Abstract
Control strategies implemented a decade ago led to a marked reduction in the prevalence of malaria in many countries. In Dienga, southeastern Gabon, the prevalence of microscopic P. falciparum infection was 7% in 2003, close to the pre-elimination threshold of 5%. The aim of this work was to determine the prevalence of P. falciparum infection in the same community a decade later. A cohort of 370 individuals aged from 3 to 85 years living in Dienga was investigated for P. falciparum infection; during six passages (P) in 15-month period. Demographic data were collected, along with behaviors and attitudes towards malaria. Plasmodium infection was diagnosed by microscopy (ME), followed by PCR to detect submicroscopic infection. The prevalence of P. falciparum infection in P1, P2, P3, P4, P5 and P6 was respectively 43.5% (25.1% ME+, 18.4% PCR+); 40.9% (27.0% ME+, 13.9% PCR+), 52.7% (26.1% ME+, 26.6% PCR+); 34.1% (14.1% ME+, 20% PCR+), 57.7% (25.4.% ME+, 32.3% PCR+); and 46.2% (21.4% ME+, 24.8% PCR+) with an overall average of 45.9% (95%CI [37.0–54.7], 23.2% ME+ and 22.7% PCR+). P4 and P5 prevalences were statically different throughout the six passages. Microscopic prevalence was significantly higher than that observed ten years ago (23% [n = 370] vs 7% [n = 323], p < 0.001). Asymptomatic infections were the most frequent (96%). Gametocytes were detected in levels ranging from 5.9% to 13.9%. Insecticide-treated nets, indoor residual insecticides, and self-medication were used by respectively 33.2% (95%CI [29.0–37.4]), 17.7% (95%CI [15.5–19.9]) and 12.1% (95%CI [10.6–13.6]) of the study population. A near-threefold increase in P. falciparum infection has been observed in a rural area of southeastern Gabon during a 10-year period. Most infections were asymptomatic, but these subjects likely represent a parasite reservoir. These findings call for urgent reinforcement of preventive measures.
Introduction
In 2013 there were 135–287 million new cases of malaria and 473 000–789 000 deaths [1]. Among the five species which can infect humans, Plasmodium falciparum and P. vivax are responsible for most morbidity and P. falciparum for most deaths, mainly among children under the age of 5 years living in sub-Saharan Africa.
Clinical malaria is defined by intermittent fever, malaise, shaking, chills, arthralgia, myalgia, vomiting and other symptoms, together with malaria parasites in blood [2]. Asymptomatic infection is the state diagnosed by the presence of the pathogen without the corresponding disease symptoms. Asymptomatic malaria can be diagnosed by blood smears showing Plasmodium on microscopic examination (ME+) and/or rapid diagnostic tests (RTD) and by PCR detecting sub microscopically infected individuals (SMI) who are negative by ME [3,4]. People living in malaria-endemic countries eventually develop immunity, enabling them to maintain a low parasite burden, below the pyrogenic threshold. Microscopic examination fails to detect as many as half of Plasmodium carriers [5]. The existence of these asymptomatically infected individuals could undermine malaria control strategies. Submicroscopic infection can also be associated with severe malaria [6,7]. Chronic untreated Plasmodium infection can negatively affect children’s health and education [8,9,10,11]. A fraction of asexual merozoites released from infected red blood cells produce sexual gametocytes that can infect mosquitoes [12] and thereby maintain human transmission [13,14,15,16,17].
More than decade ago, the international community and national partners deployed global efforts to reduce malarial morbidity and mortality. In nearly all endemic countries, including Gabon, the prevalence of P. falciparum infection declined, likely due mainly to the use of ACT and insecticide-treated bed nets [18,19,20]. Unfortunately, the prevalence of malaria is tending to increase again, including in Gabon [21,22].
The reservoir of asymptomatically infected individuals will need to be dealt with if malaria is to be eradicated. Epidemiological surveillance based on molecular tools can help to plan and monitor malaria control programs. The aim of this study was to assess the prevalence of P. falciparum infection in Dienga, a rural community in southeastern Gabon.
Materials and Methods
Study area
The study was conducted in southeast Gabon, in a rural community named Dienga (Ogooué-Lolo province). Dienga is situated near the Congo border and has around 2500 inhabitants. An equatorial climate prevails and is characterized by two rainy seasons (March-May and October-December) and two dry seasons (June-September and January-February). Malaria is highly endemic and P. falciparum (80%) is predominantly transmitted by A. gambiae. The entomological infection rate is equivalent to about 100 infective bites per human per year. Malaria in Dienga exhibits seasonal peaks of transmission coinciding with the rains, from February to June and September to December [23].
Ethical approval
The study was submitted in February 2013 to the “Comité National d’Ethique” (CNE) of Gabon together with a 2-pages questionnaire with which written consent and clinical status were obtained. A month later the study was finally approved and registered under PROT N°0018/2013/SG/CNE with the accord of the Public Health Minister, the Regional Health Director of Ogooué-Lolo province, and Dienga Traditional Chiefs. A meeting was held with the population to explain the study goals and likely benefits.
Study population
This longitudinal study was conducted from April 2013 to June 2014 during six passages. A calculated sample size of 323 was adjusted to 370, using the following formula N = t2*p (1-P)/m2, where t is the 95% confidence interval; p is alpha error which corresponds to 1.96 in the normal standard distribution, P is the recently estimated prevalence of P. falciparum malaria in Dienga (30%) and m is a 5% margin of error. The study population was composed of volunteers living permanently in Dienga; primary school children (≥3 years) and adults. A written informed consent was signed by all participants or their legal tutor before their enrolment.
Blood collection
Study participants were examined physically, and their axillary temperature and history of fever (≥37.5°C) in the previous 24 hours were recorded on a structured questionnaire. During the following passages: P1 (April 2013), P5 (March 2014) and P6 (June 2014), blood was collected by venipuncture in 5-ml EDTA tubes for thick-film preparation and PCR diagnosis. During P2 (June 2013), P3 (July 2013) and P4 (October 2013), blood samples were collected by finger prick for thick-film preparation and blotted on filter paper for PCR. During each passage the entire population of Dienga was invited to see a CIRMF physician, and any necessary laboratory tests and medications were provided free of charge.
DNA extraction
P. falciparum DNA was extracted from blots by the Chelex method [24]. Briefly, 150 μl of PBS was added to a 1.5-ml microcentrifuge tube containing three or four pieces of filter paper, then vortexed and incubated for 15 min at room temperature. The PBS was removed while keeping the filter paper at the bottom of the tube. Then 200 μl of 5% Chelex-100 was added and heated at 100°C for 15 min. The tube was vortexed every 2 minutes during the heating phase, with the Chelex pressed against the filter paper. The mixture was then centrifuged at 8000 g for 1 min and the final supernatant containing DNA was stored at −20°C until use.
DNA was extracted from whole blood by using the DNeasy Blood & Tissue kit according to the manufacturer's procedure (QIAGEN, Hilden, Germany).
Malaria diagnosis
Microscopy
Blood films were prepared as described by Planche et al. 2001 [25]. Slides were stained with 10% Giemsa solution for 15 min and examined under a microscope. Samples were considered Plasmodium-positive if at least one parasite was seen in a x100 oil-immersion field of a thick blood film (ME+) or when PCR, applied only to ME- samples, was positive (SMI). All PP+ individuals (ME+ and SMI = PCR+) received artemether-lumefantrine according to the national guideline.
Molecular diagnosis
PCR targeting Subtelomeric Variable Open Reading frame (STEVOR) genes was used in this study. Primary amplification was performed in a total reaction mixture of 25 μl containing 2.5 μl of x10 reaction buffer, 10 μM each dNTP (dATP, dGTP, dTTP and dCTP), 5 U/μl Taq DNA polymerase, 2.5 pM each primer (P5, P18, P19 and P20) and 5.0 μl of DNA extract. The PCR program consisted of denaturation at 93°C for 3 min, 93°C at 50 s, 50 s at 50°C and 50 s at 72°C followed by 25 cycles and a final extension step of 6 min at 72°C. Two microliters of the first-round PCR product was used for nested amplification in a reaction mixture of 22 μl containing 2.5 μl of ×10 reaction buffer, 10 μM each dNTP (dATP, dGTP, dTTP, and dCTP), 5 U/μl of Taq DNA polymerase, and 2.5 pM each primer (P17 and P24). The PCR program consisted of denaturation at 93°C for 3 min, 30 s at 93°C, 50 s at 50°C and 50 s at 72°C followed by 40 cycles and a final extension step of 3 min at 72°C. The primer sequences used for STEVOR PCR as previously described [26] are presented in (Table 1).
Table 1. Nucleotide primers used in STEVOR gene amplification.
| Primer Name | Nucleotide sequence |
|---|---|
| P5 | 5'-GGG AAT TCT TTA TTT GAT GAA GAT G-3' |
| P18 | 5'-TTT CA(C/T) CAC CAA ACA TTT CTT-3' |
| P19 | 5'-AAT CCA CAT TAT CAC AAT GA-3' |
| P20 | 5'-CCG ATT TTA ACA TAA TAT GA-3' |
| P17 | 5'-ACA TTA TCA TAA TGA (C/T) CC AGA ACT-3' |
| P24 | 5'-GTT TGC AAT AAT TCT TTT TCT AGC-3' |
PCR products were subjected to electrophoresis on 1% agarose gels and visualized by transillumination with ultraviolet light after staining with EZvision three DNA dye & buffer, 6X (cat. N313, Interchim, France). Fragment sizes were measured relative to a standard size marker (100 bp DNA ladder). All reactions were run in parallel with DNA from negative controls (unexposed European samples) and positive controls (40,000 P. falciparum parasites/μl on thick blood smears).
Survey of attitudes and behaviors
An oral survey was conducted in March 2014 using a questionnaire written in French and focusing on the use of insecticide-treated bed nets (ITNs), indoor residual sprays (IRS) and self-medication. Individuals were asked if they had a bed net, if they had used it the night before the interview, and if the net was treated with an insecticide. Similar questions on IRS and self-medication were posed. The parents or guardians answered for children younger than 16 years, during home by home visit. The answers to the questionnaire were stored in electronic form. Ten percent of oral responses of ITNs and IRS were checked.
Data processing and analysis
All data were recorded in an electronic database. Epi Info 5.4 software was used for conventional descriptive analysis that consisted of calculating proportions (for qualitative data), or mean ± standard deviation, median and range (for quantitative data). Comparative analyses were carried out using the Chi-square test for frequencies (proportions) and Student's t test for means. Univariate analysis was used to describe malaria infection, the dependent variable using Info 5.4 software. Multivariate analysis was performed using STATA 14.0 (Texas 77845 USA, 4905 Lakeway Drive College Station). Then, independent variables such as age, sex, use of ITNs, IRS and self-medication were considered in multivariate analysis adjusting for confounders. Significance was assumed at p<0.05.
Results
Study site
The study was conducted in southeast Gabon, in a rural community named Dienga (Ogooué-Lolo province). Dienga is situated near the Congo border and has around 2500 inhabitants. An equatorial climate prevails and is characterized by two rainy seasons (March-May and October-December) and two dry seasons (June-September and January-February). Malaria is highly endemic and P. falciparum (80%) is predominantly transmitted by A. gambiae. The entomological infection rate is equivalent to about 100 infective bites per human per year. Malaria in Dienga exhibits seasonal peaks of transmission coinciding with the rains, from February to June and September to December [23].
Sociodemographic characteristics
A total of 370 participants (schoolchildren and adult volunteers) were included in this longitudinal study from April 2013 to June 2014. The prevalence of P. falciparum infection was determined during six passages (P) (April 2013 (P1), June 2013 (P2), July 2013 (P3), October 2013 (P4), March 2014 (P5) and June 2014 (P6)). It was an open cohort and the participants were not necessarily the same number at each passage. The individuals were divided into five age groups: [3–6 y], [7–10 y], [11–14 y], [15–18 y] and [19 y≥]. There were slightly more males than females in July 2013 (53.7%) and June 2014 (54.5%) (Table 2).
Table 2. Baseline Characteristics of the study subjects.
| Apr-13 | Jun-13 | Jul-13 | Oct-13 | Mar-14 | Jun-14 | |
|---|---|---|---|---|---|---|
| Baseline characteristic | ||||||
| N | 370 | 267 | 218 | 255 | 232 | 145 |
| Means age: yrs (± SD) | 24.34 (22.65) | 24.79 (22.61) | 21.79 (19.32) | 21.88 (20.79) | 21.87 (20.77) | 17.50 (17.15) |
| Males (%) | 49.70 | 50.60 | 49.10 | 49.40 | 49.60 | 54.50 |
| Females (%) | 50.30 | 49.40 | 50.90 | 50.60 | 50.40 | 45.50 |
| Distribution by age (%) | ||||||
| 3–6 yrs. | 8.60 | 8.60 | 9.21 | 10.20 | 9.90 | 8.30 |
| 7–10 yrs. | 27.00 | 26.60 | 27.20 | 28.20 | 29.30 | 37.20 |
| 11–14 yrs. | 18.10 | 16.90 | 18.47 | 20.00 | 20.70 | 26.20 |
| 15–18 yrs. | 9.50 | 9.70 | 9.20 | 9.80 | 8.60 | 8.30 |
| 19 + yrs. | 36.80 | 38.30 | 35.92 | 31.80 | 31.50 | 20.00 |
Prevalence of P. falciparum infection
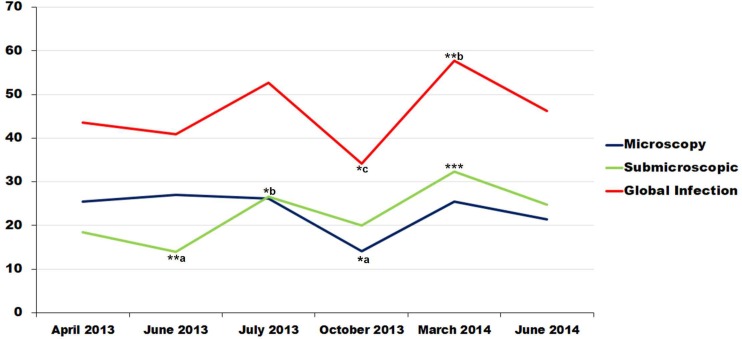
The frequency of P. falciparum infection was 161/370 (43.5%) in April 2013 (P1), comprising 93 ME + (25.1%) and 68 (18.4%) PCR+ ME—participants. The corresponding data were 109/267 (40.9%), 72 ME + (27%) and 37 PCR+ (13.9%) in June 2013 (P2); 115/218 (52.7%), 57 (26.1%) ME + and PCR+ (26.6%) in July 2013; 87/255 (37.7%), 36 ME + (14.1%) and 51 PCR+ (20%) in October 2013; 134/232 (57.7%), 59 ME + (25.4%) and PCR+ (32.3%) in March 2014 (P5); and 67/145 (46.6%), 31 ME + (21.4%) and PCR+ (24.8%) in June 2014 (P6). The microscopic prevalence was significantly higher during the first three passages (P1, P2 and P3) than in P4 (P = 0.007). There was also a significant difference between the prevalence of PCR positivity between June 2013 (P2) and July 2013 and March 2014 (p<0.005). No difference in the overall prevalence of falciparum infection (ME + plus SMI) was observed across the six passages (p = 0.13) (Fig 1 and Table 3).
Fig 1. Global prevalence of malaria infection in Dienga.
*a: Significant difference between the prevalences of October 2013 and those of other sampling periods except June 2014, in microscopic infection. ** a: Significant difference between the prevalences of June 2013 and those of other sampling periods, in submicroscopic infection. *b: Significant difference between the prevalences of July 2013 and that of June 2013, in submicroscopic infection. ***: Significant difference between the prevalence of March 2014 and those of other sampling periods except July2013 and June 2014, in submicroscopic infection. *c: Significant difference between the prevalence of October 2013 and that of March 2014, in Global infection. **b: Significant difference between the prevalence of March 2014, April and June 2013 in Global infection.
Table 3. Global P. falciparum prevalences in Dienga.
| Sampling time | n | Microscopy % (IC95%) | Submicroscopic % (IC95%) | Global Infection % (IC95%) | P value |
|---|---|---|---|---|---|
| April 2013 | 370 | 25.4 (20.9–29.9) | 18.4 (14.7–22.7) | 43.5 (38.4–48.7) | <0.001 |
| June 2013 | 267 | 27.0 (21.7–32.7) | 13.9 (9.9–18.6)**f | 40.9 (34.9–47.0) | |
| July 2013 | 218 | 26.1 (20.4–32.5) | 26.6 (20.9–33.0)*s | 52.7 (45.9–59.5) | |
| October 2013 | 255 | 14.1 (10.1–19.0) *f | 20.0 (15.3–25.4) | 34.1 (28.3–40.3) *t | |
| March 2014 | 232 | 25.4 (20.0–31.5) | 32.3 (26.4–38.8)*** | 57.7 (51.1–64.2) **s | |
| June 2014 | 145 | 21.4 (15.0–29.0) | 24.8 (18.0–32.6) | 46.2 (37.9–54.7) | |
| Mean prevalence | 23.4 (18.1–28.3) | 22.0 (15.8–29.6) | 45.4 (37.0–54.7) |
*f: Significant difference between the prevalences of October 2013 and those of other sampling periods except June 2014, in microscopic infection.
** f: Significant difference between the prevalences of June 2013 and those of other sampling periods, in submicroscopic infection.
*S: Significant difference between the prevalences of July 2013 and that of June 2013, in submicroscopic infection.
***: Significant difference between the prevalence of March 2014 and those of other sampling periods except July2013 and June 2014, in submicroscopic infection.
*t: Significant difference between the prevalence of October 2013 and that of March 2014, in the Global infection
**S: Significant difference between the prevalence of March 2014, April and June 2013 in Global infection.
The overall prevalence falciparum infection did not differ across the age groups or between genders (p>0.05). Nevertheless, a significant difference was observed between July 2013 (P3) and March 2014 (P5) among children aged 3–6 years (p<0.05); between these same children and also adults [≥19 years] in October 2013 (P4) (p<0.05), or the group of over five years older children [7–10 years] also in 2013 (P4) and [15–18 years] in June 2013 (P2) (p = 0.04) (Fig 2).
Fig 2. Mean prevalence according to the age groups.
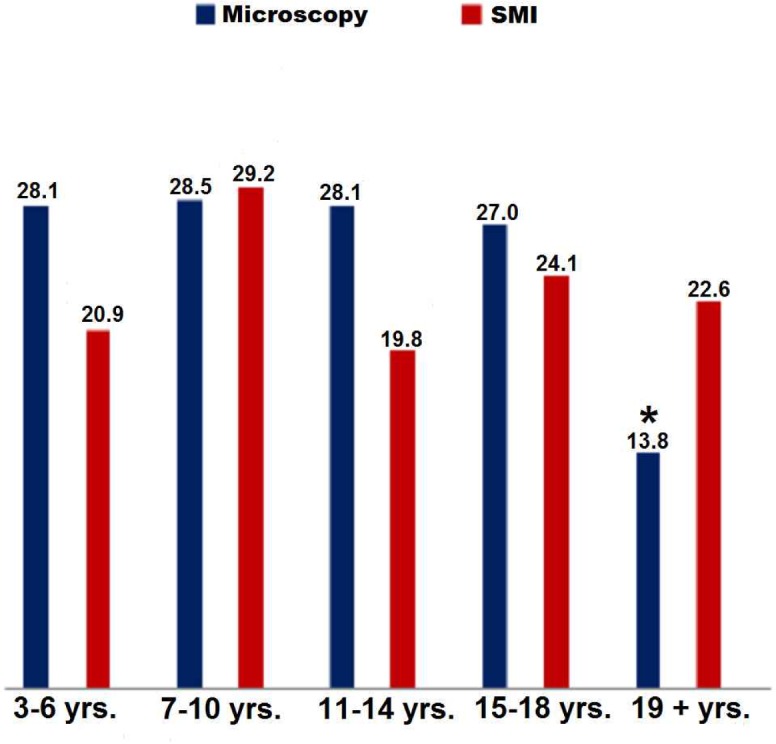
* Significant difference between the prevalence of individuals higher than 18years and other groups.
The prevalence of ME + was significantly higher in individuals of 3–14 years) and whom of 15–18 years than in adults of 19 years old and more (p<0.05) (Fig 2). The PCR+ prevalence among ME—participants did not differ significantly across the age groups. The overall prevalence of P. falciparum infection was significantly higher in individuals less than 18 years old than in older individuals (respectively 32.7% and 9.6%, p<0.001) (Fig 2).
The prevalence of both ME+ and PCR+ was slightly but not significantly higher in males than in females (Fig 3).
Fig 3. Mean prevalence according to the age groups.
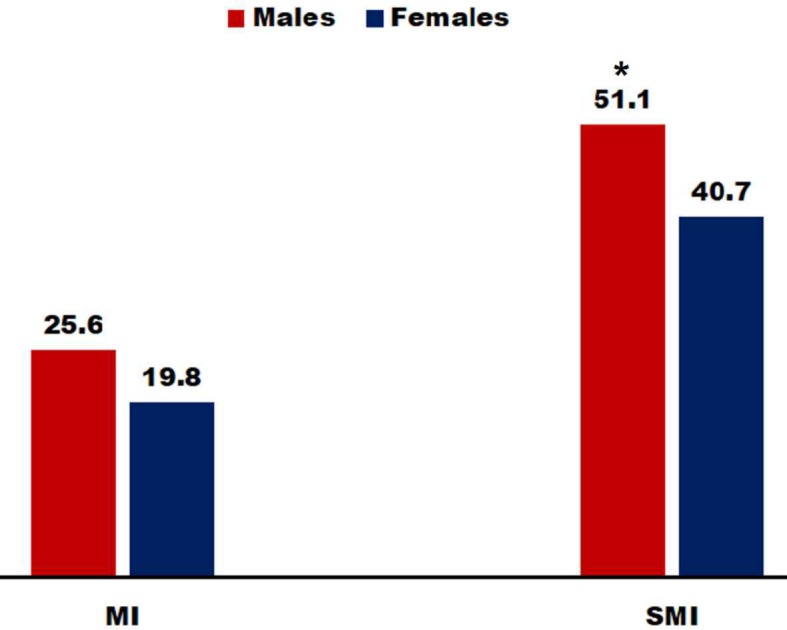
* Significant difference between the prevalence of males obtained by PCR and that observed among females.
Parasite parameters
Although peripheral blood parasites were almost all trophozoites (50 to 150 000 parasite per μl), several gametocytes were identified in 35 ME + individuals (9 symptomatic and 26 asymptomatic) during all the periods except P6, with 11 (11.8%) in P1; 5 (6.9%) in P2; 8 (14.0%) in P3; 5 (13.9%) in P4; and 3 (5.9%) in P5). Gametocyte density ranged from 50 to 700 per μl.
Malaria and fever
Thirty febrile individuals were identified: 9/370, 2.43% in P1; 1/267, 0.37% in P2; 4/218, 1.83% in P3; 1/255, 0.39% in P4; 14/232, 6.03% in P5; and 1/145, 0.68% in P6, with a mean of 1.95%. P. falciparum was detected in 23 of these 30 febrile subjects (76.7%; 17 ME + and 6 PCR+). Eight of the 17 ME + patients had at least 5000 parasites per μl (S1 Annexe). Thus, only 8 patients were considered to have clinical malaria, defined by fever associated with at least 5000 trophozoites per μl of blood.
Attitudes and behaviors concerning malaria
ITNs were used by 33.47% of individuals (95%CI [27.64–38.73]) and IRS by 18.26% (95%CI [13.8–23.8]). No significant difference was observed in the use of ITNs between P. falciparum-infected and uninfected individuals. However, adults used ITNs more than individuals ≤16years old. Self-medication was declared by 12.22% (95%CI [8.55–17.18]) of individuals, with no significant difference between clinical groups (infected and uninfected), genders or age groups (Table 4).
Table 4. Attitudes and behaviors concerning malaria.
| ITNs | P value | IRS | P value | Self-medication | P value | |
|---|---|---|---|---|---|---|
| Population follow up in March 2014 (N = 232) | 76 (32.76) | 41 (17.67) | 28 (12.00) | |||
| Positive malaria infection (N = 134) | 37 (28.46) | 0.072* | 26 (20.00) | 0.361* | 18 (13.85) | 0.293* |
| Negative malaria infection (N = 98) | 39 (39.80) | 15 (15.31) | 9 (9.28) | |||
| Males (N = 115) | 30 (26.08) | 0.929** | 21 (18.26) | 0.662** | 14 (12.17) | 0.813** |
| Females (N = 117) | 46 (39.32) | 20 (17.09) | 14 (11;97) | |||
| ≤15 (N = 159) | 38 (26.57 | 28 (19.58) | 17 (11.89) | |||
| ≥16 (N = 73) | 38 (44.71) | 0.003*** | 13 (15.29) | 0.470*** | 10 (11.90) | 0.804*** |
N: frequency
ITNs: Insecticide Treatment Nuts
IRS: Indoor Residual Spread
Data are presented as effectives and frequencies in brackets. The symbol * is the p value traducing the comparison between the positive infection malaria and negative in each rubric (INTs, IRS and self-medication). The second symbol ** is the comparison between Males and females. The last symbol *** compares the use of the INTs, IRS and the practice of self-medication between people less than 16 years and those high than 15 years
In logistic regression analysis, even if the associations are little ITNs, IRS and self-medication were associated with protection from P. falciparum infection, respective odds ratios were 0.60 (0.34–1.05), 1.38 (0.69–2.75) and 1.57 (0.68–3.59). When we analyzed according to age, protection against P. falciparum infection was associated with the use of ITNs: adults tended to be more protected than younger individuals, with an odds ratio of 2.43 (1.36–4.34) (Table 5).
Table 5. Stepwise multivariate logistic regression analysis of significant variables.
| ITNs | IRS | Self-medication | |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| N = 232 | n = 76 | n = 41 | n = 28 |
| Malaria infection | |||
| Positive (n = 134) | 1,00 | 1,00 | 1,00 |
| Negative (n = 98) | 0.60 (0.34–1.05) | 1.38 (0.69–2.75) | 1.57 (0.68–3.59) |
| Gender | |||
| Females (n = 117) | 1,00 | 1,00 | 1,00 |
| Males (n = 115) | 0.97 (0.47–2.00) | 0. 81 (0.32–2.05) | 0.83 (0.19–3.48) |
| Age (years) | |||
| ≤18 (n = 159) | 1,00 | 1,00 | 1,00 |
| ≥19 (n = 73) | 2.43 (1.36–4.34) | 0. 76 (0.36–1.60) | 0.90 (0.38–2.11) |
Discussion
The decline in the prevalence of malaria observed in many countries, including Gabon, has been attributed to artemisinin-based combination therapy (ACT), monitoring of pregnancies, and distribution of insecticide-treated nets. For example, in 2008 the proportion of Plasmodium-positive slides was reported to have fallen considerably in Libreville, the capital of Gabon [27], as well as in Franceville in the southeast [19,28]. However, these studies involved symptomatic patients usually recruited from pediatric units. In addition, there are few data on rural areas.
This longitudinal study revisited the situation in Dienga 10 years later, focusing on the hidden reservoir of P. falciparum infection. The study was designed to determine the true prevalence of P. falciparum infection during six passages (P) involving 370 individuals. The overall P. falciparum carriage rate (ME + and PCR+) ranged from 57.7% in P5 to 34.1% in P4, with an average of 45.9% (23.2% ME + and 22.7% PCR+). This study provides one of the most accurate field estimates of P. falciparum prevalence across all age groups in Gabon. Indeed, previous studies focused only on children [19,21,29] or pregnant women [30,31]. A high level of falciparum infection among asymptomatic individuals was found in Dienga, confirming the results of previous studies conducted in the same location [20], as well as in Lambaréné, central Gabon [32], and Kenya [33]. The prevalence of microscopic P. falciparum infection in Dienga (23.2%) was very similar to that found in 2011 among febrile children in Libreville (24.1%), an urban area of northwest Gabon [34]. Assuming that asymptomatic P. falciparum carriers have a five-fold increase in the risk of developing malaria [35], such carriers should be taken into account in epidemiological surveillance, notably in rural areas.
No significant difference was observed in the overall prevalence of P. falciparum across our six survey periods. In 2001, Elissa et al. reported that malaria transmission was perennial in Dienga, with a major peak in December to March and a minor peak in July to August; malaria transmission was almost undetectable during the rest of the year, owing to the disappearance of the main vector, Anopheles gambiae sensu lato, in the dry season [23,36]. It is conceivable that climate change (notably rainfall), together with other environmental and behavioral changes, influenced the abundance of A. gambiae s.l. during the 10-year period in question. Entomological investigations will be necessary to confirm this hypothesis, as well as the possible introduction of other vectors.
The mean overall prevalence of P. falciparum infection was similar in children (3–6, 7–10 and 11–14 years) and adolescents (15–18 years) but higher than in adults (Fig 3), as was the microscopic prevalence (Fig 2). Together, these data suggest that adolescents are being infected by P. falciparum as frequently as younger children, suggesting a shift in the age group at risk and therefore a change in malaria epidemiology in this region. Similar results have been obtained both in field studies [37,38] and pediatric units [21]. Children under 5 years of age are the most vulnerable group and are commonly targeted by intervention strategies, possibly explaining the higher relative prevalence in older children and adolescents. Both immunity and the attitudes of adolescents can also influence the risk of developing malaria [39]. P. falciparum carriers were slightly but not significantly more frequent among males than females (Fig 3).
Only 30 febrile individuals were identified, 8 of whom appeared to have clinical malaria, defined as fever associated with at least 5000 trophozoites per μl of blood [23]. The remaining 15 individuals had low parasitaemia, including SMI, and may have had other infections. Nevertheless, SMI (ME—PCR+) can be associated with severe malaria in areas of both discontinuous [6] and perennial transmission [40]. Submicroscopic infection has also been linked to maternal anemia and low birth weight [41,42]. The prevalence rates of microscopic and submicroscopic P. falciparum infection were both comparable, confirming that almost 50% of carriers maybe missed by microscopy alone [5].
Gametocytes were identified in 32 symptomatic or asymptomatic microscopy-positive individuals. As we did not seek to detect submicroscopic gametocyte carriage, the true prevalence of gametocyte carriage may have been underestimated. In the absence of treatment, individuals carrying asexual parasites could potentially produce sexual gametocytes that can infect mosquitoes [43]. The P. falciparum-infected adults and children identified in this study likely represent a parasite reservoir that may sustain malaria transmission [44]. Submicroscopic infection, including gametocyte carriage, must thus be taken into account in malaria control programs [5,44].
In February 2003, a prospective cohort of 319 subjects 13–70 years old was followed for malaria and hepatitis virus co-infection during a one-year period in Dienga. The overall prevalence of malaria parasitemia was 20.8% (7.2% ME + and 13.6% PCR+) [20,45]. This microscopic prevalence of 7.2% was close to the pre-elimination threshold of 5%. In the present study, 10 years later, we found by microscope a significant higher prevalence of 23% (p < 0.001). The overall prevalence of P. falciparum infection was also significantly higher in 2013 than in 2003 (45.9% versus 20.8%) (p<0.05). This increase could be due to several factors. First, in 1994 CIRMF established a fully equipped field base with three permanent workers (one nurse and two technicians) ensuring full-time cohort monitoring. In addition, the base was supplied monthly with food and drugs and was visited by a CIRMF physician. All febrile patients in Dienga were examined, and those with malaria were treated free of charge. The antimalarials used at the time were mainly chloroquine and sulfadoxine pyrimethamine (SP). In 2006, CIRMF research activities in Dienga were reduced, and in 2008 they were shifted to other topics. Antimalarial drugs were no longer distributed to the population and patient management was provided only by the local health center in Dienga, that had limited resources. Following the change in the national antimalarial policy, with a switch to ACT, in 2005 the Gabonese Malaria National Control Program (MNCP) launched a nationwide campaign of ITNs distribution, intermittent preventive treatment (IPT), and educational messages. These interventions have been evaluated in many parts of the country [27,46,47] but not in Dienga. A significant decline in the malaria burden led to a deceleration of preventive activities in 2008 [27].
The study found that ITNs and IRS were used by respectively 33.47% and 18.26% of respondents, and self-medication by 12.22%. In 2003, the rate of self-treatment was 11.3% in Dienga [45].
The comparison in the use of ITNs between youngers (3–15 years old) and individuals of 16 years old and more has shown a significant difference (p = 0.003) (Table 4), the adult individuals were using more ITNs and they were less infected. Perhaps these results can be explained by the attitude of adolescents and the absence of children less than 3 years old in the study population. Logistic regression analysis showed that the use of ITNs was a protective factor against P. falciparum malaria in Dienga (Table 5). However, the fact that the adults were less frequently infected was not necessarily due to the use of ITNs. The oral survey of ITNs and IRS use was performed in March 2014, corresponding to the major peak of malaria transmission. Of interest, the highest prevalence of P. falciparum malaria (57.7%) was observed in March 2014 (Fig 1). ITNs coverage was lower than the national average of 57% reported in 2008 [48] and this could explain the apparent lack of protection.
These results show that the MNCP campaign has not yielded the hoped-for results. The increase in malaria prevalence among febrile children observed in several pediatric units has been attributed to the decline in the preventive activities of the MNCP [21]. This may also explain the present results; although the study was performed on a cohort of asymptomatic individuals. A rebound of malaria has also been observed in Kenya [49]. In contrast, no such increase has been reported in countries where interventions have been sustained [50,51]. Finally, factors such as mosquito resistance to conventional insecticides could further undermine preventive measures. Such resistance has been documented in Libreville and Port-Gentil [52].
It would have been interesting to perform, as planned, passages every two months for a full year. Unfortunately, because of logistic constraints, it was not possible to achieve this goal. Consequently, the six passages were carried out irregularly during a 15-month period.
In conclusion, the study showed a near-threefold increase in the prevalence of P. falciparum infection in Dienga, southeastern Gabon, over a recent 10-year period. Although a large proportion of infected subjects were asymptomatic, the risk of older children and adolescents of developing falciparum infection becomes higher. These results reveal an urgent need for reinforcing intervention strategies.
Supporting Information
(DOC)
(DOC)
Acknowledgments
We thank Dr U Bisvigou for his valuable advice during the preliminary study. We are grateful to the individuals and their families who accepted to participate in the study, and to the staff of the Dienga CIRMF, especially Madame Helene Tiga and Suzan Moundemba. We also express our gratitude to Misters Justice Mayombo, Lewobo, Madames Lady Charlen Kouna, Omnella Mavoungou, Ingrid Ontsia and Dr Mpiga-Mickoto. Finally, we thank the staff of the Centre International de Recherches Médicales de Franceville (CIRMF), for help with the lab tests, particularly Jeanne Lahonko and Dr Statiana-Mbouyi.
Data Availability
All relevant data are all contained within the paper.
Funding Statement
The study was funded by Centre International de Recherches Médicale de Franceville. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2013) World malaria report World Health Organization. [Google Scholar]
- 2.Emilio V Perez-Jorge M, FACP Staff Physician, Division of Infectious Diseases, Lexington Medical Center Thomas E Herchline, MD Professor of Medicine, Wright State University, Boonshoft School of Medicine; Medical Director, Public Health, Dayton and Montgomery County, Ohio (2014) Malaria Clinical Presentation. Medscape.
- 3.Thomas CJ, Lindsay SW (2000) Local-scale variation in malaria infection amongst rural Gambian children estimated by satellite remote sensing. Trans R Soc Trop Med Hyg 94: 159–163. [DOI] [PubMed] [Google Scholar]
- 4.Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, et al. (2013) Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J 12: 221 10.1186/1475-2875-12-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okell LC, Ghani AC, Lyons E, Drakeley CJ (2009) Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200: 1509–1517. 10.1086/644781 [DOI] [PubMed] [Google Scholar]
- 6.Giha HA, IE AE, TM AE, Adam I, Berzins K, Elghazali G, et al. (2005) Cerebral malaria is frequently associated with latent parasitemia among the semi-immune population of eastern Sudan. Microbes Infect 7: 1196–1203. [DOI] [PubMed] [Google Scholar]
- 7.Berthe Amélie Iroungou JCBBE, Kassa Roland Fabrice, Nkoghé Dieudonné, Mege Jean-Louis, Ndouo Fousseyni S Touré. (2014) Submicroscopic plasmodial infection may lead to severe malaria in children. Journal of Life Science [Google Scholar]
- 8.Kurtzhals JA, Addae MM, Akanmori BD, Dunyo S, Koram KA, Appawu MA, et al. (1999) Anaemia caused by asymptomatic Plasmodium falciparum infection in semi-immune African schoolchildren. Trans R Soc Trop Med Hyg 93: 623–627. [DOI] [PubMed] [Google Scholar]
- 9.Alonzo Gonzalez M, Menendez C, Font F, Kahigwa E, Kimario J, Mshinda H, et al. (2000) Cost-effectiveness of iron supplementation and malaria chemoprophylaxis in the prevention of anaemia and malaria among Tanzanian infants. Bull World Health Organ 78: 97–107. [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando D, de Silva D, Wickremasinghe R (2003) Short-term impact of an acute attack of malaria on the cognitive performance of schoolchildren living in a malaria-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg 97: 633–639. [DOI] [PubMed] [Google Scholar]
- 11.Al Serouri AW, Grantham-McGregor SM, Greenwood B, Costello A (2000) Impact of asymptomatic malaria parasitaemia on cognitive function and school achievement of schoolchildren in the Yemen Republic. Parasitology 121 (Pt 4): 337–345. [DOI] [PubMed] [Google Scholar]
- 12.Bousema T, Okell L, Felger I, Drakeley C (2014) Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. 10.1038/nrmicro3364 [DOI] [PubMed] [Google Scholar]
- 13.Bonnet S, Gouagna LC, Paul RE, Safeukui I, Meunier JY, Boudin C (2003) Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: evaluation and comparison of several indices. Trans R Soc Trop Med Hyg 97: 53–59. [DOI] [PubMed] [Google Scholar]
- 14.Boudin C, Robert V (2003) [Plasmodium falciparum: epidemiology and man-mosquito transmission and infection in the vector]. Bull Soc Pathol Exot 96: 335–340. [PubMed] [Google Scholar]
- 15.Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P (1993) High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48: 700–706. [DOI] [PubMed] [Google Scholar]
- 16.Robert V, Boudin C (2003) [Biology of man-mosquito Plasmodium transmission]. Bull Soc Pathol Exot 96: 6–20. [PubMed] [Google Scholar]
- 17.Gamage-Mendis AC, Rajakaruna J, Carter R, Mendis KN (1991) Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am J Trop Med Hyg 45: 479–487. [DOI] [PubMed] [Google Scholar]
- 18.Bouyou-Akotet MK, Mawili Mboumba DP, Kendjo E, Mbadinga F, Obiang-Bekale N, Mouidi P, et al. (2013) Anaemia and severe malarial anaemia burden in febrile Gabonese children: a nine-year health facility based survey. J Infect Dev Ctries 7: 983–989. 10.3855/jidc.3347 [DOI] [PubMed] [Google Scholar]
- 19.Lekana-Douki JB, Pontarollo J, Zatra R, Toure-Ndouo FS (2011) [Malaria in Gabon: results of a clinical and laboratory study at the Chinese-Gabonese Friendship Hospital of Franceville]. Sante 21: 193–198. 10.1684/san.2011.0263 [DOI] [PubMed] [Google Scholar]
- 20.Toure FS, Mezui-Me-Ndong J, Ouwe-Missi-Oukem-Boyer O, Ollomo B, Mazier D, Bisser S (2006) Submicroscopic Plasmodium falciparum infections before and after sulfadoxine-pyrimethamine and artesunate association treatment in Dienga, Southeastern Gabon. Clin Med Res 4: 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mawili-Mboumba DP, Bouyou Akotet MK, Kendjo E, Nzamba J, Medang MO, Mbina JR, et al. (2013) Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malar J 12: 3 10.1186/1475-2875-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BAI Jean Claude Bitéghé Bi Essone, Lékana-Douki Jean Bernard, Ndouo Fousseyni S Touré, Onanga Richard and Benjamin and Ollomo Benjamin (2014) Submicroscopic Infection from uncomplicated Plasmodium falciparum malaria of Franceville, southeasern Gabon. IJAR 2: 117–123. [Google Scholar]
- 23.Elissa N, Migot-Nabias F, Luty A, Renaut A, Toure F, Vaillant M, et al. (2003) Relationship between entomological inoculation rate, Plasmodium falciparum prevalence rate, and incidence of malaria attack in rural Gabon. Acta Trop 85: 355–361. [DOI] [PubMed] [Google Scholar]
- 24.Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10: 506–513. [PubMed] [Google Scholar]
- 25.Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, et al. (2001) Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg 65: 599–602. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, et al. (1997) Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg 57: 495–500. [DOI] [PubMed] [Google Scholar]
- 27.Bouyou-Akotet MK, Mawili-Mboumba DP, Kendjo E, Mabika-Mamfoumbi M, Ngoungou EB, Dzeing-Ella A, et al. (2009) Evidence of decline of malaria in the general hospital of Libreville, Gabon from 2000 to 2008. Malar J 8: 300 10.1186/1475-2875-8-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zang-Edou ES, Bisvigou U, Taoufiq Z, Lekoulou F, Lekana-Douki JB, Traore Y, et al. (2010) Inhibition of Plasmodium falciparum field isolates-mediated endothelial cell apoptosis by Fasudil: therapeutic implications for severe malaria. PLoS One 5: e13221 10.1371/journal.pone.0013221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mawili-Mboumba DP, Nikiema R, Bouyou-Akotet MK, Bahamontes-Rosa N, Traore A, Kombila M (2013) Sub-microscopic gametocyte carriage in febrile children living in different areas of Gabon. Malar J 12: 375 10.1186/1475-2875-12-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouyou-Akotet MK, Mawili-Mboumba DP, Kombila M (2013) Antenatal care visit attendance, intermittent preventive treatment and bed net use during pregnancy in Gabon. BMC Pregnancy Childbirth 13: 52 10.1186/1471-2393-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouyou-Akotet MK, Nzenze-Afene S, Mawili-Mboumba DP, Owono-Medang M, Guiyedi V, Kombila M (2011) [Trends in the prevalence of malaria and anemia at delivery in Libreville from 1995 to 2011]. Sante 21: 199–203. 10.1684/san.2011.0266 [DOI] [PubMed] [Google Scholar]
- 32.Dal-Bianco MP, Koster KB, Kombila UD, Kun JF, Grobusch MP, Ngoma GM, et al. (2007) High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 77: 939–942. [PubMed] [Google Scholar]
- 33.Stresman GH, Stevenson JC, Ngwu N, Marube E, Owaga C, Drakeley C, et al. (2014) High levels of asymptomatic and subpatent Plasmodium falciparum parasite carriage at health facilities in an area of heterogeneous malaria transmission intensity in the Kenyan highlands. Am J Trop Med Hyg 91: 1101–1108. 10.4269/ajtmh.14-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mawili-Mboumba DP, Bouyou-Akotet MK, Kombila M (2014) Submicroscopic infections among children with adequate clinical and parasitological response (ACPR). Acta Trop 134: 29–32. 10.1016/j.actatropica.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 35.Le Port A, Cot M, Etard JF, Gaye O, Migot-Nabias F, Garcia A (2008) Relation between Plasmodium falciparum asymptomatic infection and malaria attacks in a cohort of Senegalese children. Malar J 7: 193 10.1186/1475-2875-7-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, et al. (1992) Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg 47: 181–189. [DOI] [PubMed] [Google Scholar]
- 37.Noland GS, Graves PM, Sallau A, Eigege A, Emukah E, Patterson AE, et al. (2014) Malaria prevalence, anemia and baseline intervention coverage prior to mass net distributions in Abia and Plateau States, Nigeria. BMC Infect Dis 14: 168 10.1186/1471-2334-14-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aina OO, Agomo CO, Olukosi YA, Okoh HI, Iwalokun BA, Egbuna KN, et al. (2013) Malariometric survey of ibeshe community in ikorodu, lagos state: dry season. Malar Res Treat 2013: 487250 10.1155/2013/487250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yimer F, Animut A, Erko B, Mamo H (2015) Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar J 14: 230 10.1186/s12936-015-0749-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthe Amélie Iroungou JCBBE, Kassa Roland Fabrice, Nkoghé Dieudonné, Mege Jean-Louis, Ndouo Fousseyni S Touré. (2014) Submicroscopic plasmodial infection may lead to severe malaria in children. Journal of Life Science. [Google Scholar]
- 41.Mayengue PI, Rieth H, Khattab A, Issifou S, Kremsner PG, Klinkert MQ, et al. (2004) Submicroscopic Plasmodium falciparum infections and multiplicity of infection in matched peripheral, placental and umbilical cord blood samples from Gabonese women. Trop Med Int Health 9: 949–958. [DOI] [PubMed] [Google Scholar]
- 42.Bouyou-Akotet MK, Nzenze-Afene S, Ngoungou EB, Kendjo E, Owono-Medang M, Lekana-Douki JB, et al. (2010) Burden of malaria during pregnancy at the time of IPTp/SP implementation in Gabon. Am J Trop Med Hyg 82: 202–209. 10.4269/ajtmh.2010.09-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins WE, Jeffery GM (1999) A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg 61: 4–19. [DOI] [PubMed] [Google Scholar]
- 44.Karl S, Gurarie D, Zimmerman PA, King CH, St Pierre TG, Davis TM (2011) A sub-microscopic gametocyte reservoir can sustain malaria transmission. PLoS One 6: e20805 10.1371/journal.pone.0020805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouwe-Missi-Oukem-Boyer O, Ndouo FS, Ollomo B, Mezui-Me-Ndong J, Noulin F, Lachard I, et al. (2011) Hepatitis C virus infection may lead to slower emergence of P. falciparum in blood. PLoS One 6: e16034 10.1371/journal.pone.0016034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mawili-Mboumba DP, Bouyou Akotet MK, Ngoungou EB, Kombila M (2010) Evaluation of rapid diagnostic tests for malaria case management in Gabon. Diagn Microbiol Infect Dis 66: 162–168. 10.1016/j.diagmicrobio.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 47.Bouyou-Akotet MK, Mawili-Mboumba DP, Tchantchou Tde D, Kombila M (2010) High prevalence of sulfadoxine/pyrimethamine-resistant alleles of Plasmodium falciparum isolates in pregnant women at the time of introduction of intermittent preventive treatment with sulfadoxine/pyrimethamine in Gabon. J Antimicrob Chemother 65: 438–441. 10.1093/jac/dkp467 [DOI] [PubMed] [Google Scholar]
- 48.WHO (2008) World malaria report World Health Organization. [Google Scholar]
- 49.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule J, Williamson JM, et al. (2011) A reversal in reductions of child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg 85: 597–605. 10.4269/ajtmh.2011.10-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chizema-Kawesha E, Miller JM, Steketee RW, Mukonka VM, Mukuka C, Mohamed AD, et al. (2010) Scaling up malaria control in Zambia: progress and impact 2005–2008. Am J Trop Med Hyg 83: 480–488. 10.4269/ajtmh.2010.10-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aregawi MW, Ali AS, Al-mafazy AW, Molteni F, Katikiti S, Warsame M, et al. (2011) Reductions in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malar J 10: 46 10.1186/1475-2875-10-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mourou JR, Coffinet T, Jarjaval F, Pradines B, Amalvict R, Rogier C, et al. (2010) Malaria transmission and insecticide resistance of Anopheles gambiae in Libreville and Port-Gentil, Gabon. Malar J 9: 321 10.1186/1475-2875-9-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are all contained within the paper.