Abstract
Pylephlebitis, or suppurative thrombophlebitis of the portal mesenteric venous system occurring in the setting of abdominal inflammatory processes, is a rare but deadly disease commonly associated with diverticulitis. We review our institutional experience in the management of patients with this condition. A retrospective review of medical records from 2002 to 2012 was performed. Patients with a portal mesenteric vein thrombosis (PMVT) within 30 days of an intra-abdominal inflammatory process were identified and evaluated. Ninety-five patients were included. The mean patient age at presentation was 57 years (range, 24–88). The most common associated processes were pancreatitis (31 %), followed by diverticulitis (19 %). Bacteremia was noted in 34 (44 %) patients. The most common organism cultured was Streptococcus viridans. Antibiotic and anticoagulation therapy was given in 86 (91 %) and 78 (82 %) patients, respectively. Overall, we report an 11 % mortality rate. Albeit rare, pylephlebitis most commonly was manifested in the setting of pancreatitis. Treatment should be individualized to culture results and extent of thrombosis. If diagnosed early and managed appropriately, a favorable outcome is possible.
Keywords: Mesenteric thrombosis, Pancreatitis, Portal vein thrombosis, Pylephlebitis, Sepsis
Introduction
Pylephlebitis, a condition characterized by suppurative thrombosis of the portal vein, is a rare and deadly complication of intra-abdominal infections.1,2 The condition was first described in 1846 by Waller, who discovered it as the source of a hepatic abscess during autopsy.3 Despite major advances in therapies over the past century, mortality rates remain high. Delays in diagnosis and management can complicate the condition, leading to a mortality rate as high as 25 % despite use of modern diagnostic imaging such as ultrasonography and computed tomography (CT) scans.4,5 A general consensus on the incidence and management of this infection is not clear in the literature, as experience is only limited to case reports, but has been estimated to be 2.7 per 100,000 person-years.4,5
Pylephlebitis results from an uncontrolled infection in the regions neighboring or drained by the portal system. Initially starting out as thrombophlebitis of small mesenteric veins, the process can spread to the portal venous system and hematogenously to the liver. Thrombosis of the mesenteric veins subsequently can lead to mesenteric ischemia, infarction, and bowel necrosis. Prior to the advent of antibiotics, pylephlebitis was most commonly seen in the setting of appendicitis; now, however, diverticulitis has become the most common cause, followed by appendicitis, inflammatory bowel disease, and other intra-abdominal infections.1,6–9 Additional risk factors for pylephlebitis include hypercoagulable states or clotting factor deficiencies.10 Albeit rare, pylephlebitis has also been reported as a complication of hemorrhoidal banding, silicone gastric banding, and CT-guided liver biopsy.11–13
The difficulty in managing pylephlebitis stems from its elusive diagnosis, as it presents with relatively nonspecific symptoms such as malaise, fever, abdominal tenderness, and nausea. Advanced stages may not be apparent until jaundice manifests due to hepatic involvement. A high clinical suspicion is of paramount importance, as presentation varies widely and diagnosis is often delayed.14 We report on our institutional experience in the management of patients with pylephlebitis and seek to examine our practice in treating this cohort. Furthermore, we present the limitations in the understanding of this disease process and propose modalities that need to be further explored to understand this phenomenon in greater depth.
Methods
Approval was obtained from our Mayo Clinic Institutional Review Board to review patient records from 2002 to 2012. Since no ICD-9 code exists for pylephlebitis, a comprehensive search was necessary and performed using a data discovery and query builder to find all patients with the ICD-9 code for portal vein thrombosis (code 452). Once this data set was obtained, it was refined by using ICD-9 codes of coexisting intra-abdominal inflammatory processes within this cohort, which included acute appendicitis with peritoneal abscess (code 540.1); appendicitis (codes 540, 541, 542); diverticulitis (codes 562.01, 562.10, 562.11, 562.13); pancreatitis (code 577); cholecystitis (codes 575, 575.10); peritonitis (codes 567, 567.1, 567.29, 567.89, 567.9); cholangitis (576.1); hepatic abscess (code 572); and duodenal ulcer (code 532). Individual review of the selected patient medical records was undertaken to ensure the accuracy of the diagnosis. Patients were excluded if they had a prior history of hepatobiliary malignancies, prior splenectomy, prior transjugular intrahepatic shunt procedure, hepatic transplant, or chronic portovenous thrombosis.
Diagnosis of Pylephlebitis
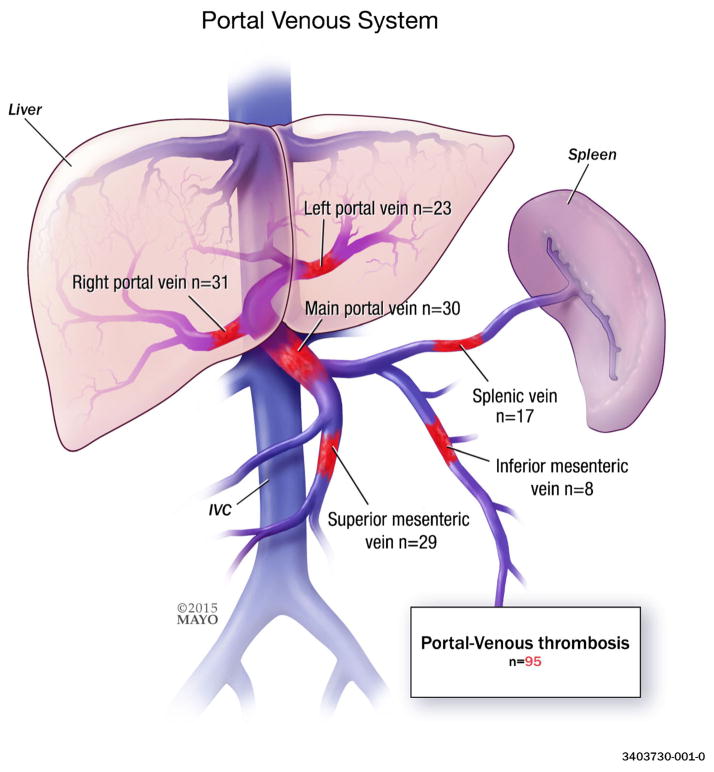
The diagnosis of pylephlebitis is challenging; short of aspiration of culture-positive fluid from the portal system, the diagnosis must be made by other clinical means. We defined pylephlebitis as the presence of portal mesenteric venous thrombosis (PMVT) with or without bacteremia within 30 days of intra-abdominal inflammatory processes, as defined by ICD-9 codes above. Typical features of PMVT on color duplex sonography include a flow defect and dilation or absent compressibility of the portal venous system. Computed tomography (CT) and magnetic resonance imaging commonly show a distinctive central hypodense portal-mesenteric-venous thrombosis compared to the peripheral hyperdense contrast media.9 Images were reviewed to confirm presence of PMVT. Data collected from the electronic medical records included patient demographics, risk factors, abdominal source, culture results, site(s) of thrombotic event(s), method(s) of diagnoses, and treatment modalities including type of antibiotic and/or anticoagulation therapy, complications, and outcomes. For the purpose of this study, anatomic sites of portal system thrombotic involvement were divided into 6 categories: (1) right portal veins, (2) left portal veins, (3) main portal vein, (4) superior mesenteric vein, (5) inferior mesenteric vein, and (6) splenic vein. Data are presented as percentages or mean±standard deviation, as appropriate. Statistical analysis included the χ2 test; a P value less than .05 was considered significant. Analysis was performed using JMP v. 9.0 (SAS Institute, Inc., Cary, NC).
Results
Patient Demographics and Etiology
A total of 95 patients met inclusion criteria, 57 (60 %) of whom were men. Mean age was 57 years ±16 (range, 24–88 years). Leading causes of pylephlebitis included pancreatitis, 31 % (n=29) and diverticulitis, 19 % (n=18) (Table 1). Thirty-two percent of patients had 1 or more risk factors for pylephlebitis (Table 2). The most common site of thrombosis was the right portal vein, 33 % (n=31) (Table 3, Fig. 1).
Table 1.
Etiology
| Etiology | Overall, No. (%) |
|---|---|
| Pancreatitis | 29 (31) |
| Diverticulitis | 18 (19) |
| Peritonitis | 14 (15) |
| Intra-abdominal abscess | 12 (13) |
| Cholangitis | 7 (7) |
| Cholecystitis | 7 (7) |
| Appendicitis | 2 (2) |
| Inflammatory bowel disease | 2 (2) |
| Hepatic abscess | 2 (2) |
| Duodenal ulcer | 1 (1) |
| Idiopathic | 1 (1) |
Table 2.
Risk factors
| Risk factors | No. (%) |
|---|---|
| Smoking | 28 (29) |
| Prior abdominal operations | 28 (29) |
| Antiplatelet | 25 (26) |
| Steroid use | 18 (19) |
| Malignancy | 16 (17) |
| Coronary artery disease | 15 (16) |
| Connective tissue disease | 14 (15) |
| Cirrhosis | 12 (13) |
| Hypercoagulable state | 5 (5) |
| Deep vein thrombosis | 3 (3) |
| Peripheral vascular disease | 3 (3) |
| Anticoagulation | 2 (2) |
| Prior cerebrovascular accident | 1 (1) |
Table 3.
Site of thrombosis
| Site of thrombosis | No. (%) involveda |
|---|---|
| Right portal vein | 31 (33) |
| Main portal vein | 30 (32) |
| Superior mesenteric vein | 29 (31) |
| Left portal vein | 23 (24) |
| Splenic vein | 17 (18) |
| Inferior mesenteric vein | 8 (8) |
Two or more sites may be involved in each category; as such, the total may be greater than the total number of patients
Fig. 1.
Most common site(s) of thrombosis
Diagnosis
A total of 90 patients (95 %) underwent CT imaging (Table 4). Blood cultures were drawn in 76 patients (80 %) and were positive in 34 (44 %). The most common organism was Streptococcus viridans in 8 (24 %), followed by Escherichia coli in 7 (21 %), and Bacteroides fragilis in 4 (12 %). Eight patients (24 %) had polymicrobial infections (Table 5). Twelve patients had intra-abdominal abscesses as the initial septic focus leading to pylephlebitis; none of these had polymicrobial bacteremia; 5 patients with pancreatic necrosis had polymicrobial bacteremia.
Table 4.
Imaging modality used to detect portal mesenteric venous thrombosis (PVMT)
| Imaging modality used | No. (%) |
|---|---|
| Ultrasound | 48 (51) |
| Computed tomography scan | 90 (95) |
| Magnetic resonance imaging | 17 (18) |
Table 5.
Blood cultures
| No. (%) | |
|---|---|
| Patients in whom blood cultures were drawn | 76 (80) |
| Bacteremia (positive blood cultures) | 34 (44) |
| Culture results | |
| Polymicrobial infection of any kind | 8 (24) |
| Streptococcus Viridans | 8 (24) |
| Escherichia coli | 7 (21) |
| Bacteroides fragilis | 4 (12) |
| Streptococcus Anginosus | 3 (8) |
Treatment
Antibiotic treatment was used in 86 patients (91 %), 66 (77 %) of whom were treated with 2 or more antibiotics. Anticoagulation therapy was used in 78 patients (82 %), with warfarin as the most common (n=62; 79 %). Sixty-four patients (67 %) were given both anticoagulation and antibiotic therapy (Table 6). When used, anticoagulation therapy was prescribed for median duration of 143 days±121. Two patients were managed conservatively without use of antibiotics or anticoagulation therapy as they cleared their septic foci early on in the course of their disease without need for intervention. No patient in our study group received thrombolytic therapy, operative exploration, or angiography to evacuate the portal venous septic thrombi.
Table 6.
Treatment
| Type of therapy used | Mortality
|
|
|---|---|---|
| No, No. (%) | Yes, No. (%) | |
| Antibiotics alone | 12 (13) | 3 (3) |
| Anticoagulants alone | 7 (7) | 0 |
| Both anticoagulant and antibiotic therapy | 64 (67) | 7 (7) |
| Neither antibiotic nor anticoagulant therapy | 2 (3) | 0 |
| Total | 85 | 10 |
Outcomes
Twenty patients in our study group developed complications from pylephlebitis, the most common of which was chronic thrombosis noted in 11 patients (12 %), followed by bowel ischemia in 4 (4 %). Only one of the four patients with bowel ischemia underwent surgical resection. The overall mortality rate was 11 % (Table 7). There was a similar rate of death when antibiotics were used in conjunction, or in isolation of, anticoagulation therapy (10 vs 20 %, P=.265). Autopsy was performed in 3 out of the 10 patients who died; 1 patient died as a result of a complication from a perihepatic abscess, whereas the other 2 died from complications of necrotizing pancreatitis. Of note, the overall sources of infection in the 10 cases which resulted in mortality are as follows: pancreatitis (4), peritonitis (3), intra-abdominal abscess (2), and cholangitis (1).
Table 7.
Complications and outcomes
| No. (%) | |
|---|---|
| Total complications | 19 (20) |
| Chronic thrombosis | 11 (12) |
| Bowel ischemia | 4 (4) |
| Hepatic abscess | 2 (2) |
| Hepatic infarction | 1 (1) |
| Splenic infarction | 1 (1) |
| Outcomes | |
| Recovery | 85 (89) |
| Death | 10 (11) |
Discussion
To the best of our knowledge, we report the largest institutional case series on pylephlebitis. Analysis of 95 cases at our institution showed that pylephlebitis can present in any age group in the adult population and is associated with multiple possible causes. As compared to the majority of existing literature showing diverticulitis and appendicitis as the most common causes of pylephlebitis, pancreatitis was the leading etiology in our study. As a tertiary referral center with expertise in pancreatic and hepatobiliary conditions, our institution tends to see a disproportionate number of patients with these conditions. As such, our results may not be reflective of the population at large and be generalizable to other centers with dissimilar referral patterns. It is also plausible that earlier diagnosis and treatment of diverticulitis and appendicitis in the high detection CT era may signify a shift in the susceptibility of the patient population affected by pylephlebitis.
The diagnosis of pylephlebitis relies on the demonstration of PMVT in the setting of suppurative bacteremia through aspiration of the portal vein. Given the invasiveness of this procedure, it is rarely performed. As a result, the use of surrogate markers such as the patient’s clinical condition, imaging, and culture results are used. Pylephlebitis commonly presents with relatively nonspecific symptoms including fatigue, fever, abdominal pain, nausea and vomiting, diarrhea, and anorexia.8 More advanced signs include hepatomegaly and jaundice, as disseminated hepatic involvement may result. Leukocytosis may be a common early finding, but both normal and decreased white blood cell counts have been noted in the literature.15–18 Thus, imaging studies demonstrating a thrombosis in the portal system are often regarded as appropriate surrogates in diagnosing this condition. Portal-venous phase imaging is the diagnostic modality of choice in an acute setting not only because of its ease of use and quality of clinical data, but also because of its ability to detect presence of sequelae such as a hepatic abscesses or intestinal ischemia if present.19 The regular use of CT imaging at our institution may have facilitated the search for occult sources of inflammation and enabled a more accurate diagnosis of pylephlebitis.
We noted the right and main portal veins to be common sites of involvement of thrombosis. An expert case analysis by Baril et al.20 echoed similar findings, showing the main portal system to be a more common site of thrombotic involvement relative to the mesenteric veins. Studies advise that blood cultures be drawn in patients with suspected pylephlebitis; however, cultures will not always demonstrate microorganism grown as case reports have documented bacteremia ranging from 23 to 88 %.15,20 Our findings showed presence of bacteremia in 44 % of patients where blood cultures were drawn. Infection in pylephlebitis is commonly polymicrobial, but when a single microorganism is cultured, it is most frequently B. fragilis.8 Bacteroides has been linked to pylephlebitis due to its facilitation of the coagulation cascade via its production of transient anticardiolipin antibodies which break down heparin, along with the presence of its capsular components that accelerate fibrin cross-linking.21,22 In contrast, our study noted S. viridans to be the most common microorganism cultured from blood samples. Kanellopoulou et al.8 noted a similar comparison, mentioning the Streptococcus spp. to be a leading isolate on culture results in his review of 100 pylephlebitis cases in the literature. Certain risk factors have been attributed to portal vein thrombosis, such as recent abdominal surgery, malignancy, hereditary thrombotic conditions, and patient immobility.4 We found many of these risk factors in our study, along with demonstration of other risk factors, such as smoking, antiplatelet and steroid use, cirrhosis, connective tissue disorders, peripheral vascular disease, and history of venous thrombosis.23
Appendicitis-associated pylephlebitis was commonly regarded as universally fatal in the preantibiotic era. As management has improved with the use of antibiotics and surgery to control the infection, there has been a noted decline in cases of appendicitis leading to mortality as a result of pylephlebitis.8,24 Many studies advocate the use of broad-spectrum antibiotics in the management of pylephlebitis, even in the absence of positive bacteremia.8,15,20 Current recommendations state that antibiotic therapy should be used in pylephlebitis for up to 4 or 6 weeks in the presence of a liver abscess.15 Randomized controlled studies evaluating empiric antibiotic regimens in the treatment of pylephlebitis have not been performed, and thus, antibiotic utilization coverage should be based on culture results when available.
The pathogenesis of appendicitis and other bowel-derived causes of pylephlebitis likely differ from pancreatitis. Given the limitations in the definition of pylephlebitis, however, there is no direct way to distinguish between pylephlebitis and portal vein thrombosis as a direct extension of pancreatitis. We suspect that the etiologies are likely different (i.e., antegrade inflammation leading to PMVT versus local pancreatic inflammation leading to PMVT). Still, an accurate timeline of events with the use of imaging would greatly discern between the two causes of PMVT.
The use of anticoagulation therapy in the management of pylephlebitis has been a controversial topic. As experience is limited to case reports, studies showing statistical significance in favor of or against the use of anticoagulation treatment are not present, resulting in much speculation. When used, the aim of anticoagulation is to reverse or prevent propagation of thrombosis and further complications. Plemmons et al.15 noted a 100 % survival rate among patients who received anticoagulation with heparin compared to 60 % survival among those who were not anticoagulated, though statistical significance was not reached. Kanellopoulou et al.8 noted that the early use of anticoagulation in portal vein thrombosis may minimize serious sequelae and speed up recanalization. Our results are concordant; we noted a lower mortality rate than that reported in the literature, which we believe can partly be attributed to early use of anticoagulation therapy. Of course, the risks of anticoagulation must be weighed against the potential benefits. There is no general consensus on duration of anticoagulation therapy use; certain studies suggest 3 to 6 months of anticoagulation treatment if no other underlying thrombotic disease is present.25 As yet, the end point of anticoagulation therapy remains elusive.
Invasive techniques such as systemic thrombolysis, catheter directed thrombolysis, and thrombectomy were not used in our cohort.14 Several case reports and small series have shown favorable outcomes, however, with thrombolysis. Given the need for multiple operations and/or percutaneous procedures, the patient population under study carried a high risk of hemorrhagic complications; thus, no patient in our series was managed in this manner. Surgical thrombectomy has been associated with higher recurrence rates of thrombosis, and thus, some studies have advised against such measures.8,25
Conclusion
In conclusion, to date, we report on the largest case series on pylephlebitis showing the lowest mortality rate currently presented in literature. We recommend early use of antibiotics and anticoagulation therapy in the course of disease concurrent with source control of the abdominal infection.
Footnotes
Presented in part at Digestive Disease Week, 2014
Level of Evidence: III
Disclosures This publication was made possible by CTSA Grant Number UL1 TR000135 and KL2 TR000136 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflict of Interest The authors declare that they have no conflicts of interest.
References
- 1.Wong K, Weisman DS, Patrice KA. Pylephlebitis: a rare complication of an intra-abdominal infection. Journal of community hospital internal medicine perspectives. 2013;3(2) doi: 10.3402/jchimp.v3i2.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh P, Yadav N, Visvalingam V, et al. Pylephlebitis—diagnosis and management. The American Journal of Gastroenterology. 2001;96(4):1312–1313. doi: 10.1111/j.1572-0241.2001.03736.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolt RJ. Diseases of the hepatic blood vessels. In: Bockus HL, editor. Gastroenterology. 4. Philadelphia: WB Saunders; 1985. pp. 3259–3277. [Google Scholar]
- 4.Abraham MN, Mathiason MA, Kallies KJ, et al. Portomesenteric venous thrombosis: a community hospital experience with 103 consecutive patients. The American Journal of Surgery. 2011;202(6):759–764. doi: 10.1016/j.amjsurg.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Acosta S, Alhadad A, Svensson P, et al. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. The British journal of surgery. 2008;95(10):1245–51. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 6.Lee BK, Ryu HH. A case of pylephlebitis secondary to cecal diverticulitis. The Journal of emergency medicine. 2012;42(4):e81–5. doi: 10.1016/j.jemermed.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Baddley JW, Singh D, Correa P, et al. Crohn’s disease presenting as septic thrombophlebitis of the portal vein (pylephlebitis): case report and review of the literature. The American journal of gastroenterology. 1999;94(3):847–9. doi: 10.1111/j.1572-0241.1999.00959.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanellopoulou T, Alexopoulou A, Theodossiades G, et al. Pylephlebitis: an overview of non-cirrhotic cases and factors related to outcome. Scandinavian journal of infectious diseases. 2010;42(11–12):804–11. doi: 10.3109/00365548.2010.508464. [DOI] [PubMed] [Google Scholar]
- 9.Falkowski AL, Cathomas G, Zerz A, et al. Pylephlebitis of a variant mesenteric vein complicating sigmoid diverticulitis. Journal of radiology case reports. 2014;8(2):37–45. doi: 10.3941/jrcr.v8i2.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rea JD, Jundt JP, Jamison RL. Pylephlebitis: keep it in your differential diagnosis. American journal of surgery. 2010;200(6):e69–71. doi: 10.1016/j.amjsurg.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Chau N, Bhatia S, Raman M. Pylephlebitis and pyogenic liver abscesses: A complication of hemorrhoidal banding. Canadian Journal of Gastroenterology & Hepatology. 2007;21(9):601–603. doi: 10.1155/2007/106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Roover A, Detry O, Coimbra C, et al. Pylephlebitis of the Portal Vein Complicating Intragastric Migration of an Adjustable Gastric Band. Obesity Surgery. 2006;16(3):369–371. doi: 10.1381/096089206776116363. [DOI] [PubMed] [Google Scholar]
- 13.Tandon R, Davidoff A, Worthington MG, et al. Pylephlebitis After CT-Guided Percutaneous Liver Biopsy. American Journal of Roentgenology. 2005;184(3_supplement):S70–S72. doi: 10.2214/ajr.184.3_supplement.01840s70. [DOI] [PubMed] [Google Scholar]
- 14.James AW, Rabl C, Westphalen AC, et al. Portomesenteric venous thrombosis after laparoscopic surgery: A systematic literature review. Archives of Surgery. 2009;144(6):520–526. doi: 10.1001/archsurg.2009.81. [DOI] [PubMed] [Google Scholar]
- 15.Plemmons RM, Dooley DP, Longfield RN. Septic Thrombophlebitis of the Portal Vein (Pylephlebitis): Diagnosis and Management in the Modern Era. Clinical Infectious Diseases. 1995;21(5):1114–1120. doi: 10.1093/clinids/21.5.1114. [DOI] [PubMed] [Google Scholar]
- 16.Dean JW, Trerotola SO, Harris VJ, et al. Percutaneous management of suppurative pylephlebitis. Journal of vascular and interventional radiology : JVIR. 1995;6(4):585–8. doi: 10.1016/s1051-0443(95)71141-1. [DOI] [PubMed] [Google Scholar]
- 17.Farin P, Paajanen H, Miettinen P. Intraoperative US diagnosis of pylephlebitis (portal vein thrombosis) as a complication of appendicitis: a case report. Abdominal imaging. 1997;22(4):401–3. doi: 10.1007/s002619900220. [DOI] [PubMed] [Google Scholar]
- 18.Duffy FJ, Jr, Millan MT, Schoetz DJ, Jr, et al. Suppurative pylephlebitis and pylethrombosis: the role of anticoagulation. The American surgeon. 1995;61(12):1041–4. [PubMed] [Google Scholar]
- 19.Castro R, Fernandes T, Oliveira MI, et al. Acute appendicitis complicated by pylephlebitis: a case report. Case reports in radiology. 2013;2013:627521. doi: 10.1155/2013/627521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baril N, Wren S, Radin R, et al. The role of anticoagulation in pylephlebitis. The American Journal of Surgery. 1996;172(5):449–453. doi: 10.1016/S0002-9610(96)00220-6. [DOI] [PubMed] [Google Scholar]
- 21.Sjobring U, Ringdahl U, Ruggeri ZM. Induction of platelet thrombi by bacteria and antibodies. Blood. 2002;100(13):4470–7. doi: 10.1182/blood-2002-01-0069. [DOI] [PubMed] [Google Scholar]
- 22.Bjornson HS, Hill EO. Bacteroidaceae in thromboembolic disease: effects of cell wall components on blood coagulation in vivo and in vitro. Infection and immunity. 1973;8(6):911–8. doi: 10.1128/iai.8.6.911-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. Journal of vascular surgery. 1994;20(5):688–97. doi: 10.1016/s0741-5214(94)70155-5. [DOI] [PubMed] [Google Scholar]
- 24.Garrett A, Carnish E, Das N, et al. Once Universally Fatal: Pylephlebitis. The American Journal of Medicine. 2014;127(7):595–597. doi: 10.1016/j.amjmed.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Allaix M, Krane M, Zoccali M, et al. Postoperative Portomesenteric Venous Thrombosis: Lessons Learned From 1,069 Consecutive Laparoscopic Colorectal Resections. World Journal of Surgery. 2014;38(4):976–984. doi: 10.1007/s00268-013-2336-7. [DOI] [PubMed] [Google Scholar]