Abstract
Development of biomarkers that detect early stage resectable premalignant lesions of colon can provide critical aid in prevention of colorectal cancer. Recent evidences advocate the utility of mucin expression to predict malignant transformation of colon pre-neoplastic lesions. In this study, we investigated the combined expression of multiple mucins and mucin-associated glycans during the adenoma-carcinoma sequence of colon cancer progression. Further, we evaluated their applicability as markers for differentiating adenomas/adenocarcinomas from hyperplastic polyps. Immunohistochemical analyses performed on colon disease tissue microarrays revealed that MUC2, MUC4 expression were downregulated (p<0.0001) and MUC1, MUC5AC expression were upregulated (p=0.01) during adenoma-adenocarcinoma progression. Expression of MUC17 was downregulated in inflamed tissues compared to normal tissues, but its increased expression differentiated adenomas (p=0.0028) and adenocarcinomas (p=0.025) from inflammation. MUC1 specific glycan-Tn/STn-MUC1 showed higher expression in hyperplastic polyps (p=0.023), adenomas (p=0.042) and adenocarcinomas (p=0.0096) compared to normal. Multivariate regression analyses indicated that a combination of MUC2, MUC5AC, and MUC17 could effectively discriminate adenoma-adenocarcinoma from hyperplastic polyps. Altogether, a combined analysis of altered mucins and mucin-associated glycans is a useful approach to distinguish premalignant/malignant lesions of colon from benign polyps.
Keywords: colonic mucin, glycan, hyperplastic polyp, adenoma, colorectal cancer
1. Introduction
For colorectal cancer (CRC), incidence and mortality rates are high worldwide [1]. CRC is the third most common cancer in both men and women, and the second leading cause of cancer deaths in the US [1]. In 2015, about 132,700 people will be diagnosed with CRC, and about 49,700 people will die of the disease [2]. Survival from CRC is associated with the stage of cancer when diagnosed, with the advanced disease having the worst outcome; the 5-year survival being 13% [3]. Only 40% of CRCs are diagnosed at early stages, due in part to the underuse of screening modalities. Thus, there is a need for specific and sensitive modalities for early diagnoses.
CRC is a heterogeneous disease. Its etiology involves modifiable, medical and hereditary risk factors, and the precise events vary from one individual to another [4]. Various pathways of neoplastic progression contribute to the molecular and biological heterogeneity exhibited by CRCs [5]. About 85% of CRCs are sporadic and progress slowly by accumulating multiple genetic mutations (APC, KRAS, p53, and DCC) in precancerous lesions (polyps/adenomas). The process is referred to as adenoma-carcinoma sequence [6]. Recent studies highlight the diagnostic potential of the mucin expression profiles in pre-neoplastic colon polyps [7, 8]. Intestinal mucosal and goblet cells produce, store, and secrete heavily glycosylated proteins termed mucins (MUCs) which are the building blocks of the gastrointestinal (GI) mucus system. Mucins provide a selective molecular barrier for luminal protection of the GI tract against factors such as food, acid, enzymes and bacteria [9, 10]. To date, 21 mucin genes have been identified and categorized into two subgroups: membrane-bound and secretory mucins [11]. The apical cell surfaces of intestinal enterocytes and colonic columnar cells anchor membrane-bound mucins (MUC1, MUC4, and MUC17) [10]. These mucins sense the intestinal environment to mediate intracellular signaling and provide a diffusion barrier [10]. In contrast, secretory mucins (MUC2, MUC5B, MUC5AC, and MUC6) form polymeric gels that facilitate lubrication and protection of GI system [10]. Various inflammatory, benign (hyperplastic polyps), premalignant (adenomas), and malignant conditions of colon are associated with alterations in mucin expression, organization, glycosylation which in turn impact their functioning. Mucin aberrations affect a variety of cellular activities, including growth, differentiation, transformation, adhesion, invasion, and immune surveillance [12]. Several studies have investigated the expression of mucins, including MUC1, MUC2, MUC4, MUC5AC, MUC6, and MUC17 and their associated glycans during the colon adenoma-carcinoma progression [13-17]. However, there has been no analysis of these mucins as a panel of markers for differentiating malignancies from normal/benign controls. The present study, for the first time, compared concurrent expression of mucins (MUC1, MUC2, MUC4, MUC5B, MUC5AC, MUC6, and MUC17) and mucin-associated glycans (Tn/STn-MUC1, TAG72 and CA 19-9) in normal, inflamed colon tissues as well as in tissues obtained from hyperplastic polyps, adenomas, and adenocarcinomas of the colon, and investigated the value of a panel of markers to differentiate premalignant and malignant lesions from benign conditions.
2. Materials and Methods
2.1 Tissue specimens
Colon tissue arrays (Cat# CO809a) having normal (9), inflamed (10), hyperplastic polyp (10), adenomas including villous, tubulovillous, tubular and serrated subtypes (30), and adenocarcinoma (16) samples were obtained from US Biomax, Rockville, MD.
2.2 Immunohistochemistry (IHC)
Following a standardized protocol, immunohistochemistry (IHC) was performed on the colon tissue arrays listed above [18]. The colon disease arrays were baked overnight at 56°C, followed by deparaffinization with xylene and rehydration with graded alcohols (5 min each). Tissues were incubated with a methanolic solution of 3% H2O2 for quenching endogenous peroxidase activity, followed by heat induced antigen retrieval with 0.05M citrate buffer (pH 6.0, 95°C) for 15 min. Sections were then blocked with 2.5% horse serum (ImmPRESS Universal antibody kit; Vector Laboratories, Burlingame, CA) for 3 hr. The tissue samples were incubated overnight at 4°C with well characterized and specific anti-mucin and anti-mucin associated glycans antibodies listed in Table 1. Next, the tissue arrays were washed and incubated with anti-rabbit/anti-mouse secondary antibody (ImmPRESS Universal antibody kit; Vector Laboratories) for 30 min. The color was developed by adding substrate chromogen, 3, 3′-diaminobenzidine solution (DAB substrate kit; Vector Laboratories). A reddish brown precipitate indicated positive expression. The slides were counterstained with Harris hematoxylin, dehydrated in graded ethanol followed by xylene and mounted with Permount (Vector Laboratories). Each tissue core in the array was reviewed, confirmed for diagnosis, and evaluated by a pathologist (Dr. Sonny L. Johansson at UNMC) to determine the staining score. The intensity of staining for each of the markers was scored on a scale of 0–3 (0-negative, 1-weak, 2-moderate, 3-intense staining) to give intensity scores (IS). The percentage of cells positive for mucin or glycan within a given lesion was scored on a scale of 1–4 as follows: 1: 0–25%; 2: 26–50%; 3: 51–75%; and 4: 76–100% cells positive. The staining intensity scores and scores corresponding to the percentage of immunoreactive cells were then multiplied to obtain a composite score (CS) representing overall expression ranging from 0-12. CS was further categorized as 0: negative, 1-3: weak, 4-6: mild, 7-9: moderate, and 10-12: strong expression. A section was considered “positive” for a particular marker if the CS was ≥1. Differences in CS for each colon tissue type were converted into a heat map for better visualization (Supplementary Fig. 1).
Table I. Panel of antibodies used for IHC analysis of various mucins and associated glycans.
2.3 Statistical Analysis
For statistical analysis, each spot on the array was considered as an individual sample. CS and IS values were compared with the Kruskal-Wallis test and Wilcoxon test for pairwise comparisons. No adjustments were made for multiple comparisons. Further, multivariate logistic regression method was used to evaluate marker combinations as predictors of hyperplastic polyps vs. adenoma/adenocarcinoma combined as well as two additional models comparing polyps vs. adenoma and polyps vs. adenocarcinoma separately. For each of the multivariate models, full models were fit, including marker (MUC17, MUC2, MUC4, MUC5AC, and Tn/STn-MUC1) composite scores as continuous variables. This model allows us to evaluate the effect of a single marker on outcome while adjusting for all the other markers. Reduced models were also considered utilizing backward variable selection with α set at 0.10 for variable inclusion in the model. Firth's penalized maximum likelihood estimation was used to reduce bias in the parameter estimates because separability occurred in some of the multivariate models. P-values <0.05 were considered to be statistically significant. All statistical analyses were performed using SAS software Version 9.3 (SAS Institute Inc., Cary, NC).
3. Results
The comprehensive immunophenotypic expression profiling of mucins and mucin-associated glycans was performed utilizing a commercial colon disease spectrum array. The primary objective was to delineate differentially expressed mucins for discriminating normal and benign tissues (hyperplastic polyps) from premalignant (adenomas) and malignant (colon adenocarcinoma) lesions. A further goal was to identify a panel of markers that could differentiate benign colon tissues from neoplastic precursor lesions and malignant lesions.
3.1 Expression of transmembrane mucins
Looking at previous reports of expression and association of transmembrane mucins (MUC1, MUC4 and MUC17) with CRC, in this study, we investigated their comprehensive expression profile during adenoma-carcinoma sequence.
MUC1
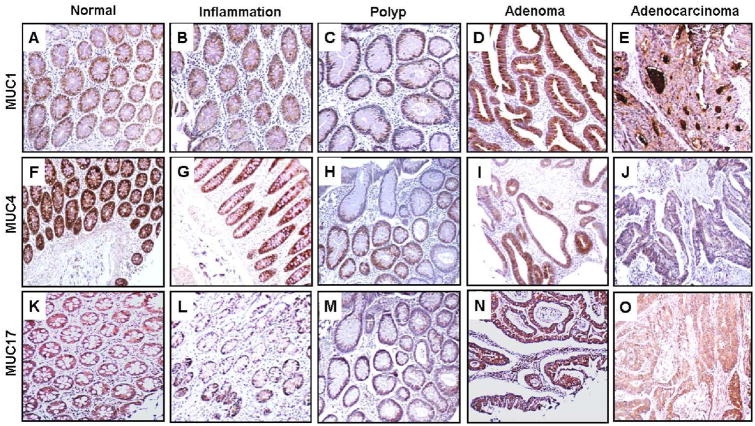
In normal colon tissues, a weak to mild MUC1 expression (mean±SE CS, 3.5±0.5; mean±SE IS, 1.7±0.2) was observed (Fig. 1A, Table II). In comparison to normal tissues, no appreciable difference in expression of MUC1 was observed in inflamed tissues (mean CS, 5.2±1.2; mean IS, 1.9±0.3), hyperplastic polyps (mean CS, 3.8±1.1; mean IS, 2.1±0.3), or adenomas (mean CS, 4.0±0.6; mean IS, 1.9± 0.2) (Fig. 1B-D, Table II). MUC1 expression was higher in adenocarcinomas (mean CS, 5.8±0.9; mean IS, 2.4±0.2) in comparison to normal tissues (Fig. 1E, Table II). Notably, a significant increase in the intensity and extent of MUC1 expression was observed in adenocarcinomas (56% of tissues having 3+ intensity scores) as compared to normal tissues (none having 3+ intensity scores).
Fig. 1. Expression analysis of transmembrane mucins (MUC1, MUC4, and MUC17) in colon disease tissue microarrays.
Colon tissue microarrays were probed with MUC1 (HMFG-2), MUC4 (8G7) monoclonal antibodies and MUC17 polyclonal antibody (SN-1132) after nonspecific blocking with horse serum. All sections were examined under microscope and the expression was evaluated on the basis of reddish brown staining by a pathologist. Representative photomicrographs are shown for MUC1 (A-E), MUC4 (F-J) and MUC17 (K-O) stained tissues of normal, Inflammation, polyp, adenoma and adenocarcinoma of colon. Elevated expression of MUC1 was observed during inflammation (B), polyp (C), adenoma (D) and adenocarcinoma (E) of colon in comparison to normal colon samples (A). Strong expression of MUC4 was observed in normal colon tissues (F) that were gradually lost during further progression to inflammation (G), hyperplastic polyps (H), adenoma (I) and adenocarcinoma (J). High immunoreactivity of MUC17 observed in normal colon (K) was downregulated in colon inflammation cases (L) and was observed to be significantly upregulated in polyp (M), adenoma (N) and adenocarcinoma (O) cases in comparison to colonic inflammation cases.
Table II. Composite and intensity scores for expression of transmembrane mucins (MUC1, MUC4, and MUC17) in normal, inflammation, polyp, adenoma and adenocarcinoma tissues of the colon.
| Diagnosis | Normal | Inflammation | Polyp | Adenoma | CAC | Overall p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Transmembrane mucins | Composite Scores | MUC1 | Mean ± SE | 3.5 ± 0.5 | 5.2 ± 1.2 | 3.8 ± 1.1 | 4.0 ± 0.6 | 5.8 ± 0.9 | 0.34 |
| Median (Min, Max) | 4 (1, 6) | 6 (0, 12) | 2.5 (0, 12) | 2 (0, 12) | 5 (0, 12) | ||||
|
| |||||||||
| MUC4 | Mean ± SE | 11.6 ± 0.3 5,8,11,14 | 7.9 ± 1 2,9,14 | 4.4 ±1.5 2,12 | 4.2 ± 07 3,4,13 | 0 .6 ± 03 3,6,7,10 | <0.0001 | ||
| Median (Min, Max) | 12 (9, 12) | 8.5 (0, 12) | 3 (0, 12) | 3.5 (0, 12) | 0 (0, 4) | ||||
|
| |||||||||
| MUC17 | Mean ± SE | 8.5 ± 1 | 5.6 ± 0.9 10,12 | 7.7 ± 1.5 | 9.5 ± 0.6 5 | 9.0 ± 0.8 4 | 0.047 | ||
| Median (Min, Max) | 8 (4, 12) | 6 (1, 9) | 9 (1, 12) | 12 (0, 12) | 8.0 (4, 12) | ||||
|
| |||||||||
| Intensity Scores | MUC1 | Mean ± SE | 1.7 ± 0.212 | 1.9 ± 0.3 | 2.1 ± 0.3 | 1.9 ± 0.212 | 2.4 ± 0.2 1,9 | 0.057 | |
| Median (Min, Max) | 2 (1, 2) | 2 (0, 3) | 2 (0, 3) | 2 (0, 3) | 3 (0, 3) | ||||
|
| |||||||||
| MUC4 | Mean ± SE | 3 ± 0 8,4,11 | 2.5 ± 0.3 7,9,14 | 1.5 ± 0.4 2,4,12 | 16 ± 02 3,4,13 | 0 5 ± 0 2 3,6,7,10 | <0.0001 | ||
| Median (Min, Max) | 3 (3, 3) | 3 (0, 3) | 2 (0, 3) | 2 (0, 3) | 0 (0, 2) | ||||
|
| |||||||||
| MUC17 | Mean ± SE | 2.4 ± 0.2 | 2 ± 0.3 | 2.3 ± 0.3 | 2.5 ± 0.1 | 2.4 ± 0.1 | 0.47 | ||
| Median (Min, Max) | 2 (2, 3) | 2 (1, 3) | 3 (1, 3) | 3 (0, 3) | 2 (1, 3) | ||||
p<0.05 vs. normal;
p<0.005 vs. normal;
p<0.0005 vs. normal;
p<0.05 vs. inflammation;
p<0.005 vs. inflammation;
p<0.0005 vs. inflammation;
p<0.05 vs. polyp;
<0.005 vs. polyp;
p<0.05 vs. adenoma;
p<0.005 vs. adenoma;
p<0.0005 vs. adenoma;
p<0.05 vs. adenocarcinoma;
p<0.005 vs. adenocarcinoma;
p<0.0005 vs. adenocarcinoma;
colon adenocarcinoma (CAC); standard error (SE); composite score (CS); intensity score (IS)
MUC4
There was strong expression of MUC4 in 89% of the normal colon samples (mean CS, 11.6±0.3; mean IS, 3±0) (Fig. 1F, Table II); its expression was reduced to moderate levels in inflamed tissues (mean CS, 7.9±1; mean IS, 2.5±0.3) (Fig. 1G, Table II). Relative to normal tissues, expression of MUC4 was significantly low in hyperplastic polyps (mean CS, 4.4±1.5; mean IS, 1.5±0.4) (Fig 1H, Table II). Further reduction in MUC4 expression in adenomas (mean CS, 4.2±0.7; mean IS, 1.6±0.2) significantly differentiated them from normal and inflammatory tissues (Fig. 1I, Table II). However, 13% of adenoma tissues exhibited strong expression of MUC4. Adenocarcinomas had drastic reduction in MUC4 expression with undetectable expression in 63% of samples (means CS, 0.6±0.3, mean IS, 0.5±0.2) (Fig. 1J, Table II). Overall, expression of MUC4 was significantly downregulated (p<0.0001) in the adenoma-carcinoma sequence.
MUC17
All normal tissues showed MUC17 positivity (mean CS, 8.5±1; mean IS, 2.4±0.2) (Fig. 1K, Table II), with mild, moderate, and strong expression distributed uniformly. Relative to normal tissues, expression of MUC17 was downregulated in inflamed tissues (mean CS, 5.6±0.9; mean IS, 2±0.3), with none of the tissues having strong expression (Fig. 1L, Table II). There was no significant differences in MUC17 expression in hyperplastic polyps compared to normal and inflamed tissues (mean CS, 7.7±1.5; mean IS, 2.3±0.3) (Fig. 1M, Table II). In adenomas, however, there was significantly higher MUC17 expression in comparison to inflamed tissues (mean CS, 9.5±0.6; mean IS, 2.5±0.1) (Fig. 1N, Table II), with 60% of adenomas showing strong expression. Further, MUC17 expression significantly differentiated adenocarcinomas from inflamed tissues (mean CS, 9±0.8; mean IS, 2.4±0.1) (Fig. 1O, Table II). Overall, higher MUC17 expression was a significant marker (p=0.047) to differentiate premalignant and malignant lesions from inflammation.
3.2 Expression of secretory mucins
Alterations in the expression of secretory mucins (MUC2, MUC5AC, MUC5B and MUC6) have been associated with CRC. Next, we examined their comprehensive expression signature during adenoma-carcinoma sequence.
MUC2
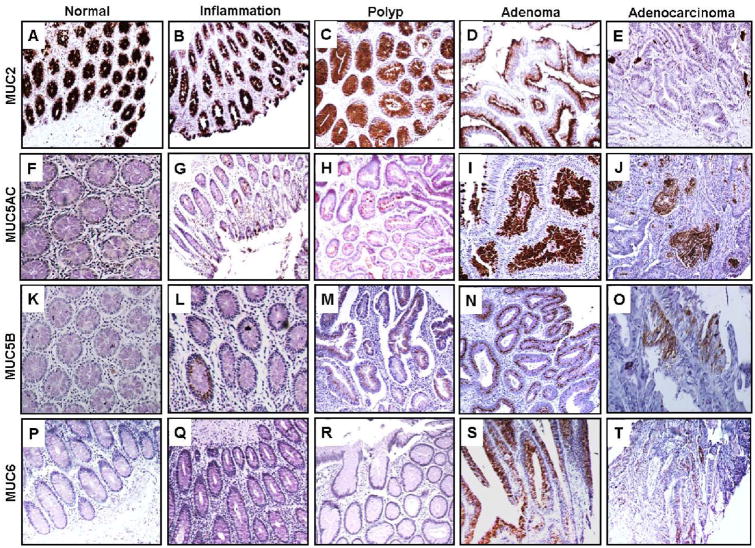
Strong expression of MUC2 was observed in all normal colon samples (mean CS, 12±0; mean IS, 3±0) (Fig. 2A, Table III). In tissues with inflammation, there was a slight decrease in MUC2 expression (mean CS, 11.4±0.4; Mean IS, 3±0) (Fig. 2B, Table III). Expression of MUC2 was significantly lower in hyperplastic polyps relative to normal tissues (mean CS, 9.7±1.1; mean IS, 2.9±0.1) (Fig. 2C, Table III), and in adenomas relative to normal, inflamed tissues and hyperplastic polyps (mean CS, 7.4±0.6; mean IS, 2.8±0.1) (Fig. 2D, Table III). Interestingly, 13% of adenomas exhibited relatively strong MUC2 expression. In adenocarcinomas, significantly lower MUC2 expression (mean CS, 3.8±0.9; mean IS, 2±0.3) was observed relative to normal, inflammation, hyperplastic polyps and adenoma tissues (Fig. 2E, Table III). Further, strong expression of MUC2 was reduced to only 6% of adenocarcinomas. Overall, MUC2 expression was significantly decreased (p<0.0001) in the adenoma-carcinoma sequence. MUC5AC: The expression of MUC5AC was undetectable in the normal colon tissues (mean CS, 0±0; mean IS, 0±0) (Fig. 2F, Table III), however 30% of inflamed tissues expressed MUC5AC at low levels (mean CS, 0.5±0.3; mean IS, 0.5± 0.3) (Fig. 2G, Table III). There was a further significant increase in expression of MUC5AC in 50% of hyperplastic polyps (mean CS, 1.8±1; mean IS, 0.9±0.4) (Fig. 2H, Table III) and in 63% of adenomas (mean CS, 3.1±0.6; mean IS, 1.5±0.2) (Fig. 2I, Table III) relative to normal tissues. For adenocarcinomas, a statistically significant upregulation of MUC5AC was found in 38% of tissues relative to normal colon (mean CS, 1.7±0.7; mean IS, 1.1±0.4) (Fig. 2J, Table III). Overall, high MUC5AC expression was a significant marker (p=0.01) that differentiated neoplastic precursors and malignant lesions from normals.
Fig. 2. Expression analysis of secretory mucins (MUC2, MUC5AC, MUC5B and MUC6) in colon disease tissue array.
Colon tissue microarrays was probed with MUC2 (EPR6145), MUC5AC (45M1), MUC5B (19.4E) and MUC6 (CLH5) monoclonal antibodies after non-specific blocking with horse serum. All sections were examined under microscope and the positive expression was evaluated on the basis of reddish brown staining by a pathologist. Representative photomicrographs are shown for MUC2 (A-E), MUC5AC (F-J), MUC5B (K-O) and MUC6 (P-T) stained tissues of normal, Inflammation, polyp, adenoma and adenocarcinoma of colon respectively. Strong expression of MUC2 observed in normal colon tissues (A). Gradual loss of expression was observed during progression to inflammation (B), hyperplastic polyps (C), adenoma (D) and adenocarcinoma (E). No immunoreactivity of MUC5AC was observed in normal colon cases (F). Expression was significantly enhanced in polyp (H), adenoma (I) and adenocarcinoma (J) of colon in comparison to normal colon (A) and inflammation cases (G). No MUC5B expression was observed in normal colon (K), while rare expression was observed during inflammation (L), polyp (M), adenoma (N) and adenocarcinoma (O). Rare expression of MUC6 was observed in cases of adenoma (S) and adenocarcinoma (T).
Table III. Composite and intensity scores for expression of secretory mucins (MUC2, MUC5B, MUC5AC, and MUC6) in tissues corresponding to normal, inflammation, polyp, adenoma and adenocarcinoma tissues of the colon.
| Diagnosis | Normal | Inflammation | Polyp | Adenoma | CAC | Overall p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Secretory mucins | Composite Scores CS) | MUC2 | Mean ± SE | 12 ± 0 7,11,14 | 11.4 ± 0.4 11,14 | 9.7±1.1 1,9,13 | 7.4 ± 0.6 3,6,7,13 | 3.8 ± 0.9 3,6,8,10 | <0.0001 |
| Median (Min, Max) | 12 (12, 12) | 12 (9, 12) | 12 (3, 12) | 9 (1, 12) | 3 (0, 12) | ||||
|
| |||||||||
| MUC5B | Mean ± SE | 0 ± 0 | 0.4 ± 0.3 | 0.8 ± 0.6 | 2.2 ± 0.7 | 0.19 ± 0.1 | 0.34 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 3) | 0 (0, 6) | 0 (0, 12) | 0 (0, 2) | ||||
|
| |||||||||
| MUC5AC | Mean ± SE | 0 ± 0 7,10,12 | 0.5 ± 0.3 9 | 1.8 ± 1 1 | 3.1 ± 0.6 2,5 | 1.7 ± 0.7 1 | 0.01 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 2) | 0.5 (0, 9) | 1.5 (0, 9) | 0 (0, 9) | ||||
|
| |||||||||
| MUC6 | Mean ± SE | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.5 ± 0.4 | 0.19 ± 0.1 | 0.29 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 12) | 0 (0, 1) | ||||
|
| |||||||||
| Intensity Scores (IS) | MUC2 | Mean ± SE | 3 ± 012 | 3 ± 012 | 2.9 ± 0.1 | 2.8 ± 0.112 | 2 ± 0.3 1,4,9 | 0.0069 | |
| Median (Min, Max) | 3 (3, 3) | 3 (3, 3) | 3 (2, 3) | 3 (1, 3) | 3 (0, 3) | ||||
|
| |||||||||
| MUC5B | Mean ± SE | 0 ± 0 | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.8 ± 0.2 | 0.2 ± 0.1 | 0.34 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 3) | 0 (0, 2) | 0 (0, 3) | 0 (0, 2) | ||||
|
| |||||||||
| MUC5AC | Mean ± SE | 0 ± 0 7,10,12 | 0.5 ± 0.3 9 | 0.9 ± 0.4 1 | 1.5 ± 0.2 2,4 | 1.1 ± 0.3 1 | 0.013 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 2) | 0.5 (0, 3) | 1 (0, 3) | 0 (0, 3) | ||||
|
| |||||||||
| MUC6 | Mean ± SE | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.29 | ||
| Median (Min, Max) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 3) | 0 (0, 1) | ||||
p<0.05 vs. normal;
p<0.005 vs. normal;
p<0.0005 vs. normal
p<0.05 vs. inflammation;
p<0.005 vs. inflammation;
p<0.0005 vs. inflammation;
p<0.05 vs. polyp;
<0.005 vs. polyp;
p<0.05 vs. adenoma;
p<0.005 vs. adenoma;
p<0.0005 vs. adenoma;
p<0.05 vs. adenocarcinoma;
p<0.005 vs. adenocarcinoma;
p<0.0005 vs. adenocarcinoma;
colon adenocarcinoma (CAC); standard error (SE); composite score (CS); intensity score (IS)
MUC5B and MUC6
None of the normal colon tissues expressed MUC5B and/or MUC6 (Fig. 2K & Fig. 2P, Table III). However, expression of MUC5B was evident in a fraction of inflamed tissues (20%) (Fig. 2L), hyperplastic polyps (20%) (Fig. 2M), adenomas (27%) (Fig. 2N), and adenocarcinomas (12.5%) (Fig. 2O). In adenomas, there was intense staining of MUC5B relative to normal tissues (mean IS, 0.8±0.2) (Table III). In contrast to MUC5B, MUC6 expression was noted in only 10% of adenomas (Fig. 2S) and in 19% of adenocarcinomas (Fig. 2T). Overall, the expression of MUC5B and MUC6 were not appreciably different between normal, inflammation, benign, and premalignant and malignant colonic tissues.
3.3 Expression of mucin-associated glycans
Altered glycosylation is one of the hallmarks of cancers. Looking at previously reported diagnostic potential of alterations in mucin-associated glycans CA 19-9, TAG72 during CRC, we examined their expression signature during adenoma carcinoma sequence.
CA 19-9
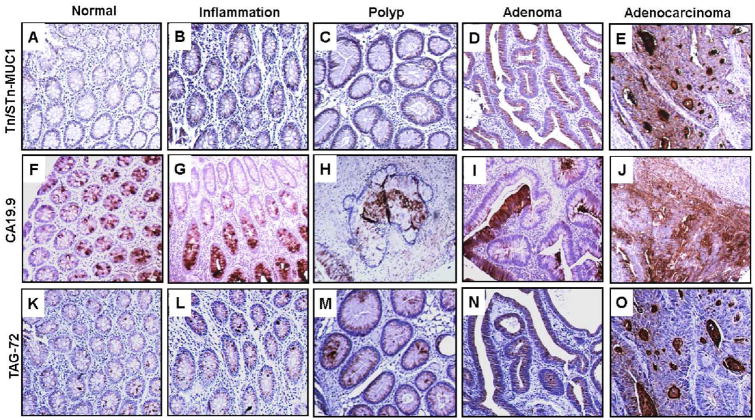
Weak expression of CA 19-9 (mean CS, 2.8±0.9; IS, 1.9±0.5) was observed in 77.8% normal tissues (Fig. 3F, Table IV) and a lack of expression in 22.2% cases. There was a similar pattern in inflamed tissues (mean CS, 3±1; mean IS, 1.6±0.5) (Fig. 3G, Table IV). Relative to normal tissues, there was a mild increase in expression of CA 19-9 in hyperplastic polyps (mean CS, 3.3±0.9; mean IS, 2.1±0.3) (Fig. 3H, Table IV) and adenomas (mean CS, 3.2±0.6; mean IS, 1.8±0.2) (Fig. 3I, Table IV). Further, there were higher levels of CA 19-9 in adenocarcinomas relative to normal and inflamed tissues (mean CS, 6.1±1.2; mean IS, 2.2±0.3) (Fig. 3J, Table IV). Interestingly, similar to normal colon, 25% of the adenocarcinomas were negative for CA 19-9 expression. In summary, CA 19-9 expression was higher in adenocarcinomas relative to normal tissues; however, this expression did not allow significant differentiation between hyperplastic polyps, adenomas, inflammatory and normal colon. TAG72: Normal colon tissues exhibited weak expression of TAG72 (mean CS, 1.7±0.3; mean IS, 1.1±0.2) (Fig. 3K, Table IV). However, there was elevated expression in inflamed tissues (mean CS, 3.5±0.3; mean IS, 1.7±0.3) (Fig. 3L, Table IV) and hyperplastic polyps (mean CS, 4.5±0.5; mean IS, 2.1±0.3) (Fig. 3M, Table IV) relative to normal tissues. Further, TAG72 expression was significantly elevated in adenomas (mean CS, 4.5±0.1; mean IS, 2.0±0.1) (Fig. 3N, Table IV) and adenocarcinomas (mean CS, 5.2±0.2; mean IS, 2.0±0.2) (Fig. 3O, Table IV), differentiating these tissues from normal tissues. Relative to normal tissues, 38% of cancers had intense staining (3+) for TAG72. Overall, there was a progressive increase in TAG-72 expression during adenoma-carcinoma sequence.
Fig. 3. Expression analysis of mucin-associated glycans (Tn/STn-MUC1, CA 19-9 and TAG72 in colon disease tissue array.
Colon tissue microarrays were probed with Tn/STn-MUC1 (5E5), CA 19-9 (NS19.9) and TAG72 (CC49) monoclonal antibodies after non-specific blocking with horse serum. All sections were examined under microscope and the positive expression was evaluated on the basis of reddish brown staining by a pathologist. Representative photomicrographs are shown for Tn/STn-MUC1 (A-E), CA 19-9 (F-J) and TAG72 (K-O) stained tissues of normal, Inflammation, polyp, adenoma and adenocarcinoma of colon. Tn/STn-MUC1 expression was elevated during colonic inflammation (B) that was further significantly enhanced in polyp (C), adenoma (D) and adenocarcinoma (E) cases of colon when compared to normal colon samples (A). An increase in CA 19-9 expression was observed in inflammation (G), hyperplastic polyps (H), adenoma (I) and adenocarcinoma (J) when compared to normal colon cases (F). Low expression of TAG72 was observed in normal colon cases (K). An increase in its expression was observed in colon inflammation (L) and polyp (M) cases that was significantly upregulated in colon adenomas (N) and adenocarcinoma (O) cases.
Table IV. Composite and intensity scores for expression of mucin-associated glycans (CA 19-9, TAG72, and Tn/STn on MUC1) in tissues corresponding to normal, inflammation, polyp, adenoma and adenocarcinoma tissues of the colon.
| Diagnosis | Normal | Inflammation | Polyp | Adenoma | CAC | Overall p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mucins associated Glycans | Composite Scores | CA 19-9 | Mean ± SE | 2.8 ± 0.9 | 3 ± 1.0 | 3.3 ± 0.9 | 3.2 ± 0.6 | 6.1 ± 1.2 | 0.3 |
| Median (Min, Max) | 3 (0, 6) | 2.5 (0, 9) | 2.5 (0, 9) | 3 (0, 12) | 6 (0, 12) | ||||
|
| |||||||||
| TAG72 | Mean ± SE | 1.7 ± 0.3 10,12 | 3.5 ± 0.3 | 4.5 ± 0.5 | 4.5 ± 0.1 2 | 5.2 ± 0.2 1 | 0.093 | ||
| Median (Min, Max) | 1(0, 4) | 2.5 (1, 9) | 4 (0, 9) | 4 (0, 12) | 6 (0, 12) | ||||
|
| |||||||||
| Tn/STn on MUC1 | Mean ± SE | 0.11 ± 0.1 7,9,13 | 1.3 ± 0.7 | 2.4 ± 1 1 | 1.8 ± 0.5 1 | 3.5 ± 1.0 2 | 0.077 | ||
| Median (Min, Max) | 0 (0, 1) | 0 (0, 6) | 1.5 (0, 9) | 0 (0, 9) | 2 (0, 12) | ||||
|
| |||||||||
| Intensity Scores | CA 19-9 | Mean ± SE | 1.9 ± 0.5 | 1.6 ± 0.5 | 2.1 ± 0.3 | 1.8 ± 0.2 | 2.2 ± 0.3 | 0.71 | |
| Median (Min, Max) | 3 (0, 3) | 2 (0, 3) | 2.5 (0, 3) | 2 (0, 3) | 3 (0, 3) | ||||
|
| |||||||||
| TAG72 | Mean ± SE | 1.1 ± 0.2 7,10,12 | 1.7 ± 0.3 | 2.1 ± 0.3 1 | 2 ± 0.1 2 | 2.0 ± 0.2 1 | 0.043 | ||
| Median (Min, Max) | 1(0, 2) | 1.5 (1, 3) | 2 (0, 3) | 2 (0, 3) | 2 (0, 3) | ||||
|
| |||||||||
| Tn/STn on MUC1 | Mean ± SE | 0.1 ± 0.1 7,9,12 | 0.5 ± 0.3 | 1.2 ± 0.4 1 | 0.9 ± 0.2 1 | 1.5 ± 0.3 1 | 0.036 | ||
| Median (Min, Max) | 0 (0, 1) | 0 (0, 2) | 1.5 (0, 3) | 0 (0, 3) | 2 (0, 3) | ||||
p<0.05 vs. normal;
p<0.005 vs. normal;
p<0.0005 vs. normal;
p<0.05 vs. inflammation;
p<0.005 vs. inflammation;
p<0.0005 vs. inflammation;
p<0.05 vs. polyp;
<0.005 vs. polyp;
p<0.05 vs. adenoma;
p<0.005 vs. adenoma;
p<0.0005 vs. adenoma;
p<0.05 vs. adenocarcinoma;
p<0.005 vs. adenocarcinoma;
p<0.0005 vs. adenocarcinoma;
colon adenocarcinoma (CAC); standard error (SE); composite score (CS); intensity score (IS)
Tn/STn-MUC1
Considering the overexpression of MUC1 during CRC, we also investigated the antigenic expression of MUC1-associated Tn/STn glycans during adenoma-carcinoma sequence. Although MUC1 was expressed in normal tissues, the presence of Tn/STn-MUC1 was not detectable in 89% samples (mean CS, 0.1±0.1; mean IS, 0.1±0.1); while positive samples exhibited weak expression (Fig. 3A, Table IV). The expression of Tn/STn-MUC1 was higher in 40% of inflamed tissues (mean CS, 1.3±0.7; mean IS, 0.5±0.3) (Fig. 3B, Table IV). Further, significantly high expression of Tn/STn-MUC1 was observed in 60% of hyperplastic polyps (mean CS, 2.4±1; mean IS, 1.2±0.4), 47% adenomas (mean CS, 1.8±0.5; mean IS, 0.9±0.2), and 63% adenocarcinomas (mean CS, 3.5±1.0; mean IS, 1.5±0.32) (Fig. 3C-E, Table IV) relative to normal tissues. Overall, Tn/STn-MUC1 is progressively increased during adenoma-carcinoma sequence and significantly differentiates normal tissues from adenomas and adenocarcinomas.
3.4 Multivariate analyses to evaluate biomarker combinations as predictors of malignancy
To determine the ability of mucins and mucin-associated glycans as biomarkers for discriminating adenoma/adenocarcinoma from hyperplastic polyp, we first performed univariate analysis. We observed that loss of MUC2 was a significant predictor (p=0.016) of adenoma/adenocarcinoma vs. hyperplastic polyps. We next investigated whether combinations of significantly altered mucins (MUC2, MUC4, MUC5AC, and MUC17) and mucin-associated glycans (Tn/STn-MUC1) can improve prediction of benign lesions (hyperplastic polyps) from neoplastic precursors (adenomas) and malignant lesions (adenocarcinoma). For this multivariate logistic regression method was utilized. In a full multivariate model, after adjusting for other variables, for each one-unit increase in CS of MUC2 it was 33% less likely to have adenoma/adenocarcinoma than hyperplastic polyp (p=0.014) (Table V). Further, after adjusting for other variables, for each one-unit increase in CS for MUC17 and MUC5AC, the likelihood of adenoma/adenocarcinoma than hyperplastic polyp was 25% and 34% respectively (Table V). In a backward selected model including only MUC4 and MUC2, after adjusting for MUC4, for each one-unit increase in CS for MUC2, it was 37% less likely to have adenocarcinoma than hyperplastic polyp (p=0.011) (data not shown). In a reduced model which includes MUC17, MUC5AC, and Tn/STn-MUC1, for each one-unit increase in CS for MUC17 after adjusting for MUC5AC and Tn/STn-MUC1, it was 31% more likely to have adenoma than hyperplastic polyp (p=0.040) (data not shown). In summary, a combination of MUC2, MUC5AC, and MUC17 expression profiles could be useful for predicting the odds of having premalignant and/or malignant lesions of the colon.
Table V. Univariate and multivariate analyses: odds of Adenoma/Adenocarcinoma vs. Hyperplastic polyp.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| Effect | OR | Lower | Upper | p-value | OR | Lower | Upper | p-value |
| MUC4 | 0.918 | 0.779 | 1.081 | 0.300 | 0.837 | 0.668 | 1.050 | 0.120 |
| MUC17 | 1.128 | 0.940 | 1.354 | 0.200 | 1.254 | 0.960 | 1.638 | 0.097 |
| MUC2 | 0.724 | 0.557 | 0.942 | 0.016 | 0.672 | 0.490 | 0.921 | 0.014 |
| MUC5AC | 1.092 | 0.861 | 1.385 | 0.470 | 1.338 | 0.962 | 1.859 | 0.083 |
| Tn/STn on MUC1 | 1.003 | 0.808 | 1.246 | 0.980 | 0.754 | 0.538 | 1.057 | 0.100 |
Confidence interval (CI); Odds Ratio (OR)
4. Discussion
Alterations in the expression of mucin proteins and in their O-glycosylation are postulated to be involved in colon carcinogenesis and are considered as molecular markers for clinical use [19]. In this context, the present study is the first to comprehensively evaluate the differential expression of mucins and mucin-associated glycans during sequential progression to colon carcinogenesis and to provide their utility in combinations for predicting the development of colon cancer.
MUC1 is a transmembrane mucin that is expressed in normal colon and overexpressed in inflammatory bowel disease (IBD) [20]. It is expressed in 19–76% of adenomas and is associated with increasing dysplasia along the adenoma-carcinoma sequence [14, 21]. Further, there is overexpression of MUC1 in most colorectal tumors, with expression in the range of 30–100% [22, 23]. Higher expression of MUC1 positively correlates with stage, metastasis, poor tumor differentiation, and worse long-term survival [22-25]. Although our results regarding MUC1 are in accordance with aforementioned findings, its expression did not serve as a statistically significant marker of tumor progression, a deficiency that could be attributed to the limited sample size. However, MUC1 can be considered as a prognostic marker for colon cancer [22].
The present study represents a comprehensive analysis of MUC4 expression in the adenoma-carcinoma sequence. MUC4 is a high-molecular-weight transmembrane mucin that is overexpressed in many carcinomas and is associated with a poor prognosis [26], especially for early stage CRCs [27]. An inverse relationship of MUC4 expression with the severity of Crohn's disease (CD) and ulcerative colitis (UC) has been established [28], and MUC4 expression is also reduced in hyperplastic polyps. Similarly, loss of MUC4 expression in serrated adenomas has been suggested to be a useful marker to identify patients with an elevated risk of developing colon cancer [13]. However, utilizing the Muc4-/- mice, it was demonstrated that during conditions of increased inflammation, loss of Muc4 renders resistance to chemical induced colitis and CRC. In addition, loss of Muc4 was shown to be compensated by elevation of Muc2 and Muc3 in this colitis induced tumor model [29]. In current study, we observed low MUC4 expression in inflammatory colon samples, suggesting that different inflammatory pathways may be involved in its regulation. In this regard, association of MUC4 with prognosis of IBD patients who develop CRC needs further validation in a large cohort of samples. Further, association between MUC4 expression and colon cancer has been variable among several studies. Shanmugam et al. reported the complete loss/decreased MUC4 expression in 75% of CRCs [27]. Biemer-Huttmann et al. demonstrated MUC4 expression in 34% of adenocarcinomas and 47% of mucinous colon cancers and suggested an inverse association between MUC4 expression and tumor stage in MUC4-positive tissues [30]. Similarly, Zhang et al. observed intense MUC4 expression in 50% of colon cancers [31]. The findings of the present study align with Shanmugam et al [27] which demonstrated strong MUC4 expression in normal colon with progressive decrease during progression to carcinoma. Identification of the molecular mechanisms underlying the downregulation of MUC4 expression during the adenoma-carcinoma process could further establish its association with colon cancer carcinogenesis.
MUC17 is another membrane-bound mucin found by our laboratory, to be highly expressed on normal colon apical epithelium and crypt columnar epithelial cells and is downregulated in ulcerative colitis, Crohn's disease, hyperplastic polyps, tubular, tubulovillous adenomas, and adenocarcinomas [17]. Consistent with our previous report, there was mild to strong expression of MUC17 in normal colon tissues, and it was reduced in inflamed colon. However, contrary to our previous report, there was higher MUC17 expression in adenomas and adenocarcinomas relative to inflamed tissues. Similar to present observation, a recent study reported that there was an 82-fold increase in MUC17 expression that differentiated sessile serrated adenomas/polyps (SSA/P) from hyperplastic polyps, adenomatous polyps, and normal tissues [32]. Further, our unpublished findings from another CRC cohort study suggested that loss of the punctate immunostaining pattern and the diffused cytoplasmic staining pattern of MUC17 were associated with aggressive behavior of CRCs and shortened patient survival (data not shown). The observation conflicts could be due to limited sample number, different site from where the tissues were obtained or different ethnicity of patient population. Therefore, these observations warrant further investigation in a larger set of subtypes of colorectal polyps and adenocarcinomas for defining MUC17 as a marker to differentiate pre-malignant lesions and to establish its conclusive association with colorectal carcinogenesis.
The gel-forming mucin, MUC2, is secreted by goblet and columnar cells of the colonic epithelium and is organized into two layers of protective mucus [33]. In CRCs, there is lower MUC2 expression at both the mRNA and protein levels and low expression correlates with reduced patient survival and a high incidence of liver and nodal metastases [34-36]. In contrast to colon adenocarcinomas, colonic mucinous carcinomas are characterized by overexpression of MUC2 [15, 37]. Consistent with these studies, we found a sequential and significant reduction of MUC2 in the adenoma-carcinoma sequence. Loss of MUC2 differentiates adenomas and adenocarcinomas from normal and benign controls. Overall, the findings suggest that a reduction of MUC2 in adenomas can be utilized as an early-stage marker to identify precursor lesions with malignant potential.
MUC5AC is not expressed in normal colon mucosa [15], but there is aberrant overexpression of MUC5AC in IBD [38], ulcerative colitis-associated dysplasia/neoplasms [39], hyperplastic polyps (HP), tubular adenomas, and SSA/Ps [8, 15, 16, 40]. It is also found in poorly differentiated adenocarcinomas located in the proximal colon independent of microsatellite instability status [41] and in mucinous CRCs. Consistent with previous studies, we found no expression of MUC5AC in normal colon tissues, but its expression was significantly higher in adenomas and adenocarcinomas. Higher MUC5AC expression differentiated adenocarcinomas from normal colon tissues. Overall, expression of MUC5AC in colon pathologies, its association with the adenoma-carcinoma sequence and its secretory nature suggests that MUC5AC combined with other relevant markers could be a useful marker for early diagnosis of CRC.
Among mucin-associated glycans, the TAG72 epitope is carried by high-molecular-weight mucins and identified as Sialyl-Tn. It is overexpressed in several malignancies, including CRC, and is considered as an early marker for CRC and other dysplastic colonic diseases [42, 43]. The present results regarding TAG72 expression are consistent with those previously reported studies. Although expression of TAG72 differentiated adenoma and adenocarcinoma from normal colon, yet it could not emerge as a significant marker during adenoma-carcinoma sequence.
The incidence of elevated serum CA 19-9 levels in CRCs ranges from 20–40% [44]. However, CA 19-9 is also expressed in normal colonic mucosa, thus limiting its diagnostic efficacy. We observed expression of CA 19-9 in 78% of normal colon tissues with weak/mild reactivity. Prominent CA 19-9 expression was observed in 94.4% of ulcerative and 72.4% of Crohn's colitis tissues previously [45]. In contrast, we did not observe appreciable alterations in CA 19-9 expression in inflammatory tissues relative to normal colon tissues. Confounding views regarding the utility and feasibility of measuring CA 19-9 to differentiate low and high-risk adenomatous polyps have been published. Despite positivity for CA 19-9 in 60% of adenomas, its utility as a marker of the adenoma-carcinoma sequence is questionable due to lack of correlation with degree of dysplasia, size of adenoma and villous component [45, 46]. These results from previous studies are corroborated by our finding that CA 19-9 was present in 90% of hyperplastic polyps and 67% of adenomas, but it could not differentiate these lesions from benign or inflammatory conditions. Although the diagnostic sensitivity and specificity of CA 19-9 for CRC has been estimated to be 70% and 61%, respectively [47], our results underscore the inability of CA 19-9 assays to differentiate normal and inflammatory colon from adenocarcinoma, despite its increase in expression in 75% of tissues. Since the elevated CA 19-9 levels correlate with tumor stage and with the highest sensitivity in patients with metastases [48], it may serve as a useful marker for advanced disease.
The Tn and sialyl-Tn epitopes appear to be colon cancer-associated antigens that act as markers of poorly differentiated adeno- and mucinous carcinomas [49]. Of colon carcinoma tissues, 82% express Tn antigens, and 73% express STn antigens on mucins in the cytoplasm or in luminal cell membranes [50]. Malignant effusions from breast, colon, gastric, and ovarian cancers have Tn antigen on MUC1 [51]. Proximity ligation assays have been applied for detection of aberrant mucin glycoforms in cancer. Tn/STn-MUC1 glycoforms are the most frequently expressed cancer biomarkers in the series of mucinous colon carcinomas [52]. Considering the overexpression of MUC1 and associated glycoforms in the adenoma-carcinoma sequence and their association with increased malignant potential of colon tissues, we investigated the antigenic expression of MUC1-associated Tn/STn glycans utilizing 5E5 antibody characterized previously [53]. The antibody 5E5 reacts with all Tn and STn glycoforms of the MUC1 tandem repeat but shows no reactivity with non-glycosylated MUC1 peptides. Our results demonstrate the utility of Tn/STn-MUC1 in differentiating hyperplastic polyps (p<0.05), adenomas (p<0.05), and adenocarcinomas (p<0.005) from normal colon. Of normal colon tissues, 89% did not express Tn/STn-MUC1 while the remaining 11% exhibited focal immunoreactivity. The incidences of Tn/STn-MUC1 in adenomas and adenocarcinomas were 47% and 63%, respectively. As Tn/STn-MUC1 is tumor associated antigen, its higher expression was observed in comparison to MUC1 in certain cases. A further analysis of histologically-defined subtypes of cancer-associated adenomas and examination of adenocarcinomas and metastatic tissues are needed to establish its utility as a diagnostic marker in the adenoma-carcinoma sequence.
In addition to determining the pattern of distribution of mucins and mucin-associated glycans individually in the adenoma-carcinoma sequence, the present study performed multivariate regression analyses to identify combinations of mucins and mucin-associated glycans for predicting colon carcinogenesis at an early stage. The results suggest that a combination of MUC2, MUC17, and MUC5AC can be utilized to assess the likelihood of adenoma and adenocarcinoma versus benign hyperplastic polyps.
While this study utilized a comprehensive panel of mucin and associated glycan antibodies, it is limited by the small sample size. The colon disease array used in this study did not include mucinous adenocarcinoma and metastatic colon cancer samples, limiting the complete picture of mucin and mucin-associated glycans expression in malignant tissues (e.g. MUC2 and MUC5AC which are highly expressed in mucinous adenocarcinomas). Additionally, the lack of information about the sites in the colon from which the tissues were procured and about associated mutations limits the interpretations with regard to prediction of malignancy.
Overall, we investigated the signature of mucins and mucin-associated glycans associated with the adenoma-carcinoma sequence and found significant alterations in expressions of MUC2, MUC4, MUC5AC, MUC17, and Tn/STn-MUC1. We also explored combinations of mucins and mucin-associated glycans for early-stage prediction of colon carcinogenesis. The combination of MUC2, MUC5AC, and MUC17 may indicate the likelihood of a colon lesion being premalignant and/or malignant versus benign. Future efforts are underway to determine utility of mucin and mucin-associated glycans profile in risk stratification of different colorectal polyp subtypes to enhance early stage management of this malignancy.
Supplementary Material
Highlights.
Comprehensive study of mucins and associated glycans during colon carcinogenesis.
Significant alteration of mucins MUC4, MUC17, MUC2, MUC5AC and glycans Tn/STn-MUC1.
MUC2/MUC5AC/MUC17 panel discriminates adenoma/adenocarcinoma from benign polyps.
Acknowledgments
This work was supported, in parts, by the grants from National Institutes of Health (Cancer Center Support Grant P30 CA036727, EDRN CA111294 and SPORE P50 CA127297).
Abbreviations
- APC
Adenomatous polyposis coli
- CA 19-9
Carbohydrate antigen 19-9
- CD
Crohn's disease
- CI
Confidence interval
- CRC
Colorectal cancer
- CS
Composite scores
- DAB
3, 3′-diaminobenzidine
- DCC
Deleted in colorectal cancer
- GI
Gastrointestinal
- H2O2
Hydrogen peroxide
- IBD
Inflammatory bowel disease
- IHC
Immunohistochemistry
- IS
Intensity scores
- KRAS
Kirsten rat sarcoma viral oncogene
- MSI
Microsatellite instability
- MUC
Mucin gene
- OR
Odds Ratio
- PLA
Proximity ligation assay
- SSA/P
Sessile serrated adenoma/Polyp
- TAG72
Tumor associated glycoprotein 72
- UC
Ulcerative colitis
Footnotes
Disclosure/Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hazewinkel Y, Dekker E. Colonoscopy: basic principles and novel techniques. Nat Rev Gastroenterol Hepatol. 2011;8:554–564. doi: 10.1038/nrgastro.2011.141. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol. 2012;4:71–75. doi: 10.4251/wjgo.v4.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolos M, Wasaznik-Jedras A, Nasierowska-Guttmejer A. Can the histological type of colorectal cancer determine the carcinogenesis pathway? Pol J Pathol. 2015;66:109–120. doi: 10.5114/pjp.2015.53003. [DOI] [PubMed] [Google Scholar]
- 6.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Molaei M, Mansoori BK, Mashayekhi R, Vahedi M, Pourhoseingholi MA, Fatemi SR, Zali MR. Mucins in neoplastic spectrum of colorectal polyps: can they provide predictions? BMC Cancer. 2010;10:537. doi: 10.1186/1471-2407-10-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Kim KJ, Rhee YY, Bae JM, Cho NY, Lee HS, Kang GH. Gastric-type expression signature in serrated pathway-associated colorectal tumors. Hum Pathol. 2015;46:643–656. doi: 10.1016/j.humpath.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15:57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1048. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 14.Percinel S, Savas B, Ensari A, Kuzu I, Kuzu MA, Bektas M, Cetinkaya H, Kursun N. Mucins in the colorectal neoplastic spectrum with reference to conventional and serrated adenomas. Turk J Gastroenterol. 2007;18:230–238. [PubMed] [Google Scholar]
- 15.Bu XD, Li N, Tian XQ, Li L, Wang JS, Yu XJ, Huang PL. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J Gastroenterol. 2010;16:4089–4094. doi: 10.3748/wjg.v16.i32.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita K, Hirahashi M, Yamamoto H, Matsumoto T, Gushima M, Oda Y, Kishimoto J, Yao T, Iida M, Tsuneyoshi M. Mucin core protein expression in serrated polyps of the large intestine. Virchows Arch. 2010;457:443–449. doi: 10.1007/s00428-010-0959-8. [DOI] [PubMed] [Google Scholar]
- 17.Senapati S, Ho SB, Sharma P, Das S, Chakraborty S, Kaur S, Niehans G, Batra SK. Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon. J Clin Pathol. 2010;63:702–707. doi: 10.1136/jcp.2010.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur S, Shimizu T, Baine MJ, Kumar S, Batra SK. Immunohistochemistry of pancreatic neoplasia. Methods Mol Biol. 2013;980:29–42. doi: 10.1007/978-1-62703-287-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szajda SD, Jankowska A, Zwierz K. Carbohydrate markers in colon carcinoma. Dis Markers. 2008;25:233–242. doi: 10.1155/2008/206510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furr AE, Ranganathan S, Finn OJ. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr Dev Pathol. 2010;13:24–31. doi: 10.2350/08-06-0479.1. [DOI] [PubMed] [Google Scholar]
- 21.Ho SB, Ewing SL, Montgomery CK, Kim YS. Altered mucin core peptide immunoreactivity in the colon polyp-carcinoma sequence. Oncol Res. 1996;8:53–61. [PubMed] [Google Scholar]
- 22.Xu F, Liu F, Zhao H, An G, Feng G. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers: A Meta-Analysis. Medicine (Baltimore) 2015;94:e2286. doi: 10.1097/MD.0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesari MV, Gaopande VL, Joshi AR, Babanagare SV, Gogate BP, Khadilkar AV. Immunohistochemical study of MUC1, MUC2 and MUC5AC in colorectal carcinoma and review of literature. Indian J Gastroenterol. 2015;34:63–67. doi: 10.1007/s12664-015-0534-y. [DOI] [PubMed] [Google Scholar]
- 24.Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. 2007;5:31. doi: 10.1186/1477-7819-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Zhang Q, Zhang Y, Lu M, Liu Y, Zheng T, Feng S, Hao M, Shi H. MUC1 Predicts Colorectal Cancer Metastasis: A Systematic Review and Meta-Analysis of Case Controlled Studies. PLoS One. 2015;10:e0138049. doi: 10.1371/journal.pone.0138049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada N, Nishida Y, Tsutsumida H, Goto M, Higashi M, Nomoto M, Yonezawa S. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer. 2009;100:344–351. doi: 10.1038/sj.bjc.6604845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanmugam C, Jhala NC, Katkoori VR, Wan W, Meleth S, Grizzle WE, Manne U. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116:3577–3586. doi: 10.1002/cncr.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterol Res Pract. 2013;2013:431231. doi: 10.1155/2013/431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S, Rachagani S, Sheinin Y, Smith LM, Gurumurthy CB, Roy HK, Batra SK. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene. 2015 doi: 10.1038/onc.2015.327. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909–1916. [PubMed] [Google Scholar]
- 31.Zhang S, Zhang HS, Cordon-Cardo C, Ragupathi G, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry: protein antigens. Clin Cancer Res. 1998;4:2669–2676. [PubMed] [Google Scholar]
- 32.Delker DA, McGettigan BM, Kanth P, Pop S, Neklason DW, Bronner MP, Burt RW, Hagedorn CH. RNA sequencing of sessile serrated colon polyps identifies differentially expressed genes and immunohistochemical markers. PLoS One. 2014;9:e88367. doi: 10.1371/journal.pone.0088367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elzagheid A, Emaetig F, Buhmeida A, Laato M, El-Faitori O, Syrjanen K, Collan Y, Pyrhonen S. Loss of MUC2 expression predicts disease recurrence and poor outcome in colorectal carcinoma. Tumour Biol. 2013;34:621–628. doi: 10.1007/s13277-012-0588-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, Kim H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann Surg Oncol. 2011;18:711–719. doi: 10.1245/s10434-010-1338-z. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsson L, Israelsson A, Oberg A, Palmqvist R, Stenlund H, Hammarstrom ML, Hammarstrom S, Lindmark G. Lymph node CEA and MUC2 mRNA as useful predictors of outcome in colorectal cancer. Int J Cancer. 2012;130:1833–1843. doi: 10.1002/ijc.26182. [DOI] [PubMed] [Google Scholar]
- 37.Melis M, Hernandez J, Siegel EM, McLoughlin JM, Ly QP, Nair RM, Lewis JM, Jensen EH, Alvarado MD, Coppola D, Eschrich S, Bloom GC, Yeatman TJ, Shibata D. Gene expression profiling of colorectal mucinous adenocarcinomas. Dis Colon Rectum. 2010;53:936–943. doi: 10.1007/DCR.0b013e3181d320c4. [DOI] [PubMed] [Google Scholar]
- 38.Shaoul R, Okada Y, Cutz E, Marcon MA. Colonic expression of MUC2, MUC5AC, and TFF1 in inflammatory bowel disease in children. J Pediatr Gastroenterol Nutr. 2004;38:488–493. doi: 10.1097/00005176-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Tatsumi N, Kushima R, Vieth M, Mukaisho K, Kakinoki R, Okabe H, Borchard F, Stolte M, Okanoue T, Hattori T. Cytokeratin 7/20 and mucin core protein expression in ulcerative colitis-associated colorectal neoplasms. Virchows Arch. 2006;448:756–762. doi: 10.1007/s00428-006-0188-3. [DOI] [PubMed] [Google Scholar]
- 40.Ban S, Mitomi H, Horiguchi H, Sato H, Shimizu M. Adenocarcinoma arising in small sessile serrated adenoma/polyp (SSA/P) of the colon: clinicopathological study of eight lesions. Pathol Int. 2014;64:123–132. doi: 10.1111/pin.12147. [DOI] [PubMed] [Google Scholar]
- 41.Imai Y. Poorly differentiated adenocarcinoma of the colon: subsite location and clinicopathologic features. Int J Colorectal Dis. 2015;30:187–196. doi: 10.1007/s00384-014-2070-0. [DOI] [PubMed] [Google Scholar]
- 42.Ding H, Carlton MM, Povoski SP, Milum K, Kumar K, Kothandaraman S, Hinkle GH, Colcher D, Brody R, Davis PD, Pokora A, Phelps M, Martin EW, Jr, Tweedle MF. Site specific discrete PEGylation of (124)I-labeled mCC49 Fab' fragments improves tumor MicroPET/CT imaging in mice. Bioconjug Chem. 2013;24:1945–1954. doi: 10.1021/bc400375f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiernan JP, Perry SL, Verghese ET, West NP, Yeluri S, Jayne DG, Hughes TA. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br J Cancer. 2013;108:662–667. doi: 10.1038/bjc.2012.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hundt S, Haug U, Brenner H. Blood markers for early detection of colorectal cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2007;16:1935–1953. doi: 10.1158/1055-9965.EPI-06-0994. [DOI] [PubMed] [Google Scholar]
- 45.Frykholm G, Enblad P, Pahlman L, Busch C. Expression of the carcinoma-associated antigens CA 19-9 and CA-50 in inflammatory bowel disease. Dis Colon Rectum. 1987;30:545–548. doi: 10.1007/BF02554787. [DOI] [PubMed] [Google Scholar]
- 46.Tucci GF, Grande M, Stroppa I, Federico F, Farinon AM. Tissue expression of carbohydrate antigen 19-9 (CA 19-9) in adenomatous lesions of the colorectum. Chir Ital. 1999;51:165–172. [PubMed] [Google Scholar]
- 47.Wang YR, Yan JX, Wang LN. The diagnostic value of serum carcino-embryonic antigen, alpha fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J Cancer Res Ther. 2014;10309(1):307. doi: 10.4103/0973-1482.151538. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Chen Y, Zhu Z, Ding Y, Ren S, Zuo Y. Differential expression of carbohydrate antigen 19-9 in human colorectal cancer: A comparison with colon and rectal cancers. Mol Clin Oncol. 2013;1:1072–1078. doi: 10.3892/mco.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holst S, Wuhrer M, Rombouts Y. Glycosylation characteristics of colorectal cancer. Adv Cancer Res. 2015;126:203–256. doi: 10.1016/bs.acr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Orntoft TF, Harving N, Langkilde NC. O-linked mucin-type glycoproteins in normal and malignant colon mucosa: lack of T-antigen expression and accumulation of Tn and sialosyl-Tn antigens in carcinomas. Int J Cancer. 1990;45:666–672. doi: 10.1002/ijc.2910450416. [DOI] [PubMed] [Google Scholar]
- 51.Freire T, Medeiros A, Reis CA, Real FX, Osinaga E. Biochemical characterization of soluble Tn glycoproteins from malignant effusions of patients with carcinomas. Oncol Rep. 2003;10:1577–1585. [PubMed] [Google Scholar]
- 52.Pinto R, Carvalho AS, Conze T, Magalhaes A, Picco G, Burchell JM, Taylor-Papadimitriou J, Reis CA, Almeida R, Mandel U, Clausen H, Soderberg O, David L. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J Cell Mol Med. 2012;16:1474–1484. doi: 10.1111/j.1582-4934.2011.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 54.Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987;47:5476–5482. [PubMed] [Google Scholar]
- 55.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 56.Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676–23685. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- 57.Reis CA, David L, Carvalho F, Mandel U, de BC, Mirgorodskaya E, Clausen H, Sobrinho-Simoes M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- 58.Sheer DG, Schlom J, Cooper HL. Purification and composition of the human tumor-associated glycoprotein (TAG-72) defined by monoclonal antibodies CC49 and B72.3. Cancer Res. 1988;48:6811–6818. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.