Abstract
Purpose of review
The endothelial cell plasma membrane is a metabolically active, dynamic and fluid microenvironment where pericellular proteolysis plays a critical role. Membrane-anchored proteases may be expressed by endothelial cells, as well as mural cells and leukocytes with distribution both inside and outside of the vascular system. Here we will review the recent advances in our understanding of the direct and indirect roles of membrane-anchored proteases in vascular biology and the possible conservation of their extravascular functions in endothelial cell biology.
Recent findings
Membrane-anchored proteases belonging to the serine or metalloprotease families contain amino- or carboxy-terminal domains which serve to tether their extracellular protease domains directly at the plasma membrane. This architecture enables protease function and substrate repertoire to be regulated through dynamic localization in distinct areas of the cell membrane. These proteases are proving to be key components of the cell machinery for regulating vascular permeability, generation of vasoactive peptides, receptor tyrosine kinase transactivation, extracellular matrix proteolysis and angiogenesis.
Summary
A complex picture is emerging of the interdependence between membrane-anchored protease localization and function that may provide a mechanism for precise coordination of extracellular signals and intracellular responses through communication with the cytoskeleton and with cellular signaling molecules.
Keywords: membrane-anchored serine protease, MT-MMP, ADAM, ACE, pro-protein convertase
Introduction
Endothelial cells regulate communication between the blood and the subendothelial tissues critical for a wide range of important physiological processes including hemostasis, the transport of solutes and macromolecules, regulation of vasomotor tone, angiogenesis, and the trafficking of leukocytes for innate and adaptive immunity [1]. The endothelial cell plasma membrane is a metabolically active, dynamic and fluid microenvironment where pericellular proteolysis plays a critical role. For many years, investigative studies have largely focused on the proteolytic enzymes that mediate pro-coagulant and anti-coagulant activities, given the critical importance of endothelial cells in maintaining hemostatic balance. The activities of secreted, pericellular coagulation proteases (e.g. thrombin, Factor Xa), the plasminogen system (e.g. tPA, uPA and plasmin) and the matrix metalloproteinases (MMPs) are well studied in this regard. Recently there has been an increase in the identification of plasma membrane-tethered proteases, such as the family of membrane-anchored serine proteases, the ADAM family, and the membrane-type MMPs (MT-MMPs), whose direct and indirect roles in vascular functions, blood vessel growth, development and structure are becoming evident through studies of murine deficiencies and dysregulation in human diseases (Table 1). The membrane-anchored proteases are widely distributed both inside and outside of the vascular system and may be expressed by endothelial cells, mural cells, and/or leukocytes. Membrane anchorage enables these proteases to initiate pericellular proteolysis in the cell microenvironment, and to interact with membrane proteins on the same cells or on nearby cells. The specific functions of many of these proteases in vascular biology, and in some cases, even their expression by endothelial and mural cells, are poorly understood. In this review, we will focus on the current knowledge of membrane-anchored proteases in vascular biology with an emphasis on recent findings, and the possible conservation of their extravascular functions in endothelial cell biology.
Table I.
Membrane-anchored proteases associated with endothelial and vascular biology and their functions.
| Protease | Common Aliases | Gene Symbol | Membrane Anchor | In Vivo Vascular and Extravascular Functions | Ref. |
|---|---|---|---|---|---|
| Serine Proteases | |||||
| Membrane-anchored Serine Proteases | |||||
| Prostasin | PRSS8, CAP1 | PRSS8 | GPI | Epidermal deficiency causes defective barrier function in mice. Tissue specific deletion in mice shows reduced fluid clearance in lung and colonic epithelium due to defects in ENaC activation. Liver specific knock-out mice develop insulin resistance associated with associated with reduced hepatic TLR-4 shedding. Implicated in blood pressure regulation in humans. | [2–8] |
| Matriptase | MT-SP1, CAP3, ST14, PRSS14, epithin | ST14 | Type II | Null and hypomorphic mice show defective global epithelial barrier function due to increased paracellular permeability. Activates the c-Met ligand pro-HGF/SF when over expressed in keratinocytes in mice. | [9–11] |
| Matriptase-2 | TMPRSS6 | TMPRSS6 | Type II | Essential for iron homeostasis by cleavage of hemajuvelin, prevents iron-refractory iron deficiency anemia in humans. Over-expression in human vascular endothelial cells inhibits tumor induced angiogenesis in a murine xenograft model. | [12,13] |
| Corin | LRP4, ATC2, TMPRSS10 | CORIN | Type II | Important regulator of blood pressure and volume via the activation of natriuretic peptides in cardiomyocytes. Promotes uterine spiral artery remodeling during pregnancy. | [14–16] |
| Proprotein convertases | |||||
| Furin | Paired basic amino acid cleaving enzyme, FUR, PACE, SPC1, PCSK3 | FURIN | Type I | Null mice are embryonic lethal due to a failure of ventral closure and large blood vessel development. Endothelial cell specific knockout mice die due to cardiovascular dysfunction. | [17,18] |
| Prolyl oligopeptidases (POP) | |||||
| DPPIV | Dipeptidyl peptidase 4, CD26 | DPP4 | Type II | Regulates vascular endothelial cell function. Inactivates stromal cell-derived factor-1 (SDF-1), a key regulator of vascular stem cell homing. Inhibition lowers blood pressure, decreases cardiovascular disease and improves outcomes after myocardial infarction in mice. | [19–22] |
| Metalloproteases | |||||
| Membrane-type | |||||
| MT1-MMP | MMP14, MT-MMP1 | MMP14 | Type I | Null mice show reduced vascular invasion of cartilage and defective corneal angiogenesis in response to basic fibroblast growth factor. | [23–25] |
| MT4-MMP | MMP17, MT-MMP4 | MMP17 | GPI | Deficiency predisposes mice to aortic aneurysm due to dysfunctional vascular smooth muscle cells. Loss of expression in patients with hereditary thoracic aortic aneurysms increases susceptibility to aneurysm rupture. | [26**] |
| ADAM Proteases | |||||
| ADAM8 | CD156, cell surface antigen MS2, human leukocyte differentiation antigen | ADAM8 | Type I | Null mice show increased branching and vessel density in pathological retinal neovascularization assays. | [27–29] |
| ADAM9 | meltrin gamma, cellular disintegrin- related protein | ADAM9 | Type I | Murine deficiency inhibits pathological retinal neovascularization in oxygen-induced retinopathy and laser-induced choroidal models. | [27,30] |
| ADAM10 | CD156c, kuzbanian protein homolog, mammalian disintegrin- metalloprotease | ADAM10 | Type I | Murine deficiency is embryonic lethal due to defective blood vessel development. Endothelial cell specific deficiency, or inhibition of ADAM10, causes increased pathological retinal neovascularization following oxygen-induced retinopathy. | [27,31, 32*] |
| ADAM12 | Meltrin alpha, metalloprotease- disintegrin 12 transmembrane | ADAM12 | Type I | Knockdown of ADAM12 in mice inhibits recovery from experimental peripheral artery disease. Inhibition of ADAM12 in mice reduces hypoxia-induced impairment of neural vascular barrier function. | [2,27,33,34**] |
| ADAM15 | Metargidin, a disintegrin and metalloproteinase domain 15 | ADAM15 | Type I | Null mice or mice carrying an active site mutation, show decreased pathological retinal neovascularization following oxygen-induced retinopathy. Null mice show a reduction in the development of atherosclerotic lesions caused by apolipoprotein E-deficiency. Mediates endothelial hyperpermeability during acute lung injury induced by lipopolysaccharide in mice. | [27,35– 37] |
| ADAM17 | TNF-alpha converting enzyme (TACE), ADAM metallopeptidase domain 18 | ADAM17 | Type 1 | Murine deficiency or mice lacking the ADAM17 Zn binding site have defective blood vessel formation causing death. Endothelial cell specific deficiency or ADAM17 inhibition causes reduced pathological retinal neovascularization. Inhibition in mice reduces hypoxia-induced impairment of neural vascular barrier function. | [2,27,32,34,38] |
| ADAM33 | disintegrin and reprolysin metalloproteinase family protein | ADAM33 | Type I | Susceptibility gene for asthma and chronic obstructive pulmonary disorder. Soluble form is found in asthmatic bronchiolar lavage fluid and promotes human endothelial cell differentiation and tube formation in vitro, and neovascularization in the chorioallantoic membrane assay. Also implicated in the pathogenesis of atherosclerosis. | [27,39– 41] |
| Angiotensin- converting enzymes | |||||
| ACE | angiotensin- converting enzyme, ACE1, CD143, carboxycathepsin, dipeptidyl carboxypeptidase 1 | ACE | Type I | Catalyzes the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor causing increased blood pressure. ACE inhibitors are the primary treatment for hypertension. Over-expression is correlated with cardiovascular diseases. | [42,43] |
| ACE2 | angiotensin- converting enzyme 2, ACEH, ACE- related carboxypeptidase | ACE2 | Type I | Acts as a counter-regulatory mechanism to ACE1 by cleaving angiotensin II to angiotensin products with vasodilator and vasoprotective properties. | [44,45] |
Abbreviations: ADAM, a disintegrin and metalloproteinase; ENaC, epithelial sodium channel; MMP, matrix metalloproteinases; MT-MMP, membrane tethered-matrix metalloprotease; pro-HGF/SF, pro-hepatocyte growth factor/scatter factor; PRSS, protease serine S1; PSCK, proprotein convertase subtilisin/kexin type; TLR, toll-like receptor; TMPRSS, transmembrane protease serine S1.
Membrane anchoring domains
The membrane-anchored proteases associated with the vasculature and discussed here all belong to the serine protease or metalloprotease families (Figure 1). The unique feature of these membrane-anchored protease families that distinguishes them from secreted soluble proteases such as MMPs and blood coagulation proteases, is that they contain domains which tether them directly to the cell surface, with their catalytic domains exposed to the extracellular environment. The manner in which they are linked to the cell surface may be through Type I or Type II single pass transmembrane domains, or via glycophosphatidylinositol (GPI)-anchors [47,49–52] (Table 1). All of these membrane-anchored proteases are synthesized with an N-terminal signal sequence which directs their trafficking to the cell surface. Type I membrane-anchored proteases are then anchored via a C-terminal hydrophobic region which spans the plasma membrane. Similarly, GPI anchored proteases possess a C-terminal hydrophobic region, which is modified by the addition of a GPI lipid moiety that anchors the protease to cholesterol rich regions in the plasma membrane. In Type II membrane-anchored proteases the N-terminal signal sequence is not removed after trafficking to the cell surface, and serves to anchor the N-terminus to the cell membrane, with the C-terminal catalytic domain localized to the extracellular space. In comparison to secreted proteases this surface localized context provides a higher degree of specificity in the substrates these proteases will encounter, with a requirement that they be either found co-localized on the cell surface or in the nearby pericellular environment. The type of membrane anchor can serve to target these proteases to specific membrane localizations (eg. apical, basolateral, cell junctions and cell protrusions) and microdomains (eg. lipid rafts), and their cytoplasmic extensions are frequently associated with membrane trafficking and/or signal transduction. In some situations, and frequently in response to inflammatory stimuli, the extracellular domains of membrane-anchored proteases are shed from the cell surface [47,49,53], which may indicate a down-regulation of the proteolytic functions of these enzymes. In addition, other mechanisms for regulation of cell surface protease activities exist, including natural inhibitors such as Kunitz-type inhibitors and serpins for the serine proteases and TIMPs for the metalloproteases, and protease endocytosis and turnover [47,54].
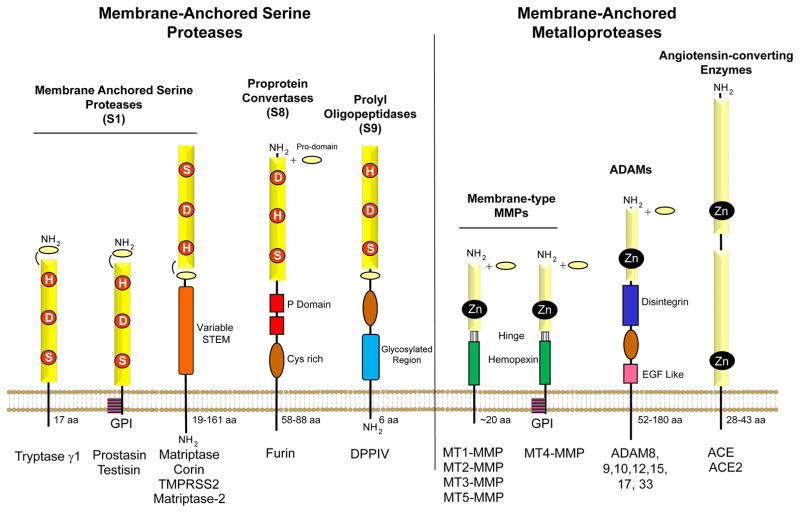
Figure 1. Domain structures of the membrane-anchored serine proteases and metalloproteases associated with endothelial and vascular biology.
The serine-type proteases are comprised of the membrane-anchored serine proteases, the proprotein convertases and the prolyl oligopeptidases. The metalloproteases consist of the membrane-type MMPs (MT-MMPs), the ADAMs (a disintegrin and metalloproteinase) and the angiotensin-converting enzymes (ACE). The catalytic domains, which function to hydrolyze the peptide bonds in protein substrates, are all located extracellularly. The catalytic domains of the membrane-anchored serine proteases are either chymotrypsin-like (S1 family), bacterial subtilisin-like (S8 family) or related to the prolyl oligopeptidases (S9 family). They all share the conserved catalytic triad amino acid residues His, Asp and Ser, albeit in different configurations. There are a total of 17 human type II transmembrane serine proteases, synthesized with a unique stem region adjacent to the plasma membrane, containing diverse protein-protein interaction domains such as scavenger receptor cysteine-rich (SRCR), CUB, SEA and Frizzled domains [46]. There are two human GPI-anchored serine proteases, testisin and prostasin. The proprotein convertases are all Type I transmembrane serine proteases containing a cysteine rich region and conserved P-domains which are essential for folding and activation. The prolyl oligopeptidases are synthesized as type II transmembrane homodimeric glycoproteins. The membrane-anchored metalloproteases differ from the membrane-anchored serine proteases in that they utilize a zinc ion to create a nucleophile for protease catalytic activity. The 6 MT-MMPs expressed in humans are either Type I (MT1-, MT2-, MT3- and MT5-MMP) or GPI-anchored (MT4- and MT6-MMP). All of the MT-MMPs share a similar hinge region and hemopexin domain, and are synthesized with an amino-terminal signal sequence and pro-domain which is cleaved by proprotein convertases during transport from the ER/Golgi to the surface, allowing the MT-MMPs to be expressed on the cell surface as active proteases [47]. The ADAM metalloproteinases share a complex domain structure containing the pro-domain followed by the metalloprotease catalytic domain, a disintegrin-like domain enabling cell-cell interactions via integrins, a cysteine-rich domain and an epidermal growth factor-like domain [48]. The ACE family of metalloproteases includes both ACE and ACE2 transmembrane proteases, which consist of an amino-terminal signal domain followed by a large extracellular domain that consists of either two independent (ACE) or one (ACE2) catalytic domains containing zinc binding motifs. The cytoplasmic extensions vary in length for these membrane-anchored proteases and are involved in membrane trafficking, and localization, with some participating in cell signaling.
Activation of membrane-anchored proteases
Most of the membrane-anchored proteases are synthesized as inactive proteins, which are converted to active protease forms by other proteases, or indirectly activated by cellular stimuli, such as sheer stress, cytokines and growth factors. The serine proteases, with the exception of the prolyl oligopeptidases, require a conformational change which generates an active site following cleavage of a propeptide that remains tethered to the catalytic domain through disulfide linkages [54]. In contrast, the MT-MMPs and the ADAMs are synthesized with a fully functional active site that is shielded by the prodomain until being processed during secretion by proprotein convertases [47,55,56]. Classically ADAMs were thought to be activated by cleavage between the pro- and catalytic domains at a canonical furin cleavage site (RXXR). However recent evidence has identified a second furin cleavage site embedded in the pro-domain that is critical for the activation and catalytic activity of ADAMs 9, 10 and 17 [57]. In addition, ADAM17 requires catalytically inactive members of the rhomboid family of proteases, iRhom1 and iRhom2, to mediate its intracellular transport and maturation, and mutations in iRhom2 may predispose patients to an inherited cancer susceptibility syndrome [58**]. Membrane-anchored serine proteases, such as matriptase, can self-activate or proteolytically activate other precursor serine protease zymogens [54], and further, non-enzymatic stimulation of matriptase activation by prostasin and activation of prostasin by the matriptase zymogen has been reported [59,60], suggesting complex interrelationships between these enzymes.
Ectodomain shedding of cell surface molecules
The ADAM proteases are recognized to play a major role in ectodomain shedding of a number of transmembrane molecules that play pivotal roles in the control of cell adhesion and cell signaling processes [61–65]. ADAM17 is a central regulator of the tumor necrosis factor (TNF) receptor and epidermal growth factor receptor (EGFR) signaling pathways that drive inflammation and injury/repair responses. Although both ADAM17 and ADAM10 have been considered the primary proteases responsible for ectodomain shedding of the Notch receptor during sprouting angiogenesis, a recent study finds that ligand-induced Notch signaling is initiated primarily by ADAM10 [32*]. During this process, ADAM17 appears to promote sprouting angiogenesis by down-regulating the inhibitor of angiogenesis thrombospondin 1 (TSP1) [32*].
Influence of membrane dynamics on substrate accessibility
The actin cytoskeleton is important for controlling membrane-anchored protease localization on the plasma membrane as well as substrate accessibility. Recent studies demonstrate that MT1-MMP and one of its substrates, the heparin-binding adhesion molecule CD44, can co-localize through anchoring to the actin cytoskeleton through ezrin/radixin/moesin (ERM) protein-mediated interactions between their cytoplasmic tails [66]. It remains to be seen whether other membrane proteases and adhesion molecules are linked to the cytoskeleton via intracellular complexes. The cytoskeleton can also mediate substrate accessibility. The transmembrane endothelial adhesion molecule CX3CL1 exists within confinement regions structured by the cortical actin cytoskeleton, which precludes its association with ADAM10. Disruption of the actin cytoskeleton in response to secreted inflammatory cytokines, leukocyte-endothelial cell interactions or changes in mechanical force exerted by the endothelium can reduce CX3CL1 sequestration and increase CX3CL1–ADAM10 interactions, resulting in enhanced shedding of soluble CX3CL1, a chemoattractant for circulating leukocytes [67**].
Influence of membrane microdomains/lipid rafts on membrane-anchored protease functions
A striking feature of the GPI-anchored proteases is their enrichment in lipid raft membrane microdomains. Testisin is a GPI-anchored serine protease expressed in microvascular endothelial cells [68] that cleaves and activates the seven-transmembrane G-protein-coupled protease-activated receptor, PAR-2, to release its tethered ligand for signal activation [69*]. Interestingly, the activation of PAR-2 by testisin induces internalization of PAR-2 and loss from the cell surface [69*], which has the potential to alter PAR-2 signal activation by soluble proteases that may be present in the extracellular microenvironment. PAR-2 can localize to lipid raft domains; indeed localization of tissue factor (TF) and PAR-2 in lipid rafts is critical for TF:FVIIa to trigger PAR-2-mediated cell signaling [70]. Lipid raft localization has also been found to regulate the cleavage specificity of PAR-1 in endothelial cells, rendering PAR-1 shielded from interaction with coagulation proteases [71]. The importance of lipid raft localization to PAR signaling responses after membrane-anchored protease activation is an interesting area for future investigation.
Vascular permeability barrier
Adjacent endothelial cells are linked by multi-protein intercellular junctional complexes composed of tight junctions, adherens junctions and gap junctions which mediate vascular integrity and control vascular permeability across the paracellular pathway [72]. Vascular permeability is controlled by the oligomerization of the membrane-spanning occludin and claudins, including claudin-5, vascular endothelial cadherin (VE-cadherin) and the endothelial junctional adhesion molecule JAM-A, which interact with cytoplasmic zona occuldens (ZO) proteins in intercellular junctional complexes and link to the actin cytoskeleton [73]. Wide variations in permeability exist across the vascular tree, with capillaries being most permeable and the blood brain barrier being relatively impermeable by comparison. Several ADAMs mediate the shedding of cell-cell adhesion molecules to modulate vascular permeability during excessive inflammation and hypoxia [27,74]. Both ADAM12 and ADAM17 were recently reported to contribute to impaired neural vascular barrier function induced by hypoxia through the degradation of claudin-5 in brain microvascular endothelial cells [34**]. Inhibition of either ADAM12 or ADAM17 was sufficient to rescue the loss of barrier function in retinal neural vasculature in vivo under hypoxic conditions. Upon hypoxia induction, it was found that the subcellular localization of ADAM12 and ADAM17 changed rapidly from cytoplasmic to cell membrane-associated, thus enabling a rapid proteolytic response. Inhibition of either ADAM12 or ADAM17 was sufficient to rescue the loss of barrier function in retinal neural vasculature in vivo under hypoxic conditions, suggesting their inhibition could offer novel treatment strategies for diseases associated with an impaired neural barriers. ADAMs may also regulate vascular permeability though mechanisms independent of junction molecule shedding, as ADAM15 is reported to induce hyper-permeability of endothelial barriers through Src/ERK1/2 signaling [75,76]. Further, a recent study has identified that the targeting of the 3' UTR of ADAM15 by the microRNA (miR)-147b attenuates ADAM15 expression. Whether miR-147b and/or other microRNAs regulate additional proteases and may be exploited to enhance endothelial barrier protection is an interesting area for further investigation.
Generation of vasoactive peptides
Several membrane-anchored proteases play key roles in cardiovascular remodeling, hypertension and angiogenesis through the cleavage and activation of vasoactive peptides. Corin present on cardiomyocytes converts the atrial natriuretic peptide (ANP) precursor, pro-ANP, to mature ANP, for the regulation of salt-water balance and blood pressure [15]. ANP also protects the endothelial cell barrier against hyperpermeability through its ability to modulate Rho-dependent actin remodeling [77]. Angiotensin converting enzyme (ACE) and ACE2 produce the vasoactive pro-inflammatory peptides Ang-II and its counter-regulatory peptide Ang-(1–7), respectively [78]. This is important because the balance between ACE/Ang II/AT1 receptor and ACE2/Ang-(1–7)/Mas receptor is critical for vascular homeostasis, especially the regulation of vasoconstriction and proliferation. Overexpression of ACE2 can protect endothelial cell function by promoting endothelial cell migration, and decreasing Ang II induced effects such as pro-inflammatory cytokines and adhesion molecules [79*].
Receptor tyrosine kinase transactivation
Various mechanisms of ligand activation and transactivation of receptor tyrosine kinases depend on membrane anchored proteases. Matriptase is an efficient proteolytic activator of pro-hepatocyte growth factor (HGF), the ligand for the c-Met receptor tyrosine kinase [10,49] that stimulates cell proliferation and promotes activation of pro-angiogenic and vasoprotective pathways in the vasculature [80]. Several of the membrane-anchored proteases, including essentially all of the known ADAMs, shed mature ligands that in turn transactivate receptor tyrosine kinases to promote pro-angiogenic pathways in endothelial cells [27,81–83]. Agonists of multiple G protein-coupled receptors (GPCR) can activate ADAMs to produce mature EGFR ligands that lead to EGFR transactivation; signaling events that may require trafficking and compartmentalization of the GPCR and ADAM providing the temporal and spatial regulation necessary for rapid and specific signal activation [84]. Further, a recent report indicates that heterotrimeric G-proteins may also directly activate membrane tethered proteases in a membrane delimited manner [85*]. MT1-MMP was shown to be activated via a direct binding interaction with activated G-protein βγ subunits, resulting in HB-EGF release and EGFR transactivation [85*]. It remains to be determined whether other membrane-anchored proteases may be activated by the same or different G-proteins in a similarly direct manner.
Membrane regulated and directed ECM proteolysis
During angiogenesis, endothelial cells initiate new blood vessel growth and invasion into the extracellular matrices (ECM). MT1-MMP functions as the major membrane-anchored collagenase, while MT1-MMP, MT2-MMP, and MT3-MMP all have fibrin degrading activities [47,56,86,87]. Shear wall stress stimulates the activation of MT1-MMP by proprotein convertases [88] and its translocation to the plasma membrane at the tips of growing vessels to promote ECM degradation [89]. MT1-MMP and most of the MT-MMPs, with the exception of MT4-MMP, have the demonstrated capability of activating pro-MMP-2 [47,90], which is able to degrade multiple ECM proteins including collagens, fibrin, fibronectin and vitronectin. Studies of MT4-MMP loss-of-function in mice [26**] show that its activity is required for vascular smooth muscle cell maturation and arterial wall function in vivo and its absence results in predisposition to thoracic aortic aneurysms. The phenotype is associated with reduced cleavage of the extracellular matrix protein, osteopontin, important for embryonic development of the aorta. MT1-MMP may also stimulate proMMP-2 activation through paracrine mechanisms. For example, activation of the endothelium by Ang II promotes aortic stiffness by triggering endothelin-1 release, which in turn acts on aortic smooth muscle cells to induce furin up-regulation, leading to increased activation of MT1-MMP and pro-MMP2 [91]. Conversely, in some contexts, MT1-MMP may down-regulate proteolytic activity, for example by proteolytically processing and inactivating MMP-11 [92]. Both hepsin and matriptase can activate latent pro-urokinase, releasing a potent plasmin-initiated proteolytic cascade that results in the degradation of fibrin and several other ECM proteins, activates MMPs, and can induce the release of growth factors like TGF β, bFGF, and VEGF from the ECM, to enhance endothelial cell migration and invasion [93].
Endothelial protease expression
Expression of genes by endothelial cells may be constitutive or inducible, or may be restricted to subsets of endothelial cell phenotypes in the vascular tree. Endothelial cells have been reported to express mRNAs encoding the membrane-anchored serine proteases testisin, hepsin, matriptase, TMPRSS2, dipeptidylpeptidase IV, and seprase [68]. Our analysis of DataSets in the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geoprofiles) reveals that several membrane-anchored proteases with no known roles in vascular biology are differentially regulated in response to various stimuli. In a study characterizing the endothelial response to laminar shear stress, TMPRSS11A and ADAM18 transcripts were found to be upregulated in response to higher shear stress levels [94]. In a separate study, hepsin transcripts were upregulated in endothelial cells isolated from cirrhotic livers in rats and neonatal murine retinal endothelial cells, which also displayed expression of TMPRSS12 [95] [96]. In another study of retinal angiogenesis utilizing laser capture microdissection of endothelial cell tips, matriptase transcripts were elevated nearly two fold as compared to the surrounding tissue [97]. Investigating transcriptional changes during capillary tube formation on a matrigel basement membrane, ADAM19 transcripts were upregulated over time [98]. TMPRSS2 transcript levels were found to be upregulated when comparing proliferative phase endometrial endothelial cells from polycystic ovary syndrome to normal endometrial endothelial cells [99]. Future studies will be required to analyze proteome expression and determine the pathophysiological functions of membrane-anchored proteases not previously associated with endothelium, in vascular functions and angiogenesis.
Conclusion
The interdependence between protease localization and function provides a mechanism for coordination of extracellular signals and intracellular responses. The membrane-anchored proteases are uniquely positioned at the endothelial plasma membrane to coordinate the release of ECM components, to proteolytically activate cell surface proteins such as PARs, growth factors, and cytokines, to interact with cell surface adhesion receptors and generate soluble ligands, and to communicate with the cytoskeleton and with cellular signaling molecules. While significant progress has been made in understanding the effects of changes in the levels and activities of some of these enzymes, more research is needed on the importance of distinct membrane and subcellular localizations in the control of vascular function under physiological conditions and how pathological states, such as inflammation, change the exposure to substrates that modulate endothelial cell functions. A better understanding of these mechanisms has the potential to provide new insights into perturbations in vascular functions associated with numerous pathophysiological states including cancer, inflammation, aging, neurological diseases, diabetes, atherosclerosis and hypertension while opening up avenues for therapeutic intervention.
Key Points.
Membrane-anchored proteases are directly linked to the cell surface via Type I or Type II single pass transmembrane domains, or via a glycophosphatidylinositol (GPI)-anchor, with their protease catalytic domains exposed to the extracellular environment.
Protease function and substrate repertoire can be regulated through localization in distinct areas of the membrane.
A better understanding of the role of membrane-anchored proteases in vascular biology may provide novel therapeutic targets for the treatment of numerous cardiovascular and angiogenesis related diseases
Acknowledgments
None
Financial Support and sponsorship
This work was supported by National Institutes of Health Grant HL118390, Department of Defense Grant PR110378, and a Veterans Administration BLR&D Merit Award 1 I01 BX001921-01.
Footnotes
Conflicts of Interest
None
References and Recommended Reading
- 1.Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitamura K, Tomita K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin Exp Nephrol. 2012;16:44–48. doi: 10.1007/s10157-011-0506-1. [DOI] [PubMed] [Google Scholar]
- 3.Maekawa A, Kakizoe Y, Miyoshi T, et al. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. J Hypertens. 2009;27:181–189. doi: 10.1097/hjh.0b013e328317a762. [DOI] [PubMed] [Google Scholar]
- 4.Zhu H, Guo D, Li K, et al. Prostasin: a possible candidate gene for human hypertension. Am J Hypertens. 2008;21:1028–1033. doi: 10.1038/ajh.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frateschi S, Keppner A, Malsure S, et al. Mutations of the serine protease CAP1/Prss8 lead to reduced embryonic viability, skin defects, and decreased ENaC activity. Am J Pathol. 2012;181:605–615. doi: 10.1016/j.ajpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Planes C, Randrianarison NH, Charles RP, et al. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med. 2010;2:26–37. doi: 10.1002/emmm.200900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leyvraz C, Charles RP, Rubera I, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchimura K, Hayata M, Mizumoto T, et al. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat Commun. 2014;5:3428. doi: 10.1038/ncomms4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzza MS, Netzel-Arnett S, Shea-Donohue T, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A. 2010;107:4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo R, Rasmussen AL, Moyer AB, et al. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30:2003–2016. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.List K, Kosa P, Szabo R, et al. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CY, Meynard D, Lin HY. The role of TMPRSS6/matriptase-2 in iron regulation and anemia. Front Pharmacol. 2014;5:114. doi: 10.3389/fphar.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb SL, Sanders AJ, Mason MD, Jiang WG. Matriptase-2 inhibits HECV motility and tubule formation in vitro and tumour angiogenesis in vivo. Mol Cell Biochem. 2013;375:207–217. doi: 10.1007/s11010-012-1544-z. [DOI] [PubMed] [Google Scholar]
- 14.Armaly Z, Assady S, Abassi Z. Corin: a new player in the regulation of salt-water balance and blood pressure. Curr Opin Nephrol Hypertens. 2013;22:713–722. doi: 10.1097/01.mnh.0000435609.35789.32. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep. 2014;16:415. doi: 10.1007/s11906-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Y, Wang W, Dong N, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roebroek AJ, Umans L, Pauli IG, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- 18.Kim W, Essalmani R, Szumska D, et al. Loss of endothelial furin leads to cardiac malformation and early postnatal death. Mol Cell Biol. 2012;32:3382–3391. doi: 10.1128/MCB.06331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solun B, Marcoviciu D, Dicker D. Dipeptidyl peptidase-4 inhibitors and their effects on the cardiovascular system. Curr Cardiol Rep. 2013;15:382. doi: 10.1007/s11886-013-0382-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015;6:477. doi: 10.3389/fimmu.2015.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauve M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacheco BP, Crajoinas RO, Couto GK, et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 23.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 24.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, Jin M, Yang F, et al. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Martin-Alonso M, Garcia-Redondo AB, Guo D, et al. Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circ Res. 2015;117:e13–e26. doi: 10.1161/CIRCRESAHA.117.305108. This study showed that MT4-MMP is a critical in vivo mediator of vascular smooth muscle cell maturation and arterial wall function through osteopontin cleavage, which is likely to be important for preventing thoracic aortic aneurysms in patients with inherited thoracic aortic aneurysms possessing a newly identified missense mutation in MT4-MMP that prevents protease expression. [DOI] [PubMed] [Google Scholar]
- 27.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol. 2012;91:472–485. doi: 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Guaiquil VH, Swendeman S, Zhou W, et al. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J Mol Med (Berl) 2010;88:497–505. doi: 10.1007/s00109-010-0591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnoli M, Mineva ND, Polmear M, et al. ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis. EMBO Mol Med. 2014;6:278–294. doi: 10.1002/emmm.201303373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guaiquil V, Swendeman S, Yoshida T, et al. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glomski K, Monette S, Manova K, et al. Deletion of Adam10 in endothelial cells leads to defects in organ-specific vascular structures. Blood. 2011;118:1163–1174. doi: 10.1182/blood-2011-04-348557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Caolo V, Swennen G, Chalaris A, et al. ADAM10 and ADAM17 have opposite roles during sprouting angiogenesis. Angiogenesis. 2015;18:13–22. doi: 10.1007/s10456-014-9443-4. This study investigated the mechanisms underlying the opposite roles of ADAM10 and ADAM17 in angiogenic sprouting. The pro-angiogenic activities of ADAM17 were found to be unrelated to Notch activation and signaling, but mediated through the down-regulation of the natural inhibitor of angiogenesis Thrombospondin 1, while ADAM10-mediated suppression of angiogenic sprouting appears to be Notch dependent. [DOI] [PubMed] [Google Scholar]
- 33.Dokun AO, Chen L, Okutsu M, et al. ADAM12: a genetic modifier of preclinical peripheral arterial disease. Am J Physiol Heart Circ Physiol. 2015;309:H790–H803. doi: 10.1152/ajpheart.00803.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Cui D, Arima M, Takubo K, et al. ADAM12 and ADAM17 are essential molecules for hypoxia-induced impairment of neural vascular barrier function. Sci Rep. 2015;5:12796. doi: 10.1038/srep12796. This study is the first to identify a proteolytic mechanism by which hypoxia induces the opening of the neural vascular barrier, identifying ADAM12 and ADAM17 as potential therapeutic targets in the treatment of neural diseases such as diabetic retinopathy and ischemic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Wu MH, Lee ES, Yuan SY. A disintegrin and metalloproteinase 15 contributes to atherosclerosis by mediating endothelial barrier dysfunction via Src family kinase activity. Arterioscler Thromb Vasc Biol. 2012;32:2444–2451. doi: 10.1161/ATVBAHA.112.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun C, Beard RS, Jr, McLean DL, et al. ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice. Am J Physiol Lung Cell Mol Physiol. 2013;304:L135–L142. doi: 10.1152/ajplung.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maretzky T, Blobel CP, Guaiquil V. Characterization of oxygen-induced retinopathy in mice carrying an inactivating point mutation in the catalytic site of ADAM15. Invest Ophthalmol Vis Sci. 2014;55:6774–6782. doi: 10.1167/iovs.14-14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weskamp G, Mendelson K, Swendeman S, et al. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holloway JW, Laxton RC, Rose-Zerilli MJ, et al. ADAM33 expression in atherosclerotic lesions and relationship of ADAM33 gene variation with atherosclerosis. Atherosclerosis. 2010;211:224–230. doi: 10.1016/j.atherosclerosis.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Holgate ST, Yang Y, Haitchi HM, et al. The genetics of asthma: ADAM33 as an example of a susceptibility gene. Proc Am Thorac Soc. 2006;3:440–443. doi: 10.1513/pats.200603-026AW. [DOI] [PubMed] [Google Scholar]
- 41.Puxeddu I, Pang YY, Harvey A, et al. The soluble form of a disintegrin and metalloprotease 33 promotes angiogenesis: implications for airway remodeling in asthma. J Allergy Clin Immunol. 2008;121:1400–6. 1406. doi: 10.1016/j.jaci.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Te RL, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 43.Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014;19:14–33. doi: 10.1177/1074248413501018. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza-Torres E, Oyarzun A, Mondaca-Ruff D, et al. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 45.Ferrao FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: What is new? World J Nephrol. 2014;3:64–76. doi: 10.5527/wjn.v3.i3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015;44–46:207–223. doi: 10.1016/j.matbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 49.Antalis TM, Bugge TH, Wu Q. Membrane–anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50. doi: 10.1016/B978-0-12-385504-6.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gass J, Khosla C. Prolyl endopeptidases. Cell Mol Life Sci. 2007;64:345–355. doi: 10.1007/s00018-006-6317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HS, Park BM, Cho Y, et al. Shedding of epithin/PRSS14 is induced by TGF-beta and mediated by tumor necrosis factor-alpha converting enzyme. Biochem Biophys Res Commun. 2014;452:1084–1090. doi: 10.1016/j.bbrc.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 54.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghajar CM, George SC, Putnam AJ. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:251–278. doi: 10.1615/critreveukargeneexpr.v18.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong E, Maretzky T, Peleg Y, Blobel CP, Sagi I. The Functional Maturation of A Disintegrin and Metalloproteinase (ADAM) 9, 10, and 17 Requires Processing at a Newly Identified Proprotein Convertase (PC) Cleavage Site. J Biol Chem. 2015;290:12135–12146. doi: 10.1074/jbc.M114.624072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.Maney SK, McIlwain DR, Polz R, et al. Deletions in the cytoplasmic domain of iRhom1 and iRhom2 promote shedding of the TNF receptor by the protease ADAM17. Sci Signal. 2015;8:ra109. doi: 10.1126/scisignal.aac5356. This study determined that genetic deletion of part of the amino-terminal cytoplasmic tails of either iRhom1 or iRhom2, which are catalytically inactive rhomboid proteases important for the transport and maturation of ADAM17, increases ADAM17 activity and TNFR shedding, resulting in resistance to TNF-induced cell death. Identified mutations in iRhom2 cytoplasmic tail in patients with a dominantly inherited cancer susceptibility syndrome may explain their predisposition to cancer through enhanced TNFR shedding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friis S, Uzzun SK, Godiksen S, et al. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem. 2013;288:19028–19039. doi: 10.1074/jbc.M113.469932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters DE, Szabo R, Friis S, et al. The membrane-anchored serine protease prostasin (CAP1/PRSS8) supports epidermal development and postnatal homeostasis independent of its enzymatic activity. J Biol Chem. 2014;289:14740–14749. doi: 10.1074/jbc.M113.541318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 62.Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- 63.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 64.Murphy G. Regulation of the proteolytic disintegrin metalloproteinases, the 'Sheddases'. Semin Cell Dev Biol. 2009;20:138–145. doi: 10.1016/j.semcdb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terawaki S, Kitano K, Aoyama M, Mori T, Hakoshima T. MT1-MMP recognition by ERM proteins and its implication in CD44 shedding. Genes Cells. 2015;20:847–859. doi: 10.1111/gtc.12276. [DOI] [PubMed] [Google Scholar]
- 67**.Wong HS, Jaumouille V, Heit B, et al. Cytoskeletal confinement of CX3CL1 limits its susceptibility to proteolytic cleavage by ADAM10. Mol Biol Cell. 2014;25:3884–3899. doi: 10.1091/mbc.E13-11-0633. This study describes a novel role for the cytoskeleton in regulating the accessibility of ADAM10 to its substrate, the transmembrane endothelial adhesion molecule CX3CL1, the proteolytic cleavage of which enhances its release in soluble form as a chemoattractant for circulating leukocytes. This observation may have important implications as a general regulatory mechanism for the cleavage of substrates by the membrane anchored proteases, which could significantly impact inflammatory processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aimes RT, Zijlstra A, Hooper JD, et al. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost. 2003;89:561–572. [PubMed] [Google Scholar]
- 69*.Driesbaugh KH, Buzza MS, Martin EW, et al. Proteolytic activation of the protease-activated receptor (PAR)-2 by the glycosylphosphatidylinositol-anchored serine protease testisin. J Biol Chem. 2015;290:3529–3541. doi: 10.1074/jbc.M114.628560. This is the first demonstration of the activation of a protease activated receptor (PAR) by a serine protease GPI-linked to the cell surface, with implications for the regulation of PAR signaling responses during angiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awasthi V, Mandal SK, Papanna V, Rao LV, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2007;27:1447–1455. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 72.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 73.McCaffrey G, Staatz WD, Quigley CA, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 74.Koenen RR, Pruessmeyer J, Soehnlein O, et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood. 2009;113:4799–4809. doi: 10.1182/blood-2008-04-152330. [DOI] [PubMed] [Google Scholar]
- 75.Sun C, Wu MH, Guo M, et al. ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling. Cardiovasc Res. 2010;87:348–355. doi: 10.1093/cvr/cvq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chatterjee V, Beard RS, Jr, Reynolds JJ, et al. MicroRNA-147b regulates vascular endothelial barrier function by targeting ADAM15 expression. PLoS One. 2014;9:e110286. doi: 10.1371/journal.pone.0110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian X, Tian Y, Gawlak G, et al. Control of vascular permeability by atrial natriuretic peptide via a GEF-H1-dependent mechanism. J Biol Chem. 2014;289:5168–5183. doi: 10.1074/jbc.M113.493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simoes e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Zhang YH, Zhang YH, Dong XF, et al. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res. 2015;64:253–260. doi: 10.1007/s00011-015-0805-1. This study highlights the importance of ACE2 and Ang-(1–7) in protecting the endothelium by countering the activities of angiotensin II and inhibiting inflammatory responses. [DOI] [PubMed] [Google Scholar]
- 80.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond) 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 81.Sahin U, Weskamp G, Kelly K, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 83.Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 85*.Overland AC, Insel PA. Heterotrimeric G proteins directly regulate MMP14/membrane type-1 matrix metalloprotease: a novel mechanism for GPCR-EGFR transactivation. J Biol Chem. 2015;290:9941–9947. doi: 10.1074/jbc.C115.647073. This study identified a previously unknown mechanism by which agonist stimulation of GPCRs can transactivate the EGFR, finding that a direct interaction of the heterotrimeric G protein βγ with MT1-MMP can induce its activation and the membrane release of HB-EGF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 87.Hotary KB, Yana I, Sabeh F, et al. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195:295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang H, Duran CL, Abbey CA, Kaunas RR, Bayless KJ. Fluid shear stress promotes proprotein convertase-dependent activation of MT1-MMP. Biochem Biophys Res Commun. 2015;460:596–602. doi: 10.1016/j.bbrc.2015.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yana I, Sagara H, Takaki S, et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007;120:1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 90.Itoh Y, Takamura A, Ito N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Otto S, Deussen A, Zatschler B, et al. A Novel Role of Endothelium in Activation of Latent Pro-Membrane Type 1 Matrix Metalloproteinase and Pro-Matrix Metalloproteinase-2 in Rat Aorta. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv256. [DOI] [PubMed] [Google Scholar]
- 92.Buache E, Thai R, Wendling C, et al. Functional relationship between matrix metalloproteinase-11 and matrix metalloproteinase-14. Cancer Med. 2014;3:1197–1210. doi: 10.1002/cam4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noel A, Maillard C, Rocks N, et al. Membrane associated proteases and their inhibitors in tumour angiogenesis. J Clin Pathol. 2004;57:577–584. doi: 10.1136/jcp.2003.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.White SJ, Hayes EM, Lehoux S, et al. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol. 2011;226:2841–2848. doi: 10.1002/jcp.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fukushima Y, Okada M, Kataoka H, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tugues S, Morales-Ruiz M, Fernandez-Varo G, et al. Microarray analysis of endothelial differentially expressed genes in liver of cirrhotic rats. Gastroenterology. 2005;129:1686–1695. doi: 10.1053/j.gastro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 98.Glesne DA, Zhang W, Mandava S, et al. Subtractive transcriptomics: establishing polarity drives in vitro human endothelial morphogenesis. Cancer Res. 2006;66:4030–4040. doi: 10.1158/0008-5472.CAN-05-3294. [DOI] [PubMed] [Google Scholar]
- 99.Piltonen TT, Chen J, Erikson DW, et al. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J Clin Endocrinol Metab. 2013;98:3765–3775. doi: 10.1210/jc.2013-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]