Abstract
Objective
To better assess the increased utilization of multiparametric magnetic resonance imaging (mpMRI) and fusion biopsy of the prostate, we compared prostate cancer detection rates among (a) men undergoing MR-ultrasound (US) fusion biopsy, (b) mpMRI cognitive-registration biopsy, and (c) conventional transrectal US-guided biopsy for the detection of prostate cancer.
Materials and Methods
We present a retrospective review of consecutive patients undergoing mpMRI of the prostate with subsequent prostate biopsy from October 2013 to September 2015. Lesions concerning for prostate cancer visualized on mpMRI were targeted with cognitive-registration or MR-US fusion biopsies. A cohort of men undergoing conventional prostate biopsy was utilized for comparison. Rates of cancer detection were compared among the 3 cohorts.
Results
A total of 231 patients underwent mpMRI-targeted biopsy (81 fusion, 150 cognitive). There was no difference in prostate specific antigen, mpMRI-defined Prostate Imaging Reporting and Data System score or number of lesions, or history of prostate cancer among the cohorts. The overall detection rate of cancer was significantly higher in the fusion cohort (48.1%) compared with both the cognitive (34.6% P = .04) and conventional (32.0%, P = .03) cohorts. Cancer detection rates were comparable in the MRI-cognitive and transrectal prostate US biopsy groups (34.6% vs 32%). MR fusion detected significantly more Gleason ≥7 cancer (61.5 vs 37.5%, P = .04) and significantly less Gleason 6 cancer (38.5 vs 62.5%, P = .04) compared with conventional biopsy.
Conclusion
Targeted biopsy of the prostate using MR-US fusion increased the cancer detection rate compared with both cognitive registration and conventional biopsy and was associated with detection of higher-grade cancer compared with conventional biopsy.
There has been a significant reduction in screening for prostate cancer in the US since the introduction of the United States Preventative Services Task Force grade D rating.1-6 One of the many criticisms of the current screening paradigm is overdiagnosis and subsequently overtreatment. Therefore, increased efforts have focused on the ability to predict the clinical behavior of prostate cancer, which can range from an indolent tumor that may not develop into clinically significant disease to an aggressive tumors with the potential to cause suffering and death.
Advances in magnetic resonance imaging (MRI) have combined conventional anatomic MRI with functional sequences, known as multiparametric MRI (mpMRI) that provides greater capability to localize regions of cancer in the prostate. Further, targeted biopsy using annotated mpMRI increases real-time functional information of prostate tissue and improves biopsy guidance of prostatic lesions.7,8 Imaging-directed biopsies can be performed using 2 approaches: cognitive registration, in which the operator biopsy lesions were predefined by radiologists according to the Prostate Imaging Reporting and Data System (PI-RADS), or software registration, in which the operator deploys biopsies under realtime fusion of transrectal prostate ultrasound (TRUS) with radiologist-annotated MR images using one of several commercially available platforms.
These technologies are quickly becoming an important component of the clinical algorithm of early prostate-cancer detection for urologists. Unfortunately, those critical of fusion biopsy technologies describe the technique as potentially more costly and time intensive than conventional TRUS biopsy without well-defined diagnostic benefits. Several studies have assessed head-to-head comparisons of different biopsy modalities, and the majority of these studies have emanated from institutions directly involved in development and testing of MRI-US fusion technologies; very few have completed institutional reviews of multiple different techniques from the same cohort.
The objective of this study is to test the hypothesis that mpMRI-US fusion targeted biopsy has a higher rate of overall and high-risk prostate-cancer detection than both cognitive-registration and conventional biopsy techniques. We compared prostate cancer detection rates among (a) men undergoing MR-US fusion biopsy, (b) mpMRI followed by cognitive-registration biopsy, and (c) conventional TRUS-guided biopsy for the detection of prostate cancer.
Methods
Study Design
All subjects were enrolled in a prospective database of patients undergoing mpMRI of the prostate at Northwestern Memorial Hospital, Chicago, Illinois. The Institutional Review Board approved this study (Study #00200770) and was Health Insurance Portability and Accountability Act compliant. All consecutive men who underwent mpMRI from October 2013 to September 2015 were retrospectively identified. The subgroup of men who underwent subsequent 12-core TRUS biopsy was then classified. Inclusion criteria for this study were an abnormal screening test with prostate-specific antigen (PSA) or digital rectal examination, and active surveillance of prostate cancer. Exclusion criteria included those men with history of radical prostatectomy or radiation therapy to the prostate. Rates of cancer detection were compared with a randomly selected cohort of 100 men undergoing conventional sextant 12-core TRUS biopsy for prostate-cancer screening or active surveillance during the same time interval. Board-certified urologists under local or monitored-anesthesia care performed all biopsies.
Imaging
All patients underwent mpMRI on 3.0-T scanners (Skyra or Verio, Siemens Medical System, Erlangen, Germany) with triplanar T2-weighted, axial dynamic contrast-enhanced, diffusion-weighted imaging, according to previously designed protocols.9 These mpMRI studies underwent blinded, dedicated urologic radiologist evaluation and lesions were assigned suspicion scores based on the standardized PI-RADS criteria scoring system: low (PI-RADS 1-2), intermediate (PI-RADS 3), and high (PI-RADS 4 of higher).9,10 Two highly experienced genitourinary radiologists (D.C. and F.M.) with significant experience in interpreting prostate MRI performed independent reviews of all studies and formed consensus reads when indicated.
Biopsy Protocol
For patients undergoing pre-biopsy mpMRI, the images were segmented, and dedicated genitourinary radiologists using the UroNav, Philips-Invivo platform recorded lesion locations. Patients with lesions identified on mpMRI underwent a targeted biopsy and standard 12-core biopsy performed by 1 of 3 urologists. All biopsies were performed transrectally under ultrasound guidance. One to 3 cores from each radiologist-defined lesion were collected into individual containers before evaluation by a dedicated genitourinary pathologist. The targeted biopsy was performed with the previously identified mpMRI lesions superimposed using the T2-weighted sequence on the real-time TRUS image. Each lesion was sampled both in axial and sagittal planes by a side-fire TRUS probe (Philips). Patients who underwent cognitive-registration biopsies had pre-biopsy mpMRI segmented by dedicated genitourinary radiologists, marking the location of areas of suspicion with corresponding PIRADS scores. The urologist then reviewed the suspicious lesions at the time of cognitive biopsy. The standard TRUS biopsy included at least 12 cores, collected in an extended sextant template of biopsies from the lateral and medial aspects of the base, mid, and apical prostate on the left and right sides. Only the TRUS imaging was used for the standard biopsy portion of the case. Two dedicated genitourinary pathologists reviewed all the pathologic specimens.
Data Analysis
The criteria described by the Standards of Reporting for MRI-Targeted Biopsies Studies (START) of the Prostate were followed in reporting this study.11 Patients were categorized by pathologic Gleason grade as clinically insignificant (Gleason 6) or clinically significant (Gleason 7 or higher). In cases where conventional or targeted biopsy detected more than 1 tumor focus, the highest Gleason score reported was used to define the risk category established by that approach.
The primary outcome was the overall detection of all cancers. Secondary outcome was detection of clinically significant cancer.
Statistical Methods
Reported statistical significance levels were all 2-sided, and the threshold of statistical significance was P <.05. Analysis of variance was used for comparing the distribution of continuous variables between the cohorts. Fisher's exact test was used to compare proportions of categorical variables. All statistical computations were performed using the SPSS statistics package 22.0 (IBM Corp, Armonk, NY).
Results
Over the 22-month study period, 1118 mpMRI of the prostate were performed. Sixty percent of all mpMRI were performed for prostate cancer screening including elevated PSA (60%) and abnormal digital rectal examination (4%). The remaining 37% were performed for radiologic or surgical intervention, clinical staging, or biochemical recurrence. Following mpMRI for abnormal screening results, 35% (231 out of 670) of men elected to undergo a prostate biopsy, either by cognitive registration (65%) or MRI-US fusion biopsy (35%, Fig. 1). Patient demographics are shown in Table 1. There was no significant difference in age, ethnicity, PSA at the time of biopsy, or history of prostate cancer among the 3 patient groups. In those who underwent mpMRI, there was no difference in the clinical suspicion for cancer based on mpMRI lesion grade or number of lesions. Significantly more patients in the MR-US fusion cohort had undergone prior biopsy procedures than in both the cognitive registration and conventional biopsy groups (13.5% vs 36%, 40%, respectively, P <.001). The median times from mpMRI to biopsy in the MRI-US fusion cohort were 40 days (interquartile range, 13-89 days) and 59 days (interquartile range, 20-90 days) in the cognitive cohort.
Figure 1.
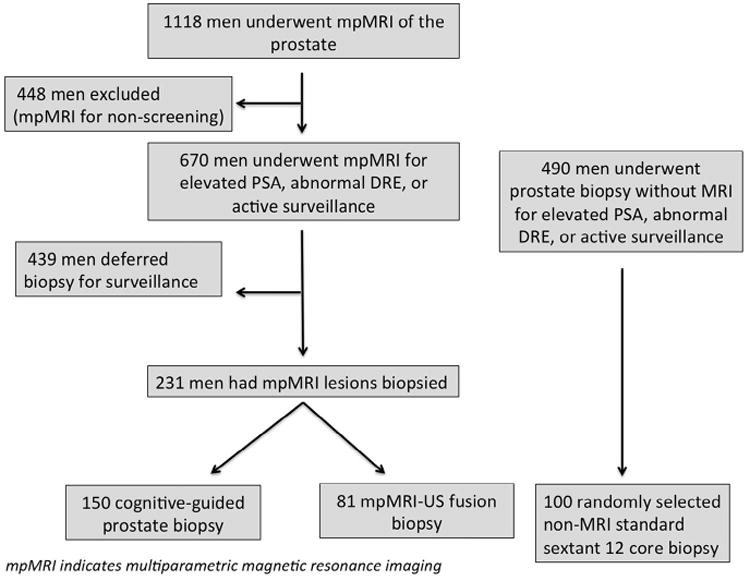
Flow chart of patients who met inclusion or exclusion criteria for the study population.
Table 1. Patient demographics among conventional sextant, cognitive registration, and MR-US fusion biopsy cohorts.
| Conventional | Cognitive Registration | MR-US Fusion | P-Value | |
|---|---|---|---|---|
| No. of patients | 100 | 150 | 81 | |
| Mean age, years (SD) | 66.0 (9.0) | 64.9 (8.1) | 64.3 (8.2) | .90 |
| Mean PSA, ng/mL (SD) | 4.2 (4) | 4.26 (4.5) | 5.1 (5) | .71 |
| Mean lesion count on MR (SD) | 1.2 (0.5) | 1.4 (0.6) | .62 | |
| MR grade (PI-RADS) | .46 | |||
| Low suspicion (1-2) | 22.1% (32) | 17.2% (14) | ||
| Intermediate (3) | 40.2% (61) | 39.5% (32) | ||
| High suspicion (4-5) | 38.2% (57) | 43.2% (35) | ||
| Prior biopsies | <.01 | |||
| 0 | 40% (40) | 36% (54) | 13.5% (11) | |
| 1 | 35% (35) | 43% (60) | 45.6% (37) | |
| 2 or greater | 25% (25) | 21% (31) | 37.0% (30) | |
| History of prostate cancer | 13% (13) | 13.4% (20) | 11.1% (9) | .69 |
MR, magnetic resonance; MR-US, magnetic resonance-ultrasound; PI-RADS, Prostate Imaging Reporting and Data System; PSA, prostate-specific antigen.
The overall prostate cancer detection rate was significantly higher in the fusion cohort (48.1%) than in both cognitive (34.6%, P = .04) and conventional biopsy (32.0%, P = .03). The use of MR without fusion biopsy did not increase the cancer detection rate compared with conventional biopsy (34.6% vs 32%, P = .87, Table 2).
Table 2. Biopsy results of convention 12-core extended sextant transrectal biopsy versus magnetic resonance-ultrasound fusion transrectal biopsy.
| Conventional | MR-US Fusion | P-Value | |
|---|---|---|---|
| Number of cores (interquartile range) | 12.1 (11.5-13) | 15.2 (13-18) | .54 |
| Prostate-cancer detection | 32% (32 of 100) | 48.1% (39 of 81) | .03 |
| Gleason score | |||
| 6 | 62.5% (20) | 38.5% (15) | .12 |
| 7 or higher | 37.5% (12) | 61.5% (24) | .04 |
Abbreviation as in Table 1.
When compared with conventional TRUS biopsy, MRUS fusion detected significantly more Gleason grade 7-10 cancer (61.5 vs 37.5%, respectively, P = .04) and significantly less Gleason 6 disease (38.4 vs 62.5%, P = .04, Table 2). There was no difference in detection of clinically significant prostate cancer between MR-US fusion and cognitive registration biopsies (Table 3).
Table 3. Biopsy results of cognitive registration versus magnetic resonance-ultrasound fusion transrectal biopsy.
| Cognitive Registration | MR-US Fusion | P-Value | |
|---|---|---|---|
| Number of cores (interquartile range) | 14.2 (12-16) | 15.2 (13-18) | .65 |
| Prostate-cancer detection | 34.6% (52 of 150) | 48.1% (39 of 81) | .04 |
| Gleason score | |||
| 6 | 53.8% (27) | 38.5% (15) | .11 |
| 7 or higher | 48.0% (25) | 61.5% (24) | .07 |
| Duration between magnetic resonance and biopsy | 59.2 | 40.1 | .61 |
Abbreviation as in Table 1.
Discussion
Increasing scrutiny of prostate-cancer screening has led to important changes in screening guidelines and even efforts to discourage PSA screening. For example, as part of a federal effort to define and reward quality in healthcare services, Medicare officials are considering a measure that would penalize physicians who order routine prostate-cancer screening tests for their patients.12 This has led to cumulative efforts to improve the detection of clinically significant prostate cancer and avoid overtreatment of clinically insignificant disease. Based on findings that the localization of both overall and high-grade or clinically significant tumors is improved with MRI guidance, there has been tremendous interest in the use of MRI to avoid unnecessary biopsies and improve the overall diagnostic yield.13 To our knowledge, this study is the first to perform a real-time comparison of 3 prostate biopsy modalities: MRI-US fusion, cognitive registration, and conventional sextant biopsy. In this study, MR-US fusion biopsy significantly increased the overall detection of prostate cancer when compared with both MR cognitive registration and conventional sextant 12-core TRUS-guided biopsy as well as the detection of clinically significant prostate cancer when compared with conventional TRUS-guided biopsies. The improvement in cancer detection of MR fusion of 16% in our study is comparable with other studies reported in the literature.13-15 In addition, we found that MR-US fusion decreased the overall detection of low-risk prostate cancer compared with conventional biopsy in similar patient cohorts.
The detection of clinically significant cancer was 24% higher with MRI-US fusion–targeted biopsy compared with conventional TRUS biopsy. These findings reinforce the results from the National Cancer Institute (Moore et al.) that mpMRI has the potential to increase the detection of high-risk prostate cancer and decreased detection of low-risk prostate cancer.11 Our study was unable to show a diagnostic benefit of targeted biopsy without fusion technologies compared with conventional biopsy.
MRI-US targeted biopsy could significantly change the distribution of risk in men newly diagnosed with prostate cancer by increasing the detection of clinically significant prostate cancer. Our study is consistent with similar reports in the literature that show the ability for MRI-guided biopsy to improve detection of prostate cancer compared with TRUS sextant biopsy.16,17
Our study is not without limitations. Because different operators performed MR-US fusion biopsies and visually directed biopsies, there may exist a work-up or operator bias with regard to the study population and cancer detection. Although the total number of biopsies performed was not statistically different, the total number of biopsies performed in the fusion cohort was clinically higher than other modalities. Even a relatively small increase in sampling could contribute to a higher cancer-detection rate. In our study, Gleason score was utilized to define the clinically significant versus insignificant prostate cancer. The most appropriate definition of clinically significant cancer for targeted biopsy has yet to be defined and, therefore, is subject to criticism and may make comparison with similar studies more difficult. Finally, our study is relatively small and lacks the level of evidence provided by a randomized trial. Despite the potential benefits to risk stratification and improved prostate-cancer detection, this study is preliminary in nature and does not contribute to assessment of benefit toward clinical endpoints including recurrence of disease and cancer-specific mortality.
Conclusion
Targeted biopsy of the prostate using MR-US fusion significantly increased the overall detection of prostate cancer when compared with both cognitive registration and conventional sextant biopsy as well as the detection of clinically significant prostate cancer when compared with conventional TRUS-guided biopsy. Additional randomized studies are needed to determine the appropriate clinical utilization of mpMRI for detection of prostate cancer.
Footnotes
Financial Disclosure: William Catalona is a consultant of Beckman Coulter, Inc. and PlatformQ Health, Inc. The remaining authors declare that they have no relevant financial interests.
References
- 1.Tabayoyong W, Abouassaly R. Prostate cancer screening and the associated controversy. Surg Clin North Am. 2015;95:1023–1039. doi: 10.1016/j.suc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 2.McGinley KF, McMahon GC, Brown GA. Impact of the US preventive services task force Grade D recommendation: assessment of evaluations for elevated prostate-specific antigen and prostate biopsies in a large urology group practice following statement revision. Rev Urol. 2015;17:171–177. [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Berkowitz Z, Hall IJ. Decrease in prostate cancer testing following the US Preventive Services Task Force (USPSTF) recommendations. J Am Board Fam Med. 2015;28:491–493. doi: 10.3122/jabfm.2015.04.150062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Nelson JB. Patients present with more advanced prostate cancer since the USPSTF screening recommendations. J Urol. 2015;194:1534–1536. doi: 10.1016/j.juro.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF Grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194:1587–1593. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 7.Recabal P, Ehdaie B. The role of MRI in active surveillance for men with localized prostate cancer. Curr Opin Urol. 2015;25:504–509. doi: 10.1097/MOU.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 8.Appayya MB, Johnston EW, Punwani S. The role of multi-parametric MRI in loco-regional staging of men diagnosed with early prostate cancer. Curr Opin Urol. 2015;25:510–517. doi: 10.1097/MOU.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 9.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging–reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544–552. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Beck M. Doctors could be penalized for ordering prostate tests. Wall St J. Available at: http://www.wsj.com/articles/doctors-could-be-penalized-for-ordering-prostate-tests-1447993920.
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendhiratta N, Rosenkrantz AB, Meng X, et al. Magnetic resonance imaging-ultrasound fusion targeted prostate biopsy in a consecutive cohort of men with no previous biopsy: reduction of over detection through improved risk stratification. J Urol. 2015;194:1601–1606. doi: 10.1016/j.juro.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 15.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound fusion–targeted prostate biopsy in men with previous negative biopsies: impact on repeat biopsy strategies. Urology. 2015;86:1192–1199. doi: 10.1016/j.urology.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannelli G, Caivano R, Rago L, et al. Diffusion-weighted magnetic resonance imaging in patients with prostate cancer treated with radiotherapy. Tumori. 2015 doi: 10.5301/tj.5000415. Epub ahead of print, September 4th, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]


