Abstract
Background
Gait disorder, a key contributor to fall and poor quality of life, represents a major therapeutic challenge in Parkinson’s disease (PD). The efficacy of acupuncture for PD remains unclear, largely due to methodological flaws and lack of studies using objective outcome measures.
Objective
To objectively assess the efficacy of electroacupuncture (EA) for gait disorders using body-worn sensor technology in patients with PD.
Methods
In this randomized pilot study, both the patients and assessors were masked. Fifteen PD patients were randomly assigned to an experimental group (n = 10) or to a control group (n = 5). Outcomes were assessed at baseline and after completion of three weekly EA treatments. Measurements included gait analysis during single-task habitual walking (STHW), dual-task habitual walking (DTHW), single-task fast walking (STFW), dual-task fast walking (DTFW). In addition, Unified Parkinson's Disease Rating Scale (UPDRS), SF-12 health survey, short Falls Efficacy Scale-International (FES-I), and visual analog scale (VAS) for pain were utilized.
Results
All gait parameters were improved in the experimental group in response to EA treatment. After adjustment by age and BMI, the improvement achieved statistical significant level for gait speed under STHW, STFW, and DTFW (9%-19%, p<0.05) as well as stride length during DTFW (9%, p = 0.037) and midswing speed during STFW (6%, p = 0.033). No significant changes were observed in the control group (p>0.110). The highest correlation between gait parameters and UPRDS scores at baseline was observed between gait speed during STFW and UPDRS II (r = -0.888, p = 0.004). The change in this gait parameter in response to active intervention was positively correlated with baseline UPDRS (r = 0.595, p = 0.057). Finally, comparison of responses to treatment between groups showed significant improvement, prominently in gait speed (effect size 0.32–1.16, p = 0.001).
Conclusions
This study provides the objective proof of concept for potential benefits of non-pharmaceutical based EA therapy on enhancing gait in patients with PD.
Trial Registration
ClinicalTrials.gov NCT02556164
Introduction
The hypokinetic rigid gait of PD is mainly characterized by a reduction of gait speed and stride length [1–3]. These symptoms progress with time in the majority of cases, leading to festination, freezing of gait, and falls [4]. Gait disorders as other axial motor symptoms such as dysphagia, fall and postural instability tend to be treatment-resistant [5]. Dopamine treatment and deep brain stimulation (DBS) have limited efficacy and in some cases even result in worsened gait in PD [4, 6, 7]. In light of the significant limitations of conventional therapy, interest in complementary and alternative therapies for PD is growing, with acupuncture emerging as one of the most popular alternative therapies [8–10].
Despite the widespread use of acupuncture in recent years, its efficacy remains unclear, largely due to methodological flaws and lack of high quality studies using objective outcome measures [11]. An open-label clinical trial of acupuncture in PD suggested that no significant changes were noted in motor or depression scores although there was subjective improvement in some symptoms such as tremor, handwriting, difficulty walking, and sleep [10]. A double-blind randomized pilot study compared acupuncture to a control with a nonacupuncture procedure, and found no significant changes in the outcomes measured, including the Unified Parkinson’s Disease Rating Scale (UPDRS) [12]. One randomized pilot study, however, did show significant improvement in the UPDRS and 30 meter walking time [13].
Recently, objective gait and balance measurement using body-worn sensors has been employed as surrogate endpoints for demonstrating clinical efficacy of new treatments, in place of counting falls from diaries, using stop-watch measures gait speed, or relying on clinical rating scales [14]. Our previous report has shown improvement in balance following electro-acupuncture (EA) using body-worn sensors in PD patients [15]. To our knowledge, gait has not been objectively quantified by using such biomechanical assessment in any PD acupuncture study. Moreover, potential acupuncture effects on attention-related motor performances, such as dual-task walking in PD, have not been evaluated, even though dual-task gait performance is strongly associated with fall risk and predicts disability in PD [16]. Finally, it is unclear if the stage of PD affects the response to acupuncture treatment [10, 17].
In this pilot study, we addressed the methodological pitfalls of earlier studies by applying objective innovative body-worn sensor technologies for assessing potential mobility-associated outcomes of PD therapy. We compared acute changes in gait that occurred after repeated administration of two interventions: A specific real EA and a sham EA. The design of our experiments uses a control that accounts for both placebo and possibly some active components of a generalized needle-insertion-based procedure. By using a sham control, we were able to fully assess whether our specific EA intervention could lead to changes in objective gait parameters, or subjective self-reported improvements that are beyond placebo induced effects and the natural course of the disease. We demonstrated that EA is safe and effective for gait disorders in patient with PD.
Methods
Standard protocol approvals, registrations, and patient consents
The protocol for this trial and supporting CONSORT checklist are available as supporting information (S1 Protocol, S1 CONSORT Checklist). Participants were consecutively recruited from May 2013 to April 2014 at the movement Disorder Clinic, the University of Arizona (HL, SS). The study was approved by the University of Arizona Institutional Review Board and written informed consent was obtained from all subjects before participation. The intervention employed routine acupuncture techniques in this clinic so the study was not registered initially. The authors confirm that all ongoing and related trials for this intervention are registered in ClinicalTrials.gov with the Identifier NCT02556164. https://clinicaltrials.gov/ct2/show/NCT02556164?term=acupuncture+and+gait&rank=1
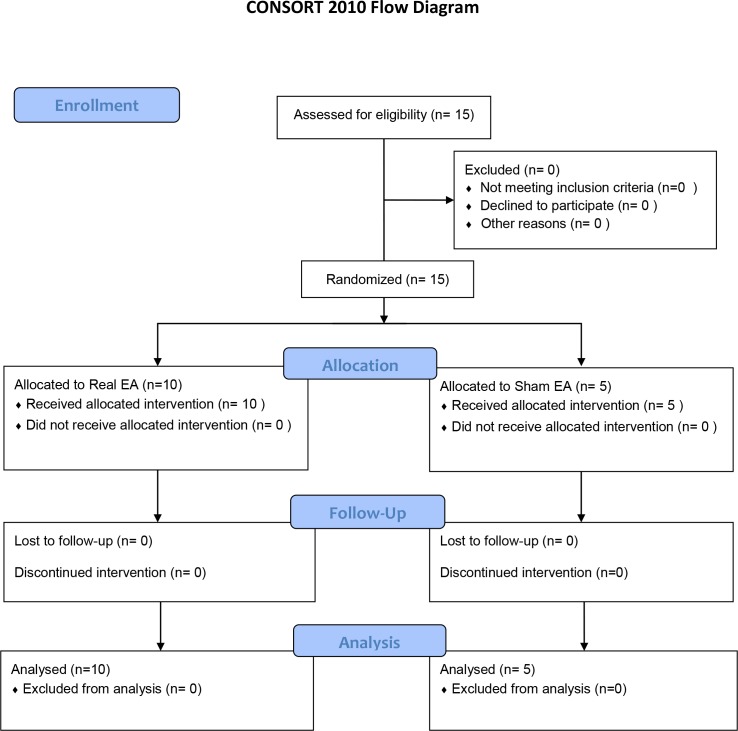
Participants were randomly assigned (NT) with a ratio of 2 to 1 to either the experimental (n = 10) or the control (n = 5) groups (Fig 1). Randomization was done by drawing pieces of paper from a bag. The patients in the experimental group received treatment at standard acupuncture points, and in the control group at placebo points. All patients underwent either real or sham acupuncture for 3 weeks in once-weekly, 30-minute sessions. Outcomes were assessed in an equal or greater than 12 hours defined off anti-Parkinsonian medication state at both baseline and 30 minutes after the completion of the final treatment. All assessors were masked to treatment allocation.
Fig 1. CONSORT Flow Diagram.
Inclusion Criteria for the study include: 1) Community-dwelling men or women ages 55 years or older with diagnosis of PD; 2) patients who have the ability to walk 20 meters without walking assistance; and 3) patients who are stable without anti-PD medication(s) change for at least 1 month. Calculation of a daily L-dopa equivalent daily dose (LEDD) was based on theoretical equivalence to L-dopa as follows (modified from Evans AH, et al., 2004 and Wüllner U, et al., 2010):[18, 19] LEDD = regular levodopa dose x 1 + levodopa continuous release dose x 0.75 + ([regular levodopa dose + continuous release levodopa dose x 0.75] x 0.25 if taking tolcapone or entacapone) + pramipexole dose x 67 + ropinirole dose x 20 + rotigotine x 30 + pergolide dose x 100 + bromocriptine dose x 10 + cabergoline dose x 50 + amantadine dose x 0.5 + selegiline dose x 10 + resagiline dose x 100. The PD diagnosis was made by movement disorder specialists based on the UK Brain Bank criteria [20], and supported by DaTscan (Ioflupane I 123 injection) when possible. Exclusion Criteria for the study include: 1) patients who have received previous acupuncture; 2) patients who have had DBS; 3) patients with any clinically significant medical condition, psychiatric condition, drug or alcohol abuse, or laboratory abnormality that would, in the judgment of the investigators, interfere with the ability to participate in the study; and 4) patients with non-PD related gait disorders.
Electro-acupuncture procedure
A standardized EA regimen, consisting of scalp and body acupuncture, with a focus on improving balance and gait for PD patients was designed and administered by a single physician (HL), who had more than 5 years of experience in acupuncture and was certified by the American Board of Medical Acupuncture and the American Board of Psychiatry and Neurology. Sterile disposable, surgical stainless steel acupuncture needles (Seirin, L type, Japan), measuring 0.25 mm in diameter and 40–50 mm in length were used for acupuncture, and 3 pocket portable electric stimulators (ITO ES-130, Japan) were used for EA stimulation. Each of the electrical stimulator has 3 independent intensity channels with a pulsed current. The pulse was asymmetric biphasic square wave with pulse width 100 microseconds (μS). Real EA was performed at standard acupuncture points with depth and frequencies as shown in Table 1. “De-qi” was achieved with needle manipulation. In the treatment EA group, the current amplitude (intensity) was adjusted and recorded as number of dial between 3.5 and 4.5, just below the level that induces visible muscle contraction. All the acupuncture points were stimulated at the same time with frequency of 100 Hz or 4 Hz. The Sham acupuncture was performed for the control group with insertion of needles less than 4mm, just under the skin at non-acupuncture points (at the same areas for all subjects) at scalp, neck, shoulder, upper and lower extremities without needle manipulation. The electric stimulation in sham acupuncture was performed in a similar fashion to the real EA but with intensity equal to zero. The treating physician was responsible for recording adverse events and safety assessments.
Table 1. Acupuncture procedure in experimental group.
| Point | Location | Insertion | Frequency (Hz) |
|---|---|---|---|
| Foot Motor Sensory Area | 1 cm left or right to the midpoint of midline, extending up to 4cm posteriorly. | Transversely | 100 |
| Balance Area | Starting at occipital protuberance, 3.5-4cm to the left or right of midline, then going downwards 4cm. | Transversely | 100 |
| GV20 (Baihui) | The midpoint of the connecting line between the auricular apices when the ears are folded. | Transversely | 4 |
| GV14 (Dazhui) | On the posterior midline, in the depression inferior to the spinous process of the 7th cervical vertebra (C7) | Perpendicularly | 4 |
| LI4 (Hegu) | On the dorsum of the first interosseus space of the hand, at the level of the midpoint of the shaft of the 2nd metacarpal bone. | Perpendicularly | 100 |
| ST36 (Zusanli) | On the superolateral aspect of the leg, 3 Cun distal to the inferior border of the patella and one finger-breath lateral to the tibial tuberosity, in a muscle groove. | Perpendicularly | 100 |
| GB34 (Yanglingquan) | On the lateral side of leg, in a depression anterior and inferior to the head of the fibula. | Perpendicularly | 100 |
| BL40 (Weizhong) | At the midpoint of the popliteal crease. | Perpendicularly | 4 |
| SP6 (Sanyinjiao) | On the medial side of the leg, posterior to the medial margin of the tibia, 3 Cun above the prominence of the medial malleolus. | Perpendicularly | 4 |
| KI3 (Taixi) | On the posteromedial aspect of the the ankle, in the depression between the prominence of the medial malleolus and Achilles tendon. | Perpendicularly | 4 |
| LR3 (Taichong) | On the dorsum of the foot, on the first and second metatarsal bones, in the depression distal to the junction of the bases of the two bones, over the dorsalis pedis artery | Perpendicularly | 100 |
Measurements
Primary outcome measure was steady-state gait speed. Secondary outcome measures were other steady-state spatio-temporal gait parameters including stride length, cadence, double support, and midswing angular velocity, the UPDRS, SF-12 health survey, short Falls Efficacy Scale-International (FES-I), and visual analog scale (VAS) for pain. Participants performed four bouts of gait test (> 25 steps) during unobstructed single-task habitual walking (STHW), dual-task habitual walking (DTHW), single-task fast walking (STFW), and dual-task fast walking (DTFW). Angular velocity of shanks, thighs and trunk were measured using wearable sensor technologies (LEGSys™ [BioSensics LLC, Boston, MA]) to derive gait outcome measures following identical procedures explained in earlier studies [21, 22]. Double support was measured as the percentage of time in each stride cycle when both feet are in contact with the ground. Midswing angular velocity was measured as the shank angular velocity peak in the middle of swing phase in each cycle and averaged cross steady state cycles. The beginning of the steady-state walking was quantified as the first stride of the group of six strides with an standard deviation (SD) below the median SD of all the analyzed strides ±6% [22]. All above measurements were performed by trained examiners at baseline and after the completion of 3 weeks’ treatment. In addition, modified Hoehn and Yahr (H&Y) staging, and Mini-Mental State Examination (MMSE) were obtained at baseline.
Statistical analysis
We used descriptive statistics to characterize demographics and performance for each group. Repeated measures analysis of covariance (ANCOVA) was used to compare the effect of the intervention on outcome parameters post intervention adjusting for age and BMI. An independent-samples t-test (or Mann–Whitney U test for non-Gaussian samples) was used for determining differences between groups except for participant’s gender, in which, a Fisher’s exact test was used. Due to small sample size, Bootstrap was also applied to Independent-samples t-test for comparison of responses to treatment between experimental and control groups. Bias-corrected and accelerated method with 1000 replications and default seed were chosen with age as strata variable. Respectively, parametric or non-parametric approach was taken for normally distributed or not normally distributed samples. To assess the normality goodness for each sample we used the Shapiro-Wilk W test. A Bivariate Correlation (Spearman’s rank) was utilized in the experimental group to assess the relationship between response to treatment (for outcomes significantly improved) and participants’ baseline characteristics such as age, H&Y staging, MMSE or UPDRS III. Cohen’s d was used for calculating effect size [23]. A p-values less than 0.05 (two-tailed) was considered statistically significant. All analyses were performed using SPSS Version 23.
Results
Baseline characteristics of participants are detailed in Table 2. No difference was observed between experimental and control groups at baseline for assessed parameters. All participants completed the three intervention sessions and tolerated procedures without significant adverse effects. However, one patient in the experimental group reported transient lightheadedness at near the end of final treatment. Subjects were not able to determine whether they received real or sham acupuncture (χ2 = 1.875, p = 0.17).
Table 2. Baseline characteristics of the study population.
| Mean (SD or percentage) | Experimental | Control | Total | p-value |
|---|---|---|---|---|
| Number (% of total) | 10 (67%) | 5 (33%) | 15 | - |
| Male (% of the group) | 6 (60%) | 2 (40%) | 8 (53%) | 0.46 |
| Age (yr) | 69.8 (4.5) | 71.0(11.7) | 70.2 (7.3) | 0.78 |
| Height (cm) | 164.6 (10.1) | 163.6 (13.5) | 164.3 (10.9) | 0.87 |
| BMI (kg/m2) | 27.5 (4.1) | 28.1 (6.5) | 27.5 (6.5) | 0.85 |
| Disease duration (yr) | 6.2 (5.9) | 5.2 (4.7) | 5.9 (5.3) | 0.75 |
| Total LEDD | 614 (381) | 324 (295) | 517 (380) | 0.17 |
| Dopamine agonist LEDD | 54 (102) | 60 (66) | 56 (90) | 0.72 |
| H&Y stage | 3.0 (1.0) | 2.9 (0.7) | 2.9 (0.9) | 0.92 |
| UPDRS I | 5.3 (3.5) | 3.3 (2.4) | 4.6 (3.2) | 0.28 |
| UPDRS II | 18 (9.7) | 17 (5.7) | 17.7 (8.4) | 0.84 |
| UPDRS III | 35.1 (15.3) | 34.2 (12.3) | 34.8 (13.9) | 0.91 |
| MMSE | 24.9 (6.7) | 27.2 (1.1) | 25.7 (5.6) | 0.47 |
LEDD = regular levodopa dose x 1 + levodopa continuous release dose x 0.75 + ([regular levodopa dose + continuous release levodopa dose x 0.75] x 0.25 if taking tolcapone or entacapone) + pramipexole dose x 67 + ropinirole dose x 20 + rotigotine x 30 + pergolide dose x 100 + bromocriptine dose x 10 + cabergoline dose x 50 + amantadine dose x 0.5 + selegiline dose x 10 + resagiline dose x 100. H&Y = Hoehn and Yahr; UPDRS = Unified Parkinson's Disease Rating Scale; MMSE = Mini-Mental State Examination. An independent-samples t-test (or Mann–Whitney U test for non-parametric samples) was used for determining differences between groups except for participant’s gender, in which, a Fisher’s exact test was used.
Gait analysis
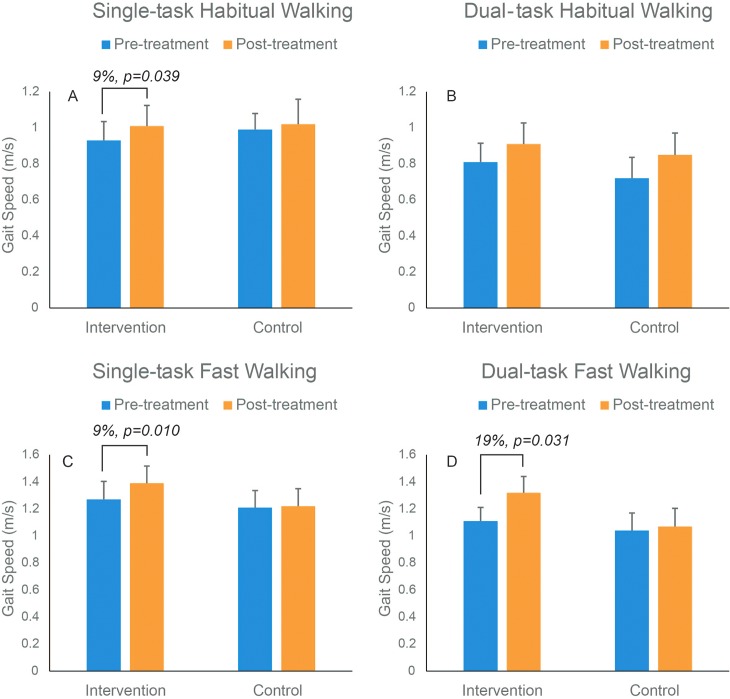
Two out of 15 participants had partial missing gait data and they were both in the experimental group. One patient refused to perform fast walking at the baseline and another patient had invalidated measurements during fast walking. These missing gait data were omitted from the statistical analysis. Table 3 shows comparison of responses to EA in gait parameters within experimental or control groups. Overall, all gait parameters were improved in the intervention group in response to EA treatment after adjustment with age and BMI. Specifically, as illustrated in Fig 2, significant improvement occurred in the experimental group compared to baseline for gait speed during STHW (9%, p = 0.039), STFW (9%, p = 0.010), and DTFW (19%, p = 0.031). Stride length was increased under all tested conditions but observed improvements were only significant for STHW (13%, p = 0.023) and DTHW (9%, p = 0.037). Similarly, a trend of improvement was noted for cadence, double support and midswing angular velocity (indicator of joint rigidity) across all conditions. But only midswing speed improvement during STFW achieved statistically significant level in our sample (6%, p = 0.033) after adjustment with age and BMI. With significantly increased cadence (12%, p = 0.048) during DTFW and increased midswing angular velocity during DTFW (16%, p = 0.029). No significant improvement was determined in any measured gait parameters in the control group compared to baseline (p>0.061). Finally, comparison of response to treatment between experimental and control groups showed significant improvement in most of the measured gait parameters (S1 Table), most prominently in gait speed during all walking conditions (effect size 0.32–1.16, p = 0.001).
Table 3. Comparison of responses to treatment in gait parameters within experimental or control groups.
| Experiment | Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait Parameter | Walking Condition | Pre-EA Mean (SD) | Post-EA Mean (SD) | % Change | 95% CI Lower | 95% CI Upper | p-value (ηp2) | Pre-EA Mean (SD) | Post-EA Mean (SD) | % Change | 95% CI Lower | 95% CI Upper | p-value (ηp2) |
| Speed (m/s) | STHW | 0.93 (0.33) | 1.01 (0.36) | 9% ↑ | -0.16 | -0.01 | .039* | 0.99 (0.20) | 1.02 (0.31) | 3% ↑ | -0.33 | 0.27 | 0.707 |
| DTHW | 0.81 (0.33) | 0.91 (0.37) | 12% ↑ | -0.25 | 0.06 | 0.182 | 0.72 (0.26) | 0.85 (0.27) | 18% ↑ | -0.51 | 0.25 | 0.285 | |
| STFW | 1.27 (0.42) | 1.39 (0.40) | 9% ↑ | -0.24 | -0.01 | .010* | 1.21 (0.28) | 1.22 (0.29) | 1% ↑ | -0.16 | 0.13 | 0.715 | |
| DTFW | 1.11(0.32) | 1.32 (0.38) | 19% ↑ | -0.4 | -0.03 | .031* | 1.04(0.29) | 1.07 (0.30) | 3% ↑ | -0.12 | 0.08 | 0.411 | |
| Stride (m) | STHW | 1.08 (0.30) | 1.13(0.27) | 13% ↑ | -0.1 | -0.01 | .023* | 1.17 (0.22) | 1.21 (0.23) | 3% ↑ | -0.2 | 0.13 | 0.424 |
| DTHW | 1.04 (0.34) | 1.09 (0.29) | 5% ↑ | -0.14 | 0.04 | 0.247 | 1.01 (0.19) | 1.10 (0.26) | 9% ↑ | -0.31 | 0.13 | 0.224 | |
| STFW | 1.21 (0.28) | 1.26 (0.30) | 4%↑ | -0.12 | 0.01 | 0.058 | 1.26 (0.25) | 1.27 (0.26) | 1% ↑ | -0.12 | 0.1 | 0.768 | |
| DTFW | 1.17 (0.19) | 1.27 (0.25) | 9% ↑ | -0.2 | -0.01 | .037* | 1.19 (0.26) | 1.20 (0.30) | .2% ↓ | -0.04 | 0.03 | 0.555 | |
| Cadence (steps/min) | STHW | 50.2 (6.92) | 52.5 (8.61) | 4% ↑ | -5.76 | 1.12 | 0.155 | 50.8 (4.64) | 49.7 (8.64) | 2% ↓ | -14.9 | 17.1 | 0.799 |
| DTHW | 45.9 (8.12) | 48.5(10.12) | 6% ↑ | -8.48 | 3.3 | 0.333 | 42.4 (9.59) | 45.6 (7.4) | 8% ↑ | -14.2 | 7.7 | 0.334 | |
| STFW | 61.3 (7.96) | 65.4 (8.05) | 7% ↑ | -9.39 | 1.26 | 0.107 | 57.2 (5.3) | 57.3 (6.5) | 0% ↑ | -2.1 | 1.9 | 0.878 | |
| DTFW | 55.7 (10.6) | 61.1(7.83) | 10% ↑ | -10.8 | 0.04 | 0.051 | 51.6 (6.8) | 53.4 (6.4) | 3% ↑ | -8.1 | 4.7 | 0.369 | |
| Double Support (%) | STHW | 27.7 (7.6) | 25.1 (7.5) | 10%↓ | -1.3 | 6.5 | 0.164 | 22.7 (2.2) | 23.3 (4.9) | 3% ↑ | -10.3 | 8.9 | 0.796 |
| DTHW | 32.2 (13.1) | 27.7 (8.7) | 14% ↓ | -1.3 | 10.1 | 0.111 | 29.0 (7.5) | 26.0 (4.6) | 10% ↓ | -11.3 | 17.2 | 0.469 | |
| STFW | 19.5 (4.7) | 16.9 (5.4) | 13% ↓ | -2 | 7.3 | 0.203 | 20.1 (5.5) | 17.9 (2.3) | 11% ↓ | -.6.2 | 10.5 | 0.382 | |
| DTFW | 21.8 (4.8) | 19.6 (6.0) | 10% ↓ | -1.5 | 6.1 | 0.186 | 24.3 (5.4) | 19.9 (2.6) | 18% ↓ | -.8.0 | 16.7 | 0.269 | |
| Midswing (degree/s) | STHW | 273 (93.8) | 287 (95.9) | 6% ↑ | -31.4 | 2.6 | 0.085 | 292 (52.3) | 304 (80.3) | 4% ↑ | -111 | 88 | 0.673 |
| DTHW | 250 (89.4) | 259 (92.6) | 4% ↑ | -43.8 | 26.2 | 0.57 | 230 (69.6) | 260 (56.4) | 13% ↑ | -119 | 60 | 0.288 | |
| STFW | 343 (111) | 365 (87.9) | 6% ↑ | -40.4 | -2.6 | .033* | 337 (62.8) | 354 (65.7) | 5% ↑ | -60 | 25 | 0.224 | |
| DTFW | 305 (88.1) | 345 (81.9) | 13% ↑ | -81.4 | 1.2 | 0.055 | 297 (60.4) | 318 (56.4) | 7% ↑ | -55 | 12 | 0.11 | |
STHW = single-task habitual walking, DTHW = dual-task habitual walking, STFW = single-task fast walking, DTFW = dual-task fast walking. CI = Confidence Intervals.
* indicates a significant difference (ANCOVA, p<0.05).
↓ indicates a reduction and,
↑ indicates an increase in each parameter following the treatment.
Fig 2. Changes in gait speed pre- and post-treatment in the intervention group (blue color) and control group (orange color) during single-task habitual, dual-task habitual, single-task fast and dual-task fast walking conditions.
Mean values and standard errors are illustrated. Only the values for those parameters, which achieved statistical significant level were illustrated.
UPDRS and subjective reports
As demonstrated in one of our previous reports [15], there was an overall improvement (S2 Table) within experimental group in mentation, behavior, and mood (UPDRS I 49%, p<0.01), in the activity of daily living (UPDRS II, 46%, p<0.0001) and motor examination (UPDRS III, 40%, p<0.01), whereas, these changes were small and non-significant in the controls (p>0.21). Although changes were not significant, short FES-I and VAS scores were reduced by 15% (p = 0.41) and 44% (p = 0.26), respectively, following treatment in the experimental group. Comparison of response to treatment between experimental and control groups showed significant improvement (See S1 Table in UPDRS I (effect size 1.82, p = 0.005), UPDRS II (effect size 1.49, p = 0.02) and UPDRS III (effect size 3.30, p<0.001).
Correlations
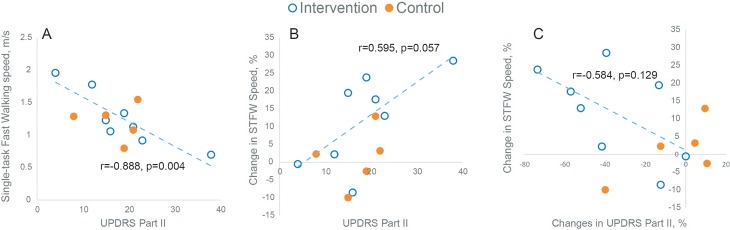
Negative correlation was observed in the intervention (real EA) group between gait speed and UPDRS scores at both baseline and follow-up with the strongest correlation between UPDRS part II and gait speed during single task fast walking condition (r = -0.888, p = 0.004, Fig 3A). A positive correlation was observed between changes in gait speed in response to EA intervention and baseline UPDRS score with the strongest correlation during single task fast walking condition (r = 0.595, p = 0.057, Fig 3B), indicating that patients with more impairment in activity of daily living may benefit more from EA treatment. Results also suggest that enhancement in gait speed in response to EA treatment, specifically during STFW, is associated with enhancement in UPDRS score, in particular for UPDRS Part II (r = -0.584, p = 0.129). However, this association didn’t achieve statistical significant level, probably due to small sample size. A negative correlation between gait speed improvement during STHW and age (r = -0.585, p = 0.049) was observed in the experimental group. No other significant correlation was observed between magnitude of gait improvement in response of EA treatment and baseline (H&Y) staging, or MMSE (p>0.153).
Fig 3.
(A) Association between baseline gait speed during Single Task Fast Walking (STFW) condition and baseline UPDRS Part II. The negative correlation suggests that those with poor UPDRS score walk slower in the intervention (real EA) group; (B) Association between changes in magnitude of gait speed during STFW in response to EA treatment and baseline UPDRS Part II. The positive correlation suggests that those with poor baseline UPDRS score may benefit more from EA treatment. (C) Association between changes in magnitude of gait speed during STFW and changes in magnitude of UPDRS. Negative correlation suggests that enhancement in gait speed in response to EA treatment is associated with enhancement in UPDRS score.
Discussion
To the best of our knowledge this is the first participants- and assessors- masked randomized control study that investigated potential benefits of EA in enhancing spatio-temporal parameters of gait in PD patients as objectively measured using wearable sensor technology. By comparing a group of patients who received real EA to those in the sham control, we found that patients in the experimental group significantly improved in gait performance after completion of the 3-week intervention. Placebo effects have been known to be caused by increased patient-physician contact or patient belief in the effectiveness of treatment, and have been postulated to relate to insufficient masking. To address these issues, we utilized sham acupuncture and masking of the patients as well as the assessors. The patients in both groups are naïve to acupuncture prior to this study. All patients received identical contact from the research team, standardized treatment and objective gait measurements. By adhering to these measures, we attempted to exclude placebo effects in the patients and contamination by the assessors. Therefore, these beneficial effects of the selected acupuncture regimen found within experimental group (comparison of pre and post EA) and between experimental and control groups can be regarded as specific to the treatment.
The results indicated an overall beneficial impact on different spatio-temporal parameters of gait such as gait speed and stride length as well as gait parameters related to dynamic postural control such as increased midswing angular velocity and decreased double support. The later results suggest an enhanced efficiency of balance control during gait [24] and are in agreement with our previous report that EA has beneficial effects on balance [15]. Moreover, evidence suggested that patients with PD usually develop cognitive decline and other non-motor problems that impair their ability to deal with multiple simultaneous tasks and revealed the insufficiency of compensatory motor mechanisms [16, 25]. Accordingly, our data showed that gait performance improved during single-task and more importantly during dual-task walking. Finally the correlation analysis suggests that patients with more impairment in activity of daily living may benefit more from EA treatment. Our clinical assessment also revealed improvement in motor and non-motor signs and symptoms, as well as subjective report of improved activity of daily living by using UPDRS and questionnaires.
Some of key well-known caveats of the clinical scales such as the UPDRS are its rater and time-of-assessment dependency. The use of wearable sensor technology could address these shortcomings by objectively measuring motor performance changes in response to treatment. The key advantages of wearable technologies are their ability to capture gait and balance characteristics irrespective of environment of test (in clinic or home) outside of gait laboratory environment with less time consumption (usually less than 20 minutes). Our results suggested that motor performance measured using wearable technologies are sensitive enough to track treatment responses to alternative therapies. Using objective modalities instead of subjective tools to evaluate treatment response to alternative therapies are particularly important when exclusion of placebo effect is difficult. All the gait measurements using body worn sensors in this study were performed in a clinical setting within a short duration of time and were well tolerated by PD patients.
Acupuncture, an alternative medicine methodology that originated in ancient China, treats patients using various techniques, including inserting small, thin needles at specific points in the body. EA, as the name implies, combines classical acupuncture and low electric current running through the needles, in order to enhance a treatment. Compared with manual manipulation in classical acupuncture, electric manipulation in EA can be standardized by selecting the type, frequency and intensity of stimulation. In the study reported here, a novel standardized EA regimen, with a focus on improving gait and balance, has been developed based on the principles of Traditional Chinese Medicine (TCM), pathophysiology of gait in PD, previous studies [13, 26–29], and our clinical experience.
The exact mechanisms behind the beneficial effects of EA in gait disorder are unclear. However, evidence from PD animal models [30, 31] suggested that acupuncture may enhance neurotransmission, trophic factor release, reduction of apoptotic and oxidative damages, improvement of synaptic plasticity and modulation of activity in the basal ganglia circuits. Moreover, human studies with functional magnetic resonance imaging (fMRI) have shown that acupuncture can influence brain networks by activating the precentral gyrus and prefrontal cortex in PD [31, 32].
We believe the beneficial effects of EA on gait observed in this study are, at least partially, due to improved motor (e.g. rigidity) and mental function after EA. In addition, EA could also improve gait through directly modulating brain networks such as locomotor and balance networks, as well as the cortex for higher-level gait disorder. Additional investigation with modern technology such as fMRI and diffusion tensor imaging could provide further insights into the mechanisms involved.
While fewer acupuncture points have been used in previously published PD related studies, in the present study we selected a total of 20 points (9 paired points and 2 single points). The procedure was well tolerated by all subjects with no serious adverse effects. No participant discontinued the study after initial consent was given. Our zero dropout rate and low adverse effects highlight the safety and feasibility of EA in PD.
There are limitations in this study: First, this pilot study has a small sample size. Although there was a trend for superior efficacy of real EA, a clinical trial with larger sample with objective randomization and further control (e.g., different freezing gait subtypes) is needed to confirm the benefits of the described intervention. Second, in this study, the number of treatment sessions was based on the combination of expected minimum treatments required in our practice and a realistic assessment of the number of sessions that a participant could attend without posing an undue hardship. It is possible that different regimens such as more frequent and/or longer treatment periods might provide more robust effect of benefits. In addition, the decision to use pre-set protocols was made in an attempt to standardize the treatment. However, the potential drawback of this approach is a possible loss in the efficacy of the acupuncture treatment due to a lack of individualized, comprehensive treatments in the way that acupuncture is traditionally performed. Third, the duration of improvements observed in gait performance was rather short after the EA treatment. Our ongoing research is extending EA therapy to ascertain whether or not there will be more sustained long term effects. Finally, randomization was done by drawing pieces of paper from a bag, which may not be fully objective. A more objective randomization order (e.g., ordering of the subjects in alphabetical order, and assigning them to treatment and control, respectively, by a random number generator with seed option) is recommended for future studies.
Conclusions
This study provides an objective proof of concept for potential benefits of non-pharmaceutical-based EA therapy on enhancing gait in patients with PD. EA used in this study is simple, safe, and effective as an alternative and complementary treatment for gait disorders in PD.
Supporting Information
(DOC)
(PDF)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Duncan RP, Leddy AL, Cavanaugh JT, Dibble LE, Ellis TD, Ford MP, et al. Comparative utility of the BESTest, mini-BESTest, and brief-BESTest for predicting falls in individuals with Parkinson disease: a cohort study. Phys Ther. 2013;93(4):542–50. Epub 2012/11/24. 10.2522/ptj.20120302 ptj.20120302 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabli D, Karachi C, Welter ML, Lau B, Hirsch EC, Vidailhet M, et al. Normal and pathological gait: what we learn from Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83(10):979–85. Epub 2012/07/04. 10.1136/jnnp-2012-302263 jnnp-2012-302263 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toosizadeh N, Mohler J, Lei H, Parvaneh S, Sherman S, Najafi B. Motor Performance Assessment in Parkinson’s Disease: Association between Objective In-Clinic, Objective In-Home, and Subjective/Semi-Objective Measures. 2015. [DOI] [PMC free article] [PubMed]
- 4.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19(8):871–84. Epub 2004/08/10. 10.1002/mds.20115 . [DOI] [PubMed] [Google Scholar]
- 5.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–83. Epub 2014/04/24. 10.1001/jama.2014.3654 1861807 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6.Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790–6. Epub 2008/03/26. 10.1002/mds.21879 . [DOI] [PubMed] [Google Scholar]
- 7.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson's disease: influence of initial stance conditions. Neurosci Lett. 2006;406(1–2):128–32. Epub 2006/08/12. doi: S0304-3940(06)00719-1 [pii] 10.1016/j.neulet.2006.07.027 . [DOI] [PubMed] [Google Scholar]
- 8.Lokk J, Nilsson M. Frequency, type and factors associated with the use of complementary and alternative medicine in patients with Parkinson's disease at a neurological outpatient clinic. Parkinsonism Relat Disord. 2010;16(8):540–4. Epub 2010/07/27. 10.1016/j.parkreldis.2010.06.007 . [DOI] [PubMed] [Google Scholar]
- 9.Rajendran PR, Thompson RE, Reich SG. The use of alternative therapies by patients with Parkinson's disease. Neurology. 2001;57(5):790–4. Epub 2001/09/12. . [DOI] [PubMed] [Google Scholar]
- 10.Shulman LM, Wen X, Weiner WJ, Bateman D, Minagar A, Duncan R, et al. Acupuncture therapy for the symptoms of Parkinson's disease. Mov Disord. 2002;17(4):799–802. Epub 2002/09/05. 10.1002/mds.10134 . [DOI] [PubMed] [Google Scholar]
- 11.Lee MS, Shin BC, Kong JC, Ernst E. Effectiveness of acupuncture for Parkinson's disease: a systematic review. Mov Disord. 2008;23(11):1505–15. Epub 2008/07/12. 10.1002/mds.21993 . [DOI] [PubMed] [Google Scholar]
- 12.Cristian A, Katz M, Cutrone E, Walker RH. Evaluation of acupuncture in the treatment of Parkinson's disease: a double-blind pilot study. Mov Disord. 2005;20(9):1185–8. Epub 2005/05/11. 10.1002/mds.20503 . [DOI] [PubMed] [Google Scholar]
- 13.Cho SY, Shim SR, Rhee HY, Park HJ, Jung WS, Moon SK, et al. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson's disease. Parkinsonism Relat Disord. 2012;18(8):948–52. Epub 2012/05/29. 10.1016/j.parkreldis.2012.04.030 S1353-8020(12)00176-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 14.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson's disease using body-worn sensors. Mov Disord. 2013;28(11):1544–51. Epub 2013/10/18. 10.1002/mds.25684 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toosizadeh N, Lei H, Schwenk M, Sherman SJ, Sternberg E, Mohler J, et al. Does integrative medicine enhance balance in aging adults? Proof of concept for the benefit of electroacupuncture therapy in Parkinson's disease. Gerontology. 2015;61(1):3–14. Epub 2014/10/25. 10.1159/000363442 000363442 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller RL, Van Winkle EP, Anderson KE, Gruber-Baldini AL, Hill T, Zampieri C, et al. Dual task performance in Parkinson's disease: a sensitive predictor of impairment and disability. Parkinsonism Relat Disord. 2013;19(3):325–8. Epub 2012/12/26. 10.1016/j.parkreldis.2012.11.011 S1353-8020(12)00444-0 [pii]. . [DOI] [PubMed] [Google Scholar]
- 17.Joh TH, Park HJ, Kim SN, Lee H. Recent development of acupuncture on Parkinson's disease. Neurological research. 2010;32 Suppl 1:5–9. Epub 2010/02/06. 10.1179/016164109X12537002793643 . [DOI] [PubMed] [Google Scholar]
- 18.Evans AH, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, et al. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19(4):397–405. Epub 2004/04/13. 10.1002/mds.20045 . [DOI] [PubMed] [Google Scholar]
- 19.Wullner U, Kassubek J, Odin P, Schwarz M, Naumann M, Hack HJ, et al. Transdermal rotigotine for the perioperative management of Parkinson's disease. J Neural Transm. 2010;117(7):855–9. Epub 2010/06/11. 10.1007/s00702-010-0425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conditions. NCCfC. Parkinson’s disease: national clinical guideline for diagnosis and management in primary and secondary care.2006. [PubMed]
- 21.Aminian K, Najafi B, Büla C, Leyvraz P-F, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. Journal of biomechanics. 2002;35(5):689–99. [DOI] [PubMed] [Google Scholar]
- 22.Grewal G, Bharara M, Menzies R, Talal T, Armstrong D, Najafi B. Diabetic peripheral neuropathy and gait: does footwear modify this association? Journal of diabetes science and technology. 2012;7(5):1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciencies. New York: Routledge; 1988. [Google Scholar]
- 24.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70(6):340–7. Epub 1990/06/01. . [DOI] [PubMed] [Google Scholar]
- 25.Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson's disease: clinical update and pathophysiology. Curr Opin Neurol. 2008;21(4):461–71. Epub 2008/07/09. 10.1097/WCO.0b013e328305bdaf 00019052-200808000-00013 [pii]. . [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Pan Y, Xue B, Wang X, Zhao F, Jia J, et al. The antioxidative effect of electro-acupuncture in a mouse model of Parkinson's disease. PLoS ONE. 2011;6(5):e19790 Epub 2011/06/01. 10.1371/journal.pone.0019790 PONE-D-11-00740 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauer K, Wendt I, Schwenk M, Rohr C, Oster P, Greten J. Stimulation of acupoint ST-34 acutely improves gait performance in geriatric patients during rehabilitation: A randomized controlled trial. Arch Phys Med Rehabil. 2011;92(1):7–14. Epub 2010/12/29. 10.1016/j.apmr.2010.09.023 S0003-9993(10)00800-2 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28.Yeo S, Lim S, Choe IH, Choi YG, Chung KC, Jahng GH, et al. Acupuncture stimulation on GB34 activates neural responses associated with Parkinson's disease. CNS Neurosci Ther. 2012;18(9):781–90. Epub 2012/09/05. 10.1111/j.1755-5949.2012.00363.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui G, Guangjian Z, Yong W, Jie F, Yanchao C, Xi J, et al. High frequency electro-acupuncture enhances striatum DAT and D1 receptor expression, but decreases D2 receptor level in 6-OHDA lesioned rats. Behav Brain Res. 2013;237:263–9. Epub 2012/10/06. 10.1016/j.bbr.2012.09.047 S0166-4328(12)00633-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 30.Zeng BY, Salvage S, Jenner P. Current development of acupuncture research in Parkinson's disease. Int Rev Neurobiol. 2013;111:141–58. Epub 2013/11/13. 10.1016/B978-0-12-411545-3.00007-9B9 78-0-12-411545-3.00007–9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31.Chae Y, Chang DS, Lee SH, Jung WM, Lee IS, Jackson S, et al. Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 2013;14(3):215–22. Epub 2013/02/12. 10.1016/j.jpain.2012.11.011 S1526-5900(12)00961-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 32.Yeo S, Choe IH, van den Noort M, Bosch P, Jahng GH, Rosen B, et al. Acupuncture on GB34 activates the precentral gyrus and prefrontal cortex in Parkinson's disease. BMC Complement Altern Med. 2014;14:336 Epub 2014/09/16. 10.1186/1472-6882-14-336 1472-6882-14-336 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.