Abstract
Metabolic activation is a common feature of many cancer cells and is frequently associated with the clinical outcomes of various cancers, including hepatocellular carcinoma (HCC). Thus, aberrantly activated metabolic pathways in cancer cells are attractive targets for cancer therapy. YAP1 and TAZ are oncogenic downstream effectors of the Hippo tumor suppressor pathway, which is frequently inactivated in many cancers. Our study revealed that YAP1/TAZ regulates amino acid metabolism by up-regulating expression of the amino acid transporters SLC38A1 and SLC7A5. Subsequently, increased uptake of amino acids by the transporters activates mTORC1, a master regulator of cell growth, and stimulates cell proliferation. We also show that high expression of SLC38A1 and SLC7A5 is significantly associated with shorter survival in HCC patients. Furthermore, inhibition of the transporters and mTORC1 significantly block YAP1/TAZ-mediated tumorigenesis in the liver. These findings elucidate regulatory networks connecting the Hippo pathway to mTORC1 through amino acid metabolism and the mechanism’s potential clinical implications for treating HCC.
Conclusion
YAP1 and TAZ regulate cancer metabolism and mTOCR1 through regulation of amino acid transportation and two amino acid transporters, SLC38A1 and SLC7A5, might be important therapeutic targets.
Keywords: HCC, Hippo pathway, mTOR pathway, Amino acid transporters, metabolism, genomics
INTRODUCTION
The Hippo pathway is a key regulator of tissue growth and cell fate and frequently deregulated in many human cancers including hepatocellular carcinoma.1–4 When Hippo signaling is deregulated, its downstream regulators YAP1 and TAZ enter the nucleus and increase transcriptional activation of genes involved in cell proliferation and survival. Activation of Yap1 in mouse livers leads to the development of hepatocellular carcinoma (HCC).3, 5, 6 Knockout of Sav1 and Mst1/2, upstream inhibitors of Yap1 and Taz, in the mouse liver also leads to the development of HCC.7–10
YAP1 and TAZ are best known for their involvement in promoting limitless cell growth and proliferation during tumor development.11 This process involves activation of cellular metabolism to support the high demand for the energy and building blocks needed for continuous cell growth and proliferation. Activating metabolism increases uptake of extracellular nutrients, accelerates aerobic glycolysis, increases biosynthesis of building blocks, and enhances anaplerosis and is principally regulated by the same oncogenes that stimulate cell proliferation signaling.12–14 However, the roles of YAP1 and TAZ in metabolic activation remain to be elucidated.
HCC is the sixth most prevalent and the second-most common cause of cancer-related death in the world.15 HCC carries a dismal prognosis and is the second-most lethal cancer type in the US, where its 5-year overall survival rate is around 11%.16 Surgical resection, potentially curative treatment, is only applicable to the small subset of patients diagnosed at the early stages. Unfortunately, conventional or targeted chemotherapies have not been fully developed to have significant impact on overall survival.17 Therefore, it will be important to have better understanding of the underlying biology involved in HCC development for improvement of therapeutic strategies for this deadly malignant disease.
In the current study, we uncovered unexpected new roles for the Hippo pathway in the regulation of cancer metabolism and the activation of mammalian target of rapamycin complex 1 (mTORC1) through the up-regulation of amino acid transporters. Our findings provide therapeutically relevant insights into molecular mechanisms of HCC development that are mediated by YAP1 and TAZ, key downstream effectors of the Hippo pathway.
MATERIALS AND METHODS
A comprehensive description of methods is found in Supporting Information.
Cell Culture and western blots
The HCC cell lines SK-Hep1 and SNU-449 were purchased from ATCC. Normal hepatocyte cell line HepaRG was purchased from ThermoFisher Scientific (HPRGC10). Cells were grown in Dulbecco’s modified essential medium supplemented with 10% fetal bovine serum. Western blot analysis performed as described previously.18 Liver tissues from 4-week-old Mst1/2−/− mutant and wild-type mice were used for Western blots. Antibody list is available in Supporting Information.
ChIP Assay
ChIP experiments were performed as described previously.18 Antibodies and primer sequences are available in Supporting Information.
Expression of YAP1-S127A, SLC7A5 and SLC38A1 in HCC Cells
Wild-type human YAP1 and constitutively active mutant human YAP1 (YAP1-S127A) were previously described19 and obtained from Addgene. Coding sequences of wild-type YAP1 and YAP1-S127A were cloned into a lentiviral vector (pCDH-EF1-MCS-T2A-Puro) at the BamH1 and Not1 sites. HCC cells were infected with purified lentiviral particles from HEK 293 cells and selected with puromycin for overexpression of YAP1-S127A. Myc-tag SLC7A5 and SLC38A1 cDNA clones were purchased from OriGene (RC207604 and RC203903; TrueORF Gold clone). The ORF region of SLC7A1 and SLC38A1 cDNA was cloned into a pCMV6 vector containing a MYC tag at the SgfI and MluI cloning sites.
Reporter Assay
To generate pGL3-SLC38A1 and SLC7A5, the promoter regions from −2,998 to +1 (SLC7A5) and −2,973 to +1 (SLC38A1) were cloned by RT-PCR from human genomic DNA. Sequences were verified by an automatic sequencer. For luciferase-based reporter assays, cells were transfected with the indicated reporter genes and plasmids (YAP1, TAZ, and TEAD1; Addgene) using FuGENE 6 (Roche) according to the manufacturer’s instructions. After 48 hours, cells were harvested for measuring luciferase activity.
Xenograft Experiments
Female athymic nude mice were purchased from the National Cancer Institute’s Frederick National Laboratory for Cancer Research and maintained according to guidelines set forth by the Association for Assessment and Accreditation of Laboratory Animal Care International and the United States Public Health Service policy on Humane Care and Use of Laboratory Animals. All mouse studies were approved and supervised by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. All mice used in the xenograft experiments were between 8 and 12 weeks of age at the time of injection. Xenograft experiments were carried out as described in Supporting Information.
Gene Expression Data
Gene expression data from cell lines and patient tissues are described in Supporting Information.
Mst1/2-Deficient Mouse Models and Rapamycin Treatment
Generation and breeding of Mst1fl/fl and Mst2fl/fl mice were done as described in a previous report.7 The mice were subsequently bred to albumin-Cre mice and then backcrossed to homozygous floxed mice. Rapamycin (LC Laboratories) was dissolved in ethanol at 50 mg/ml and aliquots stored at −80°C. A working solution was made by further dilution into an aqueous solution of 5% Tween 80 and 5% polyethylene glycol 400 to a concentration of 1 mg/ml immediately before use. Rapamycin or an equal volume of vehicle was injected intraperitoneally into Mst1/2−/− or Mst1/2fl/fl mice at a dose of 6 mg/kg of body weight. The treatment was started when the mice were 3 months of age and continued on a schedule of one injection per mouse every other day for 62 days.
Statistical Analysis
We identified genes that were differentially expressed between the two subtypes using a random-variance t-test. Differences in gene expression were considered statistically significant if p < 0.001. Receiver operating characteristic curve analyses were carried out to estimate the discriminatory power of the two amino acid transporters. We calculated the area under the curve, which ranges from 0.5 (for a noninformative predictive marker) to 1 (for a perfect predictive marker), and a bootstrap method (1000 resampling) was used to calculate the 95% CI for the area under the curve. We used multivariate Cox proportional hazards regression analysis to evaluate independent prognostic factors associated with overall survival time, using tumor stage, vascular invasion, tumor grade, and number of liver tumors as covariates.20 A p-value of <0.05 indicated statistical significance, and all statistical tests in this analysis were two-tailed.
RESULTS
Regulation of amino acid transporters by YAP1 and TAZ in HCC cells
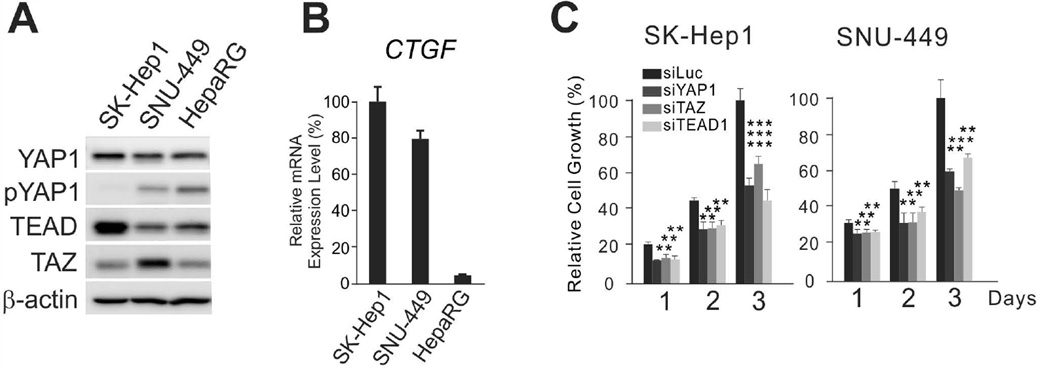
Because recent data suggest that cancer cells have a remarkably active metabolism that allows them to sustain fast proliferation,21, 22 we sought to examine how YAP1 and TAZ regulate cancer metabolism in HCC. We first selected HCC cell lines with activated YAP1 by determining YAP1 phosphorylation status. SK-Hep1 and SNU-449 cells showed higher YAP1 activity than cells from the normal hepatocyte lines HepaRG, as evidenced by the absence of phosphorylation of the S127 residue, a critical negative regulatory site for YAP1 (Fig. 1A). In SK-Hep1 (a YAP1-active cell line), YAP1 was expressed in the nucleus but not in the cytoplasm (Supporting Fig. 1A,B). In addition, expression of CTGF, the best-known downstream target of YAP1,23 was significantly higher in SK-Hep1 and SNU-449 than in HepaRG (Fig. 1B), supporting the idea that YAP1 is highly activated in HCC cell lines. Furthermore, when expression of YAP1 was silenced by small interfering RNAs (siRNAs), growth of SK-Hep1 and SNU-449 was significantly attenuated (Fig. 1C and Supporting Fig. 2), suggesting that YAP1 is necessary for maintaining the growth of SK-Hep1 and SNU-449 HCC cells. Silencing TEAD1, a key partnering transcription factor of YAP1 and TAZ,1 had a similar effect on the growth of these cells (Fig. 1C), further supporting that proliferation of SK-Hep1 and SNU-449 is largely mediated by YAP1 and TAZ.
Figure 1. YAP1 and TAZ are necessary for proliferation of HCC cells.
(A) Expression of YAP1, TAZ, and TEAD1 and phosphorylation of YAP1 were assessed in HCC (SK-Hep1 and SNU-449) and normal hepatocyte cell line (HepaRG) by Western blotting with indicated antibodies.
(B) CTGF mRNA expression was measured by quantitative RT-PCR.
(C) SK-Hep1 and SNU-449 HCC cells were transiently transfected with indicated siRNAs, and cell proliferation rates were measured by MTT assay at the indicated time points. Values shown were normalized to luciferase-specific siRNA (siLuc)-treated cells and represent mean ± standard deviation.
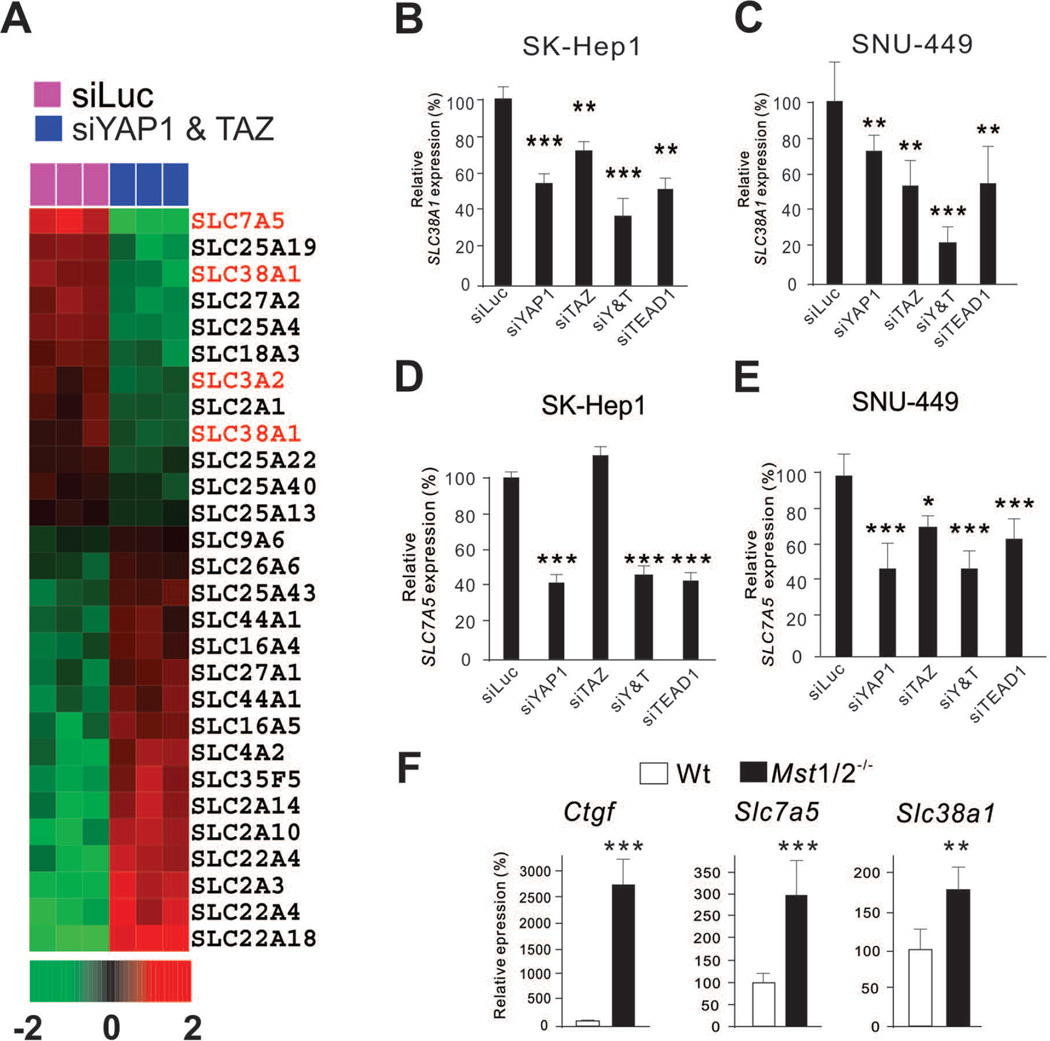
Because uptake of extracellular nutrients into cells is the first rate-limiting step for all metabolic pathways and a critical step in metabolic activation, we examined expression of metabolite transporters (solute carrier [SLC] family members) before and after silencing YAP1 and TAZ in SK-Hep1 cells. To do this, we carried out microarray experiments, extracted expression data of all SLC family members, and identified genes that were differentially expressed upon silencing of YAP1 and TAZ using a stringent cut-off (p < 0.001). Most interestingly, amino acid transporters were the most down-regulated functional group among the SLC members (Fig. 2A). In particular, expression levels of SLC7A5 and SLC38A1 were significantly down-regulated after silencing of YAP1 and TAZ (P = 0.0001 and P = 0.0002, respectively). SLC38A1 and SLC7A5 transport glutamine and essential amino acids, respectively,24 suggesting that YAP1 and TAZ regulate the first rate-limiting step of amino acid metabolism in HCC cells. Down-regulation of both SLC38A1 and SLC7A5 was confirmed by quantitative reverse transcription–polymerase chain reaction (RT-PCR) after silencing YAP1 in SK-Hep1 cells (Fig. 2B,C, and Supporting Fig. 2). Moreover, the expression of these transporters was also down-regulated by silencing of TEAD1. Silencing of YAP1, TAZ, and TEAD1 expression in another YAP1-active HCC cell line, SNU-449, further validated the tight regulation of SLC38A1 and SLC7A5 expression by YAP1 and TAZ (Fig. 2D,E). Of note, while expression of SLC38A1 was strongly affected by both YAP1 and TAZ, expression of SLC7A5 was significantly affected only by YAP1, supporting the notion that YAP1 and TAZ share similar functional roles but with slightly different specificities.25
Figure 2. YAP1 and TAZ regulate expression of SLC38A1 and SLC7A5 in HCC cells.
(A) Expression pattern of SLC family genes from microarray data. Amino acid transporters are shown in red.
(B–E) Indicated cells were transfected with indicated siRNAs. The cells were used for quantitative RT-PCR to measure SLC38A1 and SLC7A5. Expression level was normalized with siLuc samples.
(F) Quantitative RT-PCR was done with Mst1/2 knockout samples to measure CTGF, SLC38A1, and SLC7A5 mRNA expression level. *p < 0.05, **p < 0.01, ***p < 0.005 by Student t-test.
To validate the regulation of SLC38A1 and SLC7A5 by YAP1 and TAZ in vivo, we measured the expression of the transporters in livers from conditional Mst1/2−/− mice. In these mice, Yap1 and Taz are constitutively activated owing to the inactivation of Mst1 and Mst2, upstream regulators of Yap1 and Taz.7, 9, 10 Consistent with the data from cell lines, expression levels of both Slc38a1 and Slc7a5 were significantly higher in Mst1/2−/− mice than in wild-type mice (Fig. 2F). Taken together, our data strongly demonstrated that YAP1 and TAZ regulate expression of two amino acid transporters.
YAP1 and TAZ regulate glutamine metabolism through a glutamine transporter in HCC
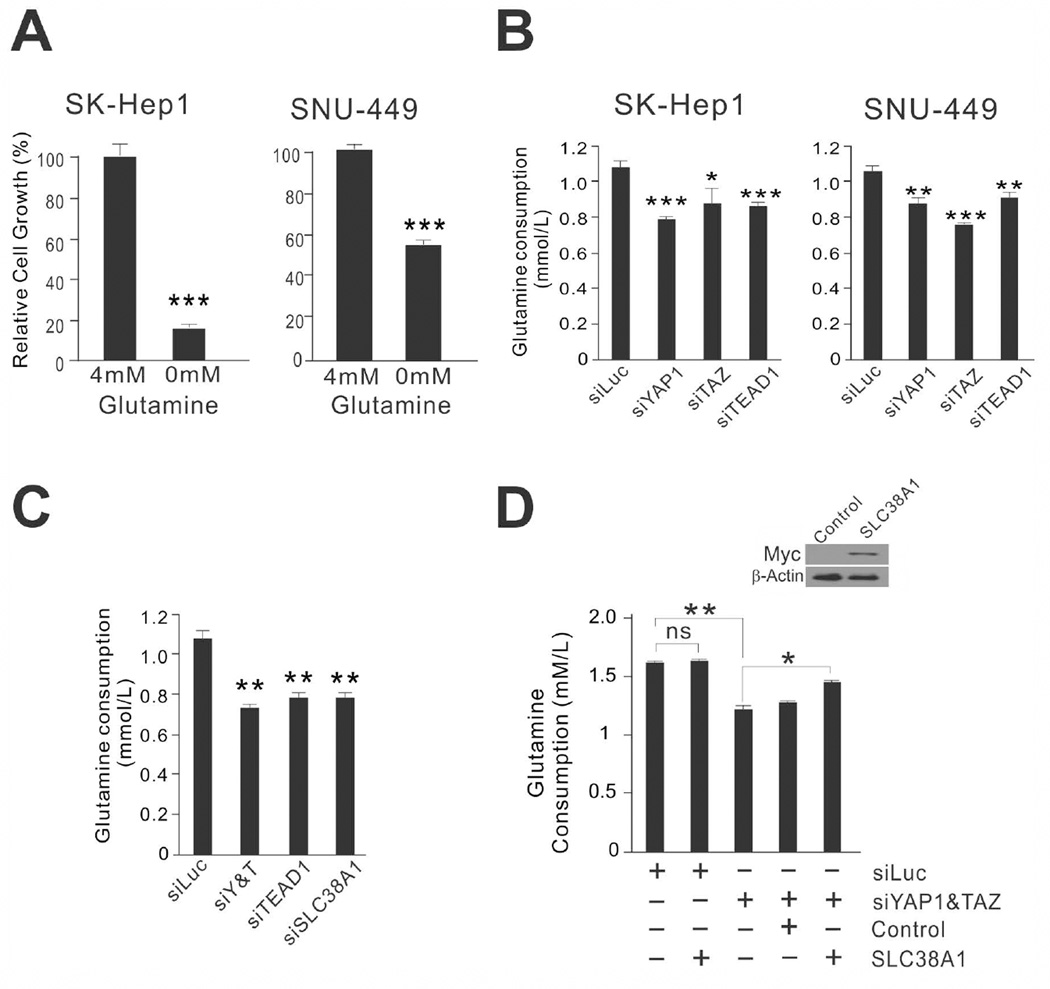
Because SLC38A1 is a glutamine transporter and glutamine is an important amino acid for both ATP generation and biosynthesis of building blocks,26, 27 we sought to test the relationship between YAP1 activation and glutamine dependency in HCC cells. Depletion of glutamine from culture media significantly reduced the growth of SK-Hep1 and SNU-449 cells (Fig. 3A), supporting the notion that glutamine is an important metabolite for proliferation of YAP1-active HCC cells.
Figure 3. Glutamine is necessary for growth of YAP1/TAZ-active HCC cells.
(A) Growth of HCC cells under conditions of glutamine abundance or deprivation. Data are the mean ± standard deviations of three independent cell cultures grown in the presence or absence of glutamine for 48 hours. *** P < 0.001 by Student t-test.
(B) SK-Hep1 and SNU-449 cells were transiently transfected with indicated siRNA for 72 hours. The medium was assayed for glutamine level. The data were normalized to cell number. *p < 0.05, **p < 0.01, ***p < 0.005 by Student t-test. ns: not significant.
(C,D) Consumed glutamine level from the medium after transfection of indicated siRNAs (C) or after expression of exogenous SLC38A1 in YAP1/TAZ-depleted SK-Hep1 cells (D). Cells were transfected with indicated siRNAs or expression vectors for 48 hours. The data were normalized to number of cells. *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test.
We next tested whether glutamine consumption is regulated by YAP1 and TAZ in SK-Hep1 and SNU-449 cells. The rate at which glutamine was consumed from the medium was significantly reduced by silencing of YAP1, TAZ, or TEAD1 expression (Fig. 3B). When expression of SLC38A1 was silenced by specific siRNA, consumption of glutamine was significantly reduced, as seen when YAP1 and TAZ were silenced (Fig. 3C), indicating that glutamine uptake in YAP1-active HCC cells is largely mediated by SLC38A1. Furthermore, glutamine consumption in YAP1/TAZ-depleted SK-Hep1 HCC cells was rescued by re-expression of exogenous SLC38A1 (Fig. 3D). Collectively, these data indicate that glutamine is necessary for the proliferation of YAP1-active HCC cells and that YAP1 actively regulates uptake of glutamine by controlling expression of the glutamine transporter SLC38A1.
SLC7A5 and SLC38A1 are necessary for YAP1/TAZ-mediated proliferation of HCC cells
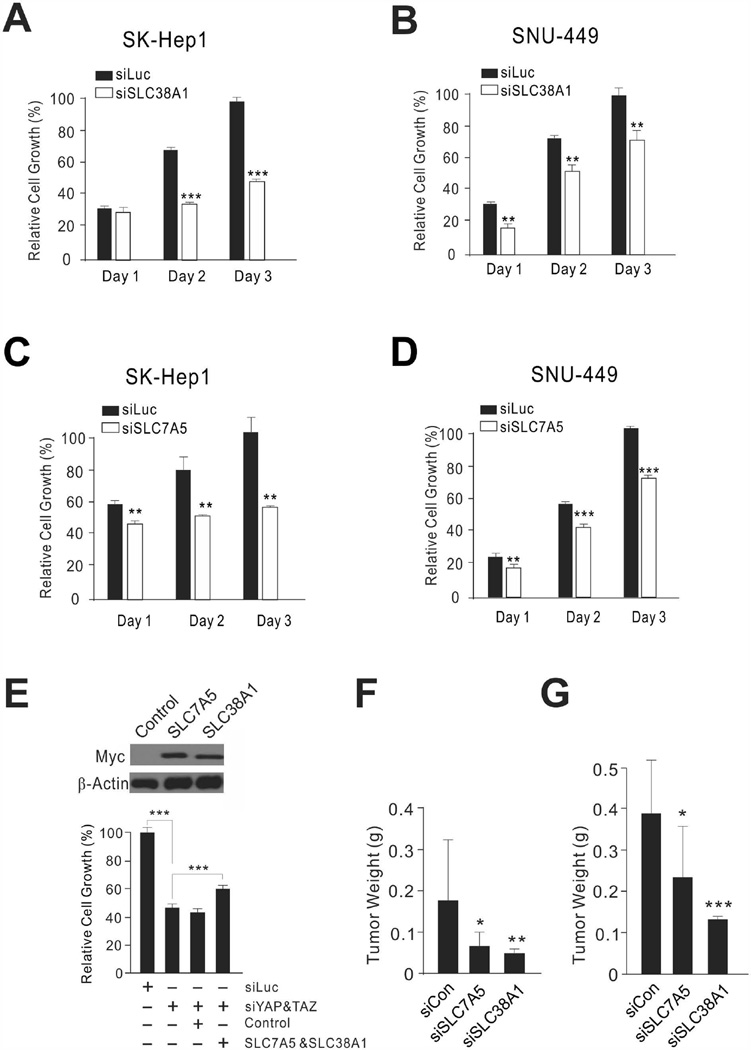
Our findings suggested that YAP1/TAZ-mediated uptake of amino acids is necessary for the proliferation of HCC cells. Thus, we tested whether expression of SLC38A1 and SLC7A5 is necessary for YAP1-mediated cell proliferation. As with silencing of YAP1, TAZ, and TEAD1, which significantly reduced the growth of SK-Hep1 and SNU-449 cells (Fig. 1C), silencing of either SLC38A1 or SLC7A5 expression significantly inhibited cell growth (Fig. 4A–D and Supporting Fig. 3). Furthermore, when exogenous SLC38A1 and SLC7A5 were introduced by expression vectors to YAP1/TAZ-depleted SK-Hep1 cells, cell growth was significantly recovered (Fig. 4E), suggesting that YAP1/TAZ-mediated cell proliferation is, in part, dependent on both uptake of glutamine by SLC38A1 and uptake of essential amino acids by SLC7A5.
Figure 4. Amino acid transporters are important for proliferation of HCC cells.
(A–D) SK-Hep1 and SNU-449 HCC cells were transiently transfected with indicated siRNAs, and cell proliferation rates were measured by MTT assay at the indicated time points. Values shown were normalized to siLuc-treated cells and represent mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test.
(E) Recovered cell growth by exogenous SLC38A1 and SLC7A5 in YAP1/TAZ-depleted SK-Hep1 cells. Myc-tagged exogenous SLC38A1 and SLC7A5 were expressed in SK-Hep1 cells. Empty expression vector pCMV6 was used as a control. Cell proliferation rates were measured by MTT assay at 72 hours after transfection of expression vectors. *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test.
(F,G) Tumor weight after treatment with siRNA-DOPC in a subcutaneous xenograft model (F) or orthotopic xenograft model (G) with SK-Hep1 HCC cells. At 6 weeks after siRNA-DOPC injection, mice were killed and tumor weights measured (n = 10 per group) Data are presented as mean ±standard error of the mean (SEM). *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test.
We next tested the in vivo effects of silencing SLC38A1 and SLC7A5 in mouse xenograft models. SK-Hep1 cells were subcutaneously transplanted into athymic nude mice, and tumor growth was monitored. Once palpable tumors were detected, mice were randomized to receive either SLC38A1-specific siRNA mixed with neutral nanoliposomes or an SLC7A5-specific siRNA mixture (n = 10 per group). In parallel, control mice were treated with unrelated siRNA. Consistent with our in vitro observations, tumor weight was significantly reduced by the silencing of SLC38A1 or SLC7A5 expression in subcutaneous xenograft models (Fig. 4F and Supporting Fig. 4A). Moreover, significant reduction of tumor growth by silencing of SLC38A1 or SLC7A5 was further demonstrated in an orthotopic liver xenograft mouse model, which is clinically more relevant to human HCC (Fig. 4G and Supporting Fig. 4B). Taken together, these results clearly demonstrated that SLC38A1 and SLC7A5 play critical roles in the growth and progression of HCC tumors. Importantly, we silenced expression of SLC38A1 and SLC7A5 by using specific siRNAs to inhibit their transporter activities even though chemical inhibitors for SLC38A1 and SLC7A5 are available because the inhibitors lack specificity for SLC38A1 and SLC7A5. For instance, α-(methylamino)isobutyric acid (MeAIB) is a competitive inhibitor of all members of the system A transporter family, including SLC38A1,28 and 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) is an inhibitor of all members of the system L transporter family, including SLC7A5.29
YAP1 and TAZ directly regulate expression of SLC38A1 and SLC7A5
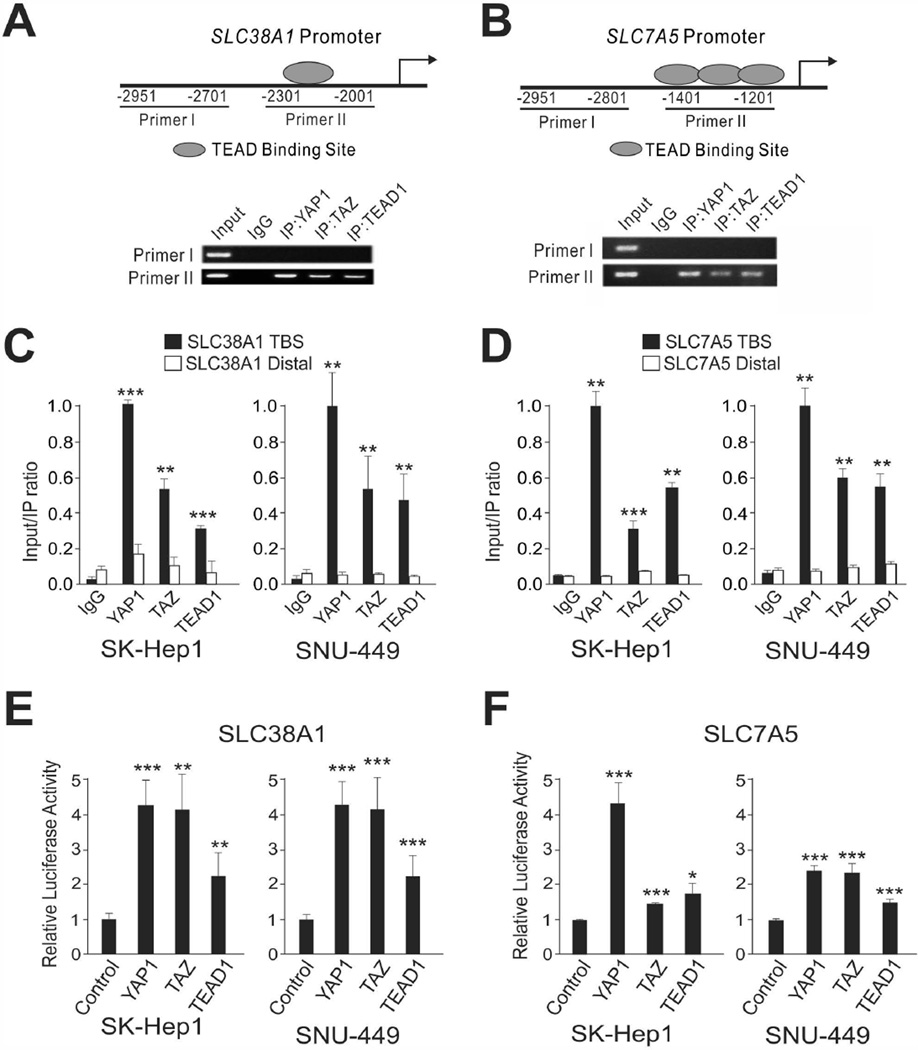
Because our data clearly demonstrated that SLC38A1 and SLC7A5 are necessary for YAP1/TAZ-mediated cell proliferation, we next sought to determine whether YAP1 and TAZ directly bind to promoter regions of SLC38A1 and SLC7A5 and thereby regulate their expression. To do this, we carried out chromatin immunoprecipitation (ChIP) assays using antibodies against YAP1, TAZ, and TEAD1. TEAD binding sites and upstream non–TEAD binding sites of SLC38A1 and SLC7A5 were measured and quantified from the immunoprecipitates (Fig. 5A–D). The results revealed that all three antibodies could enrich the TEAD binding promoters, suggesting that YAP1, TAZ, and TEAD1 directly bind to the promoters of SLC38A1 and SLC7A5 genes.
Figure 5. YAP1 and TAZ Directly Bind to the Promoters of Amino Acid Transporters.
(A) Schematic representation of SLC38A1 promoter regions for ChIP assay (top) and ChIP assay results (bottom). (B) Schematic representation of SLC7A5 promoter regions for ChIP assay (top) and ChIP assay results (bottom). ChIP assay was done in SK-Hep1 cells with indicated antibodies. Recruitment of YAP1, TAZ, and TEAD1 proteins to the SLC38A1 or SLC7A5 promoter was analyzed using primers specific to the SLC38A1 or SLC7A5 promoter. Immunoglobulin G (IgG) was used as an internal control.
(C,D) ChIP samples were used for quantitative RT-PCR for quantification of binding in indicated cell lines. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test.
(E,F) SK-Hep1 and SNU-449 cells were transiently transfected with indicated cDNA and reporter plasmids, and luciferase activity was measured by Luminometer (Promega). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005 by Student t-test. TBS: TEAD binding site. IP: immunoprecipitation.
To validate this result, we next carried out luciferase assays using a reporter carrying a DNA fragment with the SLC38A1 and SLC7A5 promoters. Consistent with the ChIP assays, the luciferase assays revealed that YAP1, TAZ, and TEAD1 activated transcription activity from both the promoters (Fig. 5E,F). Taken together, these data strongly demonstrated that YAP1 and TAZ directly regulate expression of SLC38A1 and SLC7A5 by binding to their promoters.
SLC38A1 and SLC7A5 regulate mTORC1 activity in HCC tumors
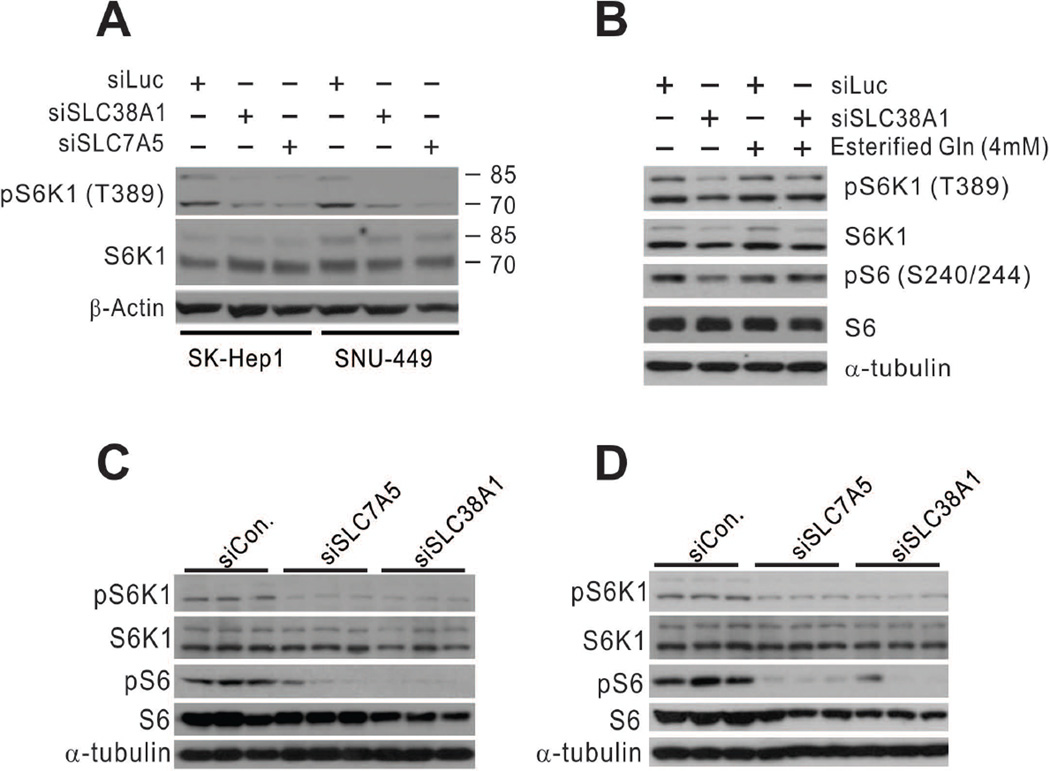
Amino acids are essential for the master growth regulator mTORC1 signaling pathway. Leucine (preferred substrate of SLC7A5) and glutamine (preferred substrate of SLC38A1) are the two most important amino acids for mTORC1 activity.30–33 Thus, we tested whether silencing of SLC38A1 and SLC7A5 expression would reduce mTORC1 activity in HCC cells. Direct silencing of SLC38A1 and SLC7A5 expression reduced mTORC1 activity in SK-Hep1 and SNU-449 cells, as evidenced by reduced phosphorylation of the T389 residue of S6K1, the best-known substrate of mTORC1 (Fig. 6A). This result agrees very well with a previous study’s finding that amino acid transporters are upstream regulators of mTORC1.32 We further tested whether mTORC1 inactivation by silencing of SLC38A1 is a direct result of reduced glutamine transporter activity in HCC cells. We treated SLC38A1-depleted SK-Hep1 cells with membrane-permeable esterified glutamine and found that phosphorylation of the T389 residue of S6K1 and S240/244 residues of S6 was recovered after the treatment (Fig. 6B), clearly indicating that uptake of glutamine is a mechanism for mTORC1 activation by SLC38A1,whose expression is regulated by YAP1/TAZ in HCC cells.
Figure 6. mTORC1 is regulated by SLC38A1 and SLC7A5 in HCC cells.
(A) SK-Hep1 and SNU-449 HCC cells were transiently transfected with indicated siRNAs for 96 hours. Phosphorylation of S6K1 was assessed by Western blotting with indicated antibodies.
(B) SK-Hep1 cells were treated with esterified glutamine (Gln) (4 mM) for 30 minutes after silencing of SLC38A1 with specific siRNA. Expression and phosphorylation of S6K1 and S6 were assessed in cell lysates by Western blotting with indicated antibodies.
(C,D) Xenografted HCC tissues treated with indicated siRNAs were used for Western blot analysis with indicated antibodies.
We next sought to examine mTORC1 activity in vivo after silencing of SLC38A1 or SLC7A5 in mouse xenograft models. Consistent with the cell line experiments, silencing of SLC38A1 or SLC7A5 by specific siRNAs attenuated mTORC1 activity, as evidenced by reduced phosphorylation of S6K1 and S6 in a subcutaneous xenograft model (Fig. 6C). Furthermore, reduced mTORC1 activity by silencing of SLC38A1 or SLC7A5 was further demonstrated in an orthotopic liver xenograft mouse model (Fig. 6D). Taken together, these results clearly demonstrated that SLC38A1 and SLC7A5 play critical roles in the regulation of mTORC1 activity in HCC tumors.
YAP1/TAZ regulates mTORC1 activity via SLC38A1 and SLC7A5
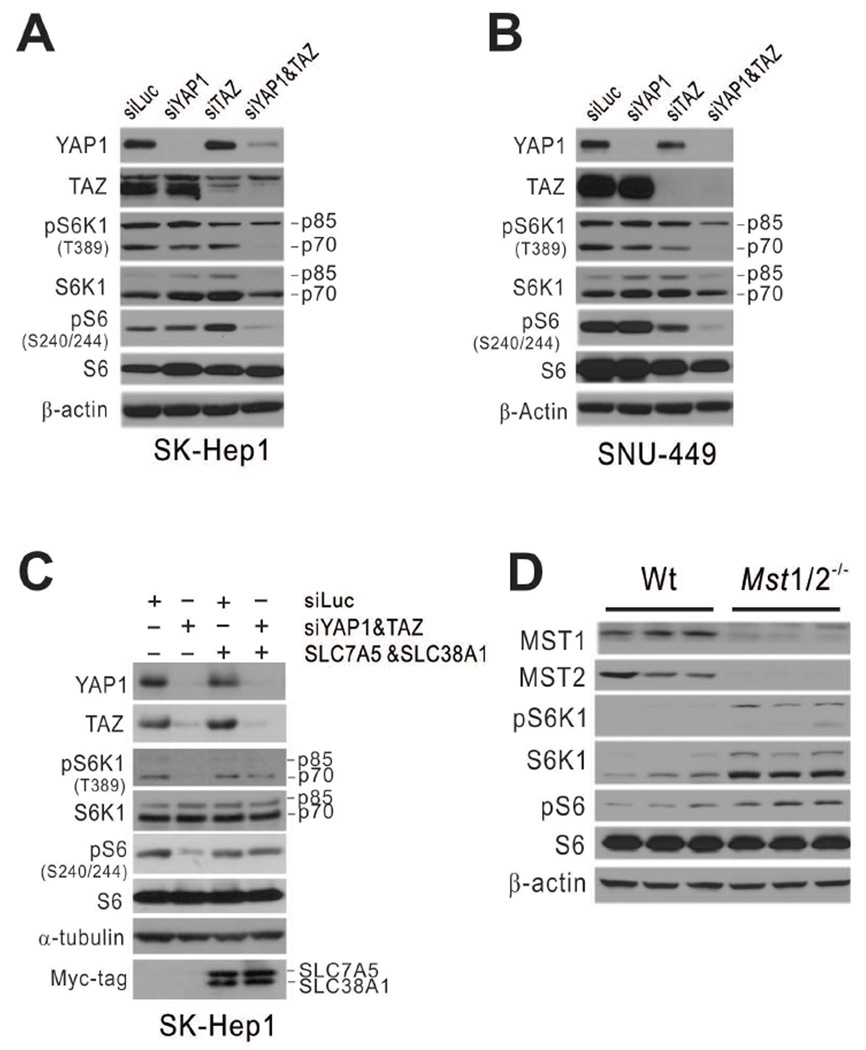
Because our data demonstrated that YAP1/TAZ regulates expression of SLC38A1 and SLC7A5, which are necessary for maintaining mTORC1 activity in HCC cells, we hypothesized that YAP1/TAZ regulates mTORC1 activity by regulating SLC38A1 and SLC7A5. When expression of both YAP1 and TAZ were silenced by specific siRNAs in SK-Hep1 cells, phosphorylation of S6K1 and S6 was substantially reduced (Fig. 7A), suggesting that YAP1/TAZ is necessary for maintaining mTORC1 activity in HCC cells. Similar reduction of mTORC1 activity upon silencing of YAP1 and TAZ expression was also observed in SNU-449 cells (Fig. 7B), further supporting the idea that YAP1/TAZ regulates mTORC1 in HCC cells.
Figure 7. YAP1 and TAZ activate mTORC1 through regulation of SLC38A1 and SLC7A5.
(A–B) SK-Hep1 (A) and SNU-449 (B) HCC cells were transiently transfected with indicated siRNA for 96 hours. Expression of YAP1 and TAZ and phosphorylation of S6K1 and S6 were assessed by Western blotting with indicated antibodies.
(C) SK-Hep1 cells were transfected with SLC38A1 and SLC7A5 cDNA after silencing YAP1 and TAZ with specific siRNAs. Expression and phosphorylation of S6K1 and S6 were assessed in cell lysates by Western blotting with indicated antibodies.
(D) Expression of Mst1, Mst2, S6K1, and S6 and phosphorylation of S6K1 and S6 were assessed in liver tissues from wild-type (Wt) and Mst1/2−/− mice (4 weeks old) by Western blotting with indicated antibodies.
To determine whether regulation of mTORC1 by YAP1/TAZ is mediated by SLC38A1 and SLC7A5 whose expression is regulated by YAP1 and TAZ, we next attempted to rescue mTORC1 activity by expressing exogenous SLC38A1 and SLC7A5 in YAP1/TAZ-depleted HCC cells. Introduction of exogenous SLC38A1 and SLC7A5 successfully recovered phosphorylation of S6K1 and S6 (Fig. 7C), suggesting that mTORC1 activation by YAP1/TAZ is mediated by the uptake of essential amino acids (including leucine and glutamine) through increased expression of SLC38A1 and SLC7A5.
To validate functional correlation of mTORC1 activation by YAP1/TAZ in vivo, we assessed the phosphorylation status of S6K1 and S6 in liver tissues from Mst1/2−/− mutant mice, in which Yap1 and Taz are constitutively activated. Consistent with the data from cell lines, mTORC1 activity in liver tissue was substantially greater in Mst1/2−/− mice than in wild-type mice, as evidenced by the increased phosphorylation of S6k1 and S6 in Mst1/2−/− mice (Fig. 7D).
Clinical Relevance and Significance of YAP1/TAZ-Mediated mTORC1 Activation in HCC
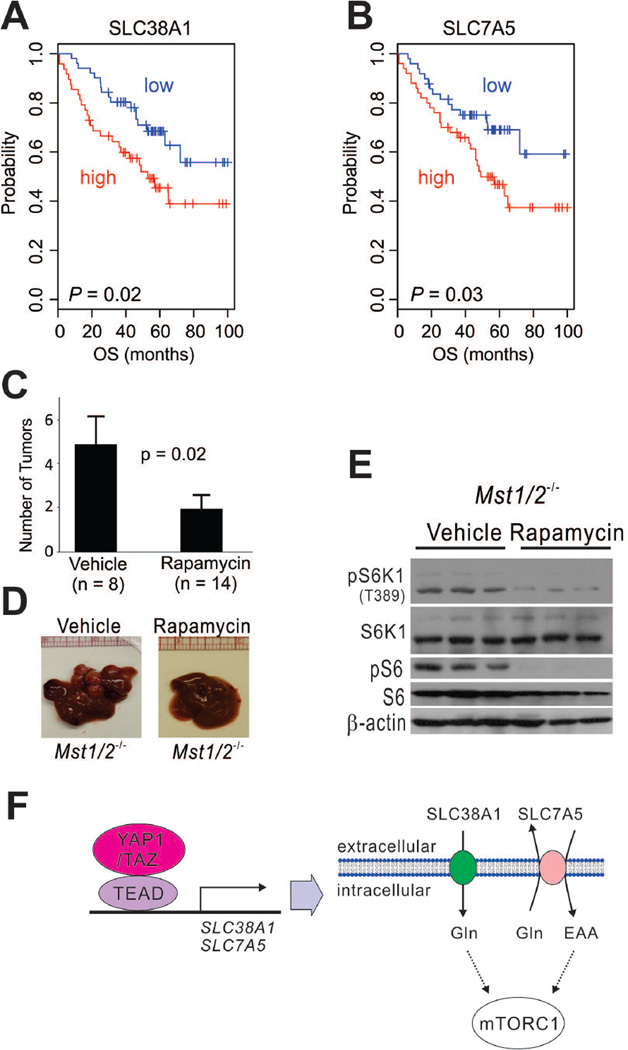
We next investigated the clinical significance of SLC38A1 and SLC7A5 in HCC patients using gene expression profile data generated from our laboratory (Cohort 1, n = 100). When patients were dichotomized according to expression of SLC38A1 using median expression value as a cut-off, we found that high expression of SLC38A1 was significantly associated with shorter overall survival (Fig. 8A). Likewise, high expression of SLC7A5 was significantly associated with shorter overall survival (Fig. 8B), suggesting that these transporters contribute to HCC development and progression. Likewise, in receiver operating characteristic analysis, which measures area under a curve to assess the prognostic power of potential markers, expression of both SLC38A1 and SLC7A5 was a significant predictor for overall survival of HCC patients. Areas under the curves plotting SLC38A1 and SLC7A5 expression levels as they relate to 5-year overall survival were 66.6% (95% confidence interval [CI], 54.6%–78.5%, P = 0.003) and 65% (95% CI, 55.7%–76.5%, P = 0.007), respectively (Supporting Fig. 5A,B). Importantly, combining data on the expression patterns of SLC38A1 and SLC7A5 substantially improved prognostication of patients, as evidenced by the facts that patients with high expression of both transporters had the worst prognosis while patients with low expression of both transporters had the best prognosis compared with those with high expression of one transporter and low expression of the other (Supporting Fig. 5C).
Figure 8. Significance of YAP1/TAZ-mediated regulation of mTORC1 in human HCC and mouse HCC models.
(A–B) Kaplan-Meier plots of overall survival (OS) of HCC patients in Cohort 1. Patients were stratified according to expression level of SLC38A1 (a) or SLC7A5 (b). P-values were calculated with the log-rank test.
(C) Liver tumor numbers after rapamycin treatment in Mst1/2−/− mice. At 62 days after rapamycin treatment, mice were killed and tumors counted. Data are presented as mean ±SEM.
(D) Representative images of liver tissue treated with rapamycin or vehicle.
(E) Expression and phosphorylation of mTOR1 downstream targets in HCC tissues assessed by Western blotting with indicated antibodies after rapamycin or vehicle treatment in Mst1/2−/− mice.
(F) Schematic diagram of direct regulation of amino acid uptake and indirect regulation of mTORC1 by YAP1 and TAZ. EAA: essential amino acids
Furthermore, the expression of the two transporters was significantly associated with prognostic subgroup as stratified by 65-gene prognostic risk scores.34 Expression levels of SLC38A1 and SLC7A5 were significantly higher in the high-risk prognostic subgroup than in the low-risk prognostic subgroup (P = 1.2 × 10−11 for SLC38A1 and P = 3.1 × 10−6 for SLC7A5; Supporting Fig. 6A,B). Cox proportional hazards regression analysis also indicated that high expression of SLC38A1 or SLC7A5 is an independent predictor for shorter survival in HCC patients (Supporting Table 1). For further validation of the prognostic significance of these transporters, we used publicly available gene expression data from HCC patients. Consistent with the data from the first cohort, expression of the two transporters remained significantly associated with prognosis in two additional cohorts of HCC patients (Supporting Fig. 5D,E, and Supporting Fig. 6C–F). Taken together with the up-regulation of Slc38a1 and Slc7a5 in the livers of Mst1/2−/− mice and the indispensability of SLC38A1 and SLC7A5 for tumor formation in xenograft models, these data strongly suggested that SLC38A1/SLC7A5-mediated activation of mTORC1 by YAP1/TAZ is a key regulatory pathway for HCC development and progression. Furthermore, our data is in good agreement with previous observation showing that higher expression of YAP1 and TAZ is significantly associated with poor prognosis in HCC.35–39
Because our data strongly suggested that mTORC1 is a major downstream effector of oncogenic activation of YAP1/TAZ during HCC development, we next tested whether inhibition of mTORC1 is sufficient to block or delay Yap1/Taz-mediated tumor development in Mst1/2−/− mice. We treated Mst1/2−/− mice with the mTORC1 inhibitor rapamycin or with a control to assess the potential therapeutic benefit of mTORC1 inhibition in Yap1/Taz-driven HCC. Mst1/2−/− mice treated with rapamycin had significantly fewer tumors than the control-treated mice (P = 0.02; Fig. 8C–E). Of note, five of the 14 rapamycin-treated mice had no tumors, suggesting that tumor development was completely blocked in some mice. These data strongly suggest that mTORC1 activity is necessary for the development of YAP1/TAZ-driven HCC, as we predicted from our cell culture experiments.
DISCUSSION
Regulation of cell cycle genes and uptake of extracellular nutrients are essential for the proliferation of cancer cells. Therefore, oncogenic signals that stimulate cell proliferation must also participate in the activation of cellular metabolism. While the roles of YAP1 and TAZ in regulation of the cell cycle have been well documented,1, 2, 4 regulation of cellular metabolism by YAP1 and TAZ has remained elusive. The present study demonstrated that YAP1 and TAZ regulate the uptake of amino acids and thereby activate mTORC1 by up-regulating expression of the amino acid transporters SLC38A1 and SLC7A5 (Fig. 8F).
Because amino acids are essential for cell proliferation, both as the building blocks of new proteins and as metabolic precursors, regulation of the activity of mTORC1, master regulator of cell growth, by amino acids is necessary for its integration of nutrients into extracellular growth signals. The two-step process for activation of mTORC1 by amino acids32 comprises first an increase in the intracellular concentration of glutamine by a Na+-dependent amino acid transporter and then use of the intracellular glutamine as an efflux substrate by a bidirectional transporter, such as SLC7A5, to stimulate uptake of extracellular essential amino acids, such as leucine. This uptake activates mTORC1, demonstrating that these amino acid transporters are upstream regulators of mTORC1. However, the molecular basis for the regulation of amino acid transporters and the clinical significance of dysregulating their expression are currently unknown. Our findings strongly suggest that YAP1 and TAZ regulate expression of the Na+-dependent amino acid transporter SLC38A1 and the bidirectional transporter SLC7A5 to regulate uptake of glutamine and leucine, the two most important amino acids for activation of mTORC1.30–33 Silencing of YAP1 and TAZ as well as SLC38A1 and SLC7A5 consistently and substantially reduced mTORC1 activity in HCC cells. High expression of these transporters in the YAP1-active HCC cells strongly supports the suggestion that up-regulation of transporter expression by YAP1 and TAZ in HCC activates mTORC1. The significantly higher phosphorylation of S6, reflecting mTORC1 activity, in Yap1/Taz-active mouse liver tissues further supports this suggestion. Furthermore, the significant association of both amino acid transporters with poor prognosis in patients with HCC agrees well with the findings of previous studies demonstrating a significant association of mTORC1 activation with poor prognosis in patients with HCC40, 41 and also supports the notion that mTORC1 activation in human HCC is mediated by SLC38A1 and SLC7A5 that are up-regulated by YAP1/TAZ.
Expression of YAP1 and SLC7A5 is also increased in regenerating liver after partial hepatectomy and intoxication with carbon tetrachloride,42, 43 further supporting the importance of YAP1-mediated SLC7A5 expression and subsequent activation of mTORC1 for cell proliferation in the liver. Our data also in good agreement with previous study demonstrating higher expression of SLC38A1 in HCC tissues than normal liver.44 Interestingly, expression of SLC7A5 was more tightly regulated by YAP1 than by TAZ, suggesting that YAP1 directly regulates mTORC1 by orchestrating uptake of extracellular leucine, whereas YAP1 and TAZ were equally important for the uptake of glutamine, which acts as an efflux substrate as well as a key anaplerotic metabolite for fast proliferation of cancer cells.27 Our data strongly support the current model postulating activation of mTORC1 by amino acid transporters32 and, furthermore, suggest that the model be updated to recognize that YAP1 and TAZ orchestrate cell proliferation by regulating both cell cycle and uptake of amino acids.
Silencing of YAP1 and TAZ significantly reduced mTORC1 activity and growth of HCC cells, and more importantly, mTORC1 inactivation and growth inhibition by depletion of YAP1/TAZ were rescued by exogenous SLC38A1 and SLC7A5 and by membrane-permeable esterified glutamine, clearly indicating that SLC38A1 and SLC7A5 are key downstream effectors of YAP1/TAZ-mediated activation of mTORC1 and cell growth. It is also important to note that growth of YAP1/TAZ-depleted cells was not fully rescued by exogenous SLC38A1 and SLC7A5, suggesting that amino acid transporters are not the only mediators of mTORC1 activation and cell growth stimulation by YAP/TAZ. Indeed, an independent study indicated cross-talk between YAP1/TAZ and the PTEN-PI3K-AKT pathway,45 suggesting that PI3K-AKT is another regulatory route for YAP1/TAZ-mediated mTORC1 activation and cell growth in HCC. However, the indispensability of SLC38A1 and SLC7A5 in tumor formation and growth in our mouse xenograft experiments clearly demonstrated that these amino acid transporters are necessary for YAP1/TAZ-driven HCC development even though they are not the sole downstream mediators of YAP1/TAZ-driven tumorigenesis. Because SLC38A1 and SLC7A5 may have additional molecular activities, it is also important to note that their non-transporter activities might be important for regulation of cell proliferation.
Because of its roles in the development of many cancers, the Hippo-YAP1/TAZ pathway has been actively pursued as a target for cancer treatment. A screening of a drug library identified the porphyrin family, including verteporfin, hematoporphyrin, and protoporphyrin IX, as inhibitors of YAP1.46 These compounds inhibit the interaction between YAP1 and TEAD. However, it remains to be elucidated whether these porphyrins are effective in the treatment of established cancers because they have relatively low affinity for YAP1. A recent study identified VGLL4 as a new negative regulator of YAP1 and proposed that peptides mimicking VGLL4 can suppress YAP1-mediated tumor growth.47 Our present study broadens the set of potential therapeutic targets of the Hippo-YAP1/TAZ pathway to include amino acid transporters, such as SLC38A1 and SLC7A5, and mTORC1 and sheds light on strategies for inhibiting this pathway for therapeutic benefit. Importantly, all of the newly identified therapeutic targets are currently druggable. The application of mTORC1 inhibitors, including rapamycin, for treatment of cancer patients is currently an area of intense clinical study.48 MeAIB, a competitive inhibitor of SLC38A1, has been used to inhibit liver regeneration in a mouse model.49 SLC7A5, another proposed target, is up-regulated in many cancers, and BCH, an inhibitor of SLC7A5, effectively inhibited the growth of many cancer cells.50 However, further studies will be necessary to determine the therapeutic potential of this approach in vivo and their safety when used for treating cancer patients because of lack of specificity of these inhibitors.
In conclusion, our data show that SLC38A1 and SLC7A5 are required for YAP1/TAZ-mediated mTORC1 activation and critical for tumor formation, growth, and progression in HCC, as evidenced by their indispensability in tumor formation and growth in mouse models and the significant association between their expression and HCC patients’ survival. Furthermore, our data suggested that SLC38A1, SLC7A5, and mTORC1 are potential therapeutic targets in HCC with activated YAP1/TAZ. However, more specific inhibitors of SLC38A1 and SLC7A5 or of antibodies that can neutralize these transporters’ activity will need to be developed to ascertain the efficacy and safety of targeting these amino acid transporters in clinical settings.
Supplementary Material
Acknowledgments
Financial Support: This research was supported in part by the 2011 and 2012 cycles of The University of Texas MD Anderson Cancer Center’s Sister Institute Network Fund (J-S.L.), 5U54 CA112970-08 (G.B.M.), 5P01CA099031-07 (G.B.M.), P30 CA016672 (G.B.M.), MD Anderson Cancer Center’s Support Grant under award number CA016672 from the National Institutes of Health, Bio R&D Program Grant M10642040002-07N4204-00210 (W.J.), and Grant 2011-0018055 (W.J.), Grant NRF-2013R1A2A2A05005990 (Y.N.P.), and NRF-2014R1A1A2053529 (Y-Y.P) from the National Research Foundation of Korea.
Abbreviations
- HCC
hepatocellular carcinoma
- YAP1
Yes-associated protein 1
- TAZ
transcriptional co-activator with PDZ-binding motif
- MST1/2
mammalian sterile 20-like ½
- SAV1
Salvador homolog 1
- LATS1/2
large tumor suppressor-like ½
- MOB1
Mps one binder kinase activator-like 1
- SLC38A1
Solute Carrier Family 38, Member 1
- SLC7A5
Solute Carrier Family 7, Member 5
- mTORC1
mammalian target of rapamycin complex 1
- TEAD
TEA domain family member
- CTGF
connective tissue growth factor
- MeAIB
alpha-(methylamino)isobutyric acid
Footnotes
The authors have no conflicts of interest.
Reference List
- 1.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011 Jan;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan D. Hippo signaling in organ size control. Genes Dev. 2007 Apr 15;21(8):886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007 Sep 21;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007 Aug;8(8):613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 5.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006 Jun 30;125(7):1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007 Dec 4;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107(4):1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013 Apr;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 12.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011 Aug 31;10(9):671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 13.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013 Mar;104(3):275–281. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher BA, Linehan WM, Helman LJ. Targeting cancer metabolism. Clin Cancer Res. 2012 Oct 15;18(20):5537–5545. doi: 10.1158/1078-0432.CCR-12-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015 Jan;61(1):191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 17.Worns MA, Galle PR. HCC therapies--lessons learned. Nat Rev Gastroenterol Hepatol. 2014 Jul;11(7):447–452. doi: 10.1038/nrgastro.2014.10. [DOI] [PubMed] [Google Scholar]
- 18.Park YY, Kim K, Kim SB, Hennessy BT, Kim SM, Park ES, et al. Reconstruction of nuclear receptor network reveals that NR2E3 is a novel upstream regulator of ESR1 in breast cancer. EMBO Mol Med. 2012 Jan;4(1):52–67. doi: 10.1002/emmm.201100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003 Aug 29;278(35):33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models with life tables. J Royal Statis Soc. 1972;34:187–220. [Google Scholar]
- 21.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011 Feb;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 22.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011 Nov 10;27:441–464. 441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 23.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008 Jul 15;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broer S, Palacin M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 2011 Jun 1;436(2):193–211. doi: 10.1042/BJ20101912. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009 Feb;87(1):77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- 26.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010 Jan 21;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010 Aug;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker GA, Ellory JC. The identification of neutral amino acid transport systems. Exp Physiol. 1990 Jan;75(1):3–26. doi: 10.1113/expphysiol.1990.sp003382. [DOI] [PubMed] [Google Scholar]
- 29.Shotwell MA, Kilberg MS, Oxender DL. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- 30.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998 Jun 5;273(23):14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 31.Nakajo T, Yamatsuji T, Ban H, Shigemitsu K, Haisa M, Motoki T, et al. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem Biophys Res Commun. 2005 Jan 7;326(1):174–180. doi: 10.1016/j.bbrc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009 Feb 6;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause U, Bertrand L, Maisin L, Rosa M, Hue L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem. 2002 Aug;269(15):3742–3750. doi: 10.1046/j.1432-1033.2002.03069.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim SM, Leem SH, Chu IS, Park YY, Kim SC, Kim SB, et al. 65-gene-based risk score classifier predicts overall survival in hepatocellular carcinoma. Hepatology. 2012 May 2;55(5):1443–1452. doi: 10.1002/hep.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Pan Q, Zhang J, Xu X, Liu X, Wang Q, et al. Functional and Clinical Evidence that TAZ is a Candidate Oncogene in Hepatocellular Carcinoma. J Cell Biochem. 2015 Feb 3;10 doi: 10.1002/jcb.25117. [DOI] [PubMed] [Google Scholar]
- 36.Xiao H, Jiang N, Zhou B, Liu Q, Du C. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015 Feb;106(2):151–159. doi: 10.1111/cas.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009 Oct 1;115(19):4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim GJ, Kim H, Park YN. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One. 2013 Sep 24;8(9):e75449. doi: 10.1371/journal.pone.0075449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014;2014:261365. doi: 10.1155/2014/261365. Epub;%2014 Apr 22.:261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008 Dec;135(6):1972–1983. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010 Jun;27(2):255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 42.Shultz VD, Campbell W, Karr S, Hixson DC, Thompson NL. TA1 oncofetal rat liver cDNA and putative amino acid permease: temporal correlation with c-myc during acute CCl4 liver injury and variation of RNA levels in response to amino acids in hepatocyte cultures. Toxicol Appl Pharmacol. 1999 Jan 1;154(1):84–96. doi: 10.1006/taap.1998.8555. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Zhang L, He Q, Feng X, Zhu J, Xu Z, et al. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Report. 2012 Feb;5(2):410–414. doi: 10.3892/mmr.2011.640. [DOI] [PubMed] [Google Scholar]
- 44.Kondoh N, Imazeki N, Arai M, Hada A, Hatsuse K, Matsuo H, et al. Activation of a system A amino acid transporter, ATA1/SLC38A1, in human hepatocellular carcinoma and preneoplastic liver tissues. Int J Oncol. 2007 Jul;31(1):81–87. [PubMed] [Google Scholar]
- 45.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012 Dec;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012 Jun 15;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A Peptide Mimicking VGLL4 Function Acts as a YAP Antagonist Therapy against Gastric Cancer. Cancer Cell. 2014 Feb 10;25(2):166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012 Apr;14(2):129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 49.Freeman TL, Ngo HQ, Mailliard ME. Inhibition of system A amino acid transport and hepatocyte proliferation following partial hepatectomy in the rat. Hepatology. 1999 Aug;30(2):437–444. doi: 10.1002/hep.510300212. [DOI] [PubMed] [Google Scholar]
- 50.Rask-Andersen M, Masuram S, Fredriksson R, Schioth HB. Solute carriers as drug targets: current use, clinical trials and prospective. Mol Aspects Med. 2013 Apr;34(2–3):702–710. doi: 10.1016/j.mam.2012.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.