Abstract
Acute ozone exposure increases circulating stress hormones and induces metabolic alterations in animals. We hypothesized that the increase of adrenal-derived stress hormones is necessary for both ozone-induced metabolic effects and lung injury. Male Wistar-Kyoto rats underwent bilateral adrenal demedullation (DEMED), total bilateral adrenalectomy (ADREX), or sham surgery (SHAM). After a 4 day recovery, rats were exposed to air or ozone (1 ppm), 4 h/day for 1 or 2 days and responses assessed immediately postexposure. Circulating adrenaline levels dropped to nearly zero in DEMED and ADREX rats relative to SHAM. Corticosterone tended to be low in DEMED rats and dropped to nearly zero in ADREX rats. Adrenalectomy in air-exposed rats caused modest changes in metabolites and lung toxicity parameters. Ozone-induced hyperglycemia and glucose intolerance were markedly attenuated in DEMED rats with nearly complete reversal in ADREX rats. Ozone increased circulating epinephrine and corticosterone in SHAM but not in DEMED or ADREX rats. Free fatty acids (P = .15) and branched-chain amino acids increased after ozone exposure in SHAM but not in DEMED or ADREX rats. Lung minute volume was not affected by surgery or ozone but ozone-induced labored breathing was less pronounced in ADREX rats. Ozone-induced increases in lung protein leakage and neutrophilic inflammation were markedly reduced in DEMED and ADREX rats (ADREX > DEMED). Ozone-mediated decreases in circulating white blood cells in SHAM were not observed in DEMED and ADREX rats. We demonstrate that ozone-induced peripheral metabolic effects and lung injury/inflammation are mediated through adrenal-derived stress hormones likely via the activation of stress response pathway.
Keywords: ozone, adrenalectomy, stress response, HPA-axis, lung injury
Epidemiological studies have demonstrated a positive association between air pollution and increases in type 2 diabetes, hyperglycemia, glucose intolerance, and increased homeostasis model assessment index (Brook et al., 2008, 2013; Hu et al., 2015; Rao et al., 2015; Thiering and Heinrich, 2015). The mechanisms by which air pollutants impact metabolic processes and change clinical indicators of insulin resistance are still largely unknown. Also not known is how air pollutants can induce changes in pathways involving lipid and amino acid metabolism (Miller et al., 2015), which can contribute to insulin resistance and steatohepatitis. It has been hypothesized that systemic inflammation, endoplasmic reticulum stress, and/or autonomic activation induced by air pollutant exposure are involved in insulin resistance (Rajagopalan and Brook, 2012).
Ambient ozone has been studied for decades for its pulmonary and cardiovascular effects. Recently, the interest in ozone health effects has been rejuvenated for 2 reasons: (1) its levels are anticipated to increase as a result of climate change (Fann et al., 2015), and (2) it has been increasingly recognized that even though ozone is less likely to translocate beyond the lung, it causes a myriad of systemic effects (Miller et al., 2015). While ambient particulate matter exposures have been linked to the onset and/or exacerbation of peripheral insulin resistance (Rajagopalan and Brook, 2012), ozone has also been associated with the incidence of diabetes (Janghorbani et al., 2014; Ren et al., 2010). In addition, new studies show that ozone exposure induces a variety of neurological effects (Akhter et al., 2015; Calderón-Garcidueñas et al., 2015; Chounlamountry et al., 2015). Ozone exposure stimulates afferent vagal sensory nerves that terminate in stress-responsive hypothalamic regions in the central nervous system (Gackière et al., 2011). Acute ozone exposure has been shown alter neuronal catecholamine biosynthesis and increase circulating stress hormones (Bass et al., 2013; Miller et al., 2015; Soulage et al., 2004; Thomson et al., 2013). Our recent studies have demonstrated that rats exposed acutely to ozone develop lung injury/inflammation, hyperglycemia, glucose intolerance, and lipidemia, which are associated with increases in circulating epinephrine and cortisol (Bass et al., 2013; Miller et al., 2015). We have also shown that acute ozone produces hypothermia and bradycardia (Gordon et al., 2014). Collectively, these data suggest that ozone peripheral metabolic effects are likely mediated by central sympathetic and hypothalamus–pituitary–adrenal (HPA) stress axis activation.
The neuronal stress response, activated as a result of bodily injury or threat, initiates physiological mechanisms that provide integrated communication between all organ systems (Smith and Vale, 2006). The initial event includes the release of hypothalamic paraventricular nucleus (PVN)-derived corticotrophin releasing hormone and the activation of catecholaminergic neurons within the locus coeruleus (LC), which results in the activation of the HPA axis and sympathetic axis, respectively (Navarro-Oliveira et al., 2000; Ulrich-Lai and Herman, 2009). The priority of the neuronal stress response is to channel energy substrates to appropriate tissues to restore equilibrium, which is mainly achieved by elevated circulating stress hormones (ie, induction of gluconeogenesis, glycogenesis, muscle proteolysis, and adipose lipolysis) (Smith and Vale, 2006). Acute ozone-induced peripheral metabolic changes we observed in humans and rats are similar to homeostatic changes in response to stress (Bass et al., 2013; Miller et al., 2016). However, it is not known if these stress hormones can contribute to pulmonary injury and inflammation induced by exposure to air pollutants.
In response to HPA activation, the adrenal cortex secretes glucocorticoids (cortisol in humans and corticosterone in rats). In contrast, the adrenal medulla can be directly stimulated by the sympathetic efferent nerves, resulting in the secretion of epinephrine and/or norepinephrine (Goldstein, 2010). Adrenalectomy has been used to examine the role of these hormones in (1) neuronal regulation of the HPA-axis (Helmreich et al., 1996; Kaminski and Watts, 2012; Weidenfeld and Feldman, 2000), (2) regeneration of adrenals over time (Rebuffat et al., 2007), and (3) endogenous corticosteroid biogenesis (Bykowski et al., 2007; Freel et al., 2007). Surgical removal of adrenal medulla can allow one to examine the role of epinephrine whereas total bilateral adrenalectomy (ADREX) can be used for examining the roles of epinephrine and cortical steroidal hormones.
In this study, we test the postulate that acute ozone-induced pulmonary and systemic metabolic effects are mediated through adrenal-derived epinephrine and corticosterone. Further, that bilateral adrenal demedullation (DEMED) will selectively dampen some effects, whereas ADREX will have more profound effect on ozone-induced systemic metabolic impairment, and pulmonary injury and inflammation. We examined pulmonary and systemic metabolic effects of ozone exposure together with an assessment of adrenal-derived hormones in rats that underwent sham surgery (SHAM), DEMED, or ADREX.
MATERIALS AND METHODS
Animals
Healthy male Wistar Kyoto (WKY) rats (250–300 g) were purchased from Charles River Laboratory (Raleigh, North Carolina) at 6–8 weeks of age. Rats were housed (n = 2/cage or as indicated below) in polycarbonate cages containing beta chip bedding in an isolated room in an animal facility maintained at 21 ± 1°C, 50% ± 5% relative humidity, and held to a 12 h light/dark cycle. The animal facility is approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals received standard (5001) Purina pellet rat chow (Brentwood, Missouri) and water ad libitum unless otherwise stated. Animal procedures were approved by the US Environmental Protection Agency (US EPA), National Health and Environmental Effects Research Laboratory (NHEERL) Animal Care and Use Committee (IACUC; Permit Number: 16-03-003). Animals were treated humanely and all efforts were made for alleviation of suffering.
Animal surgery and recovery protocol
At 12–13 weeks of age, rats underwent surgical procedures. Anesthesia was achieved using an intraperitoneal injection of ketamine/xylazine (25 mg/2 mg in 1 ml saline/kg), followed by a subcutaneous injection of buprenorphine (0.02 mg/kg) for analgesia. Eye ointment was applied to rats to prevent drying during surgery. Isoflurane was also used if any signs of movement were noticed during the procedure. Following anesthesia, rats were placed in sternal recumbency for Charles River Surgeons to perform SHAM, DEMED, or ADREX using surgical protocols established at Charles River Inc. Both (right and left) glands were removed for ADREX, whereas the DEMED involved bilateral removal of medulla only. The SHAM group underwent similar procedures as DEMED, or ADREX rats except that adrenals were not removed. The muscle wall incisions were closed with absorbable suture. Skin incisions were then closed with stainless steel surgical wound clips. Immediately following surgery, animals were placed on heated pads and observed until recovery from anesthesia. Once recovered from anesthesia, animals were injected subcutaneously with Meloxicam analgesic (0.2 mg/ml/kg). Additional 2 doses of Buprenorphine were injected (0.02 mg/ml/kg) subcutaneously every 8–12 h. All drugs used in surgery were purchased from Henry Schein Animal Health Inc. (Dublin, Ohio). Following surgery, the animals were single housed using Enviro Dry enrichment/nesting material and provided powdered food. The rats that underwent ADREX were provided with saline (0.9% NaCl) as drinking water to maintain water/salt balance. All animals were allowed 96 h to recover prior to their exposure to air or ozone. We believe that 4 days was an appropriate time as the weight gain was stabilized (Table 1), there were no clinical signs of distress noted, food and water consumption were normal and the recovery appeared near complete. Furthermore, a number of studies involving adrenalectomy previously used a 4 day recovery time (Osterlund et al., 2013; Sakakibara et al., 2014). We wanted to avoid the influence of secondary changes that might occur with a longer recovery time frame in the absence of aldosterone-mineralocorticoids, which regulate salt-fluid balance, blood volume, and cardiovascular function. The timeline of the surgery, ozone exposure, in-life testing, and necropsy are shown in Figure 1.
TABLE 1.
Effect of Surgery and Ozone Exposure on Body Weights in Rats
| Time Point of Body Weights (g) |
Prior to Surgery |
Prior to Day 1 Exposure |
After Day 1 Exposure |
Prior to Day 2 Exposure |
After Day 2 Exposure |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Air | Ozone | Air | Ozone | Air | Ozone | Air | Ozone | Air | Ozone |
| SHAM | 321 ± 32 | 318 ± 18 | 322 ± 39 | 320 ± 13 | 314 ± 31 | 307 ± 15 | 324 ± 30 | 313 ± 25 | 309 ± 26 | 299 ± 15 |
| DEMED | 318 ± 16 | 321 ± 17 | 319 ± 14 | 327 ± 15 | 310 ± 12 | 315 ± 9 | 318 ± 14 | 316 ± 17 | 305 ± 13 | 305 ± 12 |
| ADREX | 321 ± 15 | 321 ± 18 | 305 ± 16 | 299 ± 16a | 292 ± 13 | 292 ± 9 | 297 ± 13 | 296 ± 14 | 291 ± 11 | 281 ± 9a |
SHAM, sham surgery; DEMED, bilateral adrenal demedullation; ADREX, total bilateral adrenalectomy.
aSignificant (P ≤ .05) ADREX effect relative to SHAM (mean ± SEM).
FIG. 1.
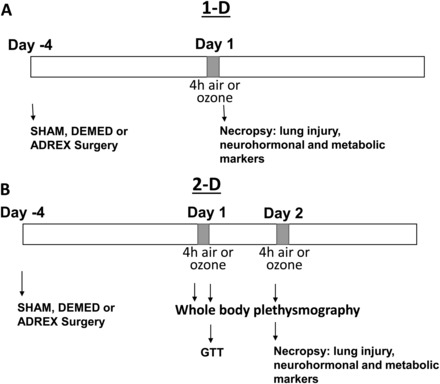
Experimental timeline. Four days prior to air or ozone exposure, WKY rats underwent SHAM, DEMED, or ADREX. After 4 days of recovery, rats were exposed to air or ozone for 4 h/day for 1 day (A, 1-D) or 4 h/day for 2 consecutive days (B, 2-D). GTT was performed in the 2-D exposure group only after the first day of exposure. Immediately following each exposure, rats were necropsied for analysis of lung injury and inflammation, neurohormones, and metabolic biomarkers. WKY, Wistar Kyoto; SHAM, sham surgery; DEMED, bilateral adrenal demedullation; ADREX, total bilateral adrenalectomy; GTT, glucose tolerance test.
Ozone generation and animal exposures
Ozone was produced from oxygen by a silent arc discharge generator (OREC, Phoenix, Arizona), and its entry into the Rochester style “Hinners” chambers was controlled by mass flow controllers (Coastal Instruments Inc., Burgaw, North Carolina). The ozone concentrations in the chambers were recorded continuously by photometric ozone analyzers (API Model 400, Teledyne Instruments; San Diego, California). Mean chamber air temperature and relative humidity were 23.3°C (74°F) and 46%, respectively. Each surgery group (SHAM, DEMED, ADREX; n = 20–24/surgery group) was randomized and subdivided into air or 1.0 ppm ozone exposure, 4 h/day for either 1 day or 2 days (n=4 for SHAM-air, n=6 for all other exposure groups). This ozone concentration is much higher than what one would expect environmentally. However, ozone concentrations as high as 0.4 ppm can be expected in tropical hot climates with high anthropogenic pollution (WHO, 1978). It has been shown that in rats once inhaled, ozone deposition in the lung is 3–4 times less than what humans will experience (Hatch et al., 1994). We have shown that humans experience metabolic effects of ozone at 0.3 ppm (Miller et al., 2016).
For the 1 day group, rats were necropsied immediately following a 1 day, 4 h exposure (1-D group), whereas for the 2 day group, rats were necropsied immediately following the second day of a 4 h ozone exposure (2-D group). Only the 2-D group underwent glucose tolerance testing (GTT) immediately following the first day of exposure. As observed in our prior study, the ozone effects are evident immediately following the first and also second day exposure. Since GTT takes approximately 3 h and a large bolus of glucose is injected in the animals, which may require several hours for blood glucose levels to return to baseline, we decided to do GTT immediately following first day exposure in rats assigned for 2-D exposure. Another group of rats, which did not undergo GTT, was necropsied immediately following first day of exposure (1-D) to correlate with changes in GTT. Since lung injury and inflammation generally takes several hours to peak after ozone exposure, and the ozone effects are maximum on the second day, we believed that 2-D exposure was necessary to determine the extent of lung damage/injury, while at the same time determining systemic responses immediately following the second day.
Glucose tolerance test
Immediately following the first day of ozone exposure, rats assigned to the 2-D exposure group underwent GTT (rats were fasted for approximately 6 h prior to testing). Baseline blood glucose concentrations were measured by pricking the distal surface of rats’ tails using a sterile needle. A Bayer Contour glucometer (Leverkusen, Germany) was used to determine blood glucose levels using test strips. After the first measurement, rats were given an intraperitoneal injection of glucose (20% D-glucose; 10 ml/kg in saline; Sigma-Aldrich, St Louis, Missouri). Measurement with the glucometer was repeated every 30 min over the course of 2 h.
Whole-body plethysmography
Pulmonary ventilatory parameters were examined prior to the first day of exposure in the morning (6:00 am), immediately following first ozone exposure (11:30 am) and in the morning prior to second day of exposure (6:00 am) in the 2-D group. A four chamber whole-body plethysmography system using Buxco BioSystem XA software (Buxco Electronics, Wilmington, North Carolina) was used to measure minute volume, tidal volume, breathing frequency, and the enhanced pause (Penh), which is presumed to provide an index of airflow limitation and often used as a surrogate for bronchoconstriction (Hamelmann et al., 1997). Each rat was placed in a plethysmograph chamber and allowed 1 minute to adapt prior to 5 min of assessing the respiratory parameters. Respiratory parameters were computed as described earlier (Kodavanti et al., 2005).
Necropsy and sample collection
The 1-D and 2-D groups were necropsied immediately following the first and second 4 h ozone exposure, respectively. Rats were weighed and anesthetized with an overdose of Nembutal (Virbac AH, Inc., Fort Worth, Texas; 50–100 mg/kg, i.p.). Blood samples were collected through an abdominal aortic puncture. Complete blood counts were performed using a Beckman-Coulter AcT blood analyzer (Beckman-Coulter Inc., Fullerton, California). All blood samples were centrifuged and aliquots of serum and plasma stored at −80°C until analysis. Bronchoalveolar lavage (BAL) was performed through tracheal tubing using 37°C phosphate buffer saline at a volume of 28 ml/kg body weight. Aliquots of BAL fluid were used to determine total cell counts with a Z1 Coulter Counter (Coulter, Inc., Miami, Florida) and cell differentials were performed on cytospin slides stained with Diff-quick (Fischer Scientific, Pittsburgh, Pennsylvania) as previously described (Bass et al., 2013). The cell-free BAL fluid (BALF) was used to analyze protein, albumin, and lactate dehydrogenase (LDH) activity as previously described (Bass et al., 2013).
Plasma and serum analysis
Adrenaline and noradrenaline plasma levels were measured using kits from Rocky Mountain Diagnostics (Colorado Springs, Colorado) per the manufacturer’s protocol. Serum corticosterone concentrations were analyzed employing an immunoassay kit (Arbor Assay, Ann Arbor, Michigan) per the manufacturer’s protocol. Total cholesterol and triglycerides were measured in serum samples using kits from TECO Diagnostics (Anaheim, California). Nonesterified free fatty acids (FFA) were measured in serum by a coupled enzymatic reaction and the resultant hydrogen peroxide detection using a colorimetric probe as per the manufacturer’s protocol (Cell Biolabs, Inc, San Diego, California). The kit protocols for measuring lipids were modified for use on the Konelab Arena 30 system (Thermo LabSystems, Espoo, Finland). Branched chain amino acids (BCAA) were measured in serum using ELISA kits and the protocol based on chemiluminescence detection (Abcam, Cambridge, Massachusetts).
Statistics
GTT data were analyzed using a 2-way repeated measure multivariate analysis of variance where serial blood glucose measurements were incorporated in the analysis (Graphpad prism 4.03 software). Area under the curve (AUC) for GTT was determined by the trapezoidal method and analyzed for statistical significance by ANOVA followed by a Duncan’s multiple range test. Other variables were analyzed using a 2-way ANOVA model (independent variables being exposure and surgical manipulation). The 2 assumptions associated with ANOVA models are equality of variances and normality of errors (residuals). We used Levene’s test to assess the equality of variances and Shapiro Wilk’s test to examine the normality of errors. The ANOVA assumptions for serum adrenaline, serum corticosterone, serum cholesterol, serum FFA, BALF protein, BALF albumin, BALF LDH, BALF neutrophils, BALF eosinophils, and PenH did not satisfy desired equal variance and/or normality. These data were then transformed to satisfy 2 assumptions (log or square root as appropriate). Pairwise comparisons were performed as subsets of the overall analysis of variance (ANOVA). The level of significance was set at 0.05. No adjustments were made for multiple comparisons.
RESULTS
Body Weights
Body weights were closely examined after surgery, throughout recovery, and during ozone exposure. The SHAM and DEMED groups did not lose body weight as a result of surgery as determined 4 days after surgery or during the course of air exposure over 2 days (Table 1). In general, ADREX group showed a slight but significant decrease in body weight gain after surgery compared with other surgery groups. Ozone exposure on the second day tended to reduce body weight in all rats; however, this effect was not significant relative to time-matched air groups.
ADREX, DEMED, and Ozone Impacted Circulating Adrenal-Derived Hormones
To determine ozone-induced changes in adrenal-derived hormones and the effectiveness of surgical intervention, we evaluated levels of circulating adrenaline, noradrenaline, and corticosterone. Concentrations of adrenaline, derived from the medulla, were markedly reduced in all DEMED and ADREX rats, regardless of exposure (Figure 2A). Ozone significantly increased adrenaline plasma levels in SHAM rats on day 1 and day 2 compared with air exposure (Figure 2A). Noradrenaline plasma levels were not altered by exposure or surgical interventions (Figure 2B). DEMED caused a slight reduction in the levels of circulating corticosterone, which was significant only in 2-D air-exposed rats. Ozone exposure in SHAM rats tended to increase levels of serum corticosterone on day 1, but the difference was statistically insignificant when compared with time-matched air group (Figure 2C). Corticosterone levels dropped to nearly zero in all ADREX rats. Ozone did not affect corticosterone levels in the DEMED and ADREX groups (Figure 3C).
FIG. 2.
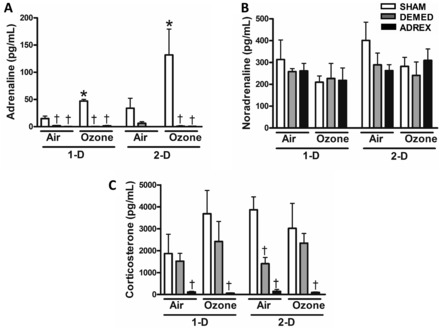
Changes in the levels of circulating hormones after air or ozone exposure in SHAM, DEMED, or ADREX rats. Adrenal cortex and medulla-derived hormones were measured in the serum and plasma, respectively, from rats exposed to air or ozone at both time points (1-D, 2-D), A, Adrenaline (Epinephrine), B, Noradrenaline (Norepinephrine), and C, Corticosterone. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group at a given time point (P ≤ .05). †Indicates surgery effect within matching exposure group at a given time point (P ≤ .05).
FIG. 3.
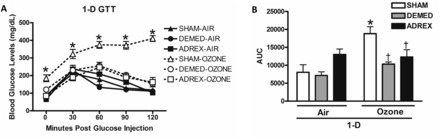
Changes in glucose tolerance after air or ozone exposure in SHAM, DEMED, or ADREX rats. GTT was performed only after the first day of exposure in the 2-D group (A). The 0 min time point shows fasting glucose levels in each group postexposure. Panel B shows the graph for the AUC for the GTT data. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group (P ≤ .05). AUC, area under the curve.
Acute Ozone-Induced Glucose Intolerance Is Inhibited by ADREX
GTT was conducted in SHAM, DEMED, and ADREX groups immediately following the first day of ozone exposure in the 2-D group to determine the relative contribution of adrenal-derived hormones to acute ozone-induced glucose metabolic effects. Glucose tolerance was not affected by ADREX or DEMED in air-exposed rats (Figure 3). The SHAM group exposed to ozone demonstrated significant fasting hyperglycemia and glucose intolerance compared with its matching air group (Figure 3A) as observed in our previous study (Miller et al., 2015). The DEMED rats showed marked suppression of ozone–induced hyperglycemia and glucose intolerance throughout the testing period (Figure 3A). There was nearly a complete reversal of ozone-induced fasting hyperglycemia and glucose intolerance in ADREX rats (Figure 3A). AUC calculations further revealed that ozone-induced glucose metabolic effects were almost completely eliminated in ADREX rats (Figure 3B).
Acute Ozone-Induced Increases in Circulating Lipids and Protein Metabolites Are Reduced in Rats With Prior ADREX
The activation of the stress response pathway and subsequent release of adrenal stress hormones are central to changes in peripheral metabolism. Our prior study demonstrated that exposure to ozone resulted in increased circulating FFA and BCAA in rats (Miller et al., 2015). Although prior metabolomic assessment showed a small increase in serum cholesterol after a 6 h ozone exposure in our previous study (Miller et al., 2015), in this study 4 h ozone exposure for 1-D or 2-D did not affect serum cholesterol levels in any group regardless of prior surgical procedure (Figure 4A). ADREX rats exposed to air had increased triglycerides at 1-D compared with SHAM rats (Figure 4B). Serum triglycerides were significantly increased after day 1 of ozone exposure in SHAM rats (Figure 4B). Ozone-induced triglyceride increase was smaller in DEMED rats whereas ADREX was associated with nearly a complete negation of ozone effect in the 1-D group (Figure 4B). Circulating FFA were not affected by DEMED or ADREX as evident in air-exposed rats. FFA tended to increase in SHAM rats (P = .15) when exposed to ozone on 1-D; however, this increase was not observed in DEMED or ADREX rats (Figure 4C).
FIG. 4.
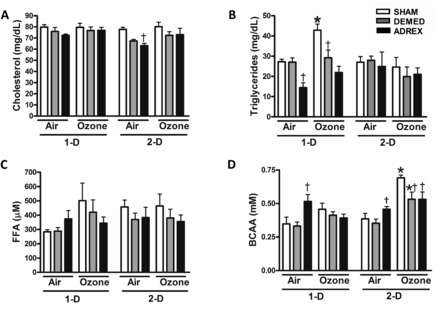
Changes in circulating metabolites after air or ozone exposure in SHAM, DEMED, or ADREX rats. Serum metabolites were measured after air or ozone exposure in rats at each time point (1-D, 2-D): A, Total cholesterol, B, triglycerides, C, FFA, and D, BCAA. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group at a given time point (P ≤ .05). †Indicates surgery effect within matching exposure group at a given time point (P ≤ .05). P = .15 for FFA air and ozone, 1-D and P = .13 for BCAA air and ozone 1-D in the SHAM rats. FFA, free fatty acids; BCAA, branched chain amino acids.
Air-exposed ADREX group showed significant increases in circulating BCAA on 1-D relative to SHAM rats. On day 1, BCAA also tended to increase (P = .13) in the SHAM ozone-exposed group but the difference remained insignificant. On day 2, however, BCAA levels significantly increased in the SHAM rats exposed to ozone. Importantly, this ozone-induced increase in BCAA did not occur in the ADREX group (Figure 4D).
Ozone-Induced Ventilatory Changes Are Reduced in Adrenalectomized Rats
Whole-body plethysmography was used to assess breathing parameters. DEMED or ADREX did not significantly affect breathing frequency, tidal volume (data not shown), minute volume, or PenH as determined prior to exposure in all rats or in the air groups postexposure (Figure 5). Ozone exposure did not change body weight-normalized minute volume in any group (Figure 5A). Immediately following 1-D of ozone exposure, PenH was markedly increased in SHAM and DEMED rats. This ozone-induced increase in PenH was significantly lower in ADREX rats compared with SHAM rats (Figure 5B). The morning after the first ozone exposure, the ozone-induced increases in PenH were still apparent in SHAM and DEMED rats but the levels were much lower compared with the measurement taken immediately after the first ozone exposure. In the morning after the first ozone exposure, the ADREX ozone-exposed group did not present significant increases in PenH compared with surgery-matched air control.
FIG. 5.
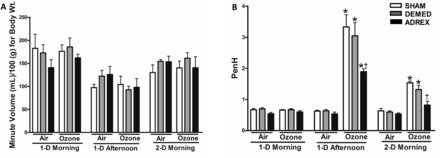
Ozone-induced changes in lung minute volume and PenH, an index of labored breathing, in SHAM, DEMED, and ADREX rats. The plethysmography was performed the morning prior to first ozone exposure (1-D Morning), immediately following first ozone exposure (1-D Afternoon), and prior to the start of second exposure (2-D Morning). A, Minute volume was calculated based on breathing frequency and tidal volume and the values were normalized to body weight. B, PenH values were computed from all ventilatory parameters measured during plethysmography. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group at a given time point P ≤ .05. †Indicates surgery effect within matching exposure group at a given time point P ≤ .5.
Ozone-Induced Lung Injury and Inflammation Are Diminished by DEMED and ADREX
Ozone exposure for 1-D increased markers of vascular protein leakage including BALF total protein and albumin in SHAM and DEMED groups compared with surgery-matched air controls (Figures 6A and B). Ozone-induced protein and albumin increases in the SHAM rats were more pronounced on 2-D when compared with 1-D. The ADREX group did not display any ozone-induced increases in protein or albumin following 1-D ozone exposure. Likewise, ozone exposure did not increase BALF protein or albumin in DEMED and ADREX rats on 2-D (Figures 6A and B). Ozone exposure led to increases in BALF LDH activity (a marker of lung cell injury) on day 1 in SHAM rats (Figure 6C). Ozone-exposed DEMED and ADREX rats did not show any increase in BALF LDH activity.
FIG. 6.
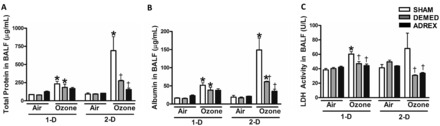
Lung injury in rats as determined by analysis of BALF after ozone exposure in SHAM, DEMED, or ADREX rats. Rats were necropsied immediately after each exposure time point (1-D, 2-D). BALF was analyzed for lung injury markers: A, Total protein, B, albumin, and C, LDH activity. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group at a given time point. †Indicates surgery effect within matching exposure group at a given time point P ≤ .05. BALF, bronchoalveolar lavage fluid; LDH, lactate dehydrogenase.
Lung inflammatory responses to ozone were assessed by determining BALF cell differentials. The number of macrophages in BALF did not change significantly as a result of DEMED or ADREX in air exposed rats. Ozone exposure also did not change the number of BALF macrophages (data not shown). The baseline levels of neutrophils were slightly higher in ADREX rats exposed to air when compared with other surgery groups (Figure 7A). Significant increases in BALF neutrophils occurred on 1-D and 2-D in SHAM rats after ozone exposure. This ozone-induced increase in neutrophils was much more pronounced on day 2 when compared with day 1 in SHAM rats (Figure 7A). The increase in neutrophils following ozone exposure was significantly reduced in DEMED (SHAM > DEMED) rats. Surprisingly, the effect of ozone on neutrophils was nearly abolished in ADREX rats, especially on 2-D. On day 1, only the ADREX rats exposed to ozone showed increases in BALF eosinophils compared with air controls (Figure 7B). On day 2, eosinophil levels in ADREX rats exposed to ozone remained higher than the air group.
FIG. 7.
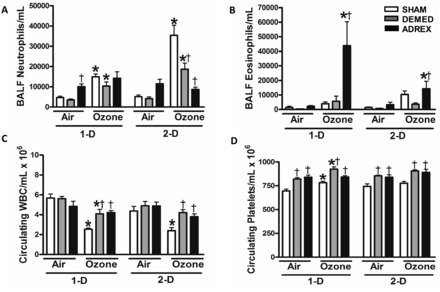
Lung inflammation as determined by analysis of cells in BALF after air or ozone exposure in SHAM, DEMED, or ADREX rats. BALF cell differentials were performed to quantify macrophages, neutrophils, and eosinophils at both time points after air or ozone exposure (1-D, 2-D). A, Neutrophils and B, Eosinophils. Data for macrophages are not shown. Circulating white blood cells (WBC) and platelets were also quantified. C, WBC and D, Platelets. Values indicate mean ± SEM (n = 4–6/group). *Indicates ozone effect when compared with matching air group at a given time point. †Indicates surgery effect within matching exposure group at a given time point P ≤ .05.
To determine systemic inflammatory response, hematological parameters were assessed. The circulating white blood cells (WBC) were significantly decreased after ozone exposure on 1-D and 2-D in SHAM rats (Figure 7C). Ozone exposure led to a small decrease in WBC on day 1 also in DEMED rats, but did not decrease WBC in ADREX rats at any time point. ADREX DEMED and ADREX caused an increase in circulating platelets (Figure 7D). Ozone exposure led to increases in circulating platelets in SHAM and DEMED rats but not in ADREX rats on 1-D.
DISCUSSION
We have recently shown that acute ozone-induced peripheral metabolic alterations are associated with elevated adrenal-derived stress hormones in humans and animals (Bass et al., 2013; Miller et al., 2016). The goal of this study was to evaluate if the adrenal-derived stress hormones are essential for mediating acute ozone-induced metabolic and pulmonary effects in rats. We used surgical DEMED to remove the medulla, which produces catecholamines in response to sympathetic stimulation, and ADREX where the medulla as well as the cortex are removed in rats. The cortex produces corticosteroids in response to pituitary-derived adrenocorticotropic hormone (ACTH) via the HPA-axis. When exposed to ozone, SHAM rats produced typical systemic metabolic effects and lung injury similar to that observed in our previous study (Miller et al., 2015). These effects were characterized by hyperglycemia, glucose intolerance, increased levels of epinephrine, corticosterone, circulating FFA, BCAA, and decreased circulating WBC, together with increased pulmonary ventilatory changes, vascular leakage, and neutrophilic inflammation. All these ozone effects were partially or fully blocked in rats with DEMED or ADREX. Thus, we demonstrate that not only ozone-induced metabolic impairment, but also pulmonary injury and inflammation are largely modulated through adrenal-derived stress hormones likely through sympathetic stimulation and activation of the HPA-axis.
Ozone produces nociceptive stimuli and activates pulmonary vagal C-fibers that trigger various cardiopulmonary reflexes (Jimba et al., 1995; Schelegle et al., 1993). Ozone activates the nucleus tractus solitarius (NTS) and also stimulates various hypothalamic stress-responsive regions-including the PVN and LC (Gackière et al., 2011). Consequently, activation of these limbic regions could lead to changes in heart rate, peripheral vasoconstriction, pulmonary arterial hypertension, and increased capillary permeability (Kubin et al., 2006; Nicolaides et al., 2015). However, these neurohormonal pathways have not been linked to ozone-induced pulmonary injury and systemic metabolic effects.
Diminution of circulating epinephrine and corticosterone confirmed successful removal of adrenals. As expected, ozone-induced increases in epinephrine and corticosterone in SHAM rats were not apparent in either DEMED or ADREX rats. This confirms our earlier findings that acute ozone increases the release of stress hormones likely through activation of sympathetic adrenomedullary and the HPA axis (Bass et al., 2013; Miller et al., 2015). Since approximately 80% of circulating noradrenaline is released from peripheral sympathetic nerve terminals and only 20% produced by the adrenal medulla (Esler et al., 1990; Patel et al., 2000), DEMED or ADREX did not affect those levels. The lack of ozone effect on noradrenaline raises the question as to the role of efferent sympathetic stimulation in mediating systemic metabolic and pulmonary effects. Because it is technically not feasible to remove the cortex and leave the medulla intact, the question about the contribution of cortex-derived hormones in response to the release of medullary epinephrine remains.
During the stress response, epinephrine initiates a transient increase in circulating glucose by the stimulation of hepatic glycogenolysis and gluconeogenesis, and inhibition of glucose disposal by insulin-dependent tissues (Sherwin and Sacca, 1984; Sherwin et al., 1980). Similarly, cortisol suppresses insulin-mediated glucose uptake through the glucocorticoid receptors (Steiner et al., 2014). The ozone-mediated increase in circulating epinephrine and cortisol in SHAM rats, together with the diminution of ozone-induced hyperglycemia and glucose intolerance in ADREX and DEMED rats, demonstrates that the adrenal-derived stress hormones are essential for the observed impairment in glucose metabolism. This corroborates with previous findings where adrenal demedullation suppressed hypoxia-induced hyperglycemia and insulin secretion (Shin et al., 2014). The stress hormones are known to prompt adipose tissue lipolysis, leading to the release of FFAs in the circulation, which are known to interfere with insulin signaling and contribute to insulin resistance (Shulman et al., 2000). We show that ozone-induced increases in FFA and triglycerides were diminished in ADREX and DEMED rats, further confirming the role of stress hormones in the observed lipidemia. Activation of glucocorticoid receptors in response to increases in stress hormones results in muscle protein catabolism and release of BCAA in the circulation (Braun and Marks, 2015). An increase in serum BCAA levels were apparent in this study in SHAM rats exposed to ozone, similar to our previous study (Miller et al., 2015), but not in ADREX and DEMED rats. These data provide further confirmation that ozone induces stress hormone-mediated muscle protein catabolism. Accumulation of BCAA has been implicated in initiating pancreatic β-cell dysfunction, inflammation, endoplasmic reticular stress, and apoptosis (Lynch and Adams, 2014). Since metabolic effects of ozone were found to be reversible upon termination of exposure (Miller et al., 2015), the long-term consequences remain to be investigated.
The contribution of pulmonary neural refluxes mediated by different areas within the NTS in ozone-induced ventilatory changes has been described (Chen et al., 2003; Schelegle and Walby, 2012; Schelegle et al., 1993; Taylor-Clark and Undem, 2010). It has been shown that ozone exposure induces reflux bronchoconstriction (Schelegle and Walby, 2012). In our study, PenH, which is often linked to airflow limitation, was increased in SHAM rats exposed to ozone. This might be due to changes in airway smooth muscle contractility. β Receptors, which are heavily distributed in large and small airways, have been shown to play a major role in regulating smooth muscle tone by epinephrine and corticosterone (Bossé, 2014). DEMED had no effect on PenH responses after ozone, whereas the reduction in ozone-induced increases in PenH by ADREX suggests that corticosterone, epinephrine and other circulating factors might be directly or indirectly playing a role in mediating the ozone-induced ventilatory changes.
There are likely a number of mechanisms influencing the development of pulmonary edema after ozone exposure in SHAM rats. First and foremost, ozone exposure can directly injure capillary walls, leading to leakage of protein in the alveoli (Banks et al., 1990; Vella et al., 2015). Pulmonary edema can also occur through acute sympathetic activation (neurogenic), which may lead to baroreflex-induced bradycardia. The resulting enhancement of venous return can cause pulmonary vascular congestion and increase hydrostatic pressure, which can damage the capillary wall causing fluid leakage (Šedý et al., 2015). Increased circulating epinephrine can also cause systemic capillary pressure increase, which may contribute to pulmonary edema after ozone exposure (Krishnamoorthy et al., 2012). Increases in circulating epinephrine and corticosterone, and ozone-induced cardiac depression together might affect the protein leakage. We have previously shown that ozone induces bradycardia and hypothermia (Gordon et al., 2014). Ozone has also been shown to decrease blood pressure (Uchiyama et al., 1986) and produce cardiac depression (Wagner et al., 2014), which may contribute to lung protein leakage. The finding that pulmonary protein leakage and lung cell injury are nearly abolished in ADREX and DEMED rats suggests that release of stress hormones, likely through sympathetic and HPA-activation, were critical in ozone-induced protein leakage.
Ozone-induced extravasation of inflammatory cells into the lung through activation of signaling mechanisms involving transendothelial migration has also been well characterized (Alexis and Carlsten, 2014; Hollingsworth et al., 2007). Consistent to these studies, ozone exposure in SHAM rats caused neutrophilic lung inflammation and depletion of circulating WBC, likely due to their extravasation into the lungs. It is generally believed that corticosteroids inhibit inflammation (Edwards, 2012); however, numerous studies have shown that acute stress-mediated increases in corticosterone and epinephrine can stimulate leukocyte trafficking and extravasation at the site of injury in an immune cell-specific manner (Dhabhar, 2009; Dhabhar et al., 2012). The precise mechanisms by which distinct types of chronic stresses modulate inflammatory processes still remain an area of intense research. The selective diminution of ozone-induced neutrophil influx but stimulation of eosinophil influx, together with the lack of an ozone-induced decrease in circulating lymphocytes in ADREX and DEMED rats suggests contribution of the dynamic and temporal changes in corticosteroids and epinephrine (Dhabhar et al., 2012). Interestingly, ozone-mediated thymus atrophy was reduced in adrenalectomized mice (Dziedzic and White, 1986), suggesting that adrenal hormones might modulate ozone-induced inflammatory response through changes in thymus function. These data together provide the insights into the contribution of central neurohormonal mechanisms in modulating pulmonary inflammatory responses induced after ozone exposure.
In this study, we show that acute ozone exposure induced a classical stress-associated systemic metabolic response and pulmonary injury/inflammation in SHAM rats, similar to what was seen in our previous studies (Bass et al., 2013; Miller et al., 2015). This response is characterized by increased levels of epinephrine and corticosterone, hyperglycemia, glucose intolerance, increased circulating FFA, increased BCAA, and decreased circulating WBC as has been observed in our previous study (Miller et al., 2015). Surgical removal of the adrenal medulla or whole adrenal glands resulted in nearly complete inhibition of these ozone-induced metabolic and pulmonary effects. Thus, we show adrenal-derived hormones mediate systemic metabolic impairment and acute pulmonary injury/inflammation likely through sympathetic and HPA-activation.
FUNDING
This work was supported by the United States Environmental Protection Agency funds, and the Co-Operative Agreement between United States Environmental Protection Agency and University of North Carolina Toxicology Training Agreement CR835152010 to D.B.M.
ACKNOWLEDGMENTS
We thank Mr. Donald Doerfler of the US EPA for statistical analysis of the data and Drs. Daniel Costa, Christopher Gordon, and Colette Miller of the US EPA for their critical review of this manuscript. We also like to thank Drs. Leslie Jarrell (SoBran, Inc., Fairfax,Virginia), Jaimie Graff (US EPA), Karen Brock (US EPA), and NHEERL animal care staff for their help in coordinating and assisting with surgeries at the US EPA, NHEERL Animal Facility and Ms. Virginia Bass (UNC, Chapel Hill,North Carolina) for her help in the experiment. We acknowledge the Charles River Inc veterinary surgeons for performing Total bilateral adrenalectomy and bilateral adrenal demedullations. Mr. Dock Terrell is acknowledged for his help in performing ozone exposures.
REFERENCES
- Akhter H., Ballinger C., Liu N., van Groen T., Postlethwait E. M., Liu R. M. (2015). Cyclic ozone exposure induces gender-dependent neuropathology and memory decline in an animal model of Alzheimer's disease. Toxicol. Sci. 147, 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis N. E., Carlsten C. (2014). Interplay of air pollution and asthma immunopathogenesis: A focused review of diesel exhaust and ozone. Int. Immunopharmacol. 23, 347–355. [DOI] [PubMed] [Google Scholar]
- Banks M. A., Porter D. W., Martin W. G., Castranova V. (1990). Effects of in vitro ozone exposure on peroxidative damage, membrane leakage, and taurine content of rat alveolar macrophages. Toxicol. Appl. Pharmacol. 105, 55–65. [DOI] [PubMed] [Google Scholar]
- Bass V., Gordon C. J., Jarema K. A., MacPhail R. C., Cascio W. E., Phillips P. M., Ledbetter A. D., Schladweiler M. C., Andrews D., Miller D., et al. (2013). Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown Norway rats. Toxicol. Appl. Pharmacol. 273, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé Y. (2014). Endocrine regulation of airway contractility is overlooked. J. Endocrinol. 222, R61–R73. [DOI] [PubMed] [Google Scholar]
- Braun T. P., Marks D. L. (2015). The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D., Cakmak S., Turner M. C., Brook J. R., Crouse D. L., Peters P. A., van Donkelaar A., Villeneuve P. J., Brion O., Jerrett M., et al. (2013). Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 36, 3313–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D., Jerrett M. P., Brook J. R. P., Bard R. L. M. A., Finkelstein M. M. (2008). The relationship between diabetes mellitus and traffic-related air pollution. J. Occup. Environ. Med. 50, 32–38. [DOI] [PubMed] [Google Scholar]
- Bykowski M. R., Smith J. C., Stricker E. M. (2007). Regulation of NaCl solution intake and gastric emptying in adrenalectomized rats. Physiol. Behav. 92, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Kulesza R. J., Doty R. L., D'Angiulli A., Torres-Jardón R. (2015). Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ. Res. 137, 157–169. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Bonham A. C., Plopper C. G., Joad J. P. (2003). Neuroplasticity in nucleus tractus solitarius neurons after episodic ozone exposure in infant primates. J. Appl. Physiol. (1985). 94, 819–827. [DOI] [PubMed] [Google Scholar]
- Chounlamountry K., Boyer B., Penalba V., François-Bellan A. M., Bosler O., Kessler J. P., Strube C. (2015). Remodeling of glial coverage of glutamatergic synapses in the rat nucleus tractus solitarii after ozone inhalation. J. Neurochem. 134, 857–864. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S. (2009). A hassle a day may keep the pathogens away: The fight-or-flight stress response and the augmentation of immune function. Integr. Comp. Biol. 49, 215–236. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S., Malarkey W. B., Neri E., McEwen B. S. (2012). Stress-induced redistribution of immune cells—from barracks to boulevards to battlefields: A tale of three hormones—Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic D., White H. J. (1986). Thymus and pulmonary lymph node response to acute and subchronic ozone inhalation in the mouse. Environ. Res. 41, 598–609. [DOI] [PubMed] [Google Scholar]
- Edwards C. (2012). Sixty years after Hench—Corticosteroids and chronic inflammatory disease. J. Clin. Endocrinol. Metab. 97, 1443–1451. [DOI] [PubMed] [Google Scholar]
- Esler M., Jennings G., Lambert G., Meredith I., Horne M., Eisenhofer G. (1990). Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol. Rev. 70, 963–985. [DOI] [PubMed] [Google Scholar]
- Fann N., Nolte C. G., Dolwick P., Spero T. L., Brown A. C., Phillips S., Anenberg S. (2015). The geographic distribution and economic value of climate change-related ozone health impacts in the United States in 2030. J. Air Waste Manag. Assoc. 65, 570–580. [DOI] [PubMed] [Google Scholar]
- Freel E. M., Bernhardt R., Ingram M., Wallace A. M., Fraser R., Davies E., Connell J. M. (2007). Endogenous corticosteroid biosynthesis in subjects after bilateral adrenalectomy. Clin. Endocrinol. 66, 659–665. [DOI] [PubMed] [Google Scholar]
- Gackière F., Saliba L., Baude A., Bosler O., Strube C. (2011). Ozone inhalation activates stress-responsive regions of the CNS. J. Neurochem. 117, 961–972. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S. (2010). Adrenal responses to stress. Cell Mol. Neurobiol. 30, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Johnstone A. F., Aydin C., Phillips P. M., MacPhail R. C., Kodavanti U. P., Ledbetter A. D., Jarema K. A. (2014). Episodic ozone exposure in adult and senescent Brown Norway rats: Acute and delayed effect on heart rate, core temperature and motor activity. Inhal. Toxicol. 26, 380–390. [DOI] [PubMed] [Google Scholar]
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G. L., Irvin C. G., Gelfand E. W. (1997). Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156, 766–775. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., Harris L. P., Mcdonnell W. F., Devlin R. B., Koren H. S., Costa D. L., Mckee J. (1994). Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 150, 676–683. [DOI] [PubMed] [Google Scholar]
- Helmreich D. L., Cullinan W. E., Watson S. J. (1996). The effect of adrenalectomy on stress-induced c-fos mRNA expression in the rat brain. Brain Res. 706, 137–144. [DOI] [PubMed] [Google Scholar]
- Hollingsworth J. W., Kleeberger S. R., Foster W. M. (2007). Ozone and pulmonary innate immunity. Proc. Am. Thorac. Soc. 4, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Ha S., Henderson B. H., Warner T. D., Roth J., Kan H., Xu X. (2015). Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ. Health Perspect. 123, 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorbani M., Momeni F., Mansourian M. (2014). Systematic review and meta analysis of air pollution exposure and risk of diabetes. Eur. J. Epidemiol. 29, 231–242. [DOI] [PubMed] [Google Scholar]
- Jimba M., Skornik W. A., Killingsworth C. R., Long N. C., Brain J. D., Shore S. A. (1995). Role of C fibers in physiological responses to ozone in rats. J. Appl. Physiol. (1985) 78, 1757–1763. [DOI] [PubMed] [Google Scholar]
- Kaminski K. L., Watts A. G. (2012). Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J. Neuroendocrinol. 24, 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti U. P., Schladweiler M. C., Ledbetter A. D., McGee J. K., Walsh L., Gilmour P. S., Highfill J. W., Davies D., Pinkerton K. E., Richards J. H., et al. (2005). Consistent pulmonary and systemic responses from inhalation of fine concentrated ambient particles: Roles of rat strains used and physicochemical properties. Environ. Health Perspect. 113, 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy V., Hiller D. B., Ripper R., Lin B., Vogel S.M., Feinstein D. L., Oswald S., Rothschild L., Hensel P., Rubinstein I., et al. (2012). Epinephrine induces rapid deterioration in pulmonary oxygen exchange in intact, anesthetized rats: A flow and pulmonary capillary pressure-dependent phenomenon. Anesthesiology 117, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L., Alheid G. F., Zuperku E. J., McCrimmon D. R. (2006). Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. (1985) 101, 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., Adams S. H. (2014). Branched-chain amino acids in metabolic signaling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Ghio A. J., Karoly E. D., Bell L. N., Snow S. J., Madden M. C., Soukup J., Cascio W. E., Gilmour M. I., Kodavanti U. P. (2016). Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am. J. Respir. Crit. Care Med. (Forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Karoly E. D., Jones J. C., Ward W. O., Vallanat B. D., Andrews D. L., Schladweiler M. C., Snow S. J., Bass V. L., Richards J. E., et al. (2015). Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol. Appl. Pharmacol. 286, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Oliveira C. M., Vassilieff V. S., Cordellini S. (2000). The sympathetic adrenomedullary system, but not the hypothalamic-pituitary-adrenal axis, participates in aorta adaptive response to stress: Nitric oxide involvement. Auton. Neurosci. 83, 140–147. [DOI] [PubMed] [Google Scholar]
- Osterlund C. D., Thompson V., Hinds L., Spencer R. l. (2013). Absence of glucocorticoids augments stress-induced Mkp1 mRNA expression within the hypothalamic-pituitary-adrenal axis. J. Endocrinol 220, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. P., Zhang K., Carmines P. K. (2000). Norepinephrine turnover in peripheral tissues of rats with heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R556–R562. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Brook R. D. (2012). Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61, 3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X., Patel P., Puett R., Rajagopalan S. (2015). Air pollution as a risk factor for type 2 diabetes. Toxicol. Sci. 143, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffat P., Macchi C., Malendowicz L. K., Nussdorfer G. G. (2007). Up-regulation of adrenomedullin gene expression in the regenerating rat adrenal cortex. Int. J. Mol. Med. 20, 551–555. [PubMed] [Google Scholar]
- Ren C., Melly S., Schwartz J. (2010). Modifiers of short-term effects of ozone on mortality in eastern Massachusetts—a case-crossover analysis at individual level. Environ. Health 9, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Katoh M., Suzuki M., Kawabe R., Iwase K., Nadai M. (2014). Effect of adrenalectomy on expression and induction of UDP-glucuronosyltransferase 1A6 and 1A7 in rats. Biol. Pharm. Bull. 37, 618–624. [DOI] [PubMed] [Google Scholar]
- Schelegle E. S., Walby W. F. (2012). Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge. Respir. Physiol. Neurobiol. 181, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle E. S., Carl M. L., Coleridge H. M., Coleridge J. C., Green J. F. (1993). Contribution of vagal afferents to respiratory reflexes evoked by acute inhalation of ozone in dogs. J. Appl. Physiol. (1985) 74, 2338–2344. [DOI] [PubMed] [Google Scholar]
- Šedý J., Kuneš J., Zicha J. (2015). Pathogenetic mechanisms of neurogenic pulmonary Edema. J. Neurotrauma. 32, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Saccà L. (1984). Effect of epinephrine on glucose metabolism in humans: Contribution of the liver. Am. J. Physiol. 247, E157–E165. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Shamoon H., Hendler R., Saccà L., Eigler N., Walesky M. (1980). Epinephrine and the regulation of glucose metabolism: Effect of diabetes and hormonal interactions. Metabolism. 29(Suppl. 1), 1146–1154. [DOI] [PubMed] [Google Scholar]
- Shin M. K., Han W., Bevans-Fonti S., Jun J. C., Punjabi N. M., Polotsky V. Y. (2014). The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 203, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I. (2000). Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Vale W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 8, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulage C., Perrin D., Cottet-Emard J. M., Pequignot J., Dalmaz Y., Pequignot J. M. (2004). Central and peripheral changes in catecholamine biosynthesis and turnover in rats after a short period of ozone exposure. Neurochem. Int. 45, 979–986. [DOI] [PubMed] [Google Scholar]
- Steiner J. L., Bardgett M. E., Wolfgang L., Lang C. H., Stocker S. D. (2014). Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J. Neurophysiol. 112, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T. E., Undem B. J. (2010). Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 588, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E., Heinrich J. (2015). Epidemiology of air pollution and diabetes. Trends Endocrinol. Metab. 26, 384–394. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Vladisavljevic D., Mohottalage S., Kumarathasan P., Vincent R. (2013). Mapping acute systemic effects of inhaled particulate matter and ozone: Multiorgan gene expression and glucocorticoid activity. Toxicol. Sci. 135, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama I., Simomura Y., Yokoyama E. (1986). Effects of acute exposure to ozone on heart rate and blood pressure of the conscious rat. Environ. Res. 41, 529–537. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y. M., Herman J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella R. E., Pillon N. J., Zarrouki B., Croze M. L., Koppe L., Guichardant M., Pesenti S., Chauvin M. A., Rieusset J., Géloën A., et al. (2015). Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes 64, 1011–124. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Allen K., Yang H. Y., Nan B., Morishita M., Mukherjee B., Dvonch J. T., Spino C., Fink G. D., Rajagopalan S., et al. (2014). Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: Effects of diet-induced metabolic syndrome. Environ. Health Perspect. 122, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenfeld J., Feldman S. (2000). Effects of adrenalectomy and corticosterone replacement on the hypothalamic-pituitary response to neural stimuli. Brain Res. 877, 73–78. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (1978). Environmental Health Criteria (7) Photochemical Oxidants. Geneva: ISBN 92-4-1540672. http://www.inchem.org/documents/ehc/ehc/ehc007.htm. [Google Scholar]


