Abstract
Background:
Psychotropic drugs increase the risk of falls, but they are still frequently prescribed to treat behavioral symptoms associated with dementia in the nursing home. We examined whether there is an acute increased risk of falls in the days following a change to an antipsychotic or benzodiazepine drug prescription.
Methods:
We collected information on 594 long-stay nursing home residents from two facilities who fell at least once between September 1, 2010 and May 31, 2013. Psychotropic drug changes were ascertained from the facilities’ computerized medication administration log. We used the case-crossover design to compare the frequency of antipsychotic and benzodiazepine drug changes during the days before a fall with the frequency of drug changes at more remote times.
Results:
Mean age was 87.5 years, and 75.1% were female. The risk of falls was higher in the 24 hours following benzodiazepine initiation compared with other times (odds ratio [OR] 3.79, 95% confidence interval [CI] 1.10, 13.00). There was no clear difference in risk following antipsychotic initiation (OR 2.42, CI 0.58, 10.06), but this could be due to the small sample size. Stopping a benzodiazepine was associated with a significantly reduced fall risk (OR 0.26, 95% CI 0.08–0.91).
Conclusions:
Benzodiazepines pose an immediate threat to fall risk, whereas it is less clear if antipsychotics also pose an immediate risk. Nursing home staff should be particularly vigilant in the days following the new prescription for a benzodiazepine in an effort to prevent injury.
Key words: Antipsychotic, Benzodiazepine, Drug change, Falls, Nursing home
In the nursing home setting, falls occur commonly at an average rate of 2.6 falls per resident per year (1). Approximately 7% of falls in the nursing home result in serious injury, such as fractures, lacerations, or head trauma (2). Injurious falls in the nursing home are associated with substantial morbidity and mortality: 36% of long stay residents with a hip fracture will die within 6 months of the fracture, and among those residents that survive 6 months, 28% will become totally disabled (3).
Drug use is one of the most modifiable risk factors for falls and fall-related injuries. Prior studies have reported that regular use of antipsychotic drugs is associated with an increased risk of falls (4–6) and hip fracture (7–9) in nursing home residents. New users of antipsychotics may have up to an 80% higher risk of hip fracture within three months (10). Similarly, regular use of benzodiazepine drugs is associated with an increased risk of falls in nursing home residents (7,11,12). New users of benzodiazeipines may have upto a 300% higher risk of falls within 1 week of starting the drug (11) and a 200% higher risk of hip fracture within 1 month of starting the drug (13).
Despite warnings to avoid using these medications in older adults (14), these drugs are commonly used off label to treat a myriad of psychiatric and behavioral symptoms, many of which may themselves be associated with falls, in nursing home residents with dementia. It is estimated that 26% of U.S. nursing home residents are prescribed an antipsychotic medication, while 13% are prescribed a benzodiazepine medication (15). Prior studies have focused largely on the association between current or chronic psychotropic drug use and falls. Less is known about whether these drugs increase the risk of falls acutely in the days following psychotropic drug initiation or increasing the dose of an existing drug, or whether more chronic exposure is required to affect risk.
The objective of our study was to determine the association between initiating, increasing, or discontinuing an antipsychotic or benzodiazepine drug and the acute risk of falls among long-stay nursing home residents. A traditional cohort design would be impractical to answer this research question given the large sample size and extensive follow-up required. Further, effect estimates can be biased using a cohort study design because treated and untreated individuals have different underlying risks of falls independent of the drug itself. For these reasons, we selected a self-controlled, case-crossover study design.
Methods
Participants
We included all long-term care residents from two nursing facilities in Boston, MA (Hebrew Rehabilitation Center and Newbridge on the Charles) that experienced one or more falls ≥ 14 days after admission to long-term care and between September 1, 2010 and May 31, 2013. We excluded residents admitted for short-term rehabilitation and residents less than 50 years of age (n = 2). We restricted the analysis to an individual’s first recorded fall from the time of admission or study entry. This study was approved by the Institutional Review Board of Hebrew SeniorLife.
Case-Crossover Study Design
The case-crossover design was developed to examine the effect of a transient exposure on an acute event (16). In this design, the frequency of exposure during a time period immediately preceding the event (hazard period) is compared with the frequency of exposure at other times (control period) for the same individual. The case-crossover study design allowed us to take advantage of the precise information recorded on the time and dose of all drugs administered in the nursing home. In addition, confounding introduced by differences between individuals in eliminated because each subject serves as his or her own control. Within-person confounding can still exist as transient changes within an individual (ie, illness severity) or an individual’s environment may impact the probability of receiving a medication and the risk of falls.
Falls
We defined a fall as accidentally coming to rest on the floor or similar lower surface. Fall reporting within nursing homes is federally mandated. In addition, the participating facilities voluntarily and publicly report patient falls and falls with injury on the quality and safety website, PatientCareLink. Falls were obtained using an electronic incident database (RL Solutions) that is updated daily by nursing staff. Staff are trained annually on fall reporting.
Psychotropic Drug Change
Antipsychotic and benzodiazepine drug administration was ascertained using the facilities’ computerized medication administration log (Meditech™), which includes detailed information on the time and dose of all drugs administered in the nursing home. Antipsychotics were categorized as typical (chlorpromazine, haloperidol, perphenazine, prochlorperazine, and thioridazine) or atypical (aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone). Benzodiazepines were categorized as short acting (alprazolam, lorazepam, oxazepam, and temazepam) or long acting (clonazepam, diazepam, and flurazepam). For each medication, we calculated the drug dose administered in each of the 24-hour periods preceding the fall by summing the total amount of drug administered whether prescribed regularly or used as needed (pro re nata). We calculated the percentiles of drug dose for each drug separately, and we then categorized the drug dose administered as none, low dose (≤25th percentile) or high dose (>25th percentile). If a resident was administered more than one medication within a psychotropic class in a 24-hour period, drug dose was categorized according to the largest dose category.
Other Characteristics
For the purposes of describing the health characteristics of the residents who experienced at least one fall, we obtained health information using the closest Minimum Data Set (MDS) assessment preceding the fall. The MDS is a federally mandated needs assessment tool that is completed on all nursing home residents at the time of their admission to the facility, and it is updated quarterly thereafter (17). There was a change from MDS version 2.0 to version 3.0 during the study period. Pain, functional status, and depression scales were unchanged between the two versions, whereas the cognitive scale was calibrated to account for subtle differences in data collection.
Cognition was ascertained using the validated Cognitive Performance Scale (0–6) (18). Cognitive status was then categorized as intact or mild impairment (scores 0–2), moderate (scores 3–4), or severe impairment (scores 5–6). Functional status was ascertained using the modified Katz Activities of daily living (ADL) short scale (19), and impairment was categorized as follows: intact or mild (scores 0–4), moderate (scores 5–11) and severe (scores 12–16). Reported pain was assessed via the validated Visual Analogue Scale (20). Pain was categorized as none (0), mild (1), or moderate to severe (2,3). Depression was ascertained using the validated Depression Rating Scale, which is sensitive to detecting depression among cognitively impaired persons (21). This scale queries whether seven depressive symptoms were never (0), occasionally (1), or always (2) present within the past 30 days. Scores from the seven items are summed, and we categorized depressive symptoms as none (0), mild (1,2), or moderate to severe (≥3).
Statistical Analysis
In the primary analysis, we compared the drug dose administered in the 24 hours before the fall to the drug dose administered in a 24-hour period exactly 1 week prior (Figure 1). Using conditional logistic regression, we compared the dose of antipsychotic and benzodiazepine drugs administered in the 24 hours before the fall to the dose administered 1 week prior. Because participants were compared with themselves, the estimates approximate the relative risk of a fall occurring following a psychotropic drug change compared to other times. We present results for three different types of drug changes: drug initiation, dose increase, or drug discontinuation. We defined drug initiation as administration of a low or high dose drug in the 24 hours prior to the fall but no drug administration 1 week prior. We defined dose increase as administration of high dose drug in the 24 hours prior to the fall with low dose administration 1 week prior. We defined drug discontinuation as administration of no drug in the 24 hours prior to the fall but low or high dose 1 week prior.
Figure 1.
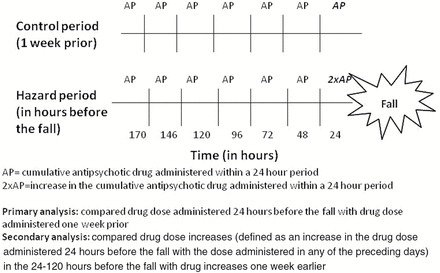
Diagram of case-crossover study design used to determine the effect of psychotropic drug changes on the risk of falls.
In a secondary analysis, we defined a drug dose increase as any increase in the drug dose administered in the 24 hours before the fall compared with the drug dose administered in any of the preceding days (ie, yes increase versus no increase). We first considered drug dose increases that occurred within 24 hours of the fall. An increase occurred if the drug dose administered in the 24 hours before the fall was greater than the dose administered in the 25–48 hours before the fall. A drug dose increase was defined identically in the control period using time exactly 1 week prior. We used conditional logistic regression models to compare the frequency of psychotropic drug dose increases in the 24 hours before the fall with the frequency of drug dose increases 1 week earlier. Because the dose of drug administered during the 24 hours preceding the hazard and control periods could differ within persons, we adjusted for the log transformed dose of drug administered in the 24 hours preceding the hazard and control period.
In order to determine when the maximal effect of a drug change on fall risk occurred, we then lengthened the hazard and control periods from 24 to 120 hours. For example, when the hazard period was 120 hours, we defined change if the drug dose administered in the 24 hours before the fall was greater than the dose administered in any of the 24 hour periods during the 25–144 hours before the fall. We used SAS version 9.4 for all analyses.
Results
Among 594 long-stay residents who experienced at least one fall, the mean age was 87.5 years (± 8.7) and 75.1% were female (Table 1). Most residents who fell were categorized as having moderate cognitive impairment (59.0%), and half had moderate functional impairment. Two-thirds of these residents were categorized as having no pain and just over half were categorized as having no depressive symptoms (54.3%).
Table 1.
Characteristics* of 594 Long-Stay Nursing Home Residents with a Fall in a Case-Crossover Study of Medication Changes and Falls
| Age (mean ± SD, y) | 87.5 (8.7) |
| Female | 75.1 (446) |
| Cognition† (n = 581) | |
| Intact/mild impairment | 29.1 (169) |
| Moderate impairment | 59.0 (343) |
| Severe impairment | 11.9 (69) |
| Functional status‡ (N=591) | |
| Mild impairment | 32.2 (190) |
| Moderate impairment | 50.6 (299) |
| Severe impairment | 17.3 (102) |
| Pain§ (N = 588) | |
| None | 66.8 (393) |
| Mild | 22.5 (132) |
| Moderate to severe | 10.7 (63) |
| Depressive symptomsǁ (N = 578) | |
| None | 54.3 (314) |
| Mild | 27.9 (161) |
| Moderate to severe | 17.8 (103) |
| Antipsychotic drug use | |
| Atypical antipsychotic use | 31.5 (187) |
| Typical antipsychotic use | 4.7 (28) |
| Benzodiazepine drug use | |
| Long acting | 7.7 (46) |
| Short acting | 20.4 (121) |
*Percent (n) unless otherwise specified.
†Cognition was ascertained using the Cognitive Performance Scale (mild impairment = scores 0–2, moderate impairment = scores 3–4, severe impairment = scores 5–6).
‡Functional status was ascertained using the modified Katz ADL short scale (intact or mild impairment = scores 0–4, moderate impairment = scores 5–11, severe impairment = scores 12–16).
§Pain was assessed via the Visual Analog Scale (none = 0, mild = 1, moderate to severe = 2–3).
ǁDepression was ascertained using the 7-item Depression Rating Scale (no symptoms = 0, mild symptoms = 1–2, moderate to severe symptoms ≥3).
Thirty-four percent of the residents who fell during the observation period were administered one or more antipsychotic drugs in the 14 days before the fall (31.5% received an atypical antipsychotic and 4.7% received a typical antipsychotic). Twenty six percent of these residents were administered one or more benzodiazepines in the 14 days before the fall (20.4% received a short acting benzodiazepine and 7.7% received a long-acting benzodiazepine). As compared with benzodiazepine users, antipsychotic users were more often men (31.2% vs 18.3%), severely impaired in their ADLs (20.2% vs 14.2%), and less likely to report any pain (33.0% vs 44.5%). Cognition and depressive severity was similar between antipsychotic and benzodiazepine users.
There was no statistically significant change in the risk of falling in the day after initiating a low dose (odds ratio [OR] 2.42, 95% confidence interval [CI] 0.58, 10.06) or high dose antipsychotic drug (OR 2.21, 95% CI 0.49, 9.90) as compared with other times (Table 2). In contrast, there was a statistically significant greater risk of falls in the day after initiating a low dose (OR 3.79, 95% CI 1.10, 13.00) or high dose benzodiazepine (OR 2.70, 95% CI 1.01, 7.20). The risk of falling was not higher in the 24 hours after increasing the dose of an antipsychotic (OR 0.91, 95% CI 0.31, 2.70) or benzodiazepine (OR 0.71, 95% CI 0.22, 2.66). It was unclear whether discontinuing low dose antipsychotics affected the acute risk of falls (OR 0.41, 95% CI 0.10, 1.72), whereas discontinuing benzodiazepines was associated with an immediately lower risk of falls (OR 0.26, 95% CI 0.08, 0.91).
Table 2.
Association Between Initiating a Low Dose or High Dose Psychotropic Drug and the Risk of Falls Among Long-Stay Nursing Home Residents Who Experienced At Least One Fall
| Dose | # Exposed in Prior 24 h | # Exposed 1 Week Earlier | # Exposed Only in the Prior 24 h | # Exposed Only 1 Week Earlier | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Antipsychotics | Low | 59 | 56 | 5 | 1 | 2.42 (0.58, 10.06) |
| High | 130 | 129 | 2 | 2 | 2.21 (0.49, 9.90) | |
| Benzodiazepines | Low | 37 | 31 | 8 | 0 | 3.79 (1.10, 13.00) |
| High | 83 | 77 | 10 | 6 | 2.70 (1.01, 7.20) |
Eight percent of residents (n = 48) received both an antipsychotic and benzodiazepine drug in the 24 hours before the fall. Four residents took no drug in the control period and both drugs in the hazard period, whereas no residents took both drugs in the control period and no drugs in the hazard period.
In our secondary analysis, there was no statistically significant change in the risk of falls in the 24 hours following an antipsychotic drug dose increase compared to 1 week prior (OR 1.36, 95% CI 0.66, 2.78) (Table 3). Results were similar when the hazard period and control periods examined the impact of drug dose increase in the 120 hours before the fall (OR 0.93, 95% CI 0.53, 1.64). For benzodiazepines, there was a higher risk of falls in the 24 hours following a drug change (OR 2.65, 95% CI 1.29, 5.44) (Table 4). This risk remained higher when the hazard period was lengthened to 120 hours (OR 2.57, 95% CI 1.41, 4.69). Results were similar when atypical antipsychotics (OR 1.84, 95% CI 0.82, 4.15) and short acting benzodiazepines (OR 2.67, 95% CI 1.26, 5.66) were considered separately.
Table 3.
Association Between New Administration or Dose Increase of an Antipsychotic Drug on the Risk of Falls in Long-Stay Nursing Home Residents Who Experienced At Least One Fall, According to Time From the Drug Change
| Exposed in Hazard Period Only | Exposed in Control Period Only | Exposed in Hazard and Control Periods | Unexposed in Hazard and Control Periods | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* | |
|---|---|---|---|---|---|---|
| Within 24 h | 18 | 16 | 4 | 556 | 1.13 (0.57, 2.21) | 1.36 (0.66, 2.78) |
| Within 48 h | 21 | 21 | 9 | 543 | 1.00 (0.55, 1.83) | 1.02 (0.55, 1.86) |
| Within 72 h | 23 | 27 | 13 | 531 | 0.85 (0.48, 1.49) | 0.92 (0.51, 1.64) |
| Within 96 h | 21 | 27 | 18 | 528 | 0.78 (0.44, 1.38) | 0.84 (0.47, 1.49) |
| Within 120 h | 25 | 27 | 20 | 522 | 0.92 (0.53, 1.61) | 0.93 (0.53, 1.64) |
*Adjusted for dose administered during the 24h preceding the hazard and control periods.
Table 4.
Association Between the New Administration or Dose Increase of a Benzodiazepine Drug on the Risk of Falls in Long-Stay Nursing Home Residents Who Experienced At Least One Fall, According to Time From the Drug Change
| Exposed in Hazard Period Only | Exposed in Control Period Only | Exposed in Hazard and Control Periods | Unexposed in Hazard and Control Periods | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* | |
|---|---|---|---|---|---|---|
| Within 24 h | 28 | 14 | 5 | 547 | 2.00 (1.05, 3.80) | 2.65 (1.29,5.44) |
| Within 48 h | 32 | 14 | 10 | 538 | 2.29 (1.22, 4.28) | 2.60 (1.32,5.18) |
| Within 72 h | 35 | 15 | 12 | 532 | 2.33 (1.27, 4.27) | 2.61 (1.38,4.94) |
| Within 96 h | 37 | 14 | 14 | 529 | 2.64 (1.43, 4.89) | 2.66 (1.44,4.92) |
| Within 120 h | 38 | 15 | 14 | 527 | 2.53 (1.39, 4.61) | 2.57 (1.41,4.69) |
*Adjusted for dose administered during the 24h preceding the hazard and control periods.
Discussion
We found no statistically significant association between initiation of an antipsychotic drug and the acute risk of falls among long-stay nursing home residents. This could be due to limited statistical power. Despite observing a large population of nursing home residents over a 3-year period, there were few falls among residents who recently initiated these medications. In contrast, residents were at a 2–4 fold higher risk of falls in the 24 hours following initiating of a benzodiazepine. Stopping a benzodiazepine was associated with an immediately lower risk of falls. Compared to other times, the risk of falls was higher in the 1–5 days following benzodiazepine drugs dose increase but not following drug dose increases of antipsychotic drugs.
A unique aspect of our study was that we focused on the short-term association between psychotropic drug changes, rather than chronic drug administration, as a risk factor for falls. We found the risk of falls was higher in the 24–120 hours following initiation of a benzodiazepine drug, but not following antipsychotic drug initiation. Huybrechts and coworkers (22) conducted a propensity score analysis to determine the risk of major adverse events following initiation of a psychotropic drug in nursing home residents. With an average follow-up of 93 days, there was a suggestion that the risk of hip fracture was greater in new users of typical antipsychotics as compared to atypical antipsychotics, but this did not reach statistical significance [incidence rate ratio (IRR) 1.49, 95% CI 0.93, 2.41]. There was no difference in the risk of hip fracture in new users of atypical antipsychotics as compared to new users of benzodiazepines (IRR 1.13, 95% CI 0.73, 1.75). The differences in our results as compared to Huybrechts may be explained by chance given our limited number of antipsychotic drug changes: only 19/556 residents received a dose change of an antipsychotic in the hazard or control periods, whereas 32/556 residents received a dose change of a benzodiazepine. Alternatively, it is possible that benzodiazepines affect falls risk immediately by impairing memory and balance, whereas antipsychotic medications need additional time to affect falls through their effects on gait and orthostasis. Different from the Huybrechts study, we focused on a very short time interval (24–120 hours) following a drug change. With more chronic administration, the effects of benzodiazepine and antipsychotic drugs on fall risk appear similar (7,23).
The mechanism whereby benzodiazepine and antipsychotic drugs impact fall risk may differ. Benzodiazepines probably contribute to fall risk through their negative effects on cognition (24) and postural control (25). Postural control is particularly impaired with higher doses of benzodiazepines in older persons (25). Studies examining the effects of antipsychotic drug use on postural control have yielded mixed results (25). Instead, it is possible that these drugs lead to falls through their negative effects on cognition, blood pressure regulation, or through their extrapyramidal motor effects including tremor, rigidity, and bradykinesia.
Benzodiazepines and antipsychotics are often used to treat similar neuropsychiatric symptoms in the nursing home setting, but they may have different consequences. Our finding of a higher fall risk following changes in benzodiazepine use but not antipsychotic administration suggests that this risk is, at least in part, due to the effects of the drug itself. Further, we found the risk of falls was slightly higher following initiation of low dose than a high dose benzodiazepine drug (OR 3.79 vs 2.70), although the confidence intervals were overlapping, making it difficult to draw firm conclusions. The suggestion that initiating a low dose benzodiazepine could be associated with a greater risk of falls as compared to initiating high dose drug could be explained if providers preferentially prescribed low dose drugs to the most frail residents at greatest risk for falls. However, if the higher risk of falls was entirely due to the underlying medical condition, one might expect a greater risk observed among residents initiating high dose drug as these residents likely have more severe acute symptoms. Treatment with antipsychotic drugs may be associated with closer staff supervision, providing less opportunity for a patient to fall, as compared to benzodiazepine drug changes. Also, providers choose between these drugs based on differences in patient characteristics such as insomnia and memory impairment; thus, the different risk observed between drug classes may relate in part to differences in the underlying neuropsychiatric or medical condition.
We found that discontinuing a low dose benzodiazepine was associated with an immediately lower risk of falls (OR 0.26, 95% CI 0.08, 0.91), whereas it was unclear whether discontinuing an antipsychotic also had an immediate effect of falls risk (OR 0.41, 95% CI 0.10, 1.72). It is possible that our noninformative findings for antipsychotic discontinuation relate to our small number of residents who were observed to discontinue these medications in the 24 hours before their fall. Larger studies should examine whether providers might expect a more immediate impact on fall risk when tapering a benzodiazepine as compared to an antipsychotic.
There are several strengths to our study. We had precise information on the dose and timing of drug administration and the timing of falls among a large population of nursing home residents. By using the case-crossover design, we were able to examine the immediate fall risks associated with dose changes, an understudied question that has implications for clinical practice. Second, the case-crossover design eliminates the concern of confounding due to unmeasured differences between users and nonusers of a drug (24).
Our study also has limitations. First, as in all observational studies, it is possible that there are transient changes in neuropsychiatric symptoms that simultaneously impact the probability of receiving an antipsychotic or benzodiazepine and also impact the risk of falls. A second limitation is that only a small proportion of the residents in the two facilities who experienced a fall had a change in the drug dose administered between the time periods under comparison. Our effect estimates, therefore, have less precision, and we were unable to separately examine the risk of falls for typical antipsychotics or individual drugs. Further, antipsychotic users in our study were more functionally impaired than users of benzodiazepines, and perhaps then, had less opportunity to fall. This may explain why we found relatively few antipsychotic dose changes preceding a fall with limited power to detect a statistical association with falls.
Finally, with our limited sample size we were unable to fully consider the relationship between concomitant drug use, drug changes, and falls. Previous work by our group suggests that initiating a non-selective serotonin reuptake inhibitor antidepressant is associated with a higher risk of falls (26) and initiating a nonbenzodiazepine hypnotic is associated with a higher risk of hip fracture in the nursing home (27). It is likely that users of multiple psychotropic agents are particularly vulnerable to the effects of a medication change.
Etiologically, falls in the nursing home can be attributed to the interaction between chronic risk factors for falls, such as impaired gait and dementia, with acute precipitating hazards, such as a wet floor or a move to an unfamiliar room. Our findings suggest that initiating a benzodiazepine drug is also an acute precipitant of falls in the nursing home setting. Providers should be very judicious when prescribing a new benzodiazepine to nursing home residents. When benzodiazepine prescription is necessary, providers should discontinue the drug as soon as possible. It may also be prudent to encourage close supervision following a new benzodiazepine prescription in an attempt to prevent falls in the nursing home. Future studies should consider whether raising staff awareness of medication changes could prevent falls in this setting.
Funding
This work was supported by a grant from the National Institute on Aging at the National Institutes of Health (K23 AG033204), funds from the Friends of Hebrew SeniorLife and the National Institutes of Health (T32 HL120505). This work was conducted with the support of a KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources), and the National Center for Advancing Translational Sciences, National Institutes of Health Award (KL2 TR001100). No funding organization had any role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Acknowledgments
This work was presented in part as an abstract at the American Geriatrics Society Annual Meeting in Orlando, FL, May 16, 2014.
References
- 1. Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–451. [DOI] [PubMed] [Google Scholar]
- 2. Thapa PB, Brockman KG, Gideon P, Fought RL, Ray WA. Injurious falls in nonambulatory nursing home residents: a comparative study of circumstances, incidence, and risk factors. J Am Geriatr Soc. 1996;44:273–278. [DOI] [PubMed] [Google Scholar]
- 3. Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hien le TT, Cumming RG, Cameron ID, et al. Atypical antipsychotic medications and risk of falls in residents of aged care facilities. J Am Geriatr Soc. 2005;53:1290–1295. [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother. 2012;10:83–94. [DOI] [PubMed] [Google Scholar]
- 6. Fraser LA, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452. [DOI] [PubMed] [Google Scholar]
- 7. Mustard CA, Mayer T. Case-control study of exposure to medication and the risk of injurious falls requiring hospitalization among nursing home residents. Am J Epidemiol. 1997;145:738–745. [DOI] [PubMed] [Google Scholar]
- 8. Jalbert JJ, Eaton CB, Miller SC, Lapane KL. Antipsychotic use and the risk of hip fracture among older adults afflicted with dementia. J Am Med Dir Assoc. 2010;11:120–127. [DOI] [PubMed] [Google Scholar]
- 9. Liperoti R, Onder G, Lapane KL, et al. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: results of a case-control study. J Clin Psychiatry. 2007;68:929–934. [DOI] [PubMed] [Google Scholar]
- 10. Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc. 2013;61:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48:682–685. [DOI] [PubMed] [Google Scholar]
- 12. Granek E, Baker SP, Abbey H, et al. Medications and diagnoses in relation to falls in a long-term care facility. J Am Geriatr Soc. 1987;35:503–511. [DOI] [PubMed] [Google Scholar]
- 13. Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–3307. [PubMed] [Google Scholar]
- 14. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevenson DG, Decker SL, Dwyer LL, et al. Antipsychotic and benzodiazepine use among nursing home residents: findings from the 2004 National Nursing Home Survey. Am J Geriatr Psychiatry. 2010;18:1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. [DOI] [PubMed] [Google Scholar]
- 17. Morris JN, Hawes C, Fries BE, et al. Designing the national resident assessment instrument for nursing homes. Gerontologist. 1990;30:293–307. [DOI] [PubMed] [Google Scholar]
- 18. Hartmaier SL, Sloane PD, Guess HA, Koch GG, Mitchell CM, Phillips CD. Validation of the Minimum Data Set Cognitive Performance Scale: agreement with the Mini-Mental State Examination. J Gerontol A Biol Sci Med Sci. 1995;50:M128–M133. [DOI] [PubMed] [Google Scholar]
- 19. Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. [DOI] [PubMed] [Google Scholar]
- 20. Fries BE, Simon SE, Morris JN, Flodstrom C, Bookstein FL. Pain in U.S. nursing homes: validating a pain scale for the minimum data set. Gerontologist. 2001;41:173–179. [DOI] [PubMed] [Google Scholar]
- 21. Koehler M, Rabinowitz T, Hirdes J, et al. Measuring depression in nursing home residents with the MDS and GDS: an observational psychometric study. BMC Geriatr. 2005;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183:E411–E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thapa PB, Gideon P, Fought RL, Ray WA. Psychotropic drugs and risk of recurrent falls in ambulatory nursing home residents. Am J Epidemiol. 1995;142:202–211. [DOI] [PubMed] [Google Scholar]
- 24. Rothberg MB, Herzig SJ, Pekow PS, Avrunin J, Lagu T, Lindenauer PK. Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61:923–930. [DOI] [PubMed] [Google Scholar]
- 25. de Groot MH, van Campen JP, Moek MA, Tulner LR, Beijnen JH, Lamoth CJ. The effects of fall-risk-increasing drugs on postural control: a literature review. Drugs Aging. 2013;30:901–920. [DOI] [PubMed] [Google Scholar]
- 26. Berry SD, Zhang Y, Lipsitz LA, Mittleman MA, Solomon DH, Kiel DP. Antidepressant prescriptions: an acute window for falls in the nursing home. J Gerontol A Biol Sci Med Sci. 2011;66:1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med. 2013;173:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]


