Abstract
Introduction:
Primate and rodent models show that peroxisome proliferator-activated receptor-alpha (PPAR-α) ligands, including fibrate medications, reduce nicotine reinforcement, reward, and related effects. We tested fenofibrate, the most common US Food and Drug Administration-approved fibrate for lipid control versus placebo for initial evidence of efficacy in smoking cessation using a validated cross-over procedure for early Phase 2 evaluations.
Methods:
Adult dependent smokers (N = 38) in this 4-week within-subjects study were those already intending to try to quit in the next 2 months. All smoked ad libitum during weeks 1 (baseline) and 3 (washout) and began fenofibrate (160mg/d; dosing approved for lipid control) or placebo near the end of weeks 1 and 3. Following each 4-day dose run-up, they were then instructed to try to quit for 4 days (Tuesday–Friday) during weeks 2 and 4, with the order of medication conditions counter-balanced and administered double-blind. Abstinence was verified daily in each 4-day quit period by self-report of no smoking in the prior 24 hours and carbon monoxide < 5 ppm. Secondary measures of acute smoking reinforcement and cue reactivity prior to quitting, and smoking reduction when trying to quit, were also assessed.
Results:
No differences between fenofibrate versus placebo were found on days quit (means ± SEM of 1.8±0.3 vs. 1.9±0.3, respectively). Similarly, there were no differences in any of the secondary measures (all P > .20).
Conclusions:
Although higher dosing or other proliferator-activated receptor-alpha agonists may show efficacy, this study indicates that fenofibrate does not aid ability to stop smoking during a brief practice quit period in dependent smokers high in current quit interest.
Introduction
Fibrate drugs are agonists of alpha-type peroxisome proliferator-activated receptors (PPARα) that have been widely used clinically for over 20 years to lower lipid levels in patients with hyperlipidemia and related disorders.1,2 Notably, drugs that activate PPARα can modulate nicotinic receptors and block nicotine-induced dopamine firing.3,4 Recent preclinical findings show that PPARα agonists, such as fibrate drugs, reduce nicotine’s actions on dopamine in the ventral tegmental area and nucleus accumbens shell, blunting rewarding effects of nicotine in the brain.5–7 In rat and monkey models, activation of PPARα via fibrates or other manipulations reduces nicotine self-administration, including post-extinction resumption of self-administration (ie, reinstatement) due to priming exposure to nicotine itself or to associated cues.8–10 These actions of PPARα agonists in nonhuman species are specific to nicotine reward and do not alter food reward.5
This research and other studies suggest the hypothesis that PPARα agonists, including fibrates, may have efficacy for smoking cessation in humans.5,11 To our knowledge, PPARα agonist drugs have not yet been tested for potential efficacy in smoking cessation. As such, an optimum candidate may be fenofibrate, by far the safest and most common fibrate medication US Food and Drug Administration (FDA) approved for lipid lowering.2,12
In this study, we tested fenofibrate versus placebo for efficacy in smoking cessation, using our efficient and validated early Phase 2 procedure for initial evaluation of a novel medication’s potential efficacy.13 This procedure’s specific objective is to quickly determine whether a novel medication does, or does not, show sufficient promise of efficacy for cessation to justify the substantial time and expense of conducting a larger randomized Phase 2 clinical trial of that medication. This approach enhances efficiency and statistical power because all participants receive both medication conditions, eliminating the individual variability between conditions and allowing for smaller sample sizes to test differences between conditions.14 In testing with model drugs, the procedure has demonstrated sensitivity by confirming efficacy in all three FDA-approved first-line cessation medications (nicotine replacement therapy patch, varenicline, and bupropion), relative to placebo.13 It has also shown specificity by verifying lack of efficacy in modafinil,15 a medication for wakefulness that was previously found ineffective for smoking cessation.16 Importantly, a key to the procedure’s sensitivity and specificity is to conduct these evaluations among smokers recruited for being high in quit interest, in that they already plan to permanently quit soon (eg, in the next few months).13
The primary outcome with this procedure is number of days abstinent during each separate week-long “practice” quit attempt on the drug conditions manipulated in the crossover design.17 In the current study, these conditions were fenofibrate and placebo, which were administered double-blind and in counter-balanced order. In secondary outcomes to explore possible mechanisms of action, we also assessed medication effects on smoking behavior and cue-induced craving (“reactivity”) just before trying to quit, based on the above-noted preclinical findings,5–10 and on reductions in daily smoking when attempting to quit.
Methods
Recruitment Procedures
As in our prior studies using this early Phase 2 procedure,13 we used online and print notices to recruit smokers from the community who reported that they already intended to quit permanently in the next 2 months. This quit intention criterion is based on our prior work showing that medication screening in this subgroup is more sensitive to effects of efficacious cessation medications, compared to placebo, while data in smokers not planning to quit soon do not show such sensitivity.13,15,17 Prospective participants were briefly screened by telephone and then again in person (to verify reliable responding) for smoking history, health, and their open-ended report on when they hoped to make a permanent quit attempt (eg, in next 2 months, in 3–6 months, etc., with study eligibility requiring next 2 months). Eligible subjects were required to be aged 18–65, smoke at least 10 cigarettes per day for at least 1 year, provide a mid-day screening carbon monoxide (CO) reading at least 10 ppm, and to not currently be taking cessation medication or receiving treatment to help quit. Screening also included assessment of alcohol use via the well-known AUDIT,18 and those with scores indicating dependence were excluded. The subsequent physical exam with study physician included bloodwork and interview to confirm no recent use of other drugs and exclude those with current major health problems (eg, heart disease, diabetes) or taking medications for lipid control (eg, statin drugs) or to treat serious psychological problems (eg, psychosis, anxiety disorders, major depression).
Although this was described as “not a treatment study,” all were told that a benefit of participation included free open-label treatment with bupropion and brief counseling to make a permanent quit attempt after completing the study. This benefit was offered specifically to attract participation by smokers high in quit interest, as in our prior study with bupropion,15 because those not wanting to quit soon would be disinterested in such a study benefit. All also agreed in writing that they would try hard to quit during the quit assessment days (weeks 2 and 4). Prior to study entry, subjects provided written informed consent for participation after the nature and consequences of the study were explained, and then they were scheduled for a physical exam by physician to confirm eligibility. This study was approved by the University of Pittsburgh Institutional Review Board.
Medication
Fenofibrate dosing was the same as that FDA-approved for lipid control (160mg/d), which is very safe and well-tolerated.2 Participants took oral fenofibrate or identically appearing placebo via one capsule per day in the AM, each for a total of 8 days. The first 4 days (Friday–Monday) provided a dose run-up before the start of the 4-day practice quit period (Tuesday–Friday), as steady-state levels of fenofibrate are achieved rapidly.2 A week of resumption of ad libitum smoking followed the first quit attempt period, to allow a subsequent test of efficacy for quitting with the other medication condition during the second quit period. This washout period was sufficient, based on fenofibrate’s elimination half-life of 20 hours.2 Compliance was greater than 99% for each condition (including placebo), assessed by pill counts at every visit (with a total of just two fenofibrate and one placebo pill missed, over all 38 participants across the two conditions).
Measures
Primary Outcome
During the “quit week” of each condition, abstinence was assessed daily on Tuesday–Friday by self-report of no smoking at all over the prior 24 hours and an expired-air CO < 5 ppm. This stringent biochemical criterion for validating smoking cessation has been shown to be more sensitive and specific than traditional (higher) CO cut-offs.19,20
Secondary Outcomes
Prequit and postquit secondary smoking outcomes were examined to explore possible mechanisms of medication effects, based on the preclinical findings with nicotine reinforcement due to fibrates.9 For the prequit outcomes, acute smoking reinforcement and cue reactivity were assessed on Mondays of weeks 2 and 4, following the dose run-up for each medication condition but just before trying to quit. For the postquit outcomes, amount of daily smoking exposure was assessed later in weeks 2 and 4 after trying to quit, to gauge potential for smoking reduction that was short of complete abstinence, the primary outcome.
Acute reinforcement was assessed via puff topography (mL per puff) and number of puffs during ad libitum smoking of one cigarette, using the portable Clinical Research Support System (“CReSS Pocket”; Borgwaldt KC, Inc, Richmond, VA). As described elsewhere in detail,21 subjects were given one cigarette of their preferred brand but under blind conditions with the brand markings covered. Told it was a “regular commercial brand,” they were instructed to smoke it as much or as little as desired and then rate it for “reward” on several subjective characteristics (adapted from22). Aside from allowing assessment of acute smoking reinforcement and reward, this brief smoking standardized recent exposure and blunted any deprivation-induced craving at baseline prior to the subsequent assessment of cue-induced craving. To do so, subjects were presented with four smoking and four neutral pictorial cues on a computer monitor (using MediaLab from Empirisoft Co, New York, NY), in counter-balanced order, and rated their craving to each using the 4-item QSU (QSU-423), with greater responses to smoking versus neutral cues indicative of cue-induced craving (ie, cue reactivity24). Each item from the smoking reward (eg, “liking,” “satisfying”22) and craving self-report measures was scored on a 0–100 visual analog scale, anchored by “not at all” (0) and “extremely” (100). These measures were obtained because of their potential applicability to preclinical research showing effects of PPARα agonists in reducing nicotine self-administration after exposure to nicotine itself or to nicotine-associated cues.9
Then, while trying to quit on Tuesday–Friday of weeks 2 and 4, participants also reported number of cigarettes over the past 24 hours, using the same prospective self-report “tally” form described in our prior similar studies.21,25 This exposure, and the CO values obtained during daily visits, were used to ascertain whether fenofibrate may aid smoking reduction, if not complete cessation.
Finally, medication blinding was assessed on Tuesday of each quit week, following the dose run-up. Subjects indicated what they perceived to be the contents of the capsule they were taking by choosing from among three response options “Fenofibrate,” “Placebo (no medication),” or “don’t know.” Side effects were rated on a 0–3 scale (none, mild, moderate, and severe) and included nausea, agitation, nervousness, constipation, dry mouth, fatigue, insomnia, headache, increased appetite, decreased appetite, etc.
Procedure
Study participation for each subject was 4 weeks, involving two 2-week phases, one for each drug condition in this double-blind, crossover procedure. For each phase, visits occurred on Tuesday and Thursday of the first week and Monday–Friday of the second week, as shown in Table 1. Each phase began with a week of ad libitum smoking (baseline, week 1), as the drug regimen was started on Friday AM while continuing to smoke (dose run-up). Acute smoking reinforcement and cue reactivity testing, described previously, were conducted on the following Monday session. Participants were then instructed to try to abstain as of that Monday PM visit through to Friday PM, and CO (as described below) was assessed at brief daily visits for the remainder of that week (ie, quit assessments on Tuesday–Friday, week 2). After Friday of week 2, all then ad libitum smoked for at least a week to repeat this 2-week phase of smoking without drug (ie, washout; week 3), starting the next dose run-up, and trying to quit during Tuesday–Friday of week 4 for the other drug condition. The duration of smoking resumption after week 2 occasionally extended for more than 1 week, prior to the start of the second 2-week phase, when holiday or vacation schedules would interfere with study visits scheduled following week 2.
Table 1.
Study Visit Timeline for Each 2-Week Phase
| Week 1 (2 visits) | Tuesday | Baseline (CO, cigs/d) | Smoke ad libitum |
| Thursday | Receive medication | ||
| Week 2 (5 visits) | Monday | Acute smoking reinforcement, Cue Reactivity testing | |
| Tuesday | Quit status (CO, cigs/d) | Try to Quit | |
| Wednesday | Quit status (CO, cigs/d) | ||
| Thursday | Quit status (CO, cigs/d) | ||
| Friday | Quit status (CO, cigs/d) |
CO = carbon monoxide.
CO was assessed upon arrival to each session via BreathCO CO monitor (Vitalograph, Inc; Lenexa, KS). To help maintain quit motivation over the Tuesday–Friday quit period in both conditions, subjects were given written suggestions on common strategies to use to avoid lapses or deal with craving or withdrawal after initiating cessation (eg, “hide all smoking materials,” “tell others you are quitting,” “stay busy,” “avoid ‘trigger’ situations,” “reward yourself for staying quit”26). Also, $15 was added to their compensation for study participation every day that subjects met the abstinence criteria of CO < 5 ppm and self-report of no smoking over the prior 24 hours (We have previously shown this modest daily monetary reinforcement does not interact with active vs. placebo conditions but rather acts independently to increase quit days.27). They were also compensated for their time at $20 for each study visit.
After study completion on Friday of week 4, all were offered a free regimen of bupropion (Zyban) and brief counseling for up to 8 weeks to help them make a permanent quit attempt. Such treatment began at a follow-up visit 1–2 weeks later.
Data Analysis
All analyses were conducted using IBM SPSS 21.0. Preliminary analysis of variances examined the effects of sex and of medication order between phases, but no significant main or interaction effects were found. The primary analysis was a repeated-measures analysis of variance of abstinent days per quit assessment week (range of 0–4), with medication (fenofibrate, placebo) as the within-subjects factor, a design that greatly increases statistical power.28 Similar analyses were conducted for the secondary outcome measures on smoking reinforcement, cue reactivity, and postquit smoking behavior (Data for cue reactivity was missing for two participants, leaving 36 for analyses of that response.).
Results
Participant Characteristics
Mean (SD) sample characteristics for these 38 adult smokers (27M, 11 F) were 30.3 (11.5) years of age, 16.4 (7.0) cigarettes per day, Fagerstrom Test of Nicotine Dependence29 score of 4.7 (2.3), and 2.8 (2.3) prior attempts to quit smoking. Participants mostly self-identified as Caucasian (86.8%), with 7.9% black, 2.6% Asian, and 2.6% as more than one ethnicity.
Quit Days
As shown in Figure 1, no significant differences were found between fenofibrate versus placebo for days quit, with means ± SEM of 1.8±0.3 versus 1.9±0.3, respectively, F(1,37) < 1.
Figure 1.
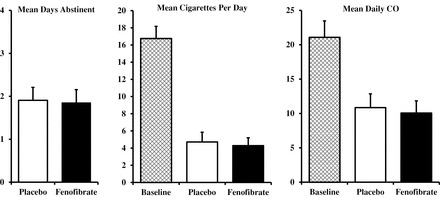
Mean (SEM) days of abstinence (left), and cigarettes per day and daily carbon monoxide (CO) (right) during each 4-day “quit” period on fenofibrate (160mg/d) and placebo (N = 38). Also shown, for comparison only, are cigarettes per day and CO during the first ad libitum smoking baseline period (BL). No differences were found on any measure between fenofibrate and placebo.
Secondary Outcome Measures
Acute Smoking Reinforcement and Cue-Induced Craving
Results for prequit acute smoking reinforcement indicated fenofibrate and placebo did not differ in total number of puffs (11.6±0.7 vs. 11.3±0.7, respectively), and volume (mL) per puff (47.9±2.2 vs. 46.9±2.6), both F(1,37) < 1. No differences between conditions were observed for smoking reward items of “liking,” “satisfying,” “how much nicotine,” and “how strong” (all P > .20). Following this smoking satiation, craving responses during the subsequent cue reactivity testing also did not differ for fenofibrate and placebo, as craving was very similar between conditions in response to the smoking cues (16.6±2.8 vs. 17.0±2.7, respectively) and to the neutral (control) cues (6.7±1.9 vs. 7.3±1.9). Yet, the main effect of cue type was highly significant, F(1,35) = 35.92, P < .001, validating the cue manipulation.
Smoking Reduction
During the 4 days of each quit attempt week, mean daily smoking intake in all 38 participants declined equally while trying to quit on fenofibrate and while on placebo, which did not differ for cigarettes per day (4.3±0.9 vs. 4.7±1.1, respectively) or CO (10.1±1.8 vs. 10.9±2.0), both F (1,37) < 1, ns. Means for each condition, and for smoking and CO during the initial ad libitum baseline week for comparison, are also shown in Figure 1.
Medication Blinding and Side Effects
The respective number (percent) of subjects identifying the medication as “fenofibrate,” “placebo”, or “don’t know” were 7 (19%), 2 (4%), and 29 (77%), respectively, for the fenofibrate condition, nearly identical to the ns of 7, 3, and 28 for respective identifications in the placebo condition. Adverse effects were mild, with most subjects responding “0” (none at all) for each effect during both drug phases. Means for all effects were 0.4 or below on the 0–3 scale except fatigue, which did not differ between fenofibrate and placebo (0.5±0.1 vs. 0.4±0.1, respectively).
Discussion
Fenofibrate did not increase days quit during a brief practice quit period in dependent smokers high in current quit interest, compared with placebo. Our cross-over procedure has demonstrated sensitivity in detecting clinical efficacy for the three first-line FDA-approved medications of nicotine patch, varenicline, and bupropion, as well as specificity in identifying lack of efficacy for modafinil,13 a drug previously found ineffective for cessation in a randomized trial.16 Therefore, despite promising preclinical findings with one fibrate,9 results of the current study indicate fenofibrate lacks clinical efficacy in aiding ability of dependent smokers to quit smoking.
Although this study tested only 38 subjects, the within-subjects crossover design should provide reasonable power to show differences between the active medication and placebo conditions, if they exist.14,28 For example, this procedure in our most recent study showed efficacy for bupropion (2.2±0.3 quit days) and lack of efficacy for modafinil (1.7±0.3), compared to placebo (1.6±0.3), with 45 smokers high in quit interest,15 very comparable to the current study’s sample size. The current study also found absolutely no differences in fenofibrate versus placebo on any of the secondary smoking behavior measures, in addition to the primary outcome of number of quit days, consistent with a lack of fenofibrate efficacy for cessation.
On the other hand, a longer duration of use or higher doses of fenofibrate may show efficacy for smoking cessation; we were limited here to testing the duration of fibrate use in decreasing nicotine reinforcement in the preclinical studies9 while maintaining the practicality of a cross-over design, as well as the clinical dose FDA-approved for lipid control. Clofibrate, the drug reducing nicotine reinforcement and related responses in animal models, noted earlier,9 was removed from the US market due to adverse side effects.30 Yet, other fibrates or PPAR-α agonists may still warrant testing for efficacy in smoking cessation.10,31
In conclusion, because of the costs and time of medication development, the efficient use of resources requires that drugs unlikely to be clinically effective be so identified in Phase 2 testing as early as possible and dropped from further consideration.32,33 Unfortunately, the track record for such tests of efficacy for smoking cessation in novel drugs suggests that negative results are far more likely than positive results.17,34 The results of the current study do not provide support for proceeding to a larger clinical trial randomizing treatment-seeking smokers to fenofibrate or placebo to test for differences in quit rates during attempts to permanently quit smoking.
Funding
This research was supported by NIH Grant P50 CA143187 (NCI). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. KAP had full access to all the data and takes full responsibility for the integrity of the data and accuracy of the data analysis.
Declaration of Interests
CL has received research funding from Pfizer that is unrelated to the current study. KAP and CL have each consulted for pharmaceutical companies that develop smoking cessation medications. CAC, KNRC, CH, JLK, VCM, and MF have no potential conflicts of interest or disclosures to report.
References
- 1. Staels B, Callongeville J, Auwerx J, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98 (19):2088–2093. doi:10.1161/01.CIR.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 2. Keating GM. Fenofibrate: a review of its lipid-modifying effects in dylipidemia and its vascular effects in Type 2 diabetes mellitus. Am J Cardiovasc Drugs. 2011;11 (4):227–247. doi:10.2165/112076900. [DOI] [PubMed] [Google Scholar]
- 3. Melis M, Carta S, Fattore L, et al. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68 (3):256–264. doi:10.1016/j.biopsych.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melis M, Scheggi S, Carta G, et al. PPAR alpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha 7 nicotinic acetylcholine receptors. J Neurosci. 2013;33 (14):6203–6211. doi:10.1523/jneurosci.4647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther. 2013;138 (1):84–102. doi:10.1016/j.pharmthera.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melis M., Carta G, Pistis M, Banni S. Physiological role of peroxisome proliferator-activated receptors type alpha on dopamine systems. CNS Neurol Disord Drug Targets. 2013;12 (1):70–77. doi:10.2174/1871527311312010012. [DOI] [PubMed] [Google Scholar]
- 7. Muldoon PP, Lichtman AH, Parsons LH, Damaj MI. The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence. Life Sci. 2013;92 (8–9):458–462. doi:10.1016/j.lfs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mascia P, Pistis M, Justinova Z, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69 (7):633–641. doi:10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panlilio LV, Justinova Z, Mascia P, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacol. 2012;37 (8):1838–1847. doi:10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. LeFoll B, DiCiano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets. 2013;14 (7):768–776. doi:10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harmey D, Griffin PR, Kenny PJ. Development of novel pharmacotherapeutics for tobacco dependence: progress and future directions. Nicotine Tob Res. 2012;14 (11):1300–1318. doi:10.1093/ntr/nts201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackevicius CA, Tu JV, Ross JS, et al. Use of fibrates in the United States and Canada. JAMA. 2011;305 (12):1217–1224. doi:10.1001/jama.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins KA, Lerman C. An efficient early Phase 2 procedure to screen medications for efficacy in smoking cessation. Psychopharmacology. 2014;231 (1):1–11. doi:10.1007/s00213-013-3364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleophas TJM. Cross-over studies: a modified analysis with more power. Clin Pharmacol Ther. 1993;53 (5):515–520. [DOI] [PubMed] [Google Scholar]
- 15. Perkins KA, Lerman C, Karelitz JL, Jao NC, Chengappa KNR, Sparks GM. Sensitivity and specificity of a procedure for early human screening of novel smoking cessation medications. Addiction. 2013;108 (11):1962–1968. doi:10.1111/add.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schnoll RA, Wileyto EP, Pinto A, et al. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98 (1–2):86–93. doi:10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkins KA. Improving efficiency of initial tests for efficacy in smoking cessation drug discovery. Expert Opin Drug Discov. 2014;9 (11):1259–1264. doi:10.1517/17460441.2014.951632. [DOI] [PubMed] [Google Scholar]
- 18. Saunders JB, Aasland OG, Babor TF, de la Fuent JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88 (6):791–804. doi:10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 19. Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100 (2):159–67. doi:10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 20. Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013;15 (2):578–582. doi:10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perkins KA, Mercincavage M, Fonte C, Lerman C. Varenicline’s effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacology. 2010;210 (1):45–51. doi:10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53 (2):309–315. [DOI] [PubMed] [Google Scholar]
- 23. Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9 (2):183–190. [DOI] [PubMed] [Google Scholar]
- 24. Conklin CA, Parzynski CS, Salkeld RP, Perkins KA, Fonte CA. Cue reactivity as a predictor of successful abstinence initiation among adult smokers. Exp Clin Psychopharmacol. 2012;20 (6):473–478. doi:10.1037/a0029599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perkins KA, Jao NC, Karelitz JL. Consistency of daily cigarette smoking amount in dependent adults. Psychol Addict Behav. 2013;27 (3):723–729. doi:10.1037/a0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkins KA, Conklin CA, Levine MD. Cognitive-Behavioral Therapy for Smoking Cessation: A Practical Guide to the Most Effective Treatments. New York, NY: Routledge; 2008. [Google Scholar]
- 27. Perkins KA, Lerman C, Stitzer ML, et al. Development of procedures for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2008;84 (2):216–221. doi:10.1038/clpt.2008.137. [DOI] [PubMed] [Google Scholar]
- 28. Cohen J. Statistical Power Analysis for the Social Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 29. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Addiction. 1991;86 (9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 30. Oliver MF. The clofibrate saga: a retrospective commentary. Brit J Clin Pharmacol. 2012;74 (6):907–910. doi:10.1111/j.1365-2125.2012.04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Melis M, Pistis M. Targeting the interaction between fatty acid ethanolamides and nicotinic receptors: therapeutic perspectives. Pharmacol Res. 2014;86 (1):42–49. doi:10.1016/j.phrs.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 32. Kola I. The state of innovation in drug development. Clin Pharm Ther. 2008;83 (2):227–230. doi:10.1038/sj.clpt.6100479. [DOI] [PubMed] [Google Scholar]
- 33. Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nature Rev Drug Discov. 2010;9 (3):203–214. doi:10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 34. Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11 (3):429–444. [DOI] [PubMed] [Google Scholar]


