Abstract
Introduction:
While much has been written about postpartum smoking relapse prevention, few have examined changes in smoking behavior from pregnancy (third trimester) through 9 months postpartum among pregnant smokers, particularly for the large number of women who decrease tobacco consumption during pregnancy but do not quit altogether.
Methods:
Data were obtained from 168 women who smoked during their pregnancy. Women were followed longitudinally from their first prenatal appointment through 9 months postpartum. Maternal substance use was assessed using the Timeline Followback and verified by maternal salivary analyses. Breastfeeding, other substance use, and partner smoking were assessed through maternal interviews at each time point and were considered as potential predictors of change in smoking.
Results:
Women returned to more than half of their levels of preconception tobacco consumption by 9 months postpartum. There was one significant predictor of changes in smoking patterns pregnancy to postpartum. Women who breastfed their infants for at least 90 days smoked far less postpartum than women who breastfed for a short time or did not breastfeed at all.
Conclusions:
As noted in previous research of pregnant quitters, postpartum relapse prevention or harm reduction interventions should ideally be timed early in the postpartum period. Additionally, promoting breastfeeding among pregnant smokers and supporting women through at least 3 months of breastfeeding may be beneficial to such interventions.
Introduction
While a large number of women quit or reduce smoking upon pregnancy recognition, many resume smoking postpartum. It is estimated that approximately 70% of women who quit smoking during pregnancy relapse within the first year postpartum,1 and of those who relapse, 67% resume smoking by 3 months, and up to 90% by 6 months.2 There is less known about the large number of smokers who decrease their tobacco consumption during pregnancy, but do not quit smoking altogether. Despite this being the case for the majority of pregnant smokers,1 the bulk of the literature on postpartum relapse relies on a dichotomous conceptualization of smoking, rather than looking at the changes in quantity of cigarettes smoked between the prenatal and postnatal periods. Postpartum relapse or increase in tobacco consumption may have deleterious effects on both the mother, and the neonate who is at higher risk of exposure to environmental tobacco smoke.
One reliable predictor of postpartum relapse is partner smoking. It is clear that having a smoking partner increases the likelihood of returning to smoking in the postpartum period.3 Based upon this evidence, intervention studies have begun to include partners in family intervention to increase efficacy.4 What is less clear is how partner smoking may impact tobacco consumption for all women, not just those who quit during pregnancy.
Though many women who quit smoking during pregnancy resume smoking in the postpartum period, there is evidence that this return to smoking is delayed in women who breastfeed.5 However, in one study 45% of women were still breastfeeding when they had their first postpartum cigarette,6 so it is important to consider the relationship between breastfeeding and quantity of tobacco consumption given that breastfeeding and postpartum smoking are not mutually exclusive.
There is also evidence that the use of other substances may increase the risks of returning to smoking postpartum. In particular, alcohol has been associated with smoking relapse in postpartum populations.6 Solomon and colleagues6 found that 23% of women had their first postpartum cigarette while using alcohol. However, the literature on postpartum smoking does not address how alcohol use is related to changes in tobacco consumption through the prenatal and postpartum periods, and does not address the role of marijuana.
The purpose of our study was to identify predictors of individual differences in smoking trajectories from the third trimester of pregnancy through 9 months postpartum. We hypothesized that partner smoking and the use of marijuana or alcohol would be associated with greater increases, while breastfeeding would be associated with slower rates of increase in smoking from pregnancy to postpartum.
Methods
Participants
The full study included 258 mother/infant dyads, with 181 infants prenatally exposed to tobacco (99 boys and 82 girls), and 77 not exposed (35 boys and 42 girls). Pregnant women were recruited at their first prenatal appointment in a local hospital and screened for eligibility. Women were eligible if they met the following criteria based on medical record review, maternal report, and maternal saliva samples taken in each trimester: more than 20 weeks gestation, single birth, 18 or more years old, no illicit drug use except cannabis, alcohol use less than 4 drinks/occasion or 1 drink/day after pregnancy recognition, marijuana use after pregnancy recognition of less than 1 joint/day, and English speaker. Each recruitment month, participating smokers were matched on maternal age and education with the closest eligible nonsmoking woman, who was then invited to participate. The smoking group was oversampled such that one nonsmoker was recruited for every two smokers.
Given the significant differences between pregnancy smokers and pregnancy nonsmokers with regard to our predictors of interest (ie, pregnancy smokers were significantly more likely to use other substances, and significantly less likely to breastfeed or have nonsmoking partners), this analysis included only women who smoked during their pregnancy. Of the 181 prenatal smokers, 13 women did not have complete data on time-invariant predictors, making the final analytic sample 168.
The prenatal smokers had an average age of 24.34 (SD = 4.96), an average education of 12.24 years (SD = 1.87), and an average of 2.25 live births (SD = 1.59). They were 47% black, 37% Caucasian, 16% Hispanic, and 10% other, with several identifying as more than one race. They smoked an average of 10.71 cigarettes per day before conception (SD = 8.01), 3.77/day in the third trimester (SD = 5.17), 5.11/day 2 months postpartum (SD = 5.06), and 6.26/day at 9 months postpartum (SD = 5.66). They drank an average of 0.62 standard drinks per day before conception (SD = 1.55), 0/day in the third trimester (SD = 0.02), 0.14/day 2 months postpartum (SD = 0.38), and 0.21/day at 9 months postpartum (SD = 0.57). They smoked an average of 1.06 joints per day before conception (SD = 2.15), 0.07/day in the third trimester (SD = 0.33), 0.16/day 2 months postpartum (SD = 0.53), and 0.65/day at 9 months postpartum (SD = 2.04). Fifty-five percent of the women did not breastfeed their infants, while an additional 11% breastfed for a week or less (M = 23.92 days, SD = 51.35). Additionally, 43% of the prenatal smokers had a smoking partner, while 25% had a nonsmoking partner.
Procedures and Instruments
Assessments were conducted once during each trimester of pregnancy, and at 2 (M = 2.51, SD = .41) and 9 (M = 8.81, SD = .87) months of infant age. Maternal tobacco, marijuana, and alcohol use was assessed at each laboratory visit using the Timeline Followback Interview.7,8 The Timeline Followback Interview yielded the average number of cigarettes smoked per day from 3 months before conception through 9 months postpartum, as well as number of joints per day, and number of standard drinks per day. Partner smoking status was ascertained at each appointment by asking women whether or not they had a partner, and if so, whether their current partner smoked cigarettes.
Data Analytic Strategy
HLM 79 was used to estimate a growth model in maternal smoking during the postnatal period.10,11 The two-level model included repeated measures of smoking at Level-1, and time invariant predictors at Level-2, and modeled both linear and quadratic trends.
All substance use variables were winsorized to within 3 standard deviations while maintaining the rank order of the variables. Time was coded as: before conception = 0, third trimester = 1, 2 months postpartum = 1.33, 9 months postpartum = 2.11 to represent the actual time in years from 3 months preconception to 9 months postpartum. Maternal age (in years), race (non-Caucasian = 0, Caucasian = 1), and education (0 = did not graduate high school, 1 = high school graduate), were determined during the first prenatal assessment and were considered time invariant predictors. Breastfeeding status was assessed at the 2- and 9-month assessments, was also considered time invariant, and was coded as the number of days the mother breastfed the infant. Marital status was assessed at each appointment and considered a time-varying predictor (0 = not married, 1 = married).
We first ran an unconditional means model, followed by an unconditional growth model. In the unconditional growth model, the intercept was set to before conception. Finally we ran the fully conditional model, in which the intercept was set to third trimester, since the focus of the study was changes from third trimester to 9 months postpartum. All predictors were grand mean centered.
Results
Potential predictors were examined in conceptually related groupings: maternal characteristics (maternal age, race, education, marital status, and breastfeeding) and substance use (maternal marijuana and alcohol use, and partner smoking), were tested in separate models. To preserve model parsimony, predictors were included in the final model only if they were significant (P < .05) in the initial testing.
On average, women returned to more than half their levels of preconception smoking (M = 10.71, SD = 8.01) by 9 months postpartum (M = 6.26, SD = 5.66). The variance components of the unconditional growth model indicated that there was significant variation in both initial status and rate of change over time (Table 1). There was only one significant predictor of changes in smoking patterns during the postpartum period. The more days women breastfed their infants, the less they smoked postpartum (Figure 1).
Table 1.
Estimates for Models of the Predictors of Postpartum Smoking Trajectories
| Parameter | Unconditional means model | Unconditional growth model | Fully conditional model |
|---|---|---|---|
| Intercept | 6.37** (0.37) | 10.70** (0.61) | −4.46* (1.98) |
| Level 1 | |||
| Time | −10.28** (0.73) | 10.73** (2.71) | |
| Time squared | 3.92** (0.28) | −2.73** (0.85) | |
| Marijuana | 0.28 (0.25) | ||
| Alcohol | 0.94 (0.64) | ||
| NP to SP | 0.20 (0.52) | ||
| NP to NSP | −0.58 (0.39) | ||
| Level 2 | |||
| Time | |||
| Breastfeeding | −0.02* (0.01) | ||
| Gestational age | −0.09 (0.26) | ||
| Level 2 | |||
| Time squared | |||
| Breastfeeding | 0.004 (0.003) | ||
| Gestational age | 0.11 (0.10) | ||
| Variance components | |||
| Intercept | 17.03** | 56.32** | 34.02 |
| Time | 65.29** | 116.31 | |
| Time squared | 7.48** | 11.17 | |
| Level-1 error | 23.97 | 6.17 | 4.08 |
Note. Predictors were grand mean centered. NSP = nonsmoking partner; NP = no partner, SP = smoking partner. The final model was: Level-1 Model: SMOKINGti = π0i + π1i*(MARIJUANAti) + π2i*(ALCOHOLti) + π3i*(NO PARTNER TO SMOKING PARTNERti) + π4i*(NO PARTNER TO NON-SMOKING PARTNERti) + π5i*(TIMEti) + π6i*(TIME2ti) + eti; Level-2 Model: π0i = β00 + r0i, π1i = β10, π2i = β20, π3i = β30, π4i = β40, π5i = β50 + β51*(BREAST FEEDINGi) + β52*(GESTATIONAL AGEi) + r5i, π6i = β60 + β61*(BREAST FEEDINGi i) + β62*(GESTATIONAL AGEi) + r6i
*P < .05, **P < .01.
Figure 1.
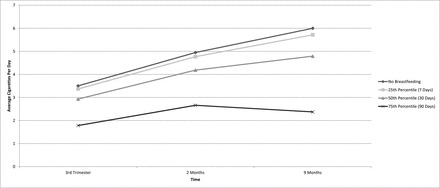
The relationship between number of breastfeeding days and average daily cigarette use in the postnatal period among prenatal smokers.
Discussion
While women decreased their tobacco consumption across the prenatal period, by 9 months postpartum they had substantially increased their smoking. There was only one significant predictor of these changes for women who smoked during their pregnancy. The more days women breastfed their infants, the less they smoked postpartum. Partner smoking status and the use of other substances were not related to changes in smoking over the postnatal period.
While previous literature has found breastfeeding delays relapse to smoking, this study adds to the literature by looking at the relationship between breastfeeding and the quantity of tobacco use postpartum for all women, not just those who quit during pregnancy. At 9 months child age, women who breastfed for 90 days or longer were smoking roughly 1/3 the number of cigarettes per day as women who did not breastfeed, and about half as much as women who breastfed for between 30 and 89 days (Figure 1).
Contrary to our hypotheses, alcohol and marijuana use were not significant predictors of postpartum smoking among prenatal smokers, and our results did not support the large body of literature on partner smoking.3,5,12 Partner smoking status was not related to postnatal smoking in our sample. It may be that the inclusion of women who continued smoking throughout pregnancy, albeit at lower rates, represents a different population of women than those who stop smoking altogether. Further research should explore this unique population of women who continue to smoke throughout their pregnancy to examine additional predictors of the changes in their smoking across the postpartum period.
One limitation of the current study is the sample, which was restricted to pregnant smokers with low levels of alcohol use, low to moderate marijuana use during pregnancy, and no other illicit substance use during pregnancy. Thus, the results are generalizable to the large majority of low-income smokers with lower levels of other substance use, but may not be applicable to pregnant smokers who have significant substance abuse problems.
Our results suggest that the timing of relapse prevention interventions for pregnant and postpartum smokers is crucial. With women returning to more than half their preconception smoking levels by 9 months postpartum, harm reduction or abstinence interventions should be targeted early in the postnatal period. In addition, breastfeeding seems to be a protective factor against increases in smoking postpartum, so interventions should educate women about breast feeding to maximize effectiveness. Supporting women through at least 3 months of breastfeeding may have long term benefits in terms of smoking reduction.
Funding
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA019632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank the mothers and infants who participated in the study as well as the research staff who were responsible for conducting and coding the assessments. A special thanks to Amol Lele and the Women and Children’s Hospital of Buffalo for their collaboration in the data collection process.
References
- 1. Scheibmeir M, O’Connell KA. In harm’s way: childbearing women and nicotine. J Obstet Gynecol Neonatal Nurs. 1997;26(4):477–484. doi:10.1111/j.1552-6909.1997.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 2. Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80(5):541–544. www.ncbi.nlm.nih.gov/pmc/articles/PMC1404636/?tool=pmcentrez Accessed October 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mullen PD, Richardson MA, Quinn VP, Ershoff DH. Postpartum return to smoking: who is at risk and when. Am J Health Promot. 1997;11(5):323–330. [DOI] [PubMed] [Google Scholar]
- 4. Wakefield M, Jones W. Effects of a smoking cessation program for pregnant women and their partners attending a public hospital antenatal clinic. Aust N Z J Public Health. 1998;22(3):313–320. doi:10.1111/j.1467-842X.1998.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 5. McBride CM, Pirie PL. Postpartum smoking relapse. Addict Behav. 1990;15(2):165–168. [DOI] [PubMed] [Google Scholar]
- 6. Solomon LJ, Higgins ST, Heil SH, Badger GJ, Thomas CS, Bernstein IM. Predictors of postpartum relapse to smoking. Drug Alcohol Depend. 2007;90(2–3):224–227. doi:10.1016/j.drugalcdep.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown RA, Burges ES, Sales SD, Whiteley JA, Evans DM, Miller I. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. doi:10.1037/0893-164X.12.2.101. [Google Scholar]
- 8. Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. Accessed September 25, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Raudenbush SW, Bryk AS, Congdon RT. HLM 7.01 for Windows [computer software]. Skokie, IL: Scientific Software International, Inc; 2013. [Google Scholar]
- 10. Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 11. Singer JD, Willett JW. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 12. Pollak KI, Mullen PD. An exploration of the effects of partner smoking, type of social support, and stress on postpartum smoking in married women who stopped smoking during pregnancy. Psychol Addict Behav. 1997;11(3):182–189. doi:10.1037/0893-164X.11.3.182. [Google Scholar]


