Abstract
Background
Herpes zoster (HZ) is a common viral disease that produces a painful vesicular rash. Early use of antiviral medications is recommended, as it reduces pain and speeds healing. A population-based observational study was conducted to evaluate the changing burden of HZ in the province of Manitoba (Canada) over a period of 17 years.
Methods
Administrative health care data including medical and hospital records were examined, and International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Clinical Modification codes were used to identify episodes of HZ between April 1, 1997 and March 31, 2014 in persons aged 20 or over. Annual age-adjusted incidence and hospitalization rates were calculated. Prescription records of HZ-diagnosed persons for acyclovir, valacyclovir, and famciclovir were used to calculate the rates and costs of antiviral treatment.
Results
There were 73,893 identified cases of HZ and 1,245 HZ-related hospitalizations between 1997 and 2013. Of these episodes, 42,270 (57.2%) were treated with antiviral medications at a total cost of $4,708,065 (CAD). The age-adjusted incidence of HZ rose from 4.67/1,000 person years in 1997/1998 to 5.67/1,000 person years in 2013/2014, a 21.9% increase. Antiviral treatment rates increased from 41.7% to 66.2% of all diagnosed episodes. Mean treatment costs per episode dropped from $127.29 in 1997/1998 to $56.06 in 2013/2014, primarily due to the introduction of generic antiviral medications. The total cost of antiviral treatment peaked in 2005/2006 at $329,935 and dropped steadily thereafter to $223,973 in 2013/2014. HZ-related hospitalization rates decreased from 3.1% to 0.9%.
Conclusion
While both the incidence of HZ and the rates of antiviral treatment have risen substantially, the economic burden from antiviral treatment has been decreasing since a peak in 2005/2006 and was only 3.2% higher in 2013/2014 than in 1997/1998. This drop in cost is attributed to the introduction of generic antiviral drugs.
Keywords: herpes zoster, burden, economics, valacyclovir, famciclovir, acyclovir
Introduction
Herpes zoster (HZ), or shingles, is a viral disease characterized by a painful, vesicular rash. It is caused by reactivation of latent varicella zoster virus (VZV), the virus responsible for varicella zoster (chicken pox) upon initial infection.1–3 VZV infection is ubiquitous in the current adult population with 95%–97% of adults over the age of 40 infected and at risk of developing HZ.4,5 It is estimated that 20%–30% of these persons will develop HZ at some time in their life.2,6 The annual incidence of HZ in the general population is estimated to be between 1.2 and 6.3 cases per 1,000 person years (PY). The incidence increases with age to 7.2–11.8 cases per 1,000 PY in persons older than 60 years.2,7,8 HZ is thus a common disease with the vast majority of adults at risk.
Antiherpetic antiviral drugs such as acyclovir, valacyclovir, and famciclovir are the cornerstone of acute treatment of HZ. Antiviral treatment reduces the duration and severity of symptoms and may decrease the risk of possible complications such as postherpetic neuralgia.9 The current recommended treatment for HZ is to begin antiviral therapy within 72 hours of the onset of symptoms and continue for 7 days.2,10,11 Antiviral drugs have been reported to account for 50% and 70% of the drug cost for treated cases of HZ and can account for a significant portion of total treatment cost.7,12,13
There have been two major changes that may impact the epidemiology of HZ. The first of these was the introduction of the varicella zoster vaccines (Varivax™; Merck Frosst Canada, Kirkland, Quebec, Canada), which became available in Canada in January 1999 and were incorporated into Manitoba’s publicly funded childhood vaccination program in 2004. Although HZ is still possible in vaccinated persons, the virulence of the vaccine strain is attenuated and this may lead to eventual decreases in the rates of HZ as these persons age into adulthood.14 The second change was the introduction of the herpes zoster vaccine (Zostavax®, Zostavax II®; Merck and Co., Inc.) in September 2009,15 which has been shown to be safe and effective in decreasing the incidence and burden of HZ.16–18 However, the impact of the HZ vaccine will be highly dependent on its uptake.
The population of Canada, including the population of Manitoba (estimated as 1.3 million in 2015), is aging.19 Due to the association of increasing age with a higher incidence of HZ, we would expect to see increasing numbers of HZ cases and an increase in the burden of disease. However, new vaccines and varying vaccine uptake coupled with other changes in the health care system make it difficult to predict the changing burden of disease for HZ. As part of a research program to determine the burden of HZ, this study examined the epidemiology of HZ and the utilization of antiviral medications in the province of Manitoba with the objectives of determining the incidence of HZ and the cost burden of acute antiviral treatments.
Methods
A retrospective, population-based cohort study of HZ was conducted using administrative data. The eligible population included all adults over the age of 20 in Manitoba (Canada) between April 1, 1997 and March 31, 2014. Data were obtained through the Manitoba Centre for Health Policy, which maintains the provincial Population Health Research Data Repository. The repository contains copies of administrative health care data of Manitoba Health. The databases used included the Drug Program Information Network (DPIN), the Medical Services database, provincial Hospital Discharge Abstracts, and the Manitoba Health Registry. These databases contain de-identified health-related information on all persons registered with Manitoba Health, virtually the entire population of Manitoba.20 Scrambled patient identifiers are shared between these databases, enabling linkage of records and allowing for the longitudinal analysis of individual patients across the entire health care system.
The DPIN system is used to administer the Manitoba Pharmacare Program, a universal prescription drug care plan available to all residents of Manitoba. DPIN is used to submit claims to Pharmacare and other third-party prescription drug programs, and captures community prescription drug use by persons registered with Manitoba Health. The Medical Services database captures fee-for-service medical claims from physicians and other health providers, and includes diagnostic codes and reimbursement costs. The Hospital Discharge Abstracts database contains information on all hospitalizations for persons registered with Manitoba Health, including both admissions in Manitoba and those of Manitoba residents hospitalized in other Canadian provinces. The Manitoba Health registry contains demographic information on all persons registered with the agency and is used to provide population counts and age.
Episodes of HZ were identified using hospital discharge abstracts and medical claim records for the period between April 1, 1995 and March 31, 2014, which included a 2-year prestudy data collection period to differentiate between incident and prevalent cases. Persons with diagnostic codes for HZ (International Classification of Diseases [ICD], Ninth Revision, Clinical Modification codes starting with 053 and ICD, Tenth Revision, Clinical Modification codes starting with B02) that were not related to HZ vaccinations were categorized as cases with episode start dates based on the earliest recorded diagnosis. Multiple episodes were allowed in cases of multiple diagnostic codes, provided that at least 2 years had elapsed from the beginning of the previous episode and there were no other diagnostic codes in the previous 180 days. Episodes with a start date prior to April 1, 1997 were excluded to ensure only incident cases were analyzed. Persons under the age of 20 years at the time of diagnosis were excluded to avoid miscoded cases of varicella zoster or varicella zoster vaccination. Hospitalizations were attributed to HZ in cases where an HZ diagnostic code appeared as either the admitting diagnosis or the diagnosis most responsible for continuing hospitalization.
Prescription records for antiviral drugs (acyclovir, valacyclovir, and famciclovir) dispensed in the period of 5 days before and up to 30 days after the appearance of the first diagnostic code for HZ were considered to be for the treatment of HZ. Prescriptions outside of this window were excluded to avoid misclassifying antiviral treatment of herpes simplex 1 or 2. Drug costs were calculated directly from the prescription records and included both cost of the drug and the dispensing fee.
The Manitoba Pharmacare fiscal year (April 1–March 31) was used in the analysis. Episodes, prescription rates, costs, and hospitalizations were all considered to have been incurred in the fiscal year in which the episode was first diagnosed. Mean treatment costs were calculated for antiviral-treated episodes by the drugs used within each year. The absolute number of HZ episodes and the incidence rates were calculated within each year. The age distribution of the 1997 Manitoba population was used to age-adjust incidence rates in subsequent years. Antiviral treatment rates were calculated as the percent of episodes associated with one or more antiherpetic prescriptions. Hospitalization rates were calculated as the percent of all episodes that resulted in HZ hospitalizations. All costs have been adjusted to 2013 Canadian dollars (CAD) using Statistics Canada’s consumer price index for prescription drug costs in Manitoba.
SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all data analyses. Approvals were granted by the University of Manitoba Health Research Ethics Board and the Manitoba Health Information Privacy Committee. These committees do not require individual consent for research conducted using de-identified administrative data when reasonable safeguards to protect confidentiality and security of personal health information are in place.
Results
Between April 1, 1997 and March 31, 2014, we identified 73,893 diagnosed episodes of HZ in Manitoba in those aged 20 years and older, with a mean unadjusted incidence rate of 4.97 episodes/1,000 PY (95% confidence interval [CI]: 4.79, 5.14) or 4.80 episodes/1,000 PY (95% CI: 4.64, 4.95) after age adjustment. Within our observation period, 6.1% of persons experienced more than one HZ episode.
A total of 45,614 prescriptions were dispensed to treat 42,270 episodes at a total cost of $4,708,065 (CAD). Overall, 57.2% of diagnosed cases were treated with antiviral medications with a mean cost per treated episode of $111.38 (95% CI: $98.59, $124.16). There were 1,245 hospitalizations where HZ was the admitting diagnosis or the most responsible diagnosis for continuing hospitalization with an incidence of 0.085/1,000 PY (95% CI: 0.053, 0.145).
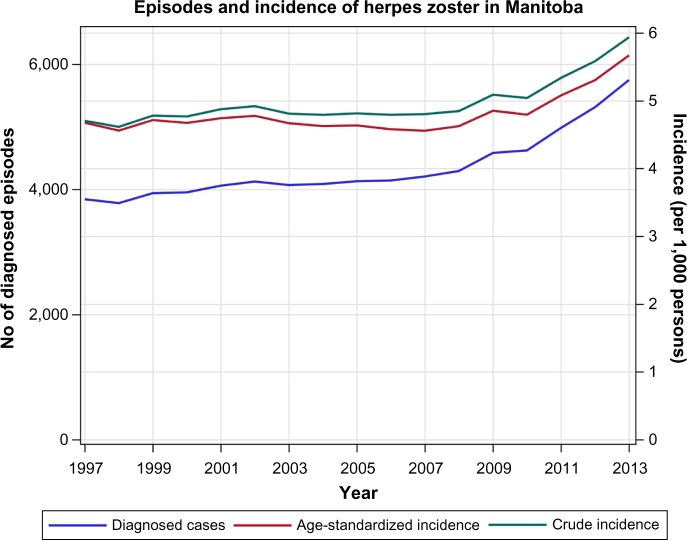
From 1997/1998 to 2008/2009, the number of HZ diagnoses climbed slowly from 3,844 to 4,295 episodes per year. However, the age-adjusted incidence of HZ diagnoses remained relatively constant at a mean of 4.65/1,000 PY (95% CI: 4.61, 4.70). The incidence rose steadily from 2009 until the end of the study period where in the year 2013/2014, the age-adjusted incidence reached 5.67/1,000 PY, an increase in incidence of 21.9% from the 1997–2008 plateau (Figure 1).
Figure 1.
Herpes zoster episode numbers with crude and age-standardized incidence rates.
Note: Population age distribution standardized to the population of Manitoba in 1997.
The use of antiviral drugs to treat HZ increased with the increased incidence of HZ, but to a greater degree as more of these episodes were treated with antivirals. Treatment rates increased from 41.7% of HZ diagnoses being prescribed antiviral drugs in 1997/1998 to 66.2% of cases in 2013/2014.
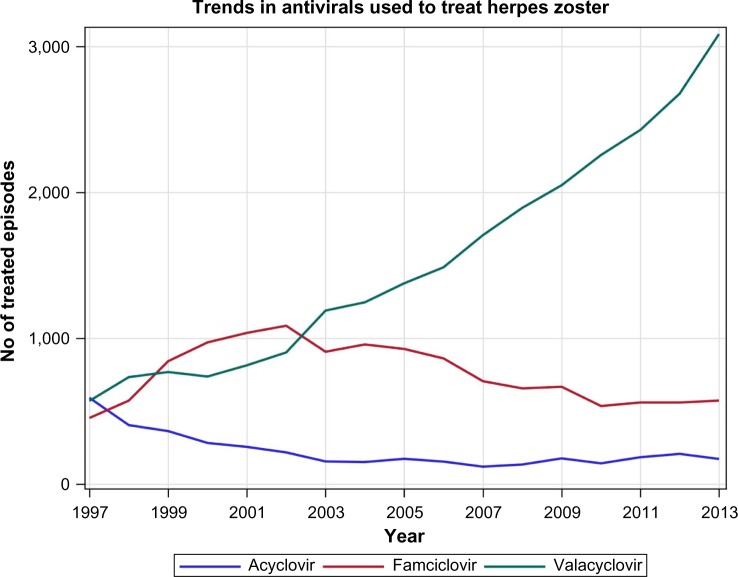
Of the 45,614 antiviral prescriptions identified, 4,263 (9.3%) were for acyclovir, 27,752 (60.8%) for valacyclovir, and 13,601 (29.8%) for famciclovir. Substantial changes in prescribing patterns of these drugs were observed over the study period. While acyclovir was the most commonly used antiviral to treat HZ at the start of the study period, its use dropped rapidly and by 2013/2014, it was used in less than 5% of treated cases. Acyclovir was replaced by famciclovir and valacyclovir, with the use of famciclovir peaking in 2002/2003 when it accounted for 49% of all HZ-related antiviral prescriptions. Since 2003/2004, valacyclovir has been the dominant antiviral treatment. By the end of the study period, valacyclovir was used to treat 50.0% of all HZ diagnoses and it accounted for 80.6% of the antiviral HZ prescriptions (Figure 2).
Figure 2.
Number of herpes zoster episodes treated with acyclovir, famciclovir, or valacyclovir by year.
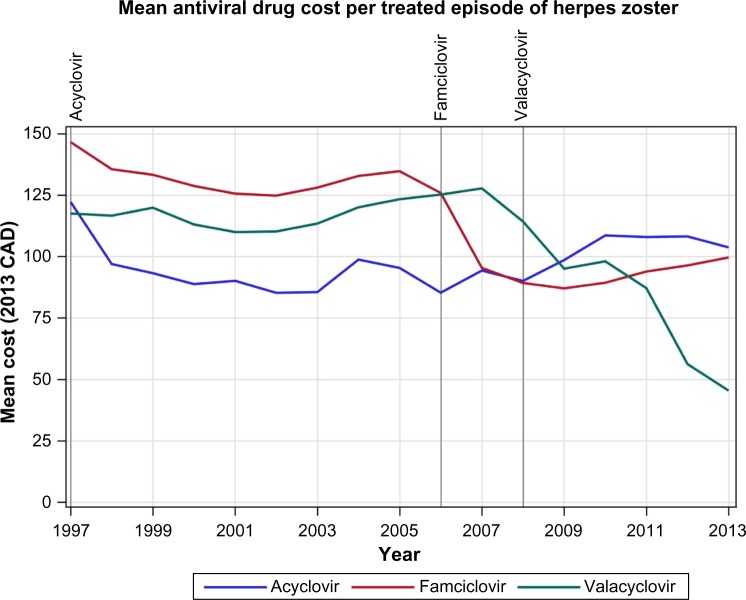
Generic acyclovir came to the Canadian market in August 1997, the first study year, generic famciclovir in August 2006, and generic valacyclovir in May 2008. Comparing the mean costs in 1997/1998 and 2013/2014, the mean cost per treated episode decreased by $18.50 (15.1%) from $122.19 to $103.69/episode for acyclovir, by $46.96 (32.0%) from $146.57 to $99.61/episode for famciclovir, and by $72.11 (61.4%) from $117.50 to $45.39 for valacyclovir (Figure 3). The mean cost per treated episode averaged across all antiviral prescriptions dropped from $127.29 to $56.06 per treated episode.
Figure 3.
Mean drug treatment cost per antiviral treated herpes zoster episode.
Note: Vertical lines indicate date generic versions of labeled drug introduced; CAD, Canadian dollars.
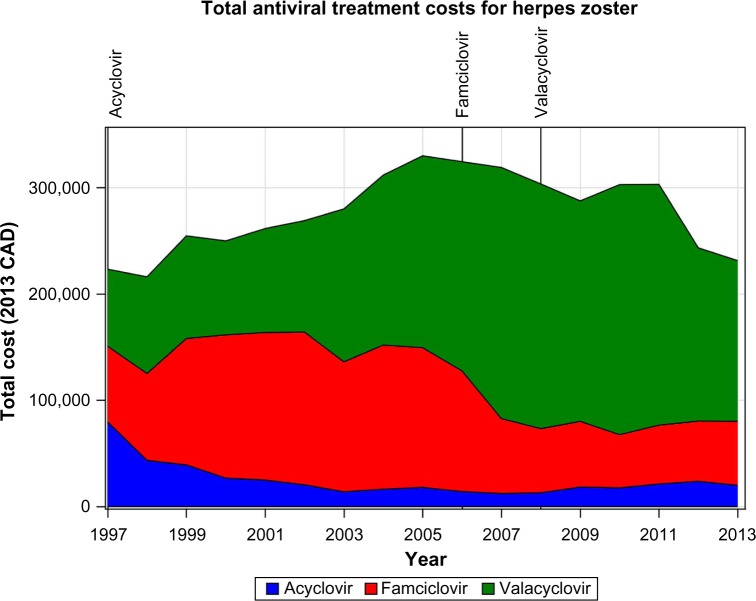
The total cost of treatment changed substantially over the study period, with the total cost for each drug dropping coincidently with the introduction of generic forms of each drug (Figure 4). Antiviral cost peaked in 2005/2006 at $329,935, which is a 47.3% increase from 1997 when the total cost was $223,973. The total cost then began a decline that continued until the end of the study period. The total cost in 2013 was $232,478, only a 3.2% increase from 1997 and a 30.0% decrease from 2005.
Figure 4.
Total costs of antiviral treatment of herpes zoster.
Note: Vertical lines indicate date generic versions of labeled drug introduced; CAD, Canadian dollars.
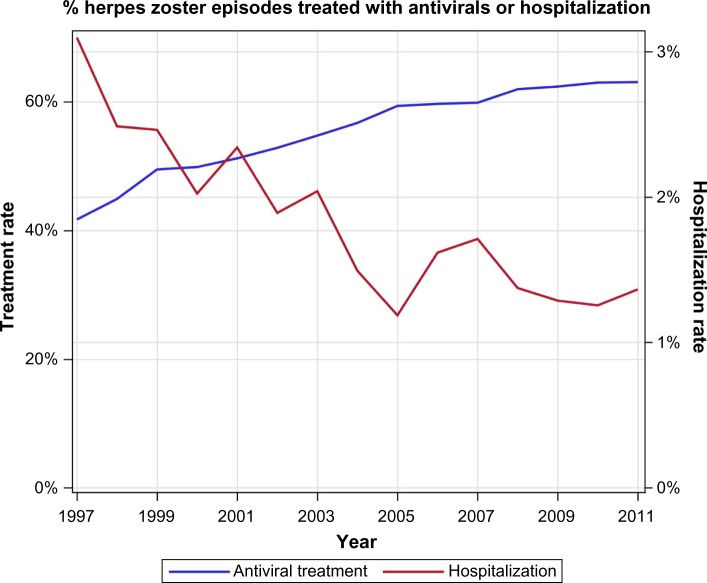
There was a dramatic decrease in the rate and number of HZ-related hospitalizations concurrently with the increased rates of antiviral treatment. The number of hospitalizations dropped by 48% between 1997 and 2013, from 119 to 51 hospital stays per year. At the same time, the percent of episodes that did not receive antiviral treatment dropped by 39% (Figure 5).
Figure 5.
Percent of episodes of herpes zoster treated with antiviral drugs, and percent of episodes resulting in hospitalization.
Discussion
It was found in the study that overall, despite an increasing incidence of diagnosed HZ cases and higher rates of treatment, the aggregate population costs of HZ antiviral treatment have declined in recent years. The increase in HZ incidence was an expected effect of the aging population. However, the age-adjusted incidence rate revealed an increase in HZ incidence independent of aging, which was unexpected. While more HZ episodes and more treatments seemed to drive an initial increase in population costs, generic pricing for antiviral medications has more than compensated for these increases and has resulted in the recent reduction in overall antiviral costs.
HZ consultation rates are frequently claimed to be an accurate estimator of the actual incidence of this disease in the population. Symptom severity is thought to drive most persons to seek medical attention and it has been estimated that up to 99% of persons with HZ do so.12,21 As such, the Manitoba-linked administrative records should provide a reliable measure of HZ incidence. The study found a relatively stable age-standardized incidence of HZ over the first two-thirds of the study period. However, starting in 2009/2010, there was a sharp and continuing increase in the incidence of HZ. A similar increase in incidence was also seen in a study conducted in Ontario (Canada) in 2009: the authors were unable to determine a cause for such an increase.22 As 2009 was also the last year of the dataset used in the study, the authors were unable to determine if it was the beginning of a trend and suggested it would be important to determine if the increase persisted. While we are no closer to determining the cause of the increase in Manitoba, it is apparent that it continued for 5 years till the end of our study period in March 2014.
One possible explanation is that the increase in incidence is an artifact of increased publicity and attention to HZ in recent years coinciding with the introduction of HZ vaccine to the Canadian marketplace in 2009. The marketing of the vaccine has raised public awareness of the signs and symptoms of HZ, possibly influencing people to seek care. Alternatively, it has been speculated that widespread varicella zoster vaccination decreases the levels of wild-type VZV in the environment, thus eliminating the natural boosting effect of exposure in a latently infected individual.23 Theoretical modeling using this hypothesis suggests we can expect an initial increase in HZ rates, eventually followed by sustained long-term declines.6 Any such increases would obviously be mitigated by HZ vaccination. While the observed increases are important and warrant continued monitoring, the cause remains speculative at this point and will be difficult to be definitively ascertained.
The study showed an increased incidence of disease and a greater likelihood of treatment with antiviral therapy, but a decrease in the economic burden of disease due to treatment. While the increases in diagnosed cases of HZ and the proportion of cases treated with antivirals drive the treatment levels up, the effects on cost are overwhelmed by the drop in the price of treatment. Both acyclovir and famciclovir dropped from a stable price point associated with the brand name product to a lower stable cost associated with the generic product. It is important to recognize that in Manitoba’s single payer system, generic substitution is mandatory and the vast majority of prescriptions are automatically switched to the generic product when it is available. As valacyclovir became the treatment of choice in Manitoba, the generic market was very competitive and there was a sustained downward trend in treatment cost with this drug. This was the major contributor to the overall drop in the cost of treatment.
The rate of HZ-related hospitalizations dropped throughout the study period. This downward trend in HZ-related hospitalization was also reported in the Ontario study where the incidence dropped by approximately half between 1992 and 2009.22 Antiviral treatment steadily increased throughout our study period, from less than 42% in 1997/1998 to over 66% in 2013/2014. Antiviral treatment has been shown to decrease both the severity and duration of symptoms.2,11 It is possible that the increasing antiviral treatment of HZ has contributed to the decreasing rates of hospitalization, but other explanations cannot be ruled out. Limitations in hospital bed availability may make it more likely that HZ is managed on an outpatient basis.
The ability to capture the entire population of the province over a time span of 17 years is a major strength of our research. The linked nature of the data allows individuals to be followed across the entire health care system. However, using administrative data also has the limitations common to all researches of this type, namely, it was created not for the purpose of research, but for managing the health care system, and cases are identified using ICD codes that are meant for billing purposes. Nevertheless, the reliability of HZ diagnoses is known to be very high due to the distinctive nature of the disease. In fact, the use of ICD codes to diagnose HZ in administrative data is both highly selective (positive predictive value 93%) and sensitive (97.5%).24
Conclusion
The aggregate cost of antiviral treatment of HZ is the product of a number of factors: the numbers of episodes, treatment rates, medication choice, and treatment cost. All these factors change with time and can alternately increase or decrease costs, making predicting the net effect very difficult. Only by directly measuring the real-world burden of a disease can the overall disease resource impacts be assessed. The results of this analysis illustrate the significant impact that generic drug pricing and mandatory substitution can have on disease management costs at the population level. Further research looking at other economic facets of HZ is needed to complete our understanding of the total burden of this disease.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project #H2014:411 (HIPC# 2014/2015-35). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Population Health Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from the data provided by Manitoba Health. We thank Heather Prior from the Manitoba Centre for Health Policy for her assistance in preliminary data extraction.
Footnotes
Disclosure
This work was supported by a grant from Merck Canada. The authors report no other conflicts of interest in this work.
References
- 1.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58(1):9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Schmader KE, Dworkin RH. Natural history and treatment of herpes zoster. J Pain. 2008;9(1 Suppl 1):S3–S9. doi: 10.1016/j.jpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Gnann JW, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347(5):340–346. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 4.Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127(2):305–314. doi: 10.1017/s0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairley CK, Miller E. Varicella-zoster virus epidemiology – a changing scene? J Infect Dis. 1996;174 Suppl(Suppl 3):S314–S319. doi: 10.1093/infdis/174.supplement_3.s314. [DOI] [PubMed] [Google Scholar]
- 6.Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol Infect. 2000;125(3):651–669. doi: 10.1017/s0950268800004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gialloreti LE, Merito M, Pezzotti P, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lionis CD, Vardavas CI, Symvoulakis EK, et al. Measuring the burden of herpes zoster and post herpetic neuralgia within primary care in rural Crete, Greece. BMC Fam Pract. 2011;12(1):136. doi: 10.1186/1471-2296-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125–132. doi: 10.2147/JPR.S57242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39(11):902–908. doi: 10.1111/j.1346-8138.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–S26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 12.Ultsch B, Köster I, Reinhold T, et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ. 2013;14(6):1015–1026. doi: 10.1007/s10198-012-0452-1. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137(1):38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 14.Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19(3):124–129. doi: 10.1016/j.spen.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drug Products Database Heal Canada Website. [Accessed November 2, 2015]. Available from: http://webprod5.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp.
- 16.Schmader KE, Johnson GR, Saddier P, et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc. 2010;58(9):1634–1641. doi: 10.1111/j.1532-5415.2010.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxman MN, Levin MJ, Shingles Prevention Study Group Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197(Suppl 2):S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 19.Statistics Canada . Annual Demographic Estimates: Canada, Provinces and Territories. Ottawa, ON: 2015. [Accessed November 25, 2015]. Available from: http://www.statcan.gc.ca/pub/91-215-x/91-215-x2014000-eng.pdf. [Google Scholar]
- 20.Roos LL, Brownell M, Lix L, Roos NP, Walld R, Macwilliam L. From health research to social research: Privacy, methods, approaches. Soc Sci Med. 2008;66:117–129. doi: 10.1016/j.socscimed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Bilcke J, Ogunjimi B, Marais C, et al. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol Infect. 2012;140(11):2096–2109. doi: 10.1017/S0950268811002640. [DOI] [PubMed] [Google Scholar]
- 22.Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29(47):8580–8584. doi: 10.1016/j.vaccine.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20(19–20):2500–2507. doi: 10.1016/s0264-410x(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 24.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86(12):1146–1153. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]