Abstract
Thoracic aortic dissection is a rare, but lethal, medical condition that is either misdiagnosed as a myocardial infarction or overlooked completely. Though thoracic aortic dissections are commonly diagnosed in patients exhibiting sharp chest pain, there are some notable cases where patients do not report the expected severity of pain. We report a unique case of a patient with a thoracic aortic dissection who was initially nearly asymptomatic for eight months, in order to heighten awareness, highlight diagnosis protocol, and improve prognosis for this commonly misdiagnosed, but fatal, condition.
Keywords: aortic dissection, long standing, diagnosis
Introduction
Thoracic aortic dissection occurs when blood flow is redirected from the aorta (true lumen) into the media of the aortic wall (false lumen), through an intimal laceration, creating a septum. Although thoracic aortic dissections are rare, resulting ruptures can lead to mortality, accounting for 60% of deaths.1 Contributing risk factors for thoracic aortic dissections include hypertension; connective tissue disorders such as Marfan or Ehlers–Danlos syndrome, bicuspid and unicommissural aortic valve; history of cardiac procedures; genetic disorders; pregnancies; and swollen blood vessels caused by arthritis or syphilis.2,3 Though men are statistically twice as more likely to be diagnosed with an aortic dissection than women, both are at equal risk.3,4 Treatments for aortic dissections vary depending on the specific condition and severity, from medical, to surgical, to nonsurgical.5 However, there are notable rare cases where a patient develops a dissection, but does not report the expected severity of pain, making it difficult to effectively detect and mend. We report a unique case of a patient with a thoracic aortic dissection who was initially nearly asymptomatic for eight months, in order to heighten awareness, highlight diagnosis protocol, and improve prognosis for this misdiagnosed, but fatal, condition.
Case Summary
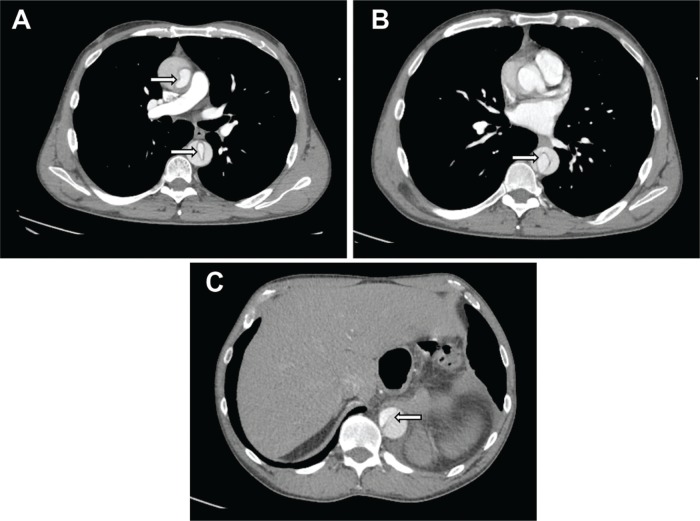
A man in his early 60s with a history of hypertension, according to him for “years,” presented with mild chest discomfort since eight months, which he attributed to his intense weightlifting regimen. He had mild retrosternal pain (4/10) radiating to both arms, which was aggravated by physical activity and relieved with rest. The patient was not taking the prescribed blood pressure medication, as he was noncompliant. Upon physical examination, his right arm blood pressure was 152/68 mm Hg, and his left arm pressure was 167/62 mm Hg, with a heart rate of 55/minute. Further examination detected a grade III/VI systolic and IV/VI diastolic murmur. Radial pulses were stronger on the left than on the right, while pedal pulses were equally bilateral. An electrocardiogram (ECG) revealed sinus bradycardia with nonspecific T-wave abnormality; a chest X-ray noted a widened mediastinum. Transthoracic echocardiography (TTE) revealed severe aortic regurgitation (AR), and grossly preserved ejection fraction and chest computed tomography (CT; Fig. 1) was consistent with dissection of the root of the thoracic aorta extending to the proximal abdominal aorta at the origin of the celiac trunk.
Figure 1.
Axial tomographic CT images showing the dissection from cranial (A) to caudal (B–C) levels (arrows).
The patient was treated with labetalol, and surgical consultation was obtained. He underwent coronary angiography prior to surgery, which showed normal coronary arteries with severe AR, a thoracic aortic dissection, and an aneurysm with a brachiocephalic origin. During surgery, an 8-cm wide section of the aorta with an intimal tear and atheroma was noted. The thoracic aorta was replaced, and the aortic valve was resuspended. Postsurgical TTE revealed a normal ejection fraction, as well as resolution of the AR. The patient was discharged in stable condition after four days of hospitalization.
Discussion
Diagnosis
Thoracic aortic dissections are rare, with estimated cases of 2 out of 10,000 patients6 yet can be lethal if not diagnosed early or treated effectively. There are two classification systems for diagnosing a thoracic aortic dissection: the Stanford type A and B, and the DeBakey type I–III. The Stanford type A is defined by the region of the dissection, which is a tear in the thoracic aorta and/or aortic arch. The comparable DeBakey I–II dissections both originate in the thoracic aorta, but DeBakey II’s point of entry is localized in the thoracic aorta, while DeBakey I may also propagate to at least the aortic arch. The Stanford type B is defined by a localized tear only in the descending aorta, while the comparable DeBakey III’s point of entry is confined in the descending aorta (Fig. 2).7,8 Common symptoms associated with thoracic aortic dissections include a sharp, tearing, stabbing, or ripping chest pain that radiates to the shoulder, neck, arm, jaw, abdomen, or hips.9 Symptoms may mimic those common in myocardial infarctions, but myocardial infarctions can be ruled out either through an ECG or cardiac biomarkers/enzymes. Chest radiography or CT angiography is the initial study of choice for evaluating acute chest pain followed by magnetic resonance angiography or transesophageal echocardiography. The challenge emerges when the patient does not report or exhibit conventional severe chest pain, resulting in an unprecedented decline in health and possible mortality. Our patient exhibited a DeBakey I aortic dissection, but presented with nearly asymptomatic conditions for which he did not initially seek medical care.
Figure 2.
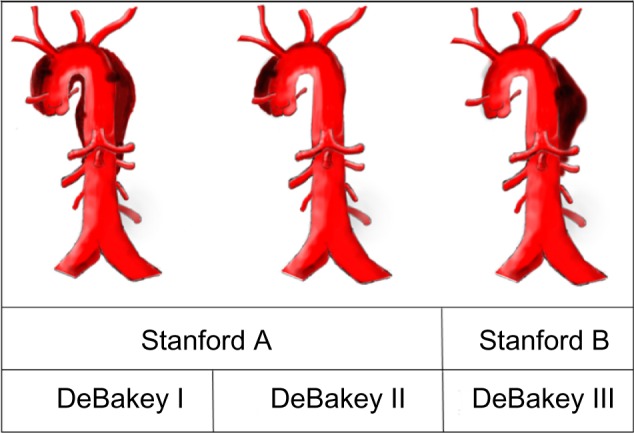
Classification of aortic dissection. The Stanford type A comparable with DeBakey I–II; Stanford type B comparable with DeBakey III.
Risk factors
There are many contributing risk factors for developing thoracic aortic dissections. A necropsy study conducted by Larson and Edwards revealed that the most common risk factor, hypertension, was present in 52% of cases with Stanford A (DeBakey I–II) and in 75% of cases with Stanford B (DeBakey III).2 Genetic syndromes, such as Marfan or Ehlers–Danlos, resulted in debilitated and deformed connective tissues in the aorta and were less common for developing a thoracic aortic dissection as 44% of Marfan cases presented with such conditions.2 Other genetic disorders, such as bicuspid and unicommissural aortic valves, also increase the risk of an aortic dissection by 9 and 18 times, respectively.2 The average age of individuals with an aortic dissection and a tricuspid, bicuspid, and unicommissural aortic valve was 63, 55, and 40 years, respectively.2 Though rare, rigorous weightlifting regimens have been noted as a contributing risk factor for a thoracic aortic dissection. A study conducted by Hatzaras et al reported that 30 male patients who were diagnosed with a thoracic aortic dissection and participated in weight lifting or similar rigorous physical exercises had most of the dissections confined in the thoracic aorta.10 Our patient’s chronic hypertension was the likely primary factor for his nearly painless thoracic aortic dissection. As our patient, in his 60s, did not present a bicuspid or unicommissural aortic valve, his age was within the common statistical range for diagnosis. His rigorous weightlifting regimen, coupled with his history of hypertension, most likely increased his likelihood for a thoracic aortic dissection presumed to have existed since the start of his symptoms eight months prior. Though Hatzaras’ statistics were analyzed on patients with conventional painful aortic dissections, similar data may be expected for those who exhibit painless aortic dissections, as the causes and features of the two dissection conditions are mainly conserved.
Presentation
Proper detection and diagnosis of painless thoracic aortic dissection is a challenging, but imperative, task for successful treatment. A study conducted by Park et al analyzed data from 977 patients in the International Registry of Acute Aortic Dissection and discovered that the frequency for painless dissections is rare, occurring in 6.4% of cases.11 Research conducted by Liu et al reported a similar rate of occurrence of painless aortic dissections, accounting for 7.26% of 482 cases.12 Even though both studies were conducted in different geographical regions, namely, U.S.A. and China, they both emphasize the statistically similar rare occurrence of asymptomatic thoracic aortic dissection. As painless aortic dissections are rare and most often overlooked, it can be presumed that conditions influencing pain-induced aortic dissections may also influence painless aortic dissections.
Patients who had painless thoracic aortic dissections were more likely to have developed a type A (DeBakey I–II) dissection, as in our case. According to Imamura et al, 81% of patients exhibiting painless dissections were diagnosed to be Stanford type A, and Liu et al reported that the ratio of type A in painless group was approximately 28% higher than in the pain group.12–15 Other medical conditions such as syncope, congestive heart failure, stroke, diabetes, aortic aneurysm, and a history of cardiovascular surgeries were more commonly associated with patients exhibiting painless aortic dissections than patients who reported pain.11 Complications such as cerebral ischemia and cardiac tamponade were more common in patients exhibiting painless aortic dissections. However, chest radiography, electrocardiography, and initial diagnostic imaging results were not significantly different between individuals with painful and painless thoracic aortic dissections.15
Prognosis and complications
Diagnosis of painless aortic dissection is challenging, as untreated/unrecognized patients with prolonged painless aortic dissection symptoms become highly susceptible to an aortic rupture or ischemia to organs, leading to mortality.6 Untreated painful aortic dissections were estimated to result in an overall mortality rate of 25% in the first 6 hours, which escalated to 48% in the first 24 hours, 53% in the first week, 70% within 3 weeks–3 months, and 85% in 1–2 years.15 Similar mortality rates are expected for painless aortic dissections, as the causes and lack of treatment are conserved in both dissection conditions. Based on the statistical range provided by Mccloy et al, our patient who had a painless aortic dissection for eight months would have been considered between the three week–three month time period and the 1–2 year time period. Based on this statistical range, his mortality rate would have been in between 70% and 85%, yielding an estimated 15%–30% chance of surviving his nearly asymptomatic thoracic aortic dissection by the time he sought treatment.
Conclusion
Painless thoracic aortic dissection is a rare, but lethal, condition that has been difficult to accurately diagnose because of the fact that it is painless. The burden of this diagnosis often falls on the shoulders of the ED physician, who is expected to consider the differential of aortic dissection at times without the classic and typical manifestations, sometimes with no symptoms at all. Though our patient lived with nearly painless thoracic aortic dissection much longer than anticipated, his specific case augments the importance for physicians to effectively treat this fatal condition. As more diagnoses and surgical procedures have been performed on symptomatic aortic dissections in the past decades, the overall mortality rate for this condition has decreased.13 Yet, many cases of painless aortic dissections have been misdiagnosed and continue to be misdiagnosed due to the absence of typical chest pain. Presentation with nascent correlating symptoms enables early detection through TTE, CT, ECG, magnetic resonance imaging, and chest X-rays. As more physicians become aware of new additional symptoms, the reported mortality rates for patients with nearly asymptomatic thoracic aortic dissections may hopefully decrease significantly.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,162 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: ANM. Analyzed the data: AK, RZ, ANM. Wrote the first draft of the manuscript: AK, RZ, ANM. Contributed to the writing of the manuscript: AK, KK, RZ, ANM. Agree with manuscript results and conclusions: AK, KK, RZ, ANM. Jointly developed the structure and arguments for the paper: AK, RZ, ANM. Made critical revisions and approved final version: AK, KK, RZ, ANM. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Clouse WD, Hallett JW, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–80. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 2.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53(6):849–55. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 3.Ramanath VS, Oh JK, Sundt TM, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc. 2009;84(5):465–81. doi: 10.1016/S0025-6196(11)60566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouse WD, Hallett JW, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280(22):1926–9. doi: 10.1001/jama.280.22.1926. [DOI] [PubMed] [Google Scholar]
- 5.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539–45. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 6.Aortic Dissection: MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine; [Accessed April 2, 2016]. Available at: https://www.nlm.nih.gov/medlineplus/ency/article/000181.htm. [Google Scholar]
- 7.DeBakey ME, Henly WS, Cooley DA, Morris GC, Crawford ES, Beall AC. Surgical management of dissecting aneurysm of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49. [PubMed] [Google Scholar]
- 8.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237–47. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 9.Cohen R, Mena D, Carbajal-mendoza R, Arole O, Mejia JO. A case report on asymptomatic thoracic aortic dissection. Int J Angiol. 2008;17(3):155–61. doi: 10.1055/s-0031-1278301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: more evidence for a connection. Cardiology. 2007;107(2):103–6. doi: 10.1159/000094530. [DOI] [PubMed] [Google Scholar]
- 11.Park SW, Hutchison S, Mehta RH, et al. Association of painless acute aortic dissection with increased mortality. Mayo Clin Proc. 2004;79(10):1252–7. doi: 10.4065/79.10.1252. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZY, Zou YL, Chai BL, Zeng HS. Analysis of clinical features of painless aortic dissection. J Huazhong Univ Sci Technolog Med Sci. 2014;34(4):582–5. doi: 10.1007/s11596-014-1319-8. [DOI] [PubMed] [Google Scholar]
- 13.Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 14.Imamura H, Sekiguchi Y, Iwashita T, et al. Painless acute aortic dissection. – Diagnostic, prognostic and clinical implications. Circ J. 2011;75(1):59–66. doi: 10.1253/circj.cj-10-0183. [DOI] [PubMed] [Google Scholar]
- 15.Mccloy RM, Spittell JA, Mcgoon DC. The prognosis in aortic dissection (dissection aortic hematoma or aneurysm) Circulation. 1965;31:665–9. doi: 10.1161/01.cir.31.5.665. [DOI] [PubMed] [Google Scholar]