requiring improved recognition and molecular characterization in order to improve individual patient management.
To explore the prevalence of visceral disease in CRPC, we examined our database of clinical trial participants. This population has been described previously[7] and all patients provided consent for data collection in IRB-approved protocols. Patients had regular 12 weekly (or 6 weekly if specified in trial protocols) computed tomography (CT) scans of the thorax, abdomen and pelvis performed for restaging or for investigation of new symptoms. Brain CT scans were performed in response to neurological symptoms. Bone metastases were assessed using standard bone scans. These prospectively collected scans were reviewed for evidence of visceral involvement, defined as disease involving liver, lungs, adrenal glands, peritoneum or pleura, brain and dura. Descriptive statistics, Kaplan Meier survival analyses and Cox regressions were performed using SPSS Statistics v20 (IBM). Quantitative Venn diagrams were constructed using BioVenn[8].
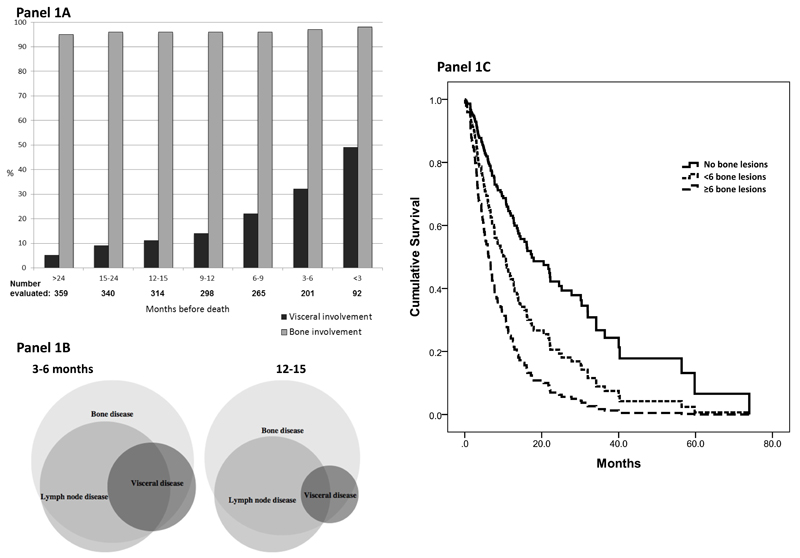
A total of 442 patients were enrolled on clinical trials and expanded access programs from June 2003 to December 2011. Of the 359 patients who died, 115 (32%) had evidence of visceral metastases on the last scan before death. We therefore related the prevalence of visceral metastases to the timing of last CT (see Figure 1A). The prevalence of visceral metastases on CT scan 9-12 months, 6-9 months, 3-6 months and within 3 months prior to death was 14%, 22%, 32% and 49%, respectively, suggesting that most patients developed visceral metastases late in the course of disease. This could reflect the natural history of the disease or be a result of current treatments proving more effective in controlling non-visceral metastases. The prevalence of visceral metastases was not increased by exposure to either abiraterone or enzalutamide (32% of 198 patients who received those agents, compared to 33% of 161 patients who did not). At detection of visceral metastases, most patients reported symptoms (see Supplemental Table 1). In 356 men with complete baseline data, metastatic disease at diagnosis was present in 192 (54%). The median intervals from cancer diagnosis or from CRPC development to development of visceral disease were 4.6 years (range 0 – 17.3) and 1.6 years (range 3.0 – 7.7) respectively.
Figure 1.
Panel A: Prevalence of visceral and bone metastases over time.
Panel B: Venn diagrams: Pattern of visceral, bone and nodal disease 3-6 months prior to death and 12-15 months prior to death.
Panel C: Survival of men with CRPC and visceral metastases, separated by degree of bone involvement.
Visceral metastases were associated with bone and nodal metastases in the majority of patients (shown in Figure 1B). The liver was the most common site of visceral involvement (71 patients), followed by lung (47), peritoneal (13), adrenal (11) and brain/dural metastases (11). In most patients only one site of visceral disease was identified. The outcome of men differed based on the degree of bone involvement at detection of visceral disease, with a median survival of 18.2 months in men with no evident bone disease (n=12; interquartile range (IR) 24.9, p = 0.001 versus ≥6 lesions), 8.1 months in those with moderate bone involvement (<6 lesions on bone scan; n=18; IR 25.6, p = 0.049 versus ≥6 lesions) and 6.1 months in those with more extensive bone involvement (≥6 lesions on bone scan; n=84; IR 8.9) (see Figure 1C). These preliminary small cohort data suggest that visceral metastases in the absence of extensive bone metastases may not represent a more aggressive disease phenotype. The association with worse outcome may instead relate to the increased prevalence of visceral disease as overall disease burden increases.
We observed heterogeneity in the clinical and biochemical behavior at diagnosis of new visceral disease. Of the 91 patients with three PSA values within the two months prior to the detection of visceral disease, 37 (41%) had confirmed PSA progression by PCWG2 criteria, 53 patients had PSA stability and one patient had a >50% PSA decline. This dissociation between PSA and radiological progression may reflect previous analyses that have failed to prove PSA as a survival surrogate[9]. Figure 2 shows two representative examples of discordant PSA kinetics at the development of visceral metastases. One patient developed biopsy-proven small cell carcinoma (Figure 2A) in the presence of a 100-fold PSA decline on docetaxel chemotherapy. Another patient had biopsy proven adenocarcinoma (Figure 2B) with negative staining for AR, PSA and ERG. Recent studies have suggested an increasing prevalence of AR negative disease in advanced prostate cancer as demonstrated in our second case (Figure 2B)[10].
Figure 2. Discordance of PSA trend at development of visceral disease in two patients with molecular work-up.
Figure 2A: Log PSA trend and computed tomography (CT) images of a patient with a 100-fold PSA decline on docetaxel-based chemotherapy. Despite very low levels of PSA the patient developed metastatic liver disease. Biopsy revealed small cell carcinoma morphology and negative IHC staining for PSA and AR (Aperio Scanscope, magnification 10x for all images).
Figure 2B: Patient developing widespread liver metastasis on treatment with abiraterone acetate in the presence of PSA stability. The archival primary prostate biopsy shows adenocarcinoma with positive IHC staining for PSA, AR and ERG and FISH confirming an underlying ERG rearrangement. At development of liver metastases in the absence of a rising PSA, a liver biopsy shows adenocarcinoma with negative IHC staining for PSA, AR and ERG despite an underlying ERG gene rearrangement (Aperio Scanscope, magnification 10x for H&E and IHC images, Ariol System for FISH pre-treatment image, magnification 40x; new liver metastasis magnification 20x)
Our data suggest that metastatic prostate cancer involves the viscera commonly, particularly in the advanced stages of disease. We show for the first time the detection of visceral disease in 49% of patients with a CT scan performed within 3 months of death. Visceral metastases in the presence of bone metastases associate with poor survival but do not predict poor response to treatment. We believe that the presence of visceral metastases should not automatically exclude patients from trial participation. In 59% evaluable patients, visceral metastases emerged in the absence of PSA progression. Timely identification of visceral disease provides opportunities to obtain fresh biopsy material for histological and molecular examination. As shown by the two examples (Figure 2A and 2B) biopsies at development of visceral disease, especially in the absence of PSA progression, may be of clinical relevance. Biopsies can be obtained safely, with low patient morbidity. The optimal choice of therapy could be informed by biopsy of individual patients, although the heterogeneity within tumors, across metastases and over time is likely to add further complexity to these analyses. If the differences in clinical characteristics are due to differences in biology, these will best be dissected by further molecular analyses and could lead to subgroup-directed treatment choices.
Supplementary Material
Supplemental Table 1: Patient characteristics.
Acknowledgements
Ulrike Naumann in the Cancer Biomarkers Group, The Institute of Cancer Research, Sutton, for statistical support.
Funding
The authors are employees of the Section of Medicine that is supported by a Cancer Research UK program grant and an Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (Ref: C51/A7401). A.O. is recipient of a 2-year bursary from the Swiss Cancer League (No. BIL KLS-02592-02-2010). R.F is funded by The Wellcome Trust (Ref: 094413/Z/10/Z). G.A. is supported by a Cancer Research UK Clinician Scientist Fellowship. The authors acknowledge NHS funding to the Royal Marsden NIHR Biomedical Research Centre.
References
- 1.Pouessel D, Gallet B, Bibeau F, Avances C, Iborra F, Senesse P, et al. Liver metastases in prostate carcinoma: clinical characteristics and outcome. BJU Int. 2007;99:807–11. doi: 10.1111/j.1464-410X.2006.06663.x. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Goodman OB, Flaig TW, Molina A, Mulders P, Fizazi K, Suttmann H, et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013 doi: 10.1038/pcan.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 6.Pezaro C, Attard G. Prostate cancer in 2011: redefining the therapeutic landscape for CRPC. Nat Rev Urol. 2012;9:63–4. doi: 10.1038/nrurol.2011.235. [DOI] [PubMed] [Google Scholar]
- 7.Omlin A, Pezaro C, Mukherji D, Mulick Cassidy A, Sandhu S, Bianchini D, et al. Improved Survival in a Cohort of Trial Participants with Metastatic Castration-resistant Prostate Cancer Demonstrates the Need for Updated Prognostic Nomograms. Eur Urol. 2013;64:300–6. doi: 10.1016/j.eururo.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collette L. Prostate-specific antigen (PSA) as a surrogate end point for survival in prostate cancer clinical trials. Eur Urol. 2008;53:6–9. doi: 10.1016/j.eururo.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.