ince the pioneering work of Lewis and Gregory [4] in 1929, fertilized rabbit ova have been cultivated in vitro from one-cell to blastocyst in various media containing serum. However, other mammalian ova in early cleavage divisions have been relatively refractory to in vitro cultivation. Hammond [3] was the first to cultivate eight-cell mouse ova through to the blastocyst stage. He used a media containing egg white. Whitten [8] demonstrated that eight-cell mouse ova will develop into blastocysts when cultivated in a balanced salt solution containing glucose and bovine serum albumin. McLaren and Biggers [6] and Tarkowski [7] confirmed this and showed that these blastocysts developed into normal young when transferred to the uterus of foster mothers. Whitten [9] substituted calcium lactate for calcium chloride in this media and obtained the development of some late two-cell ova into blastocysts. A method for relatively large-scale in vitro experiments on the early cleavage divisions of the mouse embryo has been developed in this laboratory. A particular advantage of the method is that all the embryos can be observed at any time in the experiment without disturbing the conditions. Since the method is now being used in several types of study the full details are described in this paper.
A critical factor for the in vitro cultivation of all mammalian cells is the composition of the culture media. The medium shown in Table I has been developed for in vitro cultivation of two-cell mouse ova. Large quantities of the media are prepared by dissolving the required amounts of the salts 1 to 6 in deionized H2O (80 per cent of the final volume). The volume of lactic acid necessary to achieve the final concentration is neutralized (pH 7.4) with N NaOH and added to the salt solution. Penicillin and streptomycin are prepared in a stock solution containing 100,000 U/ml of penicillin and 50,000 μg/ml of streptomycin, and the amount necessary to give the concentration in Table I is added. Crystalline bovine serum albumin is added to give a final concentration of 1 mg/ml, and the mixture is brought to final volume by adding deionized H2O. The media is sterilized by filtration and stored under 5 per cent CO2 in air. Alternatively, small quantities (1 to 20 ml) of the media are prepared from isotonic stock solutions of 1 to 7 (CaCl2—0.11 M all others 0.154 M) by mixing the quantities shown in Table I. Albumin is added to give a concentration of 1 mg/ml. Penicillin and streptomycin are added from the stock solution to give the final concentrations shown in Table I. Millipore syringe-adapted filters are used to sterilize the media, which is then stored under 5 per cent CO2 in air.
Table I.
Medium for in vitro cultivation of two-cell mouse ova.
| Concentration |
ml of isotonic stock solution |
|||
|---|---|---|---|---|
| Number | Component | g/l | mM | |
| 1 | NaCl | 6.384 | 109.23 | 9.15 |
| 2 | KC1 | 0.356 | 4.78 | 0.40 |
| 3 | CaCI2 | 0.189 | 1.71 | 0.20 |
| 4 | KH2PO4 | 0.162 | 1.19 | 0.10 |
| 5 | MgSO4 · 7H2O | 0.294 | 1.19 | 0.10 |
| 6 | NaHCO3 | 2.106 | 25.07 | 2.10 |
| 7 | Sodium lactate | 1.147 | 10.15 | 0.85 |
| 8 | Crystalline bovine albumin |
1.000 | — | — |
| 9 | Penicillin | 100 U/ml | — | — |
| 10 | Streptomycin | 50 μg/ml | — | — |
The two-cell ova used are obtained from eight-week-old random-bred Swiss mice. These mice are superovulated by an intraperitoneal injection of 10 IU of pregnant mare serum gonadotrophin (Gestyl, Organon), followed 44 hr later by 10 IU of human chorionic gonadotrophin (HCG) (Pregnyl, Organon). The injected females are placed with males at the time of the second injection and checked for vaginal plugs the following morning. Females which have mated are used the day following the observation of the vaginal plug. Since ovulation occurs 11 to 14 hr after injection of HCG [2] the age of the ova can be determined by the interval between the injection of pregnyl and harvest of the ova.
The Fallopian tubes are cut free of the ovary and uterus and placed in 0.05 ml of the culture media in a Petri dish 60 × 15 mm. A blunted 30-gauge needle attached to a 1-ml syringe is inserted into the fimbrial end of the oviduct, held in place by jeweller’s forceps, and the contents of the oviduct are flushed out. Normal two-cell ova are picked up with a finely drawn out Pasteur pipette. These ova are placed in 2 ml of culture media in a 13 × 100 mm test tube and maintained at 37°C under 5 per cent CO2 in air. Using this method 200 to 400 two-cell ova can be collected in 2 hr. After collection the storage tube is agitated and the media containing the ova is poured into an embryological watch glass. By gently rocking the dish the ova are brought together and picked up in a fine Pasteur pipette. They are then transferred to a second embryological watch glass containing 1 ml of culture media under 2 ml of light-weight paraffin oil. This transfer decreases the amount of debris with the ova and provides a storage place for them while the experiment is being set up.
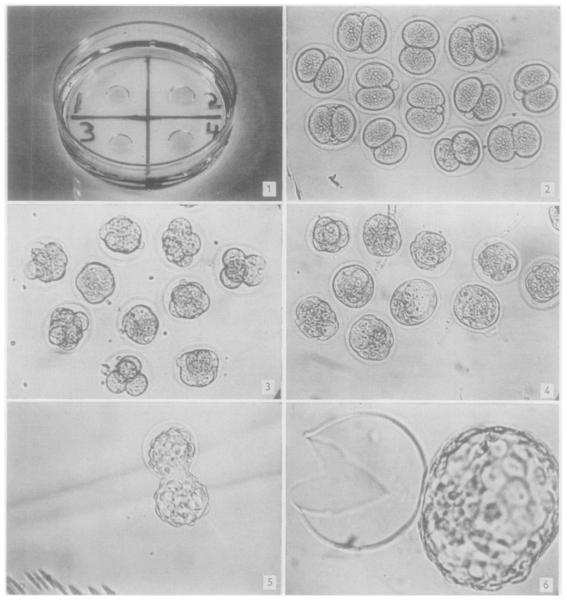
These ova are deposited in small drops under light-weight paraffin oil in a 15 × 60 mm plastic Petri dish (Fig. 1). Several days before use, the oil is equilibrated with the salt solution by bubbling 5 per cent CO2 in air through a mixture of 400 ml of sterile oil and 20 ml of sterile salt solution. Ten ml of this oil is placed in each dish, and the medium is pipetted as a microdrop (approximately 0.1 ml) under the oil by means of a finely drawn Pasteur pipette or Micro-syringe. Equal numbers of two-cell ova are placed in each drop. The Petri dishes containing the microdroplets with the ova are placed on trays in desiccating chambers at 37°C, and the chambers are gassed with 5 per cent CO2 in air bubbled through H2O [1]. The ova can be examined and photographed at any of the succeeding stages of development.
Fig. 1.—Culture dish with four microdroplets of media under light-weight paraffin oil. Approxinately original size.
Fig. 2.—Two-cell mouse ova at beginning of in vitro culture. ×60.
Fig. 3.—Eight-cell stage. Developed from two-cell mouse ova cultured in vitro for 24 hr. ×60.
Fig. 4.—Morulae and early blastocysts. Developed from two-cell ova cultured in vitro for 48 hr. ×60.
Fig. 5.—Late blastocyst escaping from zona pellucida. Developed from two-cell ova cultured in vitro for 72 hr. ×60.
Fig. 6.—Late blastocyst completely free of zona. Developed from two-cell ova cultured in vitro for 72 hr. ×120.
When two-cell ova are harvested 45 hours after injection of Pregnyl, between 60 and 100 per cent of these will develop into normal blastocysts (Figs. 2 to 4). The development occurs at the same rate as that in vivo [5], cavitation appearing late on the second day of culture. The “hatching” of the blastocyst from the zona pellucida begins on the third day and proceeds through the fourth day of cultivation (Figs. 5 and 6). The effect of omissions or additions of substances to the control media may be determined by direct observation of the developing ova at any desired time. If the effect is severe, few, if any, of the two-cell ova will undergo one cleavage division. Less severe effects are manifested by a decrease in the number of two-cell ova developing to blastocysts by the third day of cultivation.
Using the above method several thousand two-cell ova have been cultured in vitro with reproducible results. This method at present appears to be the most convenient procedure available for large scale investigation of the growth requirements of early cleavage divisions of the mouse ova and the effect on these stages of various substances.
Acknowledgments
The author would like to thank Professor John D. Biggers for his helpful suggestions. Gonadotrophins were a gift from Dr. W. J. Tindall (Organon Laboratories Ltd., England). This work was supported by a grant from the Population Council, Inc. and Grant CA-06638 from the National Cancer Institute, U.S. Public Health Service. The author is a Pennsylvania Plan Scholar at the Department of Physiology, University of Pennsylvania, Philadelphia.
REFERENCES
- 1.Biggers JD. In: Cartilage and Bone in The Biology of Cells and Tissues in Culture. Willmer EN, editor. Academic Press; New York: In press. [Google Scholar]
- 2.Edwards RG, Gates AH. J. Endocrinol. 1959;18:292. doi: 10.1677/joe.0.0180292. [DOI] [PubMed] [Google Scholar]
- 3.Hammond J., Jr. Nature. 1949;163:28. doi: 10.1038/163028b0. [DOI] [PubMed] [Google Scholar]
- 4.Lewis WH, Gregory PW. Science. 1929;69:226. doi: 10.1126/science.69.1782.226-a. [DOI] [PubMed] [Google Scholar]
- 5.Lewis WH, Wright ES. Conk. Embryol. Carneg. Inst. 1935;25:115. [Google Scholar]
- 6.McLaren A, Biggers JD. Nature. 1958;182:877. doi: 10.1038/182877a0. [DOI] [PubMed] [Google Scholar]
- 7.Tarkowski AK. Nature. 1961;190:857. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- 8.Whitten WK. Nature. 1956;176:96. doi: 10.1038/177096a0. [DOI] [PubMed] [Google Scholar]
- 9. — — ibid. 1957;177:1028. [Google Scholar]