Abstract
Photoacclimatory responses of the seagrass Zostera marina in the intertidal and subtidal zones were investigated by measuring chlorophyll a fluorescence parameters, photosynthetic pigments, leaf δ13C values, and shoot morphology in two bay systems. Intertidal plants had higher carotenoid concentrations than subtidal plants to avoid photodamage under excess light conditions during the day. The maximum relative electron transport rate (rETRmax) and minimum saturation irradiance (Ek) of the intertidal plants were higher than those of the subtidal plants, whereas photosynthetic efficiency (α) and maximum quantum yield (Fv/Fm) were higher in subtidal plants. The intertidal plants also had significantly greater Stern–Volmer non-photochemical quenching (NPQ) than that of the subtidal plants. These results suggest that the subtidal plants photoacclimated to use limited light more efficiently, and the intertidal plants exhibited photosynthetic responses to minimize photodamage at excess irradiance. The δ13C values of leaf tissues were more negative in the intertidal plants than those in the subtidal plants, suggesting that the intertidal plants used atmospheric or dissolved CO2 for photosynthesis during emersion. Effective quantum yield (ΔF/Fm´) in the intertidal plants decreased more slowly after emersion than that in the subtidal plants, indicating higher desiccation tolerance of the intertidal plants. The intertidal plants also recovered more rapidly from desiccation damage than the subtidal plants, suggesting photosynthetic adaptation to desiccation stress. The photosynthetic plasticity of Z. marina in response to variable environmental conditions most likely allows this species to occur in the intertidal and subtidal zones.
Introduction
Seagrasses occurring in the intertidal and subtidal zones are exposed to highly variable environmental conditions due to tidal changes [1,2]. Seagrasses in the intertidal zone are regularly exposed to air and consequently experience extreme high and low temperatures, high photoinhibitory irradiance, and desiccation stress relative to subtidal seagrass [2–4]. Such extreme temperatures can lead to significant seagrass dieback when seagrasses are exposed to air during low tide [5–7]. Desiccation stress during low tide has been considered the primary factor limiting seagrass distribution at the upper intertidal zone [8]. Seagrasses residing the intertidal zone are usually smaller than those in the subtidal zone to minimize the effects of emergence stress [9,10]. Intertidal seagrasses also show light-dependent responses, such as decreased photosynthetic efficiency and increased photoprotection during periods of high irradiance and air exposure [11,12].
In contrast, seagrasses in the subtidal zone adapt to reduced light conditions caused by light attenuation and scattering due to the overlaying water column and suspended particles [13,14]. Seagrasses in the deep subtidal zone generally have longer leaves and wider leaf blades than those in the shallow subtidal or intertidal zone, which allows more photosynthesis, in turn resulting in greater growth [4]. Seagrasses also respond to reduced light conditions by increasing chlorophyll content and decreasing the chlorophyll a/b ratio to enhance light absorption efficiency by using the abundant wavelengths efficiently [15–17]. As seagrasses in the intertidal and subtidal zones are under highly different light conditions, they exhibit distinctly different photoacclimatory responses to maximize photosynthetic activity and photoprotection from excess irradiance.
Seagrasses assimilate large amounts of inorganic carbon to achieve high level production [18,19]. Marine macrophytes, including seagrass, use both CO2 and HCO3− for photosynthetic carbon reduction [20–23]. Despite air exposure during low tide, seagrasses in the intertidal zone can continue to photosynthesize utilizing CO2 in the air [24]. Thus, the composition of inorganic carbon sources for seagrass photosynthesis probably varies between intertidal and subtidal plants. Because carbon stable isotope ratios of plant tissues change based on the inorganic carbon sources for photosynthesis [25,26], seagrasses in the intertidal and subtidal zones may have different carbon stable isotope ratio ranges.
Although eelgrass, Zostera marina, which is the most abundant seagrass species in the Northern Hemisphere, usually occurs in the shallow subtidal zone [27–29], intertidal Z. marina populations have also been reported from various geographical locations [2,30,31]. Despite their distribution in the intertidal and subtidal zones, the ecological and physiological differences between intertidal and subtidal Z. marina plants have rarely been studied within a bay system. Compared to morphological plasticity, photoacclimatory responses and inorganic carbon sources of intertidal and subtidal Z. marina plants are not well documented. Thus, in the present study, we examined the photoacclimatory responses of intertidal and subtidal Z. marina plants by measuring chlorophyll a fluorescence parameters. We also evaluated the variability in the inorganic carbon sources available to intertidal and subtidal plants by measuring carbon stable isotope ratios of Z. marina leaf tissues. Additionally, we conducted emersion experiments to compare desiccation tolerance and recovery ability from emersion stress between intertidal and subtidal Z. marina. We hypothesized that intertidal Z. marina would exhibit adaptive responses to an environment of harsh extremes, whereas subtidal plants would exhibit photoacclimatory responses to low irradiance. Carbon stable isotope ratios of intertidal plants are assumed to be more negative than those of subtidal plants because intertidal plants can utilize CO2 in the air during low tide. Global climate change-associated environmental disturbances, such as a rise in sea level, may lead to changes in tidal level, and consequently cause significant changes in the distribution and population structure of seagrasses in intertidal and subtidal zones. Thus, this study will provide valuable information for a better understanding of the effects of global climate change on coastal seagrass ecosystems.
Materials and Methods
Study sites
The study sites were located in Aenggang Bay (34° 46΄N, 127° 56΄E) and Koje Bay (34°48΄N, 128°35΄E) on the southern coast of Korea (Fig 1), where four Zostera species (Z. japonica, Z. marina, Z. caespitosa, and Z. caulescens) are distributed at different water depths. No specific permissions to collect research samples were required at the study sites in Aenggang Bay and Koje Bay, and the field study did not involve endangered or protected species. This study was conducted in monotypic meadows of Z. marina, which is the dominant species in both bay systems. Zostera marina was distributed continuously from the lower intertidal to the upper subtidal zones at both study sites, and thus plants at the intertidal and subtidal zones were from the same population. The plants in the lower intertidal zone were exposed to air during low tide. The emersion period for the intertidal plants ranged from 0.8 hours per day in July-September and 3.4 hours per day in March, with a mean emersion period of 1.7 hours per day. In contrast, the subtidal plants were never exposed to air in either bay system and were located at a mean water depth of about 3.0 m relative to mean lower low water. In Koje Bay, average shoot density (1140 shoots m-2) in the intertidal zone was 11.6 times higher than that (98 shoots m-2) in the subtidal zone [32,33]. Average daily underwater irradiances were 22.1 and 11.7 μmol photons m-2 d-1 in the intertidal and subtidal zones, respectively, in Koje Bay [32,33]. Water temperatures were 5.0–28.5°C, and salinity was 30.4–33.6. Sandy sediments predominated at both study sites. Water column NH4+ and PO43− concentrations were usually less than 4 μM and 2 μM, respectively; mean NO3− + NO2− concentration in the water column was about 1.7 μM [34]. The tidal amplitude at both sites varied between 4.0 m during spring tide to 1.2 m during neap tide, and the tidal regime is semi-diurnal with an approximate 3.0 m tidal range (Tide tables for Coast of Korea, National Oceanographic Research Institute of Korea).
Fig 1. Study sites in Aenggang Bay and Koje Bay on the southern coast of the Korean peninsula.
Biological measurements
Ten to fifteen mature terminal shoots were collected from each intertidal and subtidal site in the two bay systems on a seasonal basis (April, July, and October 2006 and February 2007) to measure shoot morphological characteristics. Sheath length was measured from the meristem to the top of the sheath. Shoot height was measured from the meristem to the tip of the longest leaf to the nearest 1.0 mm, and the width of the longest leaf was measured to the nearest 0.1 mm.
Concentrations of leaf pigments (chlorophylls and carotenoids) were estimated using the middle part of the youngest mature (fully grown) leaf where the chlorophyll a fluorescence parameters were measured. Six replicate samples from each site were collected and cleaned of epiphytes in the laboratory. Pre-weighed leaf tissues were extracted with 5.0 ml of N,N-dimethylformamide for 2–3 days in glass tubes following the method of Dunton and Tomasko [35]. Absorbance of the extracts was measured at 480, 647, 664, and 750 nm using a Shimadzu Model 2264 UV spectrophotometer (Tokyo, Japan). Pigment concentrations were calculated using the extinction coefficient equations [36].
Measurement of the chlorophyll a fluorescence parameters
The chlorophyll a fluorescence parameters were measured in situ using 10–15 shoots from each site. The parameters were measured at the middle part of the youngest mature leaf using a pulse amplitude modulated (PAM) fluorometer (Diving-PAM; Heinz Walz GmbH, Effeltrich, Germany) with an 8 mm fiber optic. Before measuring rapid-light curves (RLCs), maximum quantum yield (Fv/Fm) of photosystem II (PSII) was determined after a 10 min dark adaptation using the following equation: Fv/Fm = (Fm−F0) / Fm, where F0 is the minimal fluorescence of a dark-adapted leaf, in which all PSII reaction centers are open, and Fm is the corresponding maximum fluorescence measured with all PSII reaction centers closed following a short (0.8 s) saturating light period (e.g., [37]). Effective quantum yield of PSII (ΔF/Fm´) was measured on light-adapted samples according to the relationship: ΔF/Fm´ = (Fm´ –Ft)/Fm´, where Fm´ is the light-adapted maximum fluorescence and Ft is the fluorescence before a saturating pulse [38].
RLCs were produced by the Diving-PAM under control of an internal program using artificial photosynthetic photon flux density (PPFD). The samples were exposed to eight incremental steps of irradiance ranging from 0 to 1,042 μmol photons m−2 s−1. The samples were exposed to 10 s of irradiance at each incremental step during the RLC measurement. The relative electron transport rate (rETR) was determined from the following equation: rETR = ΔF/Fm´ × PPFD × 0.5 × 0.84, where ΔF/Fm´ is the effective quantum yield of PSII, 0.84 is the assumed absorption coefficient, and 0.5 is a correction for the two photosystems absorbing photons [38]. The RLC data were fitted to the model of Platt et al. [39] to obtain values of photosynthetic efficiency (α), minimum saturating irradiance (Ek), and maximum relative electron transport rate (rETRmax). Ek was calculated as Ek = rETRmax/α. Quenching coefficients were calculated according to the following equations [40].
Photochemical quenching
Non-photochemical quenching
Stern–Volmer non-photochemical quenching
Stable isotope analysis
We used the youngest mature leaf (second or third leaf) of the shoots collected for morphological characteristic to determine the carbon stable isotope ratio (δ13C) of leaf tissue. Leaf samples were dried at 60°C to a constant weight and then ground using a mortar and pestle. The ground samples were wrapped in tin capsules and combusted at high temperature (1030°C) in an elemental analyzer (Eurovector 3000 Series; Milan, Italy), and the CO2 gas was analyzed to determine the carbon stable isotope ratio using a continuous flow-through inlet system attached to an isotope ratio mass spectrometer (Isoprime; GV Instruments, Manchester, UK). Peedee belemnite marine limestone was used as the primary standard. Analytical precision was approximately 0.1–0.2‰ for δ13C.
Photosynthetic responses to emersion and recovery after re-immersion
To compare the photosynthetic responses to emersion between intertidal and subtidal Z. marina plants, we collected 10 shoots from each tidal zone in the two bay systems. The plants were maintained in 20°C aerated seawater with underwater irradiance of 150 μmol photons m−2 s−1 in the laboratory. All emersion experiments were conducted within 4 h of shoot collection. The plants were placed under air-exposed conditions within an environmentally controlled room with constant temperature (20°C), relative humidity (65%), and light intensity (300 μmol photons m−2 s−1). Effective quantum yield was measured every hour (0, 1, 2, 3, 4, 5, and 6 h) after air exposure.
Zostera marina shoots were exposed to air for 1, 2, 3, 4, 5, and 6 h periods and immediately re-immersed into seawater with constant temperature (20°C) and underwater irradiance (150 μmol photons m−2 s−1) to investigate recovery of photosynthetic efficiency from emersion stress. The effective quantum yields of the shoots were measured 0, 1, 3, 5, and 12 h after re-immersion.
Statistical analyses
All values are presented as mean ± standard error. Data were log (x + 1) or arcsin (x/1000 + 1) transformed to meet the assumptions of parametric statistics prior to analysis. Significant differences in morphological characteristics (shoot height, sheath length, and leaf width), RLC parameters (rETRmax, α, Ek, maximum quantum yield, and NPQ), photosynthetic pigments (total chlorophyll, total carotenoids, chlorophyll a/b ratio, and the total chlorophyll/total carotenoid ratio), and the carbon stable isotope ratio (δ13C) between the intertidal and subtidal plants and among seasons in each bay system were analyzed using a two-way analysis of variance (ANOVA). Significant differences in effective quantum yield between the intertidal and subtidal plants and among air exposure periods were also tested using a two-way ANOVA. When a significant difference was observed among variables, the means were analyzed using the Student–Newman–Keuls test to determine where the significant difference occurred. An alpha level of 0.05 was used for all statistical tests. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Shoot morphology and photosynthetic pigments
Shoot height, sheath length, and leaf width of Z. marina in the intertidal zone were significantly shorter or narrower (P < 0.001 for all) than those in the subtidal zone throughout the experimental period in both bay systems (Table 1; S1 Table). The greatest differences in shoot morphology between the intertidal and subtidal plants occurred during spring–summer periods when the subtidal populations exhibited their highest growth rates (Table 1).
Table 1. Morphological characteristics (shoot height, sheath length and leaf width) of intertidal and subtidal Zostera marina plants in Aenggang Bay and Koje Bay.
| Aenggang Bay | Koje Bay | ||||
|---|---|---|---|---|---|
| Season | Morphological characteristic | Intertidal | Subtidal | Intertidal | Subtidal |
| Spring | Shoot height (cm) | 34.5 ± 2.1a | 136.5 ± 6.3b | 37.2 ± 1.8a | 82.0 ± 4.4b |
| Sheath length (cm) | 7.4 ± 0.4a | 32.2 ± 1.1b | 9.9 ± 0.6a | 19.9 ± 1.0b | |
| Leaf width (mm) | 6.2 ± 0.3a | 11.6 ± 0.3b | 5.7 ± 0.2a | 9.9 ± 0.3b | |
| Summer | Shoot height | 24.8 ± 1.1a | 119.1 ± 3.8 b | 47.4 ± 3.3a | 98.8 ± 2.8b |
| Sheath length | 5.0 ± 0.2a | 15.2 ± 1.0b | 9.3 ± 0.4a | 23.3 ± 0.6b | |
| Leaf width | 4.6 ± 0.2a | 9.5 ± 0.1b | 6.9 ± 0.2a | 9.6 ± 0.2b | |
| Fall | Shoot height | 36.0 ± 2.8a | 51.2 ± 1.6b | 36.5 ± 2.0a | 65.0 ± 1.4b |
| Sheath length | 7.4 ± 0.7a | 10.6 ± 0.3b | 8.0 ± 0.5a | 13.9 ± 0.2b | |
| Leaf width | 5.8 ± 0.2a | 8.2 ± 0.2b | 6.1 ± 0.2a | 8.2 ± 0.3b | |
| Winter | Shoot height | 41.1 ± 2.2a | 55.0 ± 1.5b | 32.7 ±1.8a | 55.2 ± 1.4b |
| Sheath length | 8.0 ± 0.3a | 14.6 ± 0.8b | 8.3 ± 0.8a | 16.4 ± 0.1b | |
| Leaf width | 6.2 ± 0.3a | 9.5 ± 0.2b | 5.2 ± 0.2a | 10.0 ± 0.4b | |
Values are mean ± standard errors (n = 10–15). Values with the same letter are not significantly different (P < 0.05) between the intertidal and subtidal plants in each bay system and each season.
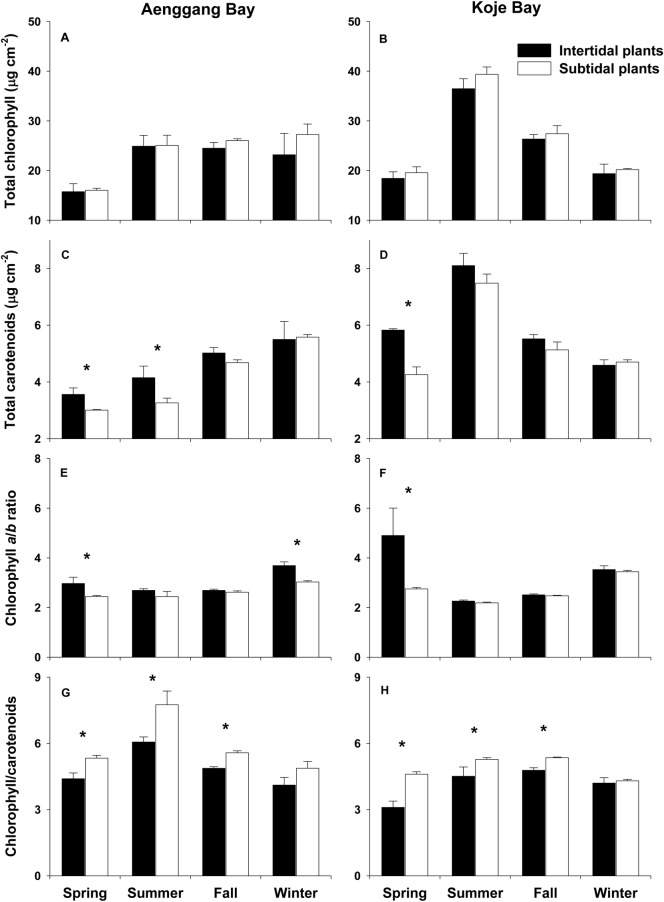
Total chlorophyll (chl. a + chl. b) content was slightly higher in the subtidal plants than in the intertidal plants, but was not significantly (P = 0.226 in Aenggang Bay; P = 0.174 in Koje Bay) different between the intertidal and subtidal plants (Fig 2A and 2B; S1 Table). Total carotenoid concentrations were significantly higher (P < 0.05 in Aenggang Bay; P < 0.01 in Koje Bay) in the intertidal plants than in the subtidal plants during spring and summer in Aenggang Bay and during spring in Koje Bay (Fig 2C and 2D; S1 Table). Chlorophyll a/b ratios of the intertidal plants were significantly higher (P < 0.01 in Aenggang Bay; P < 0.05 in Koje Bay; S1 Table) than those of the subtidal plants during spring and winter in Aenggang Bay and during spring in Koje Bay (Fig 2E and 2F; S1 Table). In contrast, the chlorophyll/carotenoid ratios were significantly higher (P < 0.05 in Aenggang Bay; P < 0.001 in Koje Bay) in the subtidal plants than in the intertidal plants during spring–fall in both bay systems (Fig 2G and 2H; S1 Table).
Fig 2. Zostera marina.
Photosynthetic pigment concentrations (total chlorophyll and total carotenoids), chlorophyll a/b ratio, and the chlorophyll/carotenoid ratio of the intertidal and subtidal plants in Aenggang Bay (A, C, E, and G) and Koje Bay (B, D, F, and H). Values are means ± standard errors (n = 6). An asterisk above the bar indicates a significant difference between the intertidal and subtidal plants in each season.
Chlorophyll a fluorescence parameters
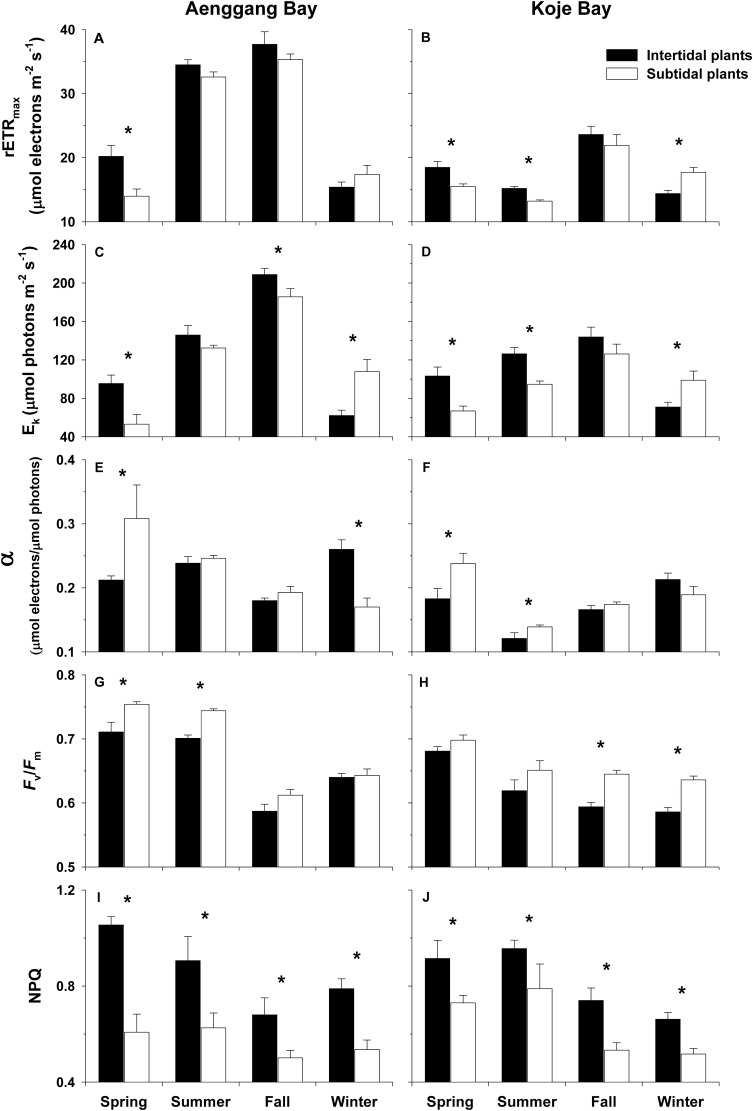
The rETRmax and Ek values of the intertidal plants tended to be higher than those of the subtidal plants during all experimental periods, except winter (Fig 3A–3D; S2 Table). The α values exhibited the opposite trend to that of rETRmax and Ek (Fig 3E and 3F; S2 Table). The α values were higher in subtidal plants than in intertidal plants during spring–fall. Subtidal plant Fv/Fm values were significantly higher (P < 0.001 in both bay systems) than those of the intertidal plants in both bay systems (Fig 3G and 3H; S2 Table). The intertidal plants had significantly greater (P < 0.001) NPQ than that of the subtidal plants in both bay systems throughout the experimental period (Fig 3I and 3J; S2 Table). Mean NPQ values (0.83 and 0.76 in Aenggang Bay and Koje Bay, respectively) in intertidal plants were 1.3–1.5 times higher than in subtidal plants (0.56 and 0.59).
Fig 3. Zostera marina.
Parameters (rETRmax, α, Ek, Fv/Fm, and NPQ) derived from the rapid light curves (RLC) of intertidal and subtidal plants in Aenggang Bay (A, C, E, G, and I) and Koje Bay (B, D, F, H, and J). Stern–Volmer non-photochemical quenching (NPQ) values were obtained at the highest actinic irradiance. Values are means ± standard errors (n = 10–15). An asterisk above the bar indicates a significant difference between the intertidal and subtidal plants in each season.
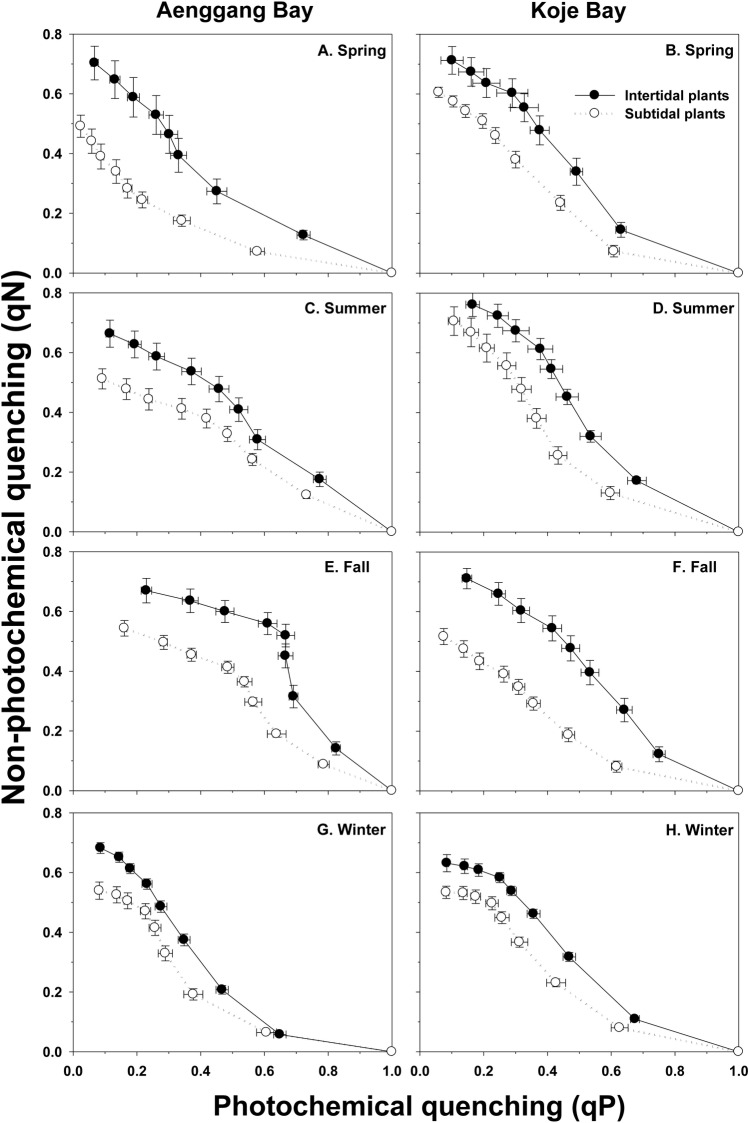
The intertidal and subtidal plants in both bay systems exhibited similar qP vs. qN relationships, and qN and qP correlated negatively throughout the experimental period (Fig 4). Although the relationship pattern between qP and qN was similar between the intertidal and subtidal plants, the negative slopes were steeper in the intertidal plants than those in the subtidal plants in both bay systems during all experimental periods (Fig 4). The values of qN in the intertidal plants were always higher than those in the subtidal plants under all light conditions in both bay systems (Fig 4).
Fig 4. Zostera marina.
Relationship between photochemical quenching (qP) and non-photochemical quenching (qN) during each season in Aenggang Bay (A, C, E, and G) and Koje Bay (B, D, F, and H). The rapid light curve (RLC) measurements were taken from the lowest to highest actinic light levels and are displayed from right to left on the x-axis. Values are means ± standard errors (n = 10–15).
Carbon stable isotope ratio
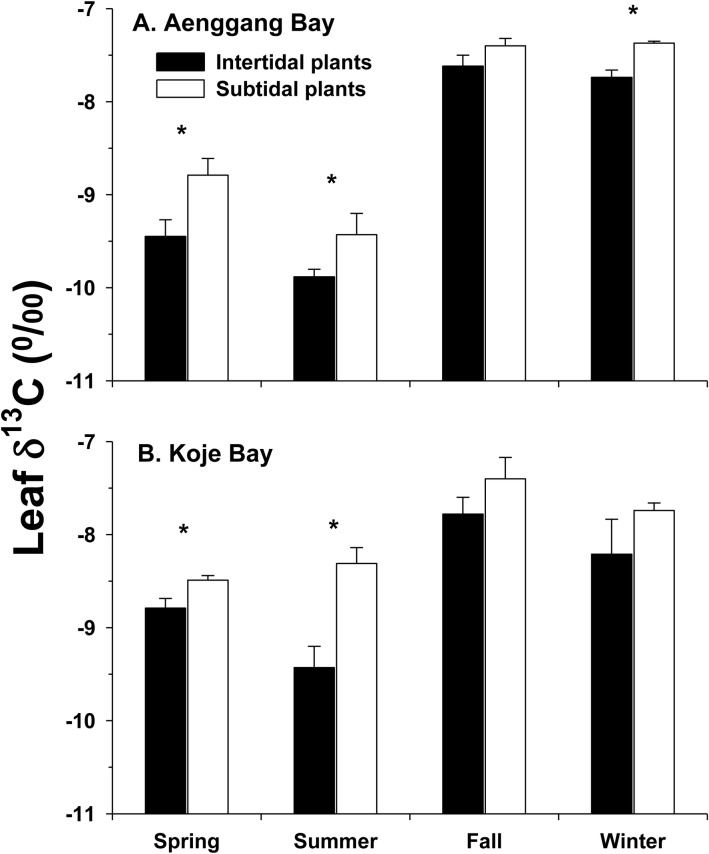
Leaf δ13C values were significantly more negative (P < 0.01) in the intertidal plants than in the subtidal plants throughout the study period in both bay systems (Fig 5). The differences in the leaf δ13C values between intertidal and subtidal plants were more distinguishable during the spring and summer growing season. Leaf δ13C values of the intertidal plants in Aenggang Bay ranged from −9.89 to −7.62‰ with a mean of −8.55‰, whereas the values for subtidal plants ranged from −9.43 to −7.37‰ with a mean of −8.38‰ (Fig 5A). Leaf δ13C values of the intertidal plants in Koje Bay ranged from −9.43 to −7.78‰ with a mean of −8.58‰, whereas values of subtidal plants ranged from −8.79 to −7.40‰ with a mean of −8.03‰ (Fig 5B).
Fig 5. Zostera marina.
Carbon stable isotope (δ13C) values of leaf tissues for the intertidal and subtidal plants in Aenggang Bay (A) and Koje Bay (B). Values are means ± standard errors (n = 6). An asterisk above the bar indicates a significant difference between the intertidal and subtidal plants in each season.
Photosynthetic responses to emersion and recovery after re-immersion
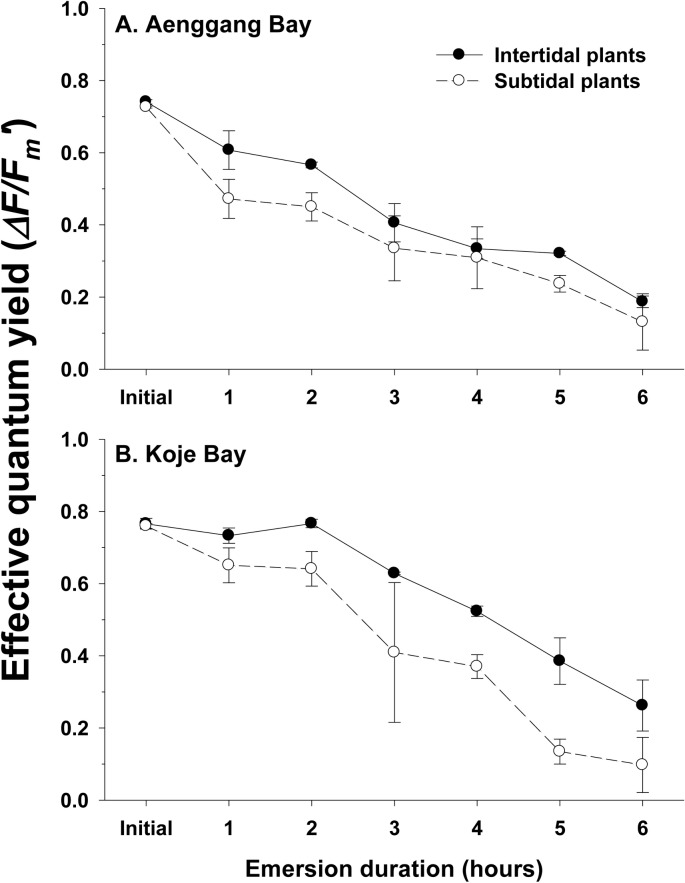
Effective quantum yield of the intertidal and subtidal plants decreased gradually with increasing emersion time, and was almost quenched after 6 hours of emersion in both bay systems (Fig 6). After 1 hour of emersion, effective quantum yield of the intertidal plants in Aenggang and Koje Bays decreased by 18% and 4%, respectively, whereas the quantum yield of the subtidal plants in Aenggang Bay and Koje Bay decreased by 35% and 14%, respectively. Effective quantum yield decreased more rapidly by emersion in subtidal plants than in intertidal plants in both bay systems (Fig 6).
Fig 6. Zostera marina.
Effective quantum yields (ΔF/Fm´) in response to emersion time for intertidal and subtidal plants in Aenggang Bay (A) and Koje Bay (B). Values are mean ± standard errors (n = 10).
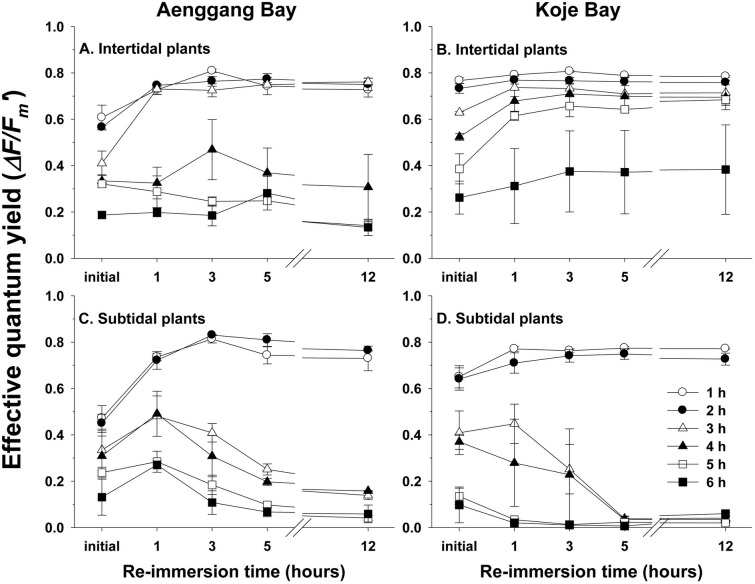
Photosynthetic recovery from emersion stress exhibited different patterns between the intertidal and subtidal plants as well as among emersion durations (Fig 7). The intertidal plants had greater ability to recover from emersion stress than did the subtidal plants in both bay systems (Fig 7). The intertidal plants in Aenggang Bay, which were exposed to air for 1–3 hours, completely recovered effective quantum yield to the undamaged level after approximately 3 hours of re-immersion, whereas the intertidal plants exposed to air for > 4 hours did not recover effective quantum yield after re-immersion (Fig 7A). The intertidal plants in Koje Bay, which were exposed to air for 1–5 hours, completely recovered effective quantum yield after re-immersion (Fig 7B). Only the subtidal plants in both bay systems, which were exposed to air for < 2 hours, recovered quantum yield after approximately 3 hours of re-immersion (Fig 7C and 7D). The effective quantum yields of the subtidal plants were irreversibly damaged by air exposure for > 2 hours (Fig 7C and 7D).
Fig 7. Zostera marina.
Recovery of effective quantum yield after 0, 1, 3, 5, and 12 h of re-immersion for the intertidal (A and C) and subtidal plants (B and D) previously exposed to air for 1, 2, 3, 4, 5, and 6 h emersion periods. Values are means ± standard errors (n = 10).
Discussion
Morphology and photosynthetic pigments
Shoot length and leaf width of Z. marina in the intertidal zone were significantly smaller than those in the subtidal zone. Reduced shoot size in the intertidal zone has been reported for many temperate and tropical seagrasses [10,41,42]. The temperate seagrasses Z. marina and Phyllospadix iwatensis in the intertidal zone have shorter canopy heights and narrower leaf widths than subtidal plants due to desiccation stress during low tide [2,43]. When the plants were transplanted from the subtidal zone to the intertidal zone, leaf sizes of the tropical seagrasses Cymodocea rotundata and Thalassia hemprichii decreased approaching the sizes of intertidal shoots [10]. The reduction in seagrass shoot size in the intertidal zone has been considered an adaptation for better survival under desiccation stress [9,10]. In the present study, Z. marina plants in the intertidal zone were regularly exposed to high desiccation stress and excessive light conditions during low tide, which may have resulted in permanent cellular and tissue damages.
The composition of photosynthetic pigments in seagrass leaves varies with light conditions [9,44]. Under reduced light conditions, chlorophyll content in seagrass leaf tissues increases and chlorophyll a/b ratios decrease [15,16,45]. A higher chlorophyll a/b ratio has been reported in intertidal C. nodosa plants, compared to that of subtidal plants [4]. In the present study, total chlorophyll concentrations in subtidal plants were slightly higher than those in intertidal plants, whereas the chlorophyll a/b ratio was significantly higher in the intertidal plants than in the subtidal plants. Subtidal Z. marina appeared to adapt to absorb more light under limited light conditions, in contrast to the intertidal plants.
The main role of carotenoids in plants is to protect chlorophyll from photodamage under excess light [46,47]. Dramatic increases in total carotenoid concentrations have been observed in terrestrial and marine plants exposed to high light conditions [48,49]. In the present study, the intertidal plants had higher carotenoid contents than did the subtidal plants during all experimental periods, except winter, in both bay systems. Mean carotenoid concentration of intertidal plants during spring–fall was 1.2 times higher than that of subtidal plants. The intertidal plants were periodically exposed to air during low tide, and thus were probably faced with excess light stress, which increased carotenoid content to avoid damage to the photosystems.
Photosynthetic characteristics
In the present study, the RLC parameters (rETRmax, α, Ek, Fv/Fm, and NPQ) of Z. marina varied significantly between intertidal and subtidal plants. The rETRmax and Ek values of intertidal plants were significantly higher than those of subtidal plants, whereas the α values were higher in subtidal plants than those in intertidal plants. Reductions in rETRmax and Ek with increasing water depth have been reported in several seagrass species [3,50]. Reduced light availability with increasing water depth was attributed to reduced ETRmax and Ek, and increased α of tropical seagrass species, indicating a photoacclimatory response to low light conditions for more efficient light use [14, 51].
However, rETRmax, Ek, and Fv/Fm were higher in subtidal plants than in intertidal plants during winter. Dramatic declines in maximum and effective quantum yields at extreme temperatures, which are linked to chronic inhibition of photosynthesis, have been observed in H. ovalis [52]. Mean air temperature at both bay systems in the present study was approximately 2°C during December–February [53]. Thus, the intertidal plants probably experienced extremely low air temperature when they were exposed to air during low tide in winter. Lower rETRmax, Ek, and Fv/Fm values in the intertidal plants during winter may have been caused by the low temperatures they encountered during low tide.
Reduced Fv/Fm under high irradiance has been observed in various marine plants [3,54,55]. Declines in Fv/Fm under high light fluxes are considered a photoprotective response to PSII photodamage [3,56,57]. Plant leaves adapted to high light environments generally have high photosynthetic capacity and active photoprotective mechanisms [58]. Ralph and Gademann [59] demonstrated that high-light adapted Z. marina leaves have a high rETR capacity and show active NPQ. In the present study, the intertidal plants had lower Fv/Fm but higher NPQ than those of the subtidal plants. Additionally, the qP and qN responses of the intertidal and subtidal plants also indicated that the intertidal plants were phenotypically adapted to high ambient light. Thus, the photosynthetic responses exhibited by the intertidal plants were probably associated with photoprotection to minimize photodamage due to excessive irradiance.
Inorganic photosynthetic carbon source
In the present study, the δ13C values of leaf tissues were more negative in the intertidal plants than those in the subtidal plants throughout the experimental period in both bay systems. Seagrasses are adapted to use HCO3−, which is the most abundant form of dissolved inorganic carbon in seawater, as well as dissolved CO2 [60]. As the δ13C of seawater HCO3− is less negative than those of atmospheric and dissolved CO2 [26], marine plants, which can use HCO3− for photosynthesis, usually have heavier δ13C values, compared to those of terrestrial C3 plants [61]. The heavier δ13C values in tropical seagrasses are attributed to greater use of HCO3− due to a low supply of CO2 in seawater [62]. Additionally, lighter δ13C values are observed in intertidal H. ovalis plants than in subtidal plants [63]. The lighter carbon isotope values in intertidal plants have been suggested to be caused by a sufficient CO2 supply in seawater due to air mixing during tidal changes [63]. Thus, the lighter carbon isotope values of the intertidal plants in the present study were probably due to direct use of atmospheric or dissolved CO2 for photosynthesis during emersion [63–65]. Although the δ13C values of intertidal plants were more negative than those of the subtidal plants, intertidal plants in the present study had much heavier carbon isotope values than those of atmospheric and dissolved CO2 or terrestrial C3 plants [61], suggesting that the intertidal plants in the present study primarily used HCO3− in seawater for photosynthesis during immersion.
Emersion stress and recovery after re-immersion
The desiccation tolerance of intertidal seagrasses has been explained by changes in morphological characteristics and higher shoot density [41,43]. Reduced shoot size, such as shorter canopy height and narrower leaf width in intertidal seagrasses, has been suggested as a morphological adaptation to desiccation stress during low tide [2,9,10,43]. Higher seagrass shoot density in the intertidal zone has also been suggested as an adaptation to reduce desiccation stress through mutual shading of the shoots [43]. In the present study, effective quantum yield of the intertidal plants decreased more slowly than that of the subtidal plants with increasing emersion time, suggesting higher desiccation tolerance in intertidal plants than in subtidal plants. Thus, the intertidal Z. marina plants in the present study showed photosynthetic adaptation to desiccation stress. The intertidal plants also possessed a greater ability to recover from emersion stress than did the subtidal plants in this study. The intertidal plants that were exposed to air for < 3–5 hours completely recovered photosynthetic ability after re-immersion, but only the subtidal plants that were exposed to air for 1–2 hours recovered photosynthetic ability. This higher desiccation tolerance and recovery ability from photosynthetic damage by intertidal plants may contribute to survival and distribution of this seagrass species in the intertidal zone.
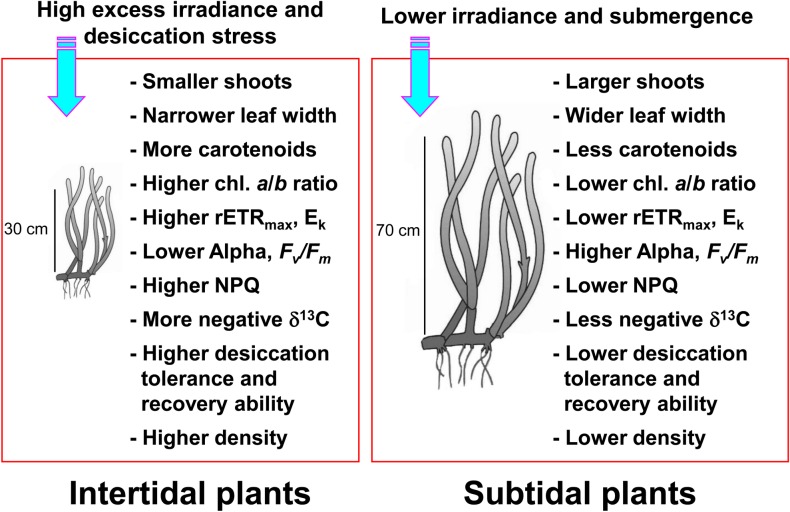
In conclusion, Z. marina shoot size, such as shoot height and leaf width, was significantly smaller in plants inhabiting the intertidal zone than in plants inhabiting the subtidal zone (Fig 8). The smaller size of the intertidal plants appeared to be caused by high desiccation stress in the intertidal zone. Lower chlorophyll contents, higher chlorophyll a/b ratios, and higher carotenoid contents were observed in the intertidal Z. marina plants than in those in the subtidal zone (Fig 8). We considered the variability in photosynthetic pigment contents between the intertidal and subtidal plants to be a photo-adaptation to effectively use in situ light and prevent photodamage. Reductions in rETRmax and Ek were observed in subtidal plants. However, the α values were higher in subtidal plants than in intertidal plants. Reduced rETRmax and Ek and increased α in the subtidal plants are suggested photoacclimatory responses to low light conditions (Fig 8). The intertidal plants exhibited higher desiccation tolerance and recovery ability from desiccation damage than did the subtidal plants (Fig 8). The δ13C value of leaf tissues was more negative in the intertidal plants than in the subtidal plants, suggesting that the intertidal plants utilized atmospheric or dissolved CO2 for photosynthesis during low tide (Fig 8). The morphological and photosynthetic plasticity exhibited by the intertidal and subtidal plants appeared to contribute to the distribution of Z. marina in both intertidal and subtidal zones. A better understanding of photoacclimatory responses of seagrass plants along the tidal height gradient will promise more accurate prediction of changes in coastal seagrass ecosystems due to global climate associated environmental disturbances, such as a sea level rise.
Fig 8. Schematic summary of the morphological and photoacclimatory responses of intertidal and subtidal Zostera marina plants.
Supporting Information
Values are mean ± standard errors.
(XLSX)
All data were transformed by log(x+1) to meet the assumption of parametric statistics prior to analysis.
(DOC)
All data were transformed by log(x+1) to meet the assumption of parametric statistics prior to analysis.
(DOC)
Acknowledgments
We thank HJ Song, JH Kim, and SH Kim for their many hours of field and lab assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Ministry of Oceans and Fisheries, Korea (Project title: Long-term changes in structure and function in the marine ecosystems of Korea) to SRP, KSL, and CKK, and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST; NRF–2015R1A2A2A01004850) to KSL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Silva J. Santos R. Daily variation patterns in seagrass photosynthesis along a vertical gradient. Mar Ecol Prog Ser. 2003; 257: 37–44. [Google Scholar]
- 2.Boese BL, Robbins BD, Thursby G. Desiccation is a limiting factor for eelgrass (Zostera marina L.) distribution in the intertidal zone of a northeastern Pacific (USA) estuary. Bot March 2005; 48: 274–283. [Google Scholar]
- 3.Durako MJ, Kunzelman JI, Kenworthy WJ, Hammerstrom KK. Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens. Mar Biol. 2003; 142: 1219–1228. [Google Scholar]
- 4.Olivé I, Vergara JJ, Pérez-Lioréns JL. Photosynthetic and morphological photoacclimation of the seagrass Cymodocea nodosa to season, depth and leaf position. Mar Biol. 2013; 160: 285–297. [Google Scholar]
- 5.Hemminga MA, Durate CM. Seagrass ecology Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 6.Seddon S, Cheshire AC. Photosynthetic response of Amphibolis antarctica and Posidonia australis to temperature and desiccation using chlorophyll fluorescence. Mar Ecol Prog Ser. 2001; 220: 119–130. [Google Scholar]
- 7.Hirst A, Ball D, Heislers S, Young P, Blake S, Coots A. Baywide Seagrass Monitoring Program, Milestone Report No. 2 (2008). Fisheries Victoria Technical Report No. 29, January 2009. Queenscliff, Victoria: Department of Primary Industries; 2009.
- 8.Koch EW. Beyond light: physical, geological and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries. 2001; 24: 1–17. [Google Scholar]
- 9.Abal EG, Loneragan NR, Bowen P, Perry CJ, Udy JW, Dennison WC. Physiological and morphological responses of the seagrass Zostera capricorni Aschers to light intensity. J Exp Mar Biol Ecol. 1994; 178:113–129. [Google Scholar]
- 10.Tanaka Y, Nakaoka M. Emergence stress and morphological constraints affect the species distribution and growth of subtropical intertidal seagrasses. Mar Ecol Prog Ser. 2004; 284: 117–131. [Google Scholar]
- 11.Björk M, Uku J, Weil A, Beer S. Photosynthetic tolerances to desiccation of tropical intertidal seagrasses. Mar Ecol Prog Ser. 1999; 191: 121–126. [Google Scholar]
- 12.Petrou K, Jimenez-Denness I, Chartrand K, McCormack C, Rasheed M, Ralph PJ. Seasonal heterogeneity in the photophysiological response to air exposure in two tropical intertidal seagrass species. Mar Ecol Prog Ser. 2013; 482: 93–106. [Google Scholar]
- 13.Schwarz AM, Björk M, Buluda T, Mtolera M. Beer S. Photosynthetic utilization of carbon and light by two tropical seagrass species as measured in situ. Mar Biol. 2000; 137: 755–761. [Google Scholar]
- 14.Campbell SJ, McKenzie LJ, Kerville SP, Bité JS. Patterns in tropical seagrass photosynthesis in relation to light, depth and habitat. Estuar Coast Shelf S. 2007; 73: 551–562. [Google Scholar]
- 15.Lee K-S, Dunton KH. Effects of in-situ light reduction on the maintenance, growth, and partitioning of carbon resources in Thalassia testudinum Banks ex Konig. J Exp Mar Biol Ecol. 1997; 210: 53–73. [Google Scholar]
- 16.Longstaff BJ, Dennison WC. Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat Bot. 1999; 65: 105–121. [Google Scholar]
- 17.Collier CJ, Lavery PS, Ralph PJ, Masini RJ. Physiological characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Mar Ecol Prog Ser. 2008; 353: 65–79. [Google Scholar]
- 18.Lee K-S, Park SR, Kim YK. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. J Exp Mar Biol Ecol. 2007; 350: 144–175. [Google Scholar]
- 19.Nayar S, Collings GJ, Miller DJ, Bryars S, Cheshire AC. Uptake and resource allocation of inorganic carbon by the temperate seagrasses Posidonia and Amphibolis. J Exp Mar Biol Ecol. 2009; 373: 87–95. [DOI] [PubMed] [Google Scholar]
- 20.Beer S. Photosynthesis and respiration of marine angiosperms. Aquat Bot. 1989; 34: 153–166. [Google Scholar]
- 21.Larkum AWD, James PL. Towards a model for inorganic carbon uptake in seagrasses involving carbonic anhydrase In Kuo J, Phillips RC, Walker DI, Kirkman H, editors. Seagrass biology: Proceedings of an International Workshop. Nedlands: The University of Western Australia; 1996. pp. 191–196. [Google Scholar]
- 22.Beer S, Rehnberg J. The acquisition of inorganic carbon by the seagrass Zostera marina. Aquat Bot. 1997; 56: 277–283. [Google Scholar]
- 23.Invers O, Zimmerman RC, Alberte RS, Pérez M, Romero J. Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. J Exp Mar Biol Ecol. 2001; 265: 203–217. [Google Scholar]
- 24.Silva J, Santos R, Calleja ML, Duarte CM. Submerged versus air-exposed intertidal macrophyte productivity: from physiological to community-level assessments. J Exp Mar Biol Ecol. 2005; 317: 87–95. [Google Scholar]
- 25.O’Leary MH. Carbon isotopes in photosynthesis. BioScience. 1988; 38: 328–336. [Google Scholar]
- 26.Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, et al. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct Plant Biol. 2002; 29: 355–378. [DOI] [PubMed] [Google Scholar]
- 27.Lee K-S, Lee SY. The seagrasses of the Republic of Korea In: Green EP, Short FT, Spalding MD, editors. World Atlas of seagrasses: present status and future conservation. Berkeley: University of California Press; 2003. pp. 193–198. [Google Scholar]
- 28.Borum J, Duarte CM, Krause-Jensen D, Greve TM. European seagrasses: an introduction to monitoring and management Copenhagen: The M&MS Project; 2004. [Google Scholar]
- 29.Micheli F, Bishop MJ, Peterson CH, Rivera J. Alteration of seagrass species composition and function over two decades. Ecol Monogr. 2008; 78: 225–244. [Google Scholar]
- 30.Collin A, Long B, Archambault P. Salt-marsh characterization, zonation assessment and mapping through a dual-wavelength LiDAR. Remote Sens Environ. 2010; 114: 520–530. [Google Scholar]
- 31.Kim M-S, Lee S-M, Kim H-J, Lee S-Y, Yoon S-K, Shin K-H. Carbon stable isotope ratios of new leaves of Zostera marina in the mid-latitude region: implications of seasonal variation in productivity. J Exp Mar Biol Ecol. 2014; 461: 286–296. [Google Scholar]
- 32.Lee K-S, Park SR, Kim J-B. Production dynamics of the eelgrass, Zostera marina in two bay systems on the south coast of the Korean peninsula. Mar Biol. 2005; 147: 1091–1108. [Google Scholar]
- 33.Kim YK, Kim SH, Lee K-S. Seasonal growth responses of the seagrass Zostera marina under severely diminished light conditions. Estuar Coast. 2015; 38: 558–568. [Google Scholar]
- 34.Park SR, Kim YK, Kim J-H, Kang C-K, Lee K-S. Rapid recovery of the intertidal seagrass Zostera japonica following intense Manila clam (Ruditapes philippinarum) harvesting activity in Korea. J Exp Mar Biol Ecol. 2011; 407: 275–283. [Google Scholar]
- 35.Dunton KH, Tomasko DA. In situ photosynthesis in the seagrass Halodule wrightii in a hypersaline tropical lagoon. Mar Ecol Prog Ser. 1994; 107: 281–293. [Google Scholar]
- 36.Wellburn AL. The spectral determination of chlorophylls a and b, as well as total carotenoids, Using various solvents with spectrophotometers of different resolutions. J Plant Physiol. 1994; 144: 307–313. [Google Scholar]
- 37.Van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosyn Res. 1990; 25: 147–150. doi: 10.1007/BF00033156 [DOI] [PubMed] [Google Scholar]
- 38.Genty B, Briantais J-M, Baker NR. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochem Biophys. 1989; 990: 87–92. [Google Scholar]
- 39.Platt T, Gallegos CL, Harrison WG. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res. 1980; 38: 687–701. [Google Scholar]
- 40.Schreiber U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview In: Papageorgiou GC, Govindjee, editors. Chlorophyll fluorescence: a signature of photosynthesis. Dordrecht: Kluwer Academic Publishers; 2004. pp. 279–319. [Google Scholar]
- 41.Boese BL, Alayan KE, Gooch EF, Robbins BD. Desiccation index: a measure of damage caused by adverse aerial exposure on intertidal eelgrass (Zostera marina) in an Oregon (USA) estuary. Aquat Bot. 2003; 76: 329–337. [Google Scholar]
- 42.Apichanangkool P, Prathep A. Changes in seagrass leaf reddening and morphology in response to emersion. 2014; Bot March 57: 433–440. [Google Scholar]
- 43.Yabe T, Ikushima I, Tsuchiya T. Production and population ecology of Phyllospadix iwatensis Makino. II. Comparative studies on leaf characteristics, foliage structure and biomass change in an intertidal and subtidal zone. Ecol Res. 1996; 11: 291–297. [Google Scholar]
- 44.Macauley JM, Clark JR. Price WA. Seasonal changes in the standing crop and chlorophyll content of Thalassia testudinum Banks ex Konig and its epiphytes in the northern Gulf of Mexico. Aquat Bot. 1988; 31: 277–287. [Google Scholar]
- 45.McPherson BF, Miller RL. The vertical attenuation of light in Charlotte Harbor, a shallow, subtropical estuary, southwestern Florida. Estuar Coast Shelf S. 1987; 25: 721–737. [Google Scholar]
- 46.Armstrong GA, Hearst JE. Genetics and molecular biology of carotenoid pigment biosynthesis. Faseb J. 1996; 10: 228–237. [DOI] [PubMed] [Google Scholar]
- 47.Demming-Adams B, Adams WW III. The role of xanthophyll cycle carotenoids in the protection of photosystems. Trends Plant Sci. 1996; 1: 21–26. [Google Scholar]
- 48.Härtel H, Grimm B. Consequences of chlorophyll deficiency for leaf carotenoid composition in tobacco synthesizing glutamate 1-semialdehyde aminotransferase antisense RNA: dependency on developmental age and growth light. J Exp Bot. 1998; 49: 535–546. [Google Scholar]
- 49.Ralph PJ, Gademann R, Larkum AWD, Kühl M. Spatial heterogeneity in active fluorescence and PSII activity of coral tissues. Mar Biol. 2002; 141: 639–646. [Google Scholar]
- 50.Schwarz A-M, Hellblom F. The photosynthetic light response of Halophila stipulacea growing along a depth gradient in the Gulf of Aqaba, the Red Sea. Aquat Bot. 2002; 74: 263–272. [Google Scholar]
- 51.Bité JS, Campbell SJ, McKenzie LJ, Coles RG. Chlorophyll fluorescence measures of seagrasses Halophila ovalis and Zostera capricorni reveal differences in response to experimental shading. Mar Biol. 2007; 152: 405–414. [Google Scholar]
- 52.Ralph PJ. Photosynthetic response of Halophila ovalis (R. Br) Hook f. to thermal stress. Mar Ecol Prog Ser. 1998; 171: 123–130. [Google Scholar]
- 53.Lee SY, Kim JB, Lee SM. Temporal dynamics of subtidal Zostera marina and intertidal Zostera japonica on the southern coast of Korea. Mar Ecol. 2006; 27: 133–144. [Google Scholar]
- 54.Hader DP, Lebert M, Figueroa FL, Jimanez C, Viaegla B, Perez-Rodriguez E. Photoinhibition in Mediterranean macroalgae by solar radiation measured on site by PAM fluorescence. Aquat Bot. 1998; 61: 225–236. [Google Scholar]
- 55.Yakoleva IM, Titlyanov EA. Effect of high visible and UV irradiance on subtidal Chondrus crispus: stress, photoinhibition and protective mechanisms. Aquat Bot. 2001; 71: 47–61. [Google Scholar]
- 56.Ralph PJ, Burchett MD. Photosynthetic responses of the seagrass Halophila ovalis (R. Br.) Hook. f. to high irradiance stress, using chlorophyll a fluorescence. Aquat Bot. 1995; 51: 55–66. [Google Scholar]
- 57.Major KM, Dunton KH. Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: evidence for photoacclimation. J Exp Mar Biol Ecol. 2002; 275: 173–189. [Google Scholar]
- 58.Demmig-Adams B, Adams W III, Ebbert V, Logan BA. Ecophysiology of the xanthophyll cycle In: Frank HA, Young AJ, Britton G, Cogdell RJ, editors. The Photochemistry of Carotenoids. the Netherlands: Kluwer Academic Publishers; 1999. pp. 245–269. [Google Scholar]
- 59.Ralph PJ, Gademann R. Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot. 2005; 82: 222–237. [Google Scholar]
- 60.Beer SM, Björk M, Hellblom F, Axelsson L. Inorganic carbon utilization in marine angiosperms (seagrasses). Funct Plant Biol. 2002; 29: 349–354. [DOI] [PubMed] [Google Scholar]
- 61.Fry B, Sherr EB. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems In: Ehleringer J, Rundel P, editors. Stable isotopes in ecological research. New York: Springer-Verlag; 1989. pp. 196–229. [Google Scholar]
- 62.Hemminga MA, Mateo MA. Stable carbon isotopes in seagrasses: variability in ratios and use in ecological studies. Mar Ecol Prog Ser. 1996; 140: 285–298. [Google Scholar]
- 63.Yamamuro M, Umezawa Y, Koike I. Seasonality in nutrient concentrations and stable isotope ratios of Halophila ovalis growing on the intertidal flat of SW Thailand. Limnology. 2001; 2: 199–205. [Google Scholar]
- 64.Clough BF, Attiwill PM. Primary productivity of Zostera muelleri Irmisch ex Aschers. in Westernport Bay (Victoria, Australia). Aquat Bot. 1980; 9: 1–13. [Google Scholar]
- 65.Leuschner C, Rees U. CO2 gas exchange of two intertidal seagrass species, Zostera marina L. and Zostera noltii Hornem., during emersion. Aquat Bot. 1993; 45: 53–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are mean ± standard errors.
(XLSX)
All data were transformed by log(x+1) to meet the assumption of parametric statistics prior to analysis.
(DOC)
All data were transformed by log(x+1) to meet the assumption of parametric statistics prior to analysis.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.