Abstract
We have previously shown that patients with the major depressive disorder (MDD) exhibited elevated phosphorylation of the lymphocyte glucocorticoid receptor (GR) at serine 226 (S226). Here, we further analyse potential alterations of GR signalization in lymphocytes of MDD patients, i.e. the cytoplasmic/nuclear distribution of GR, levels of FK506-binding protein 5 (FKBP5) and glucocorticoid-induced leucine zipper (GILZ). The FKBP5 acts as an important regulator of GR activation, by decreasing ligand binding and impeding translocation of the receptor to the nucleus, while GILZ mediates glucocorticoid anti-inflammatory effects. Our result showed that the depressed patients had significantly higher GR levels in the cytoplasm compared to controls, which was accompanied by higher FKBP5 levels. Linear regression model demonstrated significantly higher correlation between FKBP5 and cytoplasmic GR than the presence of MDD itself or phosphorylation of nuclear GR at S226. There were no differences in the levels of GILZ isoforms. Therefore, the results suggest that accumulation of the GR in cytoplasm is related to the elevation of FKBP5, adding one more step in understanding altered GR signalling in lymphocytes, and potentially brain tissue, of MDD patients.
Keywords: Major depressive disorder, Glucocorticoid receptor, FKBP5, GILZ
Introduction
Major depressive disorder (MDD) is a complex psychiatric disorder which pathogenesis involves multiple biological systems, including disturbed glucocorticoid signalling (Anacker et al. 2011; Zunszain et al. 2011). The reduced glucocorticoid receptor (GR) function is believed to underline alterations in hypothalamic–pituitary–adrenal (HPA) axis activity frequently observed in MDD patients, but it is also well documented in peripheral blood cells (Calfa et al. 2003; Cattaneo et al. 2013; Menke et al. 2012; Pariante and Lightman 2008; Pariante and Miller 2001). The peripheral tissues, except for being an easy accessible model for analysing GR signalization in MDD patients, have a potential to be used as biomarkers for diagnosis and following up the treatment of depression (Cattaneo et al. 2013; Hepgul et al. 2013; Menke et al. 2012). However, studies of GR function in leukocytes could also be helpful in understanding crosstalk of glucocorticoids and immune system, for a long been recognized to be disturbed in depression (Maes 1999; Miller et al. 2009; Zunszain et al. 2011).
The well-known mode of GR action is as follows: in the absence of glucocorticoids, GR predominantly resides in the cytoplasm in a multiprotein complex consisting of heat shock proteins and immunophilins, and upon ligand binding, it translocates to the nucleus where it regulates transcription of numerous genes (Anacker et al. 2011; Heitzer et al. 2007). One of the important players in regulating this process is FK506-binding protein 5 (FKBP5), an immunophilin that when bound to the GR complex lowers the receptor affinity for its ligand and interfere with its translocation to the nucleus (Denny et al. 2000; Storer et al. 2011; Wochnik et al. 2005). Furthermore, FKBP5 expression is induced by glucocorticoids, as a part of an intracellular ultra-short negative feedback loop restraining GR activity (Binder 2009; Vermeer et al. 2003). Increased expression of FKBP5 has already been linked with susceptibility to depressive and anxiety disorders, implicating the importance of further exploring the function of this protein in depression (Binder 2009; Suzuki et al. 2014; Tatro et al. 2010).
In response to glucocorticoids, GR stimulates a wide variety of gens, including glucocorticoid-induced leucine zipper (GILZ), one of the key mediators of glucocorticoid anti-inflammatory actions (Ayroldi and Riccardi 2009; D'Adamio et al. 1997). GILZ inhibits the activation of nuclear factor kB (NF-kB) and activator protein 1 (AP-1), and it may regulate apoptosis in T lymphocytes (Ayroldi et al. 2001; Ayroldi and Riccardi 2009). In spite of the mentioned importance of GILZ's role in mediating GR anti-inflammatory effects, it has not been so extensively analysed in depressed patient or animal models of depression.
We have previously shown altered phosphorylation of nuclear GR in lymphocytes of patients with a current depressive episode (Simic et al. 2013b), and here, we further analyse potential alterations in GR signalling by assessing GR nuclear/cytoplasmic distribution and levels of FKBP5 and GILZ, in lymphocytes of these subjects.
Materials and Methods
Subjects
The subjects were already described in our previous study (Simic et al. 2013b). Briefly, the study protocol was approved by the Medical Ethics Committee of Clinical Centre of Serbia. Thirty patients were selected from the University Clinic for Psychiatry, Clinical Centre of Serbia, while 35 control subjects were recruited by local advertisements. All subjects gave their written informed consent prior to entering the study. ICD X and DSM IV (M.I.N.I.) criteria were used for diagnosing the current major depressive episode, confirmed by two trained psychiatrists. All patients had neither a history of ongoing significant medicine nor neurological conditions, although a number of subjects had sub-threshold diagnoses of anxiety disorder. The intensity of depressive symptomatology was evaluated by Hamilton Rating Scale for Depression (HAMD-21), and the recruited patients had moderate to very severe depression (HAMD>14). All patients were under medical treatment at the time of blood sampling, namely, combined therapy comprising of antidepressants (88.5 % of patients), benzodiazepines (96.2 % of patients), mood stabilizers (30.8 % of patients) and antipsychotics (50 % of patients). The patients were adherent to aforementioned psychiatric drugs for at least 1 week before the interview. The lifetime exposure to antidepressants and benzodiazepines varied from several months to several years.
Blood Sample Collection, Purification of Lymphocytes and Preparation of the Cell Extracts
Blood sampling, purification of peripheral blood mononuclear cells (PBMC) and preparation of cellular extract were done as already described (Simic et al. 2013a, b).
All blood samples for determining cortisol levels and molecular biology analyses were obtained between 0800 and 0900 h. For obtaining PBMC, blood (10 ml) was drawn in Na2EDTA BD Vacutainer tubes. PBMC were purified using lymphocyte separation medium (LSM, MP Biomedicals) according to the manufacturer's protocol. After washing with cold phosphate-buffered saline (PBS), PBMC pellets were stored at −80 °C until further preparation.
The cytoplasmic and nuclear extracts from PBMC were prepared according to mini-extraction protocol with slight modifications (Sheehan et al. 1998). Briefly, PBMC pellets were suspended in 500 μl of ice-cold buffer A (10 mM HEPES pH 7.9) containing 10 mM KCl, 0.1 mM Na2EDTA, 0.1 mM Na2EGTA, 1 mM DTT and protease and phosphatase inhibitors as defined in our previous papers. The cells were incubated on ice for 15 min, after which 75 μl of 10 % solution of Tween 20 was added, the tubes were vortexed vigorously for 20 s and finally PBMC homogenates were centrifuged at 10,000×g for 7 min at 4 °C. After pouring off the supernatant, which was used as cytoplasmic extract, nuclear pellets were suspended in 150 μl of ice-cold buffer C (20 mM HEPES 7.9 pH) containing 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and the same protein and phosphatase inhibitors as in the buffer A. The samples were incubated at 4 °C for 15 min on shaking platform followed by centrifugation at 10,000×g for 10 min at 4 °C. The obtained supernatants were used as nuclear extracts (the purity of cellular compartments is presented in the Supplemental Material). After determining the protein concentration by BCA Assay kit (SERVA Electrophoresis), the nuclear extracts were boiled in sample loading buffer according to Laemmli for 5 min.
Western Blot Analysis
For Western blot analysis, equal amounts of nuclear proteins (60 μg) were loaded into each well of SDS polyacrylamide gel (10 or 12 %), separated by electrophoresis and transferred onto PVDF membrane (Immobilon-P membrane, Millipore). After blocking for 1 h in a 5 % non-fat dry milk in PBS, membranes were incubated with appropriate primary and secondary antibodies. The following antibodies were used: GR M-20 (Santa Cruz Biotechnology), FKBP51 H-100 (Santa Cruz Biotechnology) and GILZ D-2 (Santa Cruz Biotechnology), for detecting the respective proteins. The β-actin was used as a loading control, detected by specific antibody (Abcam). The signals were detected using enhanced chemiluminescence substrate Pico or Femto (Pierce) and exposing the membrane to an X-ray film. Densitometry of protein bands on X-ray film was performed by ImageJ analysis PC software. In order to compare the protein levels between different blots, an internal reference sample (IRS) was run on each gel. The protein levels from all subjects were represented as the percentages of respective IRS set as 100 %. All samples were analysed at least twice. Non-representative signals of the examined proteins were excluded from further analyses.
Statistical Analyses
Comparisons between patients with MDD and healthy subjects were performed by t test for continuous variables or by χ2 test for categorical variables. Logarithmic transformations of variables with non-parametric distributions were carried out. The effect of potential covariates was analysed using a general linear model (GLM). The Pearson's correlation and linear regression analyses were used to describe relations between desired variables. The statistical significance was accepted at p<0.05. The statistical analyses were performed using SPSS.
Results
Demographic and Clinical Data
The demographic and clinical data of healthy and MDD subjects are already described in our previous study (Simic et al. 2013b) but also shown here in Table 1. There was no statistical difference regarding the gender contributions in healthy and MDD groups (Table 1). However, the controls were significantly younger than MDD patients, and there were more smokers in MDD than in the healthy group (Table 1). The concentrations of cortisol were also slightly higher in depressed patients (Table 1). Indeed, in further analyses, age, cortisol levels and smoking were considered as covariates.
Table 1. The main sociodemographic and clinical characteristics of the subjects.
| Characteristics | Healthy subjects (n=35) | MDD patients (n=30) | Test values |
|---|---|---|---|
| Age (years) | 39.49±9.64 | 44.77±7.58 | t=−2.423; p=0.018 |
| Gender (% of women) | 54.3 % | 56.7 % | χ2=0.037; p=0.847 |
| Cortisol (nmol/l) | 348±144 | 469±157 | t=−1.921; p=0.059 |
| HAMD-17 | 24.91±7.03 | ||
| smoking (% of daily smokers) | 31.40 % | 70.00 % | χ2=9.615; p=0.002 |
Data are represented as mean±SD or percentage
MDD major depressive disorder
Increased GR Levels in the Cytoplasm of PBMC of MDD Patients
Firstly, we compared nuclear and cytoplasmic levels of GR in the patients and control subjects (Fig. 1a). There was a significant increase of GR in cytoplasm of PBMC of depressed patients compared to controls (t=−2.51, p=0.015), while there were no differences in nuclear levels between the groups, as already reported in our previous paper (Simic et al. 2013b). After adjustment for age, gender, cortisol and smoking, MDD still exhibited significant effect on cytoplasmic GR levels (Table 2), and effects of the covariates were not significant. Additionally, we analysed if smoking status could contribute to variances in cytoplasmic/nuclear GR levels in controls and MDD group separately, but no statistically significant differences were found. Further, we examined if treatments by antipsychotics and mood-stabilizers could contribute as well to variations in GR levels in MDD group (since half the patients were treated with antipsychotics and about a third with mood stabilizers). However, patients did not differ in cytoplasmic/nuclear GR levels depending on weather they were treated with these medications or not.
Fig. 1.
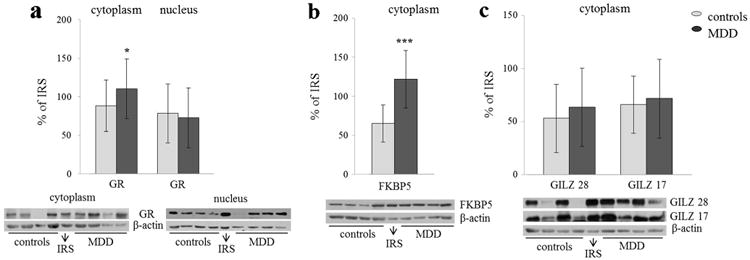
Quantified levels and representative Western blots of a cytoplasmic and nuclear GR, b FKBB5 and c GILZ in PBMC of healthy and MDD subjects. The levels of all proteins were adjusted to β-actin, which served as a loading control, and represented as the percentage of IRS of each protein. Significant differences were detected applying t test for MDD versus healthy subjects: *p<0.05; ***p<0.001
Table 2. The effects of MDD on the levels of GR and FKBP5 in cytoplasm, adjusted for age, gender, cortisol and smoking.
| Effect of MDD | GR | FKBP5 |
|---|---|---|
| Model 1 (no adjustment) | F=6.301 | F=54.481 |
| p=0.015 | p<0.001 | |
| Model 2 (adjusted for age) | F=5.963 | F=55.764 |
| p=0.018 | p<0.001 | |
| Model 3 (adjusted for gender) | F=5.844 | F=50.858 |
| p=0.019 | p<0.001 | |
| Model 4 (adjusted for cortisol) | F=5.580 | F=54.490 |
| p=0.021 | p<0.001 | |
| Model 5 (adjusted for smoking) | F=4.050 | F=42.661 |
| p=0.049 | p<0.001 |
F and p values from GLM univariate analyses are given
MDD major depressive disorder, GR glucocorticoid receptor, FKBP5 FK506-binding protein 5
Increased FKBP5 in the Cytoplasm of PBMC of MDD Patients
There was statistically significant increase in FKBP5 in MDD subjects compared to controls (t=−7.381, p<0.001; Fig. 1b). Indeed, after adjustment for age, gender, cortisol and smoking, the effect of the disorder on the levels of FKBP5 was still significant (Table 2). The effects of any of the covariates on FKBP5 levels were not significant as well. As for GR levels, we also examined if differences between smokers and non-smokers contributed to the variations in FKBP5 levels in control and patient groups separately but found no statistically significant effects. Likewise, we analysed if treatments by antipsychotics and mood stabilizers could affect the variance of FKBP5 levels in MDD group. There was no statistically significant difference in FKBP5 levels between patients treated and non-treated with mood stabilizers. However, patients treated with antipsychotics did have higher FKBP5 levels than those not treated with these medications (t=−2.241, p=0.035), but still, the latter group had higher FKBP5 levels than controls (t=−4.33, p<0.001).
Secondly, we investigated how the changes in FKBP5 were related to the changes in nuclear GR (published in our previous study), since FKBP5 is strongly induced by glucocorticoids. FKBP5 levels positively correlated with increased levels of GR phosphorylated at S211 and S226 (FKBP5 and pGR-S211, r=0.324, p=0.012; FKBP5 and pGR-S226, r=0.555, p<0.001) and negatively with the ratio of pGR-S211/pGR-S226 (r=−0.262, p=0.044), while there was no correlation with nuclear GR levels.
No Changes in GILZ Levels in the Cytoplasm of PBMC of MDD Patients
There were no differences in the levels of GILZ, neither in isoform of 17 nor 28 kDa between MDD and control subjects (Fig. 1c). Subsequently, we investigated whether there were possible associations between GILZ levels and alterations in nuclear GR, since GILZ expression is known to be stimulated by glucocorticoids. However, levels of GILZ correlated neither with nuclear GR levels nor with changes in GR phosphorylation.
Accumulation of GR in the Cytoplasm Is Primarily Related to the Elevation of FKBP5
Finally, we analysed whether the accumulation of GR in cytoplasm, found in MDD patients, was related to increased cytoplasmic FKBP5 levels. Additionally, we assumed that elevated phosphorylation of nuclear GR at serine 226, demonstrated in our previous study (Simic et al. 2013b), could also contribute to the accumulation of GR in the cytoplasm, since it is known that phosphorylation of this amino acid enhances GR export from nucleus to cytoplasm (Itoh et al. 2002). Indeed, the elevation of cytoplasmic GR levels was positively related to elevation of cytoplasmic FKBP5 levels, on one hand, and nuclear pGR-S226 levels, on the other hand (cytoplasmic GR and FKBP5, r=0.508, p<0.001; cytoplasmic GR and nuclear pGR-S226, r=0.409, p=0.002; Fig. 2, note that, on the graphic, the truth levels of analysed proteins are represented, while in Pearson's correlations, the transformed values were used). When patient and control groups were analysed separately, in both groups, there were also positive correlations between cytoplasmic GR and nuclear pGR-S226 (controls, r=0.267, p=0.17; patients, r=249, p=0.17) and between cytoplasmic GR and FKBP5, however, only correlation of cytoplasmic GR and FKBP5 in patients was significant (controls, r=0.316, p=0.116; patients, r=0.560; p=0.002).
Fig. 2.
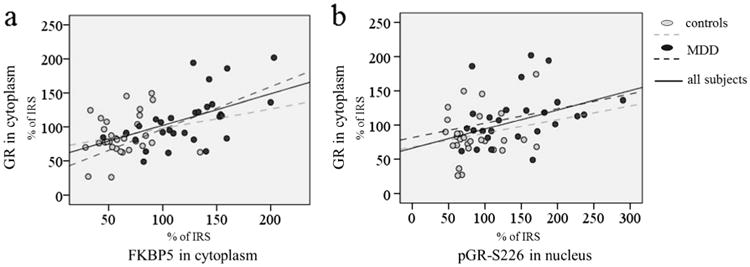
Relations of GR levels in cytoplasm with cytoplasmic FKBP5 levels (a) and nuclear levels of GR phosphorylated at S226 (b) in control and MDD subjects. The levels of GR and FKBP5 in cytoplasm were adjusted to β-actin, while the levels of pGR-S226 were adjusted to total GR levels in the nucleus. The levels of all proteins are represented as percentages of IRS for respective protein
Then, we run a linear regression model to identify the relative contributions of the levels of FKBP5 in the cytoplasm, pGR-S226 in the nucleus and the presence of MDD to predict GR levels in the cytoplasm (Table 3). It was shown that only FKBP5 had a significant effect on GR levels in the cytoplasm, and standardized coefficient was by far higher for FKBP5 than for other predictors.
Table 3. Standardized coefficients and p values of linear regression model that assessed relative contributions of the levels of FKBP5 in the cytoplasm, pGR-S226 in the nucleus and presence of MDD to predict GR levels in the cytoplasm.
| Predictors | Effect of each predictor on GR levels in cytoplasm |
|---|---|
| FKBP5 | β=0.588, p<0.001 |
| pGR-S226 | β=0.089, p=0.420 |
| MDD | β=−0.070, p=0.596 |
Discussion
MDD is known to be characterized by glucocorticoid resistance in both brain and periphery, which in turn could lead to numerous physiological disturbances, including hyperactivity of HPA axis and increased inflammation (Anacker et al. 2011; Zunszain et al. 2011). Among previous studies analysing GR function in lymphocytes, it was demonstrated that GR binding was reduced or unchanged in cytoplasm or whole cell extracts of depressed patients (Calfa et al. 2003; Pariante and Miller 2001). Herein, we documented that in lymphocytes of MDD patients, there is an accumulation of the GR in cytoplasm. Bearing in mind that GR detection in our samples was achieved by the Western blot technique, our results cannot be directly compared with results of GR binding studies. Namely, while Western blot enables us to detect total GR protein levels, binding assays give information about the ability of the GR to bind ligand, a feature that could be dependent on other factors such as its interactions with chaperones. However, in light of this, our results of elevated cytoplasmic GR could indicate the reduced capacity of the receptor ligand binding. Indeed, the elevation of FKBP5 in PBMC cytoplasm of MDD patients, which had been shown to reduce GR affinity for cortisol and its subsequent activation (Davies et al. 2002; Denny et al. 2000; Wochnik et al. 2005), could support this assumption.
We assumed that this accumulation of GR in the cytoplasm could be due to decreased import of the receptor to the nucleus and/or its increased export from the nucleus, although the changes in nuclear levels of GR were not detected. As it is already mentioned, elevation of FKBP5 could mediate reduced GR import to the nucleus (Davies et al. 2002; Wochnik et al. 2005), while, on the other hand, elevated GR phosphorylation at S226 could contribute to enhanced nuclear export of the GR (Itoh et al. 2002). Indeed, it was shown that increased phosphorylation of GR at S226 contributed to impaired GR translocation in PBMC of severe asthma patients (Mercado et al. 2011). Nevertheless, according to our results, it seems that the accumulation of GR in cytoplasm of lymphocytes from MDD patients is more likely related to high FKBP5 levels than to the elevation of nuclear pGR-S226. Similarly, chronic mild stress model of depressive-like behaviour in laboratory animals revealed accumulation of cytoplasmic GR in ventral hippocampus and prefrontal cortex which was related to FKBP5 elevation, rather than increased phosphorylation of GR at S246 (rat ortholog of S226) (Guidotti et al. 2013). Therefore, we could suggest that retention of GR in cytoplasm due to high FKBP5 levels could be one of the mechanisms contributing glucocorticoid resistance in brain tissue of depressed patients as well.
In our previous studies, we showed that increased phosphorylation of GR at S226 indicating reduced GR function was related to increased reports of depression, anxiety and stress in healthy subjects (Simic et al. 2013a), and further elevation of pGR-S226 could contribute to further disruption of GR function in patients with MDD (Simic et al. 2013b). The present study reveals one more possible mechanism attributing to disturbed GR signalling in MDD patients, that is the retention of the GR in the cytoplasm related to the increased FKBP5 levels in depressed subjects (correlations of FKBP5 or cytoplasmic GR and current reports of depression, anxiety and stress were not significant in healthy subjects; data not showed). Certainly, it is not surprising that in MDD patients, there are multiple points at which GR signalling could be disrupted.
A further possibility that could lead to elevated cytoplasmic GR levels is reduced degradation of GR in lymphocytes of MDD patients. However, to our best knowledge, there are no evidences about the altered degradation of GR related to MDD. Bering in mind that on one side, agonist binding markedly reduces GR half-life (Hoeck et al. 1989; Webster et al. 1997) and, on the other, as already mentioned, the findings of reduced GR binding in MDD patients (Calfa et al. 2003; Pariante and Miller 2001), we could hypothesize that indeed, there might be reduced degradation of GR in lymphocytes of MDD patients. However, this presumption should indeed be confirmed by additional experiments.
Our findings of elevated FKBP5 in lymphocytes of MDD patents are in agreement with previous studies of increased messenger RNA (mRNA) for FKBP5 in depressed patients (Cattaneo et al. 2013). Additionally, increased FKBP5 at the levels of both mRNA and protein was found in the prefrontal cortex of post-mortem tissue of depressed subjects (Tatro et al. 2009). Concerning the efforts in finding easily accessible biomarkers that could mirror the changes in brain functioning, recent data from animal models emphasize the value of FKBP5, since it was shown that the enhancements of its expression are comparable between blood and hippocampus in response to increasing concentrations of corticosterone (Ewald et al. 2014). It is also well documented that several gene variants of FKBP5 gene are related to augmented induction of FKBP5 upon stress exposure (Ising et al. 2008; Luijk et al. 2010), and the same alleles are associated with increased susceptibility to depression and anxiety disorders (Binder et al. 2004; Menke et al. 2013; Minelli et al. 2013; Suzuki et al. 2014; Szczepankiewicz et al. 2014). Therefore, although we did not do genotyping in our sample, it is possible that some of these polymorphisms contributed to higher FKBP5 levels observed in MDD patients in this study as well.
Also, it should be noted that the elevation of FKBP5 in lymphocytes of MDD patients occurred in spite of increase in pGR-S226 and decrease in pGR-S211/p-GR-S226 ratio which suggest reduced GR transcription activity. However, increased phosphorylation of GR at S211 still might contribute to higher FKBP5 levels in MDD patients. Additionally, it is probable that some other mechanisms, beyond the GR phosphorylation, are responsible for sustaining high FKBP5 levels, such as epigenetic changes (Klengel et al. 2013; Lee et al. 2010), or stability of its mRNA molecule (Tatro et al. 2009).
There were no changes in GILZ protein levels between controls and the patients, either in isoform of 17 or 28 kDa, and changes in GR phosphorylation did not lead to altered GILZ levels. No differences in GILZ mRNA levels were detected in one other previous study, but in the depressed group of subjects, it was showed that GILZ expression was negatively related to hippocampal atrophy (Frodl et al. 2012). It is possible that alterations in GILZ levels are linked to some specific morphological/molecular changes in MDD patients that we did not examine in this study and, considering the crosstalk of glucocorticoids and immunity system, further research of GILZ related to depression we believe is required.
It could be deemed that a limitation of this study is that all patients were on medications, at the time of blood sampling. Our results showed that treatment with antipsychotics could indeed lead to more elevated FKBP5 levels in MDD patients, but this treatment did not account for the overall observed differences between MDD patients and controls. Besides that, all patients had a significant intensity of depressive symptoms at the time of their evaluation, implicating limited effects of the medications. Actually, successful long-term antidepressant treatments have rather been associated with decreased than to increased FKBP5 levels (Cattaneo et al. 2013).
In conclusion, our results revealed that accumulation of GR in the cytoplasm was related to elevation of FKBP5 which could be one more aspect of disturbed GR signalling in lymphocytes of MDD patients. Therefore, together with our previous findings, alterations in GR signalization in PBMC, and possibly brain tissue of MDD patients, could be related not only to the altered phosphorylation of GR but also to the increased FKBP5 contributing the retention of the GR in cytoplasm.
Supplementary Material
Acknowledgments
We are grateful to the Ministry of Education and Sciences of Serbia (Grant III41029) and NIH grant 1R21MH098793-01A1 for supporting the study.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12031-014-0451-z) contains supplementary material, which is available to authorized users.
Conflict of Interest There is no conflict of interest to report concerning this research.
Contributor Information
Iva Lukic, Email: iwa@vinca.rs, Laboratory of Molecular Biology and Endocrinology, VINCA, Institute of Nuclear Sciences, University of Belgrade, P.O. Box-522-MBE090, 11001 Belgrade, Serbia.
Milos Mitic, Laboratory of Molecular Biology and Endocrinology, VINCA, Institute of Nuclear Sciences, University of Belgrade, P.O. Box-522-MBE090, 11001 Belgrade, Serbia.
Ivan Soldatovic, Faculty of Medicine, University of Belgrade, Dr Subotica 8, 11000 Belgrade, Serbia.
Milica Jovicic, Clinic for Psychiatry, Clinical Centre of Serbia, Pasterova 2, 11000 Belgrade, Serbia.
Nadja Maric, Faculty of Medicine, University of Belgrade, Dr Subotica 8, 11000 Belgrade, Serbia; Clinic for Psychiatry, Clinical Centre of Serbia, Pasterova 2, 11000 Belgrade, Serbia.
Jelena Radulovic, Department of Psychiatry and Behavioral Sciences, The Asher Center for Study and Treatment of Depressive Disorders, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA.
Miroslav Adzic, Laboratory of Molecular Biology and Endocrinology, VINCA Institute of Nuclear Sciences, University of Belgrade, P.O. Box-522-MBE090, 11001 Belgrade, Serbia.
References
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The gluco-corticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. doi: 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- Ayroldi E, Migliorati G, Bruscoli S, et al. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood. 2001;98:743–753. doi: 10.1182/blood.v98.3.743. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamio F, Zollo O, Moraca R, et al. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity. 1997;7:803–812. doi: 10.1016/s1074-7613(00)80398-2. [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Ewald ER, Wand GS, Seifuddin F, et al. Alterations in DNA methylation of Fkbp5 as a determinant of blood–brain correlation of glucocorticoid exposure. Psychoneuroendocrinology. 2014;44:112–122. doi: 10.1016/j.psyneuen.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Hughes MM, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. 2013;38:616–627. doi: 10.1038/npp.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Rev Endocr Metab Disord. 2007;8:321–330. doi: 10.1007/s11154-007-9059-8. [DOI] [PubMed] [Google Scholar]
- Hepgul N, Cattaneo A, Zunszain PA, Pariante CM. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med. 2013;11:28. doi: 10.1186/1741-7015-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeck W, Rusconi S, Groner B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem. 1989;264:14396–14402. [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk MP, Velders FP, Tharner A, et al. FKBP5 and resistant attachment predict cortisol reactivity in infants: gene-environment interaction. Psychoneuroendocrinology. 2010;35:1454–1461. doi: 10.1016/j.psyneuen.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Menke A, Arloth J, Putz B, et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37:1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Klengel T, Rubel J, et al. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Mercado N, To Y, Kobayashi Y, Adcock IM, Barnes PJ, Ito K. p38 mitogen-activated protein kinase-gamma inhibition by long-acting beta2 adrenergic agonists reversed steroid insensitivity in severe asthma. Mol Pharmacol. 2011;80:1128–1135. doi: 10.1124/mol.111.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Maffioletti E, Cloninger CR, et al. Role of allelic variants of FK506-binding protein 51 (FKBP5) gene in the development of anxiety disorders. Depress Anxiety. 2013;30:1170–1176. doi: 10.1002/da.22158. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- Simic I, Adzic M, Maric N, et al. A preliminary evaluation of leukocyte phospho-glucocorticoid receptor as a potential biomarker of depressogenic vulnerability in healthy adults. Psychiatry Res. 2013a;209:658–664. doi: 10.1016/j.psychres.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Simic I, Maric NP, Mitic M, et al. Phosphorylation of leukocyte glucocorticoid receptor in patients with current episode of major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013b;40:281–285. doi: 10.1016/j.pnpbp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22:481–490. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Matsumoto Y, Sadahiro R, Enokido M, Goto K, Otani K. Relationship of the FKBP5 C/T polymorphism with dysfunctional attitudes predisposing to depression. Compr Psychiatry. 2014;55:1422–1425. doi: 10.1016/j.comppsych.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz A, Leszczynska-Rodziewicz A, Pawlak J, et al. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J Affect Disord. 2014;164:33–37. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Masliah E, et al. Differential expression of immunophilins FKBP51 and FKBP52 in the frontal cortex of HIV-infected patients with major depressive disorder. J Neuroimmune Pharmacol. 2009;4:218–226. doi: 10.1007/s11481-009-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatro ET, Nguyen TB, Bousman CA, et al. Correlation of major depressive disorder symptoms with FKBP5 but not FKBP4 expression in human immunodeficiency virus-infected individuals. J Neurovirol. 2010;16:399–404. doi: 10.3109/13550284.2010.504248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003;88:277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


