Abstract
Species within the toxic marine diatom genus Pseudo-nitzschia coexist in coastal and estuarine waters globally and are difficult to distinguish by microscopy. Here, we describe a sensitive, high throughput PCR-based Automated Ribosomal Intergenic Spacer Analysis (ARISA) approach to determine the relative abundance of Pseudo-nitzschia species within natural communities over space and time. The method was quantitatively validated using simplified mixtures of DNA or ITS1 standards from isolates of P. pungens, P. multiseries, and P. delicatissima. Relative abundance calculations based on ARISA profiles from these mixtures reflected input ratios, with minor deviations resulting from intraspecific variability. ARISA was used to identify and quantify at least eight species within Puget Sound and the eastern Strait of Juan de Fuca, Washington, USA: P. americana, P. australis/P. seriata, P. cuspidata, P. delicatissima, P. fraudulenta, P. fryxelliana, P. multiseries, and P. pungens; genotypes corresponding to P. pungens var. pungens and P. pungens var. cingulata were identified by environmental sequencing. The different species were significantly correlated with physical (temperature, salinity), biological (chlorophyll a fluorescence, oxygen), and/or chemical (ammonium, nutrient ratios) factors. The ability to determine shifts in the relative abundance of Pseudo-nitzschia species over spatial and temporal scales relevant to dispersion and selection facilitates dissection of the varied mechanisms driving vertical and horizontal species distribution patterns in hydrographically complex systems.
INTRODUCTION
The pennate diatom genus Pseudo-nitzschia is distributed worldwide across oceanic, coastal, and estuarine environments (Hasle 2002, Lundholm et al. 2004, Trainer et al. 2012). Interest in Pseudo-nitzschia taxonomy and ecology has increased over the past few decades largely because at least 14 species are capable of producing the neurotoxin, domoic acid (DA). Toxin production among these species varies over ~9 orders of magnitude (Trainer et al. 2012, Fernandes et al. 2014). DA accumulates in filter feeding shellfish and planktivorous fish and can cause Domoic Acid Poisoning in marine vertebrates that feed on contaminated prey (Lefebvre et al. 2010). Human consumption of DA-contaminated seafood produces the syndrome Amnesic Shellfish Poisoning (ASP), which can result in permanent short-term memory loss or death (Perl et al. 1990). Production of DA is enhanced in Pseudo-nitzschia cultures by manipulating abiotic and biotic factors (summarized in Lelong et al. 2012, Bates 1998). Although Pseudo-nitzschia species commonly occur in phytoplankton assemblages, DA production is not constitutive in the environment, and can vary in response to environmental stimuli such as nitrogen speciation and concentration, iron availability, chlorophyll a biomass, pH, and salinity (Armstrong-Howard et al. 2007, Lelong et al. 2012). Recent studies have identified synergistic effects when cultures are simultaneously exposed to multiple stressors (Fuentes et al. 2012, Tatters et al. 2012), highlighting the taxonomic and ecological complexity underlying DA production in the environment (Trainer et al. 2012, Terseleer et al. 2013).
Among the >40 Pseudo-nitzschia species currently recognized, complexes composed of species with cryptic (i.e. identical) and pseudo-cryptic (i.e. finely differentiated) morphologies have been identified (Trainer et al. 2012, Lim et al. 2012, Lim et al. 2013, Orive et al. 2013). The use of phylogenetics, electron microscopy, and mating studies has greatly facilitated the recognition of new species within these complexes (Amato et al. 2007, Quijano-Scheggia et al. 2009, Lundholm et al. 2012). The characterization of region-specific as well as cosmopolitan Pseudo-nitzschia diversity (Hasle 2002, Orsini et al. 2004, Hubbard et al. 2008, Amato et al. 2007, Casteleyn et al. 2009) suggests that environmental and ecological processes act on multiple spatial (local, regional, and global) and temporal (event, seasonal, annual, decadal) scales to generate and maintain diversity in this genus (Lundholm et al. 2010, Hasle 2002, Ribalet et al. 2010). The emerging message from culture and field studies is that examination of Pseudo-nitzschia at appropriate taxonomic scales is necessary to determine the ecological significance of spatiotemporal factors that influence the assembly, growth, maintenance, and toxicity of communities in this harmful algal bloom (HAB) genus.
We previously developed an ARISA approach that discriminates a majority of known Pseudo-nitzschia species in both laboratory and field studies (Hubbard et al. 2008). The approach employs genus-specific PCR primers to amplify a variable region of the internal transcribed spacer 1 (ITS1). Previous work (Hubbard et al., 2008; Marchetti et al. 2008) demonstrates that the nucleotide sequence and length of the PCR amplicon is diagnostic of different species. Here, we develop a more quantitative ARISA approach for determining relative abundance of the different species, following methodology employed for bacterial community fingerprinting (Brown et al. 2005, Lueders and Friedrich 2003) and taking into account heterogeneity in Pseudo-nitzschia species ITS1 copy numbers per genome (e.g. Penna et al. 2013). We use this approach to examine Pseudo-nitzschia species diversity in simplified laboratory communities consisting of isolate DNA, and in environmental communities sampled during June 2006 in the Puget Sound estuarine system, encompassing five interconnected, hydrographically distinct, basins and the eastern Strait of Juan de Fuca. The sensitive ARISA approach revealed dynamic shifts in Pseudo-nitzschia species composition across horizontal and vertical environmental gradients in Puget Sound, permitting greater insight into the complexity of ecological and physical drivers that contribute to the spatial and temporal distribution of phytoplankton species in estuarine systems.
METHODS
Culture conditions and estimation of DNA and ITS1 copy number per cell
Non-axenic diatom isolates of Pseudo-nitzschia pungens, P. multiseries, P. delicatissima, and Ditylum brightwellii from Puget Sound and the outer coast of Washington were provided by the Center for Environmental Genomics, University of Washington, Seattle, USA (Table 1). Cultures were maintained in autoclaved Puget Sound water amended with f/2 nutrients (Guillard 1975) and grown at 13°C as semi-continuous batch cultures on a 16:8 light:dark cycle of 30–50 μmol photons m−2 s−1 cool-white light. Increases in chlorophyll a fluorescence were monitored with a Turner Designs 10-AU fluorometer (Sunnyvale, CA, USA). Five mL of mid-to-late exponential cultures were fixed with Lugol’s solution for cell counts and cell volume estimates (see below). A 10 mL aliquot of the culture was also diluted into 1000 mL of sterile Puget Sound seawater. Replicate 505 mL subsamples were taken from the diluted culture to simulate environmental sub-sampling from a Niskin bottle, with five mL aliquots from each subsample fixed with Lugol’s and the remaining 500 mL filtered onto a 25 mm diameter mixed cellulose filter with 0.45 μm pore-size (Millipore, Billerica, MA), and stored at −80 °C until DNA extraction.
Table 1.
Summary of species affiliation and origin of isolates utilized to generate genomic DNA and ITS1 standards for ARISA. ARISA fragment size was predicted from sequence and ARISA data. Cell length and width were determined microscopically for isolates of P. pungens and P. multiseries from Puget Sound and were estimated for P. delicatissima, using genetically similar temperate strains. For cellular DNA content, standard deviations reflect the variability of cell count data, and for ITS1 copies, the variability of 27–36 qPCRs generated for each isolate. The DNA content for P. delicatissima isolate PNWH2O_604 was used in calculations of ITS1 copy number cell−1 for PNWH2O_609.
| Species | Isolate | Date of isolation | Location of isolation | ARISA fragment size (DNA bp) | Average cell length (μm ± SD) | Average cell width (μm ± SD) | Average cell volume (μm3 ± SD) | DNA content (pg cell−1) | ITS1 copy number cell−1 ± SD |
|---|---|---|---|---|---|---|---|---|---|
| P. pungens var. pungens | PNWH2O_GGC3 | 8/2010 | Golden Gardens, WA | 142 | 133.1±1.5 | 6.0±1.0 | 3877±1191 | 0.92±0.62 | 149±44 |
| PNWH2O_PnC2 | 7/2009 | Friday Harbor, WA | 142 | 62.1±2.6 | 5.0±0.9 | 1286±403 | 0.78±0.24 | 227±128 | |
| P. pungens var. cingulata | PNWH2O_GGA1 | 8/2010 | Golden Gardens, WA | 142 | 84.7±3.9 | 3.9±0.8 | 1063±446 | 0.86±0.30 | 404±134 |
| PNWH2O_GGA3 | 8/2010 | Golden Gardens, WA | 142 | 92.6±4.3 | 4.1±0.6 | 1298±399 | 0.61±0.21 | 150±44 | |
| PNWH2O_608 | 10/2006 | Whidbey Basin, WA | 142 | n/a | n/a | n/a | 0.93±0.47 | n/a | |
| P. multiseries | PNWH2O_PC9 | 7/2010 | Penn Cove, WA | 144 | 90.8±2.3 | 3.8±0.7 | 1107±415 | 1.48±0.45 | 748±203 |
| PNWH2O_GGB1 | 8/2010 | Golden Gardens, WA | 144 | 100.3±4.4 | 4.1±0.7 | 1348±442 | 1.63±0.60 | 406±105 | |
| P. delicatissima | PNWH2O_604 | 6/2006 | N. Hood Canal | 168 | n/a | n/a | n/a | 0.47±0.30 | 26±7 |
| PNWH2O_609 | 6/2006 | N. Hood Canal | 168 | n/a | n/a | n/a | n/a | 16±11 | |
| WA coast1, Bay of Fundy2 | 1682 | 17–66 | 1–2.3 | 39–243 | n/a | n/a |
For each Lugol’s-fixed sample (n = 3 per isolate), cells were enumerated using a Nikon Eclipse TS100 inverted light microscope to count at least 30 fields of view or 1000 cells on a Sedgewick-Rafter slide. Apical and transapical axes (in valve view) were measured for 20 randomly selected cells of each isolate with ImageJ software (United States National Institute of Health, Bethesda, Maryland, USA; http://imagej.nih.gov/ij/). Calculations of average cell volume considered the linear (60%) and tapered (40%) portions of Pseudo-nitzschia cells, and used width as a proxy for depth (Lundholm et al. 2004):
A subset of the examined cells (n=7) was measured in girdle view. These cells were actively dividing and were excluded from further analysis.
Isolate and environmental (see below) DNA samples were extracted from filtered cells as described in Hubbard et al. (2008) using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). Genomic DNA from isolates was quantified using a Nanodrop 1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) and PicoGreen® assays (Invitrogen, Carlsbad, CA, USA) with a SpectraMax M2 microplate reader (Danaher Medical Technologies, Washington D.C., USA). The DNA content cell−1 was calculated by dividing average cell abundance (n=3) by the total DNA (using PicoGreen measurements) extracted from each culture.
To generate ITS1 sequence standards, 10–30 ng of P. pungens, P. multiseries, or P. delicatissima isolate DNA were added to 20 μL PCRs containing 0.4 mM dNTPs, 0.8 μM 18S-euk F primer, 0.8 μM 5.8S-euk R primer, 2.5 mM MgCl2, 1x standard reaction buffer and 0.75 U taq polymerase (Genechoice, Apex™ Bioresearch Products, San Diego, CA). Primers and cycling parameters are described in Hubbard et al. (2008). PCR products were excised from a 1% agarose gel, purified using the QIAquick PCR purification kit (Qiagen), ligated into the pCR® 4-TOPO® vector and used to transform the Top 10 strain of Escherichia coli (Invitrogen). For each species, DNA was isolated from four to six clones using the QIAprep Spin Miniprep Kit (Qiagen) and the ITS1 inserts from at least four clones were sequenced in both directions on an ABI 3730XL high-throughput capillary DNA analyzer. Standards for each species were generated by linearizing the ITS1 plasmid with the restriction enzyme SpeI (Invitrogen). Real-time qPCR assays determined ITS1 copy numbers for all Pseudo-nitzschia isolates except PNWH2O 608 (due to insufficient DNA). Triplicate 20 μL qPCR amplifications contained 5 μL of isolate DNA or water, 0.8 μM PnallF (5′-TCTTCATTGTGAATCTGA-3′) and 0.8 μM Pnall R (5′-CTTTAGGTCATTTGGTT-3′) (Hubbard et al. 2008), and iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Assays were performed with the iCycler iQ Real-Time PCR detection system (Bio-Rad Laboratories) using the following cycling parameters: 95°C for 3 minutes followed by 50 cycles of 95°C for 10 seconds, 50.6°C for 50 seconds, and 72 °C for 50 seconds. Separate runs were conducted for each species and included a dilution series of the corresponding linearized ITS1 standard. The threshold cycle (CT) was determined for each dilution and compared to the standard curve using the iCycler iQ Optical System Software (Bio-Rad Laboratories) to quantify copy number for ITS1 target DNA. Copy number was normalized to DNA concentration and corresponding cell numbers for each reaction.
Cellular estimates of size (volume), DNA content, and ITS1 copy number for Pseudo-nitzschia isolates were examined for normality using Shapiro-Wilk tests (Shapiro et al. 1968) and homogeneity of variances using Levene’s test (Levene 1960) in SPSS (IBM software, Chicago, IL, USA). Differences in the mean values for each metric were analyzed using a one-way analysis of variance (ANOVA) in conjunction with post-hoc analyses.
Puget Sound sample collection
Five Puget Sound basins and the eastern Strait of Juan de Fuca were sampled on 26–29 June 2006 from the R/V Thomas G. Thompson during the Puget Sound Regional Synthesis Model (PRISM) biannual hydrographic survey (Fig. 1). Salinity, temperature, and potential density measurements were obtained at 40 stations using a SBE, Inc. 911plus® CTD profiler (Bellevue, WA, USA). Potential density (CTD) was plotted with Ocean Data View (ODV; Schlitzer 2012) to identify pycnoclines at vertically profiled stations. Discrete water samples were collected with Niskin bottles mounted on a rosette, generally at 2.5, 5, 10, 20, 30, 40, 50, 100, 150, and 200 m, and near-bottom. For determination of nutrient concentrations, 35 mL of seawater were syringe-filtered through 0.22 μm Whatman filter units (GE Healthcare Biosciences Corp., Piscataway, NJ, USA), frozen onboard, and subsequently analyzed with a Technicon AutoAnalyzer II (UNESCO 1994) at the University of Washington Marine Chemistry Lab. For each chlorophyll sample, 65 mL of whole seawater were filtered through 25 mm Whatman glass fiber filters (GF/F), extracted with acetone, and analyzed with a Turner Designs 10-AU fluorometer. Dissolved oxygen samples were collected in glass bottles and analyzed using modified Winkler titration (Carpenter 1965). Environmental data is available on the Nanoos server: http://nvs.nanoos.org/CruisePrism
Figure 1.
Regional map of Washington coast and estuaries. The Puget Sound study region (dotted rectangle) is expanded (right panel) to indicate ARISA sampling sites during June 26–28, 2006. Surface samples (open circles) and a vertical profile of samples (closed circles) were collected at a subset of PRISM stations. Three transects are indicated: the Strait of Juan de Fuca transect through Whidbey Basin towards Admiralty Inlet (gray line); the Strait transect to Hood Canal (dotted line), and the Strait transect through Main Basin to South Sound (black line).
A surface net tow (33 μm mesh size) was conducted at 20 stations and phytoplankton communities were examined onboard the ship with a Nikon Eclipse TS100 inverted light microscope. Water samples for DNA were collected from the surface (i.e. 2.5 m) at the same 20 stations and from 3–4 additional depths at the following stations: P24, Strait of Juan de Fuca (5, 50, and 115 m); P30, Main Basin (10, 50, and 80 m); P4, Whidbey Basin (5, 50, and 87 m); P11, Hood Canal (5, 13, 50, and 85.6 m); P37, South Sound (6.5, 10, 30, 52 m); and P38, South Sound (7, 10, and 50 m) (Fig. 1). Subsurface community sample depths were adaptively selected to target distinct water masses, based on pycnoclines identified in the downward CTD cast. For each DNA sample, 500 mL of seawater were filtered onto two replicate 0.45 μm HA filters (Millipore) and stored at −80°C until extraction. Following extraction, environmental DNA was quantified as described above and diluted with sterile water to a concentration of 2 ng μL−1.
Amplification for ARISA
Isolate and environmental DNA was amplified for ARISA using the Pseudo-nitzschia specific ITS1 Pnall primer set (Hubbard et al. 2008). Template for ARISA PCRs contained 10 ng of DNA consisting of mixtures of different proportions (2–98%) of P. pungens, P. delicatissima or P. multiseries genomic DNA with or without the addition of 5ng of D. brightwellii genomic DNA or environmental genomic DNA. A dilution series of ITS1 sequence standards, derived from target species (P. delicatissima, P. multiseries, or P. pungens), were added to PCRs to evaluate ARISA limits of detection. Standards of ITS1 DNA were used as template in PCRs containing 1, 10, 100, 500, 1000, and 10,000 ITS1 copies of each species (with D. brightwellii DNA added to obtain 10 ng template). Similar concentrations of ITS1 standards from each species were individually added to PCRs containing a cocktail of 5 ng environmental DNA and varying amounts of D. brightwellii DNA to obtain 10 ng. For ARISAs containing only genomic environmental template, 10 ng of DNA were added to each PCR.
For each ARISA sample, triplicate 20 μl PCRs contained 0.4 mM dNTP’s, 0.4 μM of primer PnallF, 0.4 μM of FAM-labeled primer Pnall R, 0.75 U taq polymerase, 2.5 mM of MgCl2, 1x standard buffer (Genechoice), and 10 ng template DNA. PCRs consisted of the following cycling parameters: a 2-minute denaturing step at 94° C, followed by 32 cycles of 30 s at 95° C, 30 s at 50.6° C, and 60 s at 72° C, with a final 10 minute extension at 72° C. PCR products were purified using MultiScreen PCRμ96 filter plates (Millipore). Triplicate PCRs were pooled, quantified with PicoGreen®, and diluted to 0.1 ng μL−1. For each sample, 1 ng DNA was ethanol precipitated and resuspended with 0.078 μL 10% Tween 20, 9.77 μL sterile water, and 0.15 μL fluorescent size standard Et-ROX 400 (GE Healthcare). Samples were analyzed on a Megabace 1000 capillary sequencer (GE Healthcare). Electropherograms were analyzed using DAx software (Van Mierlo Software, Eindhoven, Netherlands). Fluorescent peak height data were extracted from DAx using the online fragment binner Dakster (http://rocaplab.ocean.washington.edu/cgi/dakster/index.html) with 0.1 base pair (bp) discrimination, and were manually grouped into 1-bp bins. The proportion of total peak height attributed to each ARISA peak was computed for isolate and environmental samples. Peaks ranging between 100 and 400 bp that represented greater than 3% total ARISA fluorescence and 15x the signal:noise ratio were considered in further analyses. Heat maps were used to display community profiles from individual filters (http://www.bioinformatics.ubc.ca/matrix2png/bin/matrix2png.cgi).
Raw and relative ARISA peak height data generated from mixed templates of species DNA were tested for normality using a Shapiro-Wilk test. Linear regressions were calculated for peak height data (raw and relative) versus relative concentration of Pseudo-nitzschia species DNA added to PCRs (in SPSS).
Environmental clone libraries
Two samples were selected for environmental sequencing, based on ARISA composition, to best capture both species diversity as well as intraspecific diversity within P. pungens. Genomic DNA samples from surface waters at stations P4 (Whidbey Basin) and P30 (Main Basin) were PCR-amplified using the Pnall primers as in Hubbard et al. (2008). PCR products were ligated into the pCR® 4-TOPO® vector and used to transform E. coli, Top 10 strain (Invitrogen). At least 78 transformants were randomly chosen from each library and the Templi-phi DNA Amplification Kit (GE Healthcare) was used to amplify DNA for sequencing with the DYEnamic ET Dye Terminator Kit (GE Healthcare) on a Megabace 1000 capillary sequencer. Both strands of the Pnall ITS1 amplicon were sequenced to completion with M13 vector primers. Sequences were compiled, edited, and aligned in Sequencher® 4.9 (Gene Codes Corporation, Ann Arbor, MI), and deposited in GenBank (accession numbers HM007359-HM007523). Pseudo-nitzschia species and intraspecific genotypes (defined by nucleotide polymorphisms) were identified based on ITS1 sequence similarity to sequences in the BLAST nucleotide (nr/nt) database as of March 2013. Rarefaction analyses were conducted with EstimateS software (Colwell 2005).
Comparative community analyses
For environmental ARISA profiles, the non-parametric statistic ANalysis Of SIMilarities (ANOSIM) was used to test for Pseudo-nitzschia community structure according to basin origin (PRIMER-E, Clarke and Warwick 2001); relative fluorescence for individual species was averaged across replicate samples. The Bray-Curtis similarity index (Bray and Curtis 1957, Magurran 2004) considered ARISA fragment composition and the relative abundance of each ARISA fragment in a sample and was utilized to characterize community similarity between replicate filters and to identify spatial and ecological patterns in Pseudo-nitzschia communities (in PC-ORD, McCune and Grace 2002). Associations among environmental parameters and Pseudo-nitzschia species, based on ARISA data, were determined using the non-parametric Spearman’s correlation analysis (in SPSS).
RESULTS
Interspecific variability in cell size, DNA content, and ITS1 copies
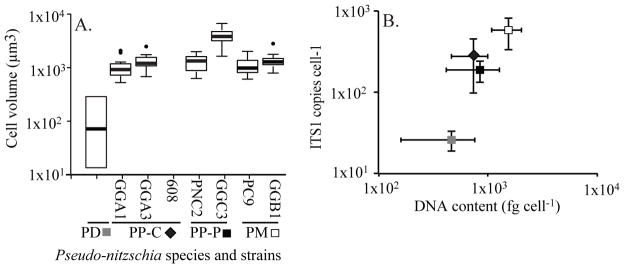
Cellular dimensions were similar among P. pungens var. cingulata isolates GGA1 and GGA3 (3–7 μm wide and 56–100 μm long), P. pungens var. pungens isolate PnC2 (3–7 μm wide and 56–66 μm long), and P. multiseries isolates PC9 and GGB1 (3–6 μm wide and 85–106 μm long). One isolate of P. pungens var. pungens isolate was considerably larger (4–8 μm wide and 131–136 μm long) (Table 1, Fig. 2A). Size estimates could not be made before the local P. delicatissima isolates (604, 609) were lost. Instead, strains from temperate regions with ITS1 sequences identical to 604 and 609 (Kaczmarska et al. 2008, Stehr et al. 2002) were used for P. delicatissima size estimates (17–66 μm long, 1–2.3 μm wide) (Table 1, Fig. 2A). Statistically significant differences in cell volumes across isolates were identified (one-way ANOVA F=64.516, df=6, p<0.001), although isolates of P. multiseries and P. pungens were not statistically different (Fig. 2A) with the exception of Pseudo-nitzschia pungens isolate GGC3, which was three times greater in volume than other isolates and was significantly distinct from other isolates (post-hoc Games-Howell test, p<0.01). Isolates from both species were estimated to be one to two orders of magnitude larger in volume than P. delicatissima (Table 1).
Figure 2.
Comparison of A. cell volume (μm3), B. DNA content (fg cell−1) and ITS1 copies (cell−1) for isolates of Pseudo-nitzschia delicatissima (PD), P. pungens var. cingulata (PP-C), P. pungens var. pungens (PP-P), and P. multiseries (PM). Symbols corresponding to each species/variety are the same for panels A and B. In 2A, literature cellular dimensions are used to estimate volume for PD isolates; for other isolates, estimates are derived from 20 measured cells. In 2B, PP-C isolate 608 was used for DNA content and ITS1 copy estimates, but not for cell volume estimates. In all boxplots, bars = mean, boxes = 1st and 3rd quartiles, whiskers = 10th and 90th percentiles, and dots = outliers based on variation in estimates of cellular abundance (n=3 per isolate for DNA content) or variation in qPCRs (n = 27–36 PCRs per isolate for ITS1 copies per cell).
The average DNA content per cell for the five P. pungens isolates ranged from 0.61–0.93 pg DNA cell−1, despite the larger cell volume of GGC3. Pseudo-nitzschia multiseries isolates on average contained twice as much DNA (1.48–1.63 pg DNA cell−1) as P. pungens isolates and three times that of the P. delicatissima isolates (0.47 pg DNA cell−1; Table 1, Fig. 2B). Isolate differences in DNA per cell were significant (one-way ANOVA F=100.1, df=7, p<0.001), and DNA per cell was significantly different between isolates of P. multiseries and P. delicatissima, and P. multiseries and P. pungens (post-hoc Tukey test, p<0.01), but not among isolates from the same species or between certain isolates of P. delicatissima and P. pungens. A ratio of 3:2:1 of P. delicatisima: P. pungens: P. multiseries was used to normalize DNA per cell between species. A log transformation for normality was applied to estimates of ITS1 copies per cell. Species differences in log-transformed ITS1 copies per cell were significant (one-way ANOVA F=353.8, df = 2, p<0.001) and each species was significantly different from the other species examined (post-hoc Games-Howell test, p<0.01). A positive relationship between DNA per cell and ITS copies per cell was observed (Fig. 2B), however DNA per cell was not significantly correlated with cell length, width or volume across isolates or species. On a per cell basis, P. multiseries possessed about 30 times as many ITS1 copies as P. delicatissima and about 2.5 times as many as P. pungens. On a per DNA basis, this disparity was significantly reduced and P. multiseries possessed 6.5 times more ITS1 copies pg−1 DNA than P. delicatissima, and 1.5 times more than P. pungens (Table 1, Fig. 2B).
Species quantification with ARISA
Cloned ITS1 DNA standards or genomic DNA from P. delicatissima, P. pungens, or P. multiseries isolates were added to PCRs to evaluate ARISA performance across a range of cellular equivalents (<1 cell to 104 cells). When the template mix was strongly skewed towards P. delicatissima genomic DNA (>80%), additional peaks (239 and 280 bp) representing <12% of ARISA fluorescence were detected. These fragment sizes do not correspond to known Pseudo-nitzschia species and were not observed in PCRs containing high proportions of cloned P. delicatissima ITS1 DNA; these peaks were removed and proportions were recalculated.
Whereas absolute ARISA peak heights were not correlated with the amount of species DNA in mixed samples (R2 < 0.2, not significant), relative ARISA peak heights were positively correlated with relative proportions of P. delicatissima, P. pungens and/or P. multiseries DNA in mixed samples (Fig. 3; linear regression, R2 = 0.92, 0.88, 0.87 for each species respectively, p<0.01; R2 = 0.87 for all species, p<0.01). Pseudo-nitzschia multiseries comprised a greater proportion of the ARISA signal in mixed species templates relative to similar DNA concentrations of P. pungens or P. delicatissima (Fig. 3). A bias towards P. multiseries was expected as this species has about 1.5 times as many ITS1 copies fg−1 DNA as P. pungens and about 6.5 times as many as P. delicatissima (Table 1). The observed relative ARISA peak heights were consistent with expected proportions and differed by up to an additional 10%. To evaluate the impact of known differences in the ITS1 fragment length and sequence on ARISA signal, equivalent numbers (101, 102, 103, 104 or 105) of ITS1 standards from P. multiseries, P. pungens, and P. delicatissima were combined for PCRs. Slight differences (1.4–5.5%) were observed in ITS1 amplification efficiency between species, with P. pungens amplifying the most efficiently. Thus, the relative ARISA peak height was determined to be a robust indicator of the relative contribution of ITS1 copy numbers or DNA to the total signal for the different species.
Figure 3.
The proportion of P. delicatissima (gray), P. pungens (black), and/or P. multiseries (white) DNA added to PCRs and corresponding relative ARISA fluorescence. The linear regression (solid line; y = 0.78x+0.12; R2 = 0.87, p<0.01) represents data derived from all three species. Dashed line indicates the 1:1 line.
ARISA characterization of field assemblages
Surface net tow samples collected throughout Puget Sound and the Strait of Juan de Fuca (Fig. 1) mostly consisted of mixed phytoplankton assemblages. Pseudo-nitzschia species were visually observed at all stations, except station P38 in Carr Inlet (South Sound), where a bloom of the diatom Rhizosolenia sp. dominated the phytoplankton community.
Pseudo-nitzschia species were identified with ARISA in each of the 39 discrete locations sampled, spanning surface and subsurface waters. Ten ARISA fragments were detected. Amplicon lengths 142, 144, 150, 168, 196, 202, 207, and 233, respectively, corresponded to P. pungens, P. multiseries, P. australis/P. seriata (which share an ARISA amplicon size), P. delicatissima, P. americana, P. fraudulenta, P. fryxelliana, and P. cuspidata (Fig. 4A–C). Environmental ITS1 DNA sequences (from P4 and P30) confirmed the presence of five species: P. pungens, P. multiseries, P. seriata, P. delicatissima, and P. americana. Rarefaction estimates indicated that species diversity was well, but not fully, sampled by sequencing (not shown). Genotypes for P. delicatissima, P. multiseries, P. americana, P. seriata, P. pungens var. pungens and P. pungens var. cingulata were identical to Genbank sequences from North Atlantic and/or North Pacific isolates. A single 150 bp sequence corresponded to P. seriata, and further discrimination between P. australis/P. seriata was not conducted. Additional fragments (140 and 257 bp) were identified in a few ARISA profiles (Fig. 4 A–C) but did not correspond to known Pseudo-nitzschia species and contributed less than 8% to total ARISA fluorescence; these peaks were excluded from subsequent analyses.
Figure 4.
ARISA profiles for A. Station P24 (Strait of Juan de Fuca), B. Station P19 (Admiralty Inlet), and C. Station P30 (Main Basin). Genbank accession numbers for representative sequences are listed for identified peaks in the ARISA profiles; peak position for P. cuspidata is indicated. Species in bold and associated sequences were generated from EM-identified strains, and species with asterisks (*) have been identified in regional environmental sequence databases (this study, Hubbard et al. 2008). Pie charts below each profile represent the relative proportion of Pseudo-nitzschia species in the ARISA profiles, based on relative ARISA peak heights (top: all species; middle: subset of P. delicatissima, P. pungens, and P. multiseries) or data that has been transformed (bottom: same subset), by applying a ratio of 6.5:1.5:1 respectively (predicted from ITS1 copies fg−1 P. sp. DNA) to the ARISA signal to estimate relative species abundance.
Relative ARISA peak heights indicated that three species dominated field samples: P. delicatissima, P. pungens, and to a lesser extent, P. multiseries (Fig. 4D–F, top and middle panels; Fig. 5). Replicate surface samples collected from the same Niskin bottle (Fig. 5) displayed an average Bray-Curtis similarity of 0.92 (ranging from 0.77–0.99), with an average species discrepancy of 2% (up to 16.6%). Greater variability was detected between replicates collected at subsurface depths (average Bray-Curtis similarity 0.79, ranging from 0.56 to 0.99), especially below 20 m (Fig. 5). When relative cell abundances for P. delicatissima, P. pungens, and P. multiseries were estimated by normalizing to ITS1 copies fg−1 DNA (1:1.5:6.5 for P. multiseries: P. pungens: P. delicatissima), the estimates for P. delicatissima accordingly increased by an average of 2.5x in each sample (Fig. 4D–F, bottom panel). Communities dominated by either the small-celled species, P. delicatissima, or the larger-celled species, P. pungens, were similar regardless of whether they were based on the relative ARISA signal or the transformed ARISA signal that approximates relative species abundance (Fig. 4). Because ITS1 copy number fg−1 DNA is unknown for most species, the more conservative proportions of ITS1 copies calculated directly from ARISA data were utilized in subsequent descriptions of communities within Puget Sound, unless otherwise noted.
Figure 5.
Heat maps of relative ARISA fluorescence for replicate filters, shown as pairs of circles, from stations along the three Puget Sound transects. A. Strait of Juan de Fuca through Whidbey Basin towards Admiralty Inlet (gray line, Fig. 1), B. Strait to Hood Canal (dotted line, Fig. 1), and C. Strait through Main Basin to South Sound (black line, Fig. 1). Solid lines above the heat maps indicate basin location of samples. Subsurface Pseudo-nitzschia profiles within different basins are indicated by gray arrows.
Environmental and community transitions in Puget Sound
Surface and subsurface samples for ARISA-based species identification were collected from waters that ranged across >9 salinity units, 12°C, >30 μM nitrate, ~3 μM ammonium, >75 μM silicate, >3.5 μM phosphate, and ~20 μg L−1 chlorophyll a (Table S1). In general, the chlorophyll a maxima occurred at either the surface (top 2.5 m) or the nitracline (top 20 m), and decreased below 20 m to less than 1 μg L−1. Pseudo-nitzschia pungens and P. delicatissima were found at most sampling locations, whereas other species (P. cuspidata, P. fraudulenta, P. multiseries, P. australis/P. seriata, P. fryxelliana, and P. americana) displayed more patchy distributions. Samples from the same basin generally exhibited similar species composition (global ANOSIM value of 0.416, p < 0.01).
Stations in the Strait and in northern Admiralty Inlet were generally saltier (29.4–30.5), colder (10.6–11.1 °C), less stratified (Fig. 6A–C), and had higher nutrient concentrations and lower biomass than most of Puget Sound (Table S1). Surface and subsurface Pseudo-nitzschia communities from the Strait of Juan de Fuca and the northern Admiralty Inlet sill (station P19) were dominated by P. delicatissima (>60%; Fig. 5A, 5C). This area was populated by all species except P. cuspidata, and represented the only location where P. fraudulenta was detected. Proportions of P. fraudulenta and P. fryxelliana were highest at station P24, at 50 m (19% and 46% respectively; Fig. 5C).
Figure 6.
Potential density along Puget Sound transects. A. Strait of Juan de Fuca to Whidbey Basin, top 100 m; B. Strait of Juan de Fuca to Hood Canal, top 100 m; and C. Strait of Juan de Fuca to Main Basin/South Sound, with bathymetry. Vertical CTD profiles (shown by lines) were collected at stations where DNA and environmental samples were collected (indicated by numbers) and where environmental samples only were collected (asterisks).
Sharp transitions to Puget Sound’s more estuarine conditions occurred at the northern shallow sills, Admiralty Inlet and Deception Pass (Fig. 6A–C). A ~2 kg m−3 decrease below 50 m depth and a 2.7 kg m−3 decrease in surface density occurred respectively at the northern and southern Admiralty Inlet sills (Fig. 6C). Density on either side of Deception Pass, a narrow, shallow passage connecting the Strait of Juan de Fuca to Whidbey Basin, varied by >3 kg m−3 at the surface and 1.5 kg m−3 at depth (Fig. 6A). These transitions occurred across <20 km, and were complemented by corresponding shifts in nutrient concentrations, biomass, and the dominant Pseudo-nitzschia species (Table S1, Fig. 5A, C). Communities near these well-mixed sill regions were composed of more similar proportions of P. pungens and P. delicatissima.
Much of Puget Sound was dominated by P. pungens, including Whidbey Basin, Main Basin, and parts of South Sound and Hood Canal (Fig. 5A–C). At the surface, Whidbey Basin outflows into the central Main Basin and then into Admiralty Inlet (transect WB; Figs. 1, 5A, and 6A). The highest chlorophyll a biomass was observed east of Deception Pass, in Whidbey Basin at station P4 (20 μg L−1), where surface communities were populated by at least seven Pseudo-nitzschia species including P. cuspidata (Fig. 5A). Nearly the warmest (15.8 °C) and coldest (9.1 °C) waters sampled within Puget Sound were observed at this stratified station (Table S1, Fig. 6A). Fewer species were observed below the thermocline, where silicate concentrations were in excess of 80 μM (nearly twice the levels detected at less stratified locations), and nitrate concentrations were 8–10 μM greater than at less stratified stations (Table S1). The other Whidbey and Main Basin stations along the WB transect were similarly dominated by P. pungens and populated by P. delicatissima, P. americana, and P. multiseries (Fig. 5C).
Surface waters in South Sound and the Main Basin generally flow seaward (transect MB/SS). Within South Sound, Carr and Case Inlets were respectively dominated by P. delicatissima or P. pungens (Fig. 5C). Neither inlet was strongly stratified (Fig. 6C). In Carr Inlet (P38), a Rhizoselenia bloom was observed and P. delicatissima represented >90% of surface and subsurface ARISA profiles there (Fig. 5C). In nearby Case Inlet (P37), P. pungens instead dominated. Species P. australis/P. seriata (5–25%) and P. americana (9–24%) were also present. These species comprised a greater fraction of the community at 7 and 10 m (Fig. 5C), where ammonium concentrations were nearly 3 μM, compared to the near-zero values observed in most of Puget Sound (Table S1). Near the Tacoma Narrows sill (P39, P34, P35), roughly equal proportions of P. pungens and P. delicatissima and low proportions of P. australis/P. seriata (Fig. 5C) were observed and were clearly distinct from the P. pungens-dominated communities observed further north in the Main Basin. In the central Main Basin, weak vertical stratification was observed (Fig. 6C), and communities were generally similar across different depths at station P30 (Fig. 5C); the only observance of P. australis/P. seriata was at 80 m.
The surface waters the westernmost basin, Hood Canal (transect HC; Fig. 1), were dominated by P. delicatissima (>40%) at the northern (P17) and southern (P11) stations (Fig. 5B); P. pungens and P. australis/P. seriata were also observed at both locations. A Phaeocystis sp. bloom occurred mid-canal (P13), with single cells of a small cell-type species of Pseudo-nitzschia suspended within the gelatinous matrix surrounding Phaeocystis colonies. At P13, P. delicatissima comprised >90% of the Pseudo-nitzschia ARISA fluorescence (Fig. 5B). In central and south Hood Canal a relatively warm (≤19.8°C), fresh (<24.9 psu) surface layer (top 20 m; Fig. 6B) overlaid denser waters that were >10 °C colder, >5 saltier, and more nutrient-rich (Table S1). The only occurrence of P. fryxelliana within Puget Sound was at the northern Hood Canal station (P17) and at the subsurface chlorophyll a maximum (13 m) at station P11 (Fig. 5B). The only occurrence of P. multiseries in Hood Canal was at 86 m.
Species were significantly correlated with 1–8 of the 15 possible environmental factors examined, except for P. cuspidata, which was only detected in one sample (Table 2). Pseudo-nitzschia delicatissima dominated low biomass assemblages at both ends of the observed temperature/salinity/nutrient gradient, and increased relative to other species below the euphotic zone in every basin (Fig. 5). This species was negatively correlated with biomass, whereas P. pungens and P. multiseries were positively correlated (Table 2). Pseudo-nitzschia delicatissima, P. fryxelliana, and P. fraudulenta were significantly correlated with high salinity and low oxygen concentrations. With the exception of P. australis/P. seriata, which was positively associated with ammonium concentration, correlations between individual species and standing stock nutrient concentrations were not detected (Table 2). Five species demonstrated varied associations with the ratio of nitrate or silicate to other nutrients, especially phosphate (Table 2).
Table 2.
Significant Spearman’s correlation coefficients for bivariate comparisons of species and environmental parameters are shown for p < 0.05 (*) and for p < 0.01 (**).
| P. pungens | P. multiseries | P. australis/P. seriata | P. delicatissima | P. fryxelliana | P. fraudulenta | P. americana | P. cuspidata | |
|---|---|---|---|---|---|---|---|---|
| Oxygen | .43** | .32* | - | −.38* | −.46** | −.42** | - | - |
| Salinity | −.52** | - | - | .39* | .66** | .55** | - | - |
| Temperature | .36* | - | - | - | −.50** | −.45** | - | - |
| Chlorophyll a | .63** | .48** | - | −.59** | - | - | - | - |
| Nitrate:silicate | - | −.33* | - | - | - | - | - | - |
| Nitrate:phosphate | - | .37* | - | - | .42** | .42** | .37* | - |
| Nitrate:ammonium | −.34* | - | −.40* | .39* | - | - | - | - |
| Phosphate:ammonium | - | - | −.37* | - | - | - | - | - |
| Silicon:phosphate | - | - | −.41** | - | −.48** | −.42** | - | - |
| Silicon:ammonium | - | .32* | −.32* | - | - | - | - | - |
| Silicon:nitrate | - | - | - | - | - | −.44** | - | - |
| Ammonium | - | - | .39* | - | - | - | - | - |
| Ammonium:silicate | - | - | .41** | - | - | - | - | - |
| Ammonium:nitrate | - | - | .41* | - | - | - | - | - |
| Ammonium:phosphate | - | - | .39* | - | - | - | - | - |
Similar associations with environmental conditions (Table 2) were detected for P. pungens, P. multiseries and P. delicatissima regardless of whether relative ARISA signal or relative cell abundance estimated from ITS1 copies fg-1 DNA (to account for species variability, e.g. Fig. 4) data were utilized. More specifically, P. pungens and P. delicatissima displayed opposing, significant associations with oxygen, salinity, biomass, and the ratio of nitrate:ammonium.
INTRASPECIFIC DIVERSITY
The environmental ITS1 sequences from two stations (P4 and P30) with high proportions of P. pungens in ARISA profiles (Fig. 5, Table 3) were examined to determine the extent of diversity within the P. pungens complex. Seventeen distinct P. pungens ITS1 genotypes were identified, and rarefaction analyses (not shown) were not saturated for instraspecific diversity. Five or more sequences were obtained for each of three P. pungens genotypes. Genotype “A” was identical to sequences from P. pungens var. pungens (with a T at position 59 of the Pnall amplicon), and genotype “B” was identical to P. pungens var. cingulata (with an A at position 59). A third genotype “C” differed by one SNP from P. pungens var. cingulata (A/G at position 8). The proportion of genotype C in Whidbey Basin (P4) greatly exceeded that found in the Main Basin (Table 2). Two sequences from Whidbey Basin were most similar to genotype C but contained an additional SNP (Table 3). Thirteen other P. pungens genotypes, represented by SNPs scattered throughout the ITS1 amplicon, were each detected only once, in either library (E-Q) (Table 2).
Table 3.
Species affiliation, biogeography, Genbank accession numbers, and sequence polymorphism of Pseudo-nitzschia genotypes detected in ITS1 sequences from station P4 and P30 clone libraries. Reference sequences for globally distributed genotypes of P. pungens and P. multiseries are shown in the top row for those species; Puget Sound (PS) sequences are listed separately. Numbers indicate polymorphic sites in clone sequences; numbering starts following the Pnall F primer. The appropriate nucleotide is indicated for sites where clone sequences deviated from the reference sequence, and dots indicate sites that matched the reference sequence. Genotypes listed in bold were identified more than once in sequence libraries; non-bold genotypes represent singletons, and the polymorphic sites differentiating singletons are indicated for each genetically distinct species or variety. Pseudo-nitzschia pungens sequences are divided into groups A, B, and C, based on nucleotide composition at sites 8 and 59, shown in bold.
| Species | Genotype | Distribution | # P4 seq. | # P30 seq. | Genbank accession numbers | Location of nucleotide polymorphisms on Pnall amplicon | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 19 | 20 | 21 | 41 | 53 | 56 | 59 | 62 | 83 | 92 | 94 | 98 | ||||||
|
|
||||||||||||||||||
| P. pungens var. pungens | A | Global | 13 | 29 | HM007359-400 | G | T | T | T | A | A | G | T | A | T | A | T | A |
| SINGLETONS | PS | 2 | 3 | HM007492 | A/T | • | • | C | • | • | C | • | C | • | • | A | • | |
| P. pungens var cingulata | B | Pacific NW | 19 | 41 | HM007401-60 | • | • | • | • | • | • | • | A | • | • | • | • | • |
| SINGLETONS | PS | 4 | 1 | HM007493 | • | C | C | • | T | G | • | A | • | • | • | • | • | |
| P. pungens, novel | C | PS | 22 | 5 | HM007461-87 | A | • | • | • | • | • | • | A | • | • | • | • | • |
| SINGLETONS | PS | 2 | 0 | HM007488-89 | A | • | • | • | • | • | • | A | • | C | T | • | G/C | |
|
|
||||||||||||||||||
| 2 | 13 | 51 | ||||||||||||||||
|
|
||||||||||||||||||
| P. multiseries | A | Global | 10 | 5 | HM007503-17 | C | C | G | ||||||||||
| SINGLETONS | PS | 1 | 0 | HM007518 | T | A | A | |||||||||||
| P. seriata | Pacific NW | 1 | 0 | HM007521 | ||||||||||||||
| P. americana | Pacific NW | 1 | 0 | HM007522 | ||||||||||||||
| P. delicatissima | Global | 0 | 1 | HM007523 | ||||||||||||||
DISCUSSION
The ability of ARISA to distinguish a majority of Pseudo-nitzschia species as well as novel diversity (Hubbard et al. 2008) and the high throughput nature of this approach allows sampling frequency and analyses to more closely match the temporal and local to global spatial scales upon which advection and selection can act. The modified ARISA approach performed well in quantifying the relative abundance of ITS1 PCR fragments from different Pseudo-nitzschia species. The identification and relative proportions of individual species based on ITS1 copies were highly reproducible when used with the standardized protocols developed for amplification of culture and environmental templates. The sensitivity of the ARISA was demonstrated through successful detection of dilution series of Pseudo-nitzschia DNA or ITS1 standards spanning five orders of magnitude. Cell size, DNA content, and ITS1 copy number varied across the three species most prevalent in Puget Sound during sampling – P. multiseries, P. delicatissima, and P. pungens. Despite this variation, normalizing ITS1 abundance relative to any of these factors provided relatively little significant improvement in determining trends in proportional abundances across environmental gradients in the present study. Because cellular DNA content and ITS1 copy numbers are not yet known for most Pseudo-nitzschia species, the more conservative estimator of relative ITS1 copies can be used as a proxy for relative cell abundance in environmental ARISA. The transition to using more quantitative ARISA data allowed greater insight into the ecology of individual Pseudo-nitzschia species in Puget Sound.
Pseudo-nitzschia delicatissima was detected with ARISA in nearly every sample. The Puget Sound P. delicatissima was genetically identical to strains isolated from the temperate waters of Denmark (Quijano-Sheggia et al. 2009) and the Bay of Fundy (Kaczmarska et al. 2008). In our study, this species was distributed throughout the water column, and comprised >90% of the Pseudo-nitzschia community at locations where nearly monospecific phytoplankton blooms were observed. For example, the cells embedded within Phaeocystis colonies in Hood Canal were identified as P. delicatissima, as has also been described by Sazhin et al. (2007). The colonial matrix of Phaeocystis cells may provide protection from grazers, and accumulates certain nutrients and trace metals (see Schoemann et al. 2005). Isolates of P. delicatissima appear able to use organic and inorganic substances produced by other phytoplankton to maintain their growth rate (Loureiro et al. 2009). Thus, it is possible that diverse processes (e.g. reduced grazing, flexible nutrient requirements) could contribute to the spatial and/or temporal persistence of P. delicatissima in Puget Sound and other temperate estuaries (Klein et al. 2010, Kaczmarska et al. 2008).
Pseudo-nitzschia pungens was also detected throughout Puget Sound. Environmental ITS1 sequences corresponding to the globally distributed P. pungens var. pungens and the more regionally localized P. pungens var. cingulata were both detected in two adjoining Puget Sound basins, based on ITS1 sequencing of environmental DNA. An additional ITS1 variant of P. pungens var. cingulata was detected and appeared to be more prevalent in Whidbey Basin than Main Basin. Sexual hybridization between the P. pungens varieties has been documented both in culture (Casteleyn et al. 2009) and the field (Holtermann et al. 2010), and the three dominant P. pungens var pungens/P. pungens var. cingulata genotypes observed in Puget Sound were detected in one presumably hybridized strain of P. pungens isolated from the Washington coast (NA233, Casteleyn et al. 2009). Pseudo-nitzschia pungens var. pungens and P. pungens var. cingulata have been previously detected together in summer samples from the coast of British Columbia and the offshore Juan de Fuca eddy (Casteleyn et al. 2009). In contrast, a November 2004 sample from Puget Sound contained only genotypes corresponding to P. pungens var. cingulata (Hubbard et al. 2008). The coexistence of at least three distinct ITS1 types within summer communities in Puget Sound, and the potential maintenance of only P. pungens var. cingulata within winter communities demonstrates that intraspecific diversity in P. pungens is dynamic in space/time. Furthermore, the genotypes associated with P. pungens likely reflect physiologically distinct populations, as has been observed in Puget Sound populations of the diatom Ditylum brightwellii (Rynearson et al. 2006). Although P. multiseries and P. pungens were similarly correlated with some environmental variables, less intraspecific diversity has been observed, regionally and globally, within P. multiseries (this study, Hubbard et al. 2008).
The community at depth in the Strait of Juan de Fuca was distinct from other locations in Puget Sound. Pseudo-nitzschia multiseries, although present in surface waters, was absent at depth, and the relative contribution from P. pungens was minimal (observed in only one replicate at 115 m depth). Instead, relatively high proportions of P. fraudulenta and P. fryxelliana were observed; these species were significantly correlated with salinity, and were likely advected inland from coastal waters via estuarine circulation. The detection of the corresponding ITS1 fragment (207 bp) for P. fryxelliana in previously generated ARISA data from the coastal and offshore waters of the eastern Pacific (Hubbard et al. 2008, Ribalet et al. 2010) highlights the sensitivity of ARISA to specifically detect known and novel species diversity. Although P. fryxelliana was positively correlated with salinity, its presence in the relatively fresh 13 m Hood Canal sample (station P11), and in summer communities from the offshore iron-limited waters of the Pacific (described as P. sp. 207; Ribalet et al. 2010) and the west coast of Vancouver Island (Hubbard et al. 2008), suggests that this recently described species can persist across a broad salinity range. Pseudo-nitzschia fraudulenta has previously been observed in estuarine waters north of our survey locations (Horner et al. 1997, Rines et al. 2002), in Puget Sound (Stehr et al. 2002), as well as off the coast of Vancouver Island, British Columbia, Canada (Hubbard et al. 2008). This species, observed only in the Strait and northern Admiralty Inlet in the present study, may have a lower tolerance for estuarine conditions than other Pseudo-nitzschia species, in which case the distribution of this species may have been restricted by the lower-than-average salinity in Puget Sound during 2006 (Sutherland et al. 2011, Hickey et al. 2010).
Pseudo-nitzschia australis or P. cuspidata typically dominate toxic Pseudo-nitzschia blooms in Puget Sound and WA coastal waters (Trainer et al. 2007), although regional isolates of P. multiseries produce high DA levels in culture (Guannel et al. 2011) and P. seriata isolates elsewhere are toxic (Fehling et al. 2004, Fernandes et al. 2014). Our cruise samples from June 2006 were collected nine months after separate toxic blooms of P. cuspidata and P. australis in Puget Sound resulted in shellfish harvest closures (Trainer et al. 2007). During the cruise, P. cuspidata was only detected in Whidbey Basin and represented a minor fraction of the community. The P. australis/P. seriata complex was detected at regions of enhanced mixing, and at depth in Main Basin, and in South Sound’s Case Inlet, where ammonium concentrations were elevated during the cruise and can exceed 39 μmol L−1 due to anthropogenic input (www.ecy.wa.gov/). The abundance of P. australis in the San Francisco Bay (Howard-Armstrong et al. 2007) and P. seriata in Scottish waters (Fehling et al. 2006) was positively associated with ammonium concentration, similar to our observations in Puget Sound. During our survey, low DA concentrations (1-ppt) were detected only in shellfish from a harbor in southern Main Basin nearest P34 (Quartermaster Harbor on Vashon Island; pers. communication with Jerry Borchert, Washington State Department of Health). It is not clear whether the low levels of DA in shellfish could be attributed to low abundance and/or toxicity of P. australis/P. seriata or to less toxic species such as P. pungens or P. delicatissima.
It is interesting to consider the spatial and temporal maintenance of Pseudo-nitzschia species within Puget Sound, given the influx of coastal waters at depth that provide a source of new diversity into this estuarine system, and the surface outflow of estuarine waters and diversity that can become entrained in alongshore (Hickey et al. 1991) or cross-shelf coastal currents (Mac Fadyen et al. 2005) at the mouth of the Strait of Juan de Fuca. In contrast to the typical inland flow that occurs at depth, surface coastal waters and their associated phytoplankton communities can be transported into Puget Sound when strong onshore winds cause an overturn of estuarine circulation in the Strait of Juan de Fuca (Hickey et al. 1991). Within a Danish fjord, a genetically stable population of the diatom Skeletonema marionoi—differentiated from the oceanic S. marinoi population—has been maintained across thousands of generations, despite a lack of strong physical dispersal barriers (Härnström et al. 2011). Cells of the diatom Skeletonema marinoi form resting stages, which can remain viable within sediment for 100 years of more (Härnström et al. 2011). Sexual reproduction has been demonstrated for numerous Pseudo-nitzschia species, and thin layers of Pseudo-nitzschia spp. have been observed in a northern Puget Sound inlet in and below the euphotic zone (Rines et al. 2002), however, limited information is available on the formation and/or viability of sediment resting stages by different species (Zhang et al. 2010, Lelong et al. 2012).
Although stratified, estuarine circulation dominates throughout Puget Sound and the Strait, vertical tidal mixing causes recirculation of waters at shallow sills that separate some basins and sub-basins. Accordingly, residence times for basins and sub-basins in Puget Sound are estimated to vary from less than 1 day to over 180 days (Babson et al. 2006, Sutherland et al. 2011). Some species, including P. pungens, P. delicatissima, P. multiseries, P. australis/P. seriata, and P. fryxelliana were detected in the deep waters of basins such as Hood Canal and Whidbey Basin that experience persistent stratification with residence times that span several seasons. Assuming the deep (>50 m) subsurface cells remain viable until reintroduction to the euphotic zone, they have the potential to continuously reseed the species (and genetic) pool in Puget Sound, counteracting the strong winds, tidal and freshwater exchange that over the course of a few days can drive extensive horizontal advection of planktonic communities into or out of Puget Sound (Holbrook et al. 1980, Hickey et al. 1991, Edwards et al. 2007, Moore et al. 2008). Thus, it is likely that species assembly and coexistence throughout Puget Sound is controlled by a combination of stochastic and resource-driven processes. The relative contribution of these different processes can now be considered over broader temporal and spatial scales to better model the physical connectivity of Pseudo-nitzschia communities and populations in WA coastal and inland waters and their roles in the initiation and maintenance of toxic blooms.
The sensitive molecular detection capabilities implemented here allowed the characterization of diverse Pseudo-nitzschia assemblages and a dynamic range of associations between species and environmental conditions from a comprehensive sampling snapshot of Puget Sound. Our study adds to a growing database of studies that identify hypothesized factors related to succession in Pseudo-nitzschia species composition across varied spatial or temporal gradients (Almondoz et al. 2008, Kaczmarska et al. 2007, Klein et al. 2010, Caroppo et al. 2005, Ribalet et al. 2010, Olson and Lessard, 2010). The integration of species-specific, quantitative approaches such as ARISA into monitoring and research programs may be sufficient to tease apart taxa-specific associations with a matrix of factors involved in the growth and ecology of individual taxa of interest, including greater resolution of the bottom-up and top-down processes that promote HAB formation, toxin production, as well as bloom decline. Given the taxonomic diversity observed in planktonic field assemblages and even within this single genus, it is interesting to consider the respective ecological and biogeochemical implications of size, DNA content, or cell-based abundance estimates. A compelling question that requires more in depth consideration is whether some phytoplankton species or genotypes act as biomarkers of distinct physical or chemical environmental conditions which could be informative especially for early HAB detection.
Supplementary Material
Acknowledgments
We acknowledge the crew of the R.V. Thompson and the University of Washington Puget Sound Regional Synthesis Model (PRISM) program, funded by the University of Washington Initiative Fund (UIF), for sampling assistance. We thank E. Ostlund Lin for help with DNA sequencing, M. Guannel for isolates and assistance in the field, and F. Ribalet, M. Logsdon, C. Frazar, D. Sutherland, and T. Connolly for assistance with data visualization and/or analysis. We also thank M. Parker, J. Koester, and G. Rocap for helpful discussions about the research. This work was supported by the Pacific Northwest Center for Human Health and Ocean Sciences (National Institute of Environmental Health: P50 ES012762 and National Science Foundation: OCE-0434087); a NOAA Oceans and Human Health Graduate Traineeship award and a WHOI Doherty Postdoctoral Scholar award to K.A. Hubbard; and a Gordon and Betty Moore Foundation Marine Microbiology Investigator Award to E. V. Armbrust.
LITERATURE CITED
- Amato A, Kooistra WHCF, Ghiron JHL, Mann DG, Pröschold T, Montresor M. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist. 2007;158:193–207. doi: 10.1016/j.protis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Armstrong-Howard MD, Cochlan WP, Ladizinsky N, Kudela RM. Nitrogenous preference of toxigenic Pseudo-nitzschia australis (Bacillariophyceae) from field and laboratory experiments. Harmful Algae. 2007;6:206–217. [Google Scholar]
- Babson AL, Kawase A, MacCready P. Seasonal and interannual variability in the circulation of Puget Sound, Washington: A box model study. Atmosphere-Ocean. 2006;44:29–45. [Google Scholar]
- Bates SS. Ecophysiology and metabolism of ASP toxin production. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Heidelberg: Springer-Verlag; 1998. pp. 405–426. [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr. 1957;27:325–349. [Google Scholar]
- Brown MV, Schwalbach MS, Hewson I, Fuhrman JA. Coupling 16S-ITS rDNA clone libraries and automated ribosomal intergenic spacer analysis to show marine microbial diversity: development and application to a time series. Environ Microbiol. 2005;7:1466–1479. doi: 10.1111/j.1462-2920.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- Carpenter JH. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol Oceanogr. 1965;10:141–143. [Google Scholar]
- Casteleyn G, Evans KM, Backeljau T, D’Hondt S, Chepurnov V, Sabbe K, Vyverman W. Lack of population genetic structuring in the marine planktonic diatom Pseudo-nitzschia pungens (Bacillariophyceae) in a heterogeneous area in the Southern Bight of the North Sea. Mar Biol. 2009;156:1149–1158. [Google Scholar]
- Clarke KR, Warwick RM. Changes in marine communities: an approach to statistical analysis and interpretation. Plymouth: PRIMER-E; 2001. [Google Scholar]
- Colwell RK. EstimateS: Statistical estimation of species richness and shared species from samples. 2005 http://purl.oclc.org/estimates.
- Edwards K, Kawase M, Sarason C. Circulation in Carr Inlet, Puget Sound, during Spring 2003. Estuaries Coasts. 2007;30:945–958. [Google Scholar]
- Fehling J, Davidson K, Bolch C, Tett P. Seasonality of Pseudo-nitzschia spp. (Bacillariophyceae) in western Scottish waters. Mar Ecol Prog Ser. 2006;323:91–105. [Google Scholar]
- Fehling J, Davidson K, Bolch CJ, Bates SS. Growth and domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) under phosphate and silicate limitation. J Phycol. 2004;40:674–683. [Google Scholar]
- Fernandes LF, Hubbard KA, Richlen M, Smith J, Bates SS, Ehrmann J, Leger C, Mafra L, Kulis D, Quilliam M, Libera K, McCauley L, Anderson DM. Deep-Sea Research Part II. 2014. Diversity and toxicity of the diatom Pseudo-nitzschia Peragallo in the Gulf of Maine, Northwestern Atlantic. in press. special edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes MS, Wikfors GH. Control of domoic acid toxin expression in Pseudo-nitzschia multiseries by copper and silica: Relevance to mussel aquaculture in New England (USA) Marine Environmental Research. 2013;83:23–28. doi: 10.1016/j.marenvres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Guannel ML, Horner-Devine MC, Rocap G. Characterization of bacterial communities co-existing with species of the toxigenic diatom Pseudo-nitzschia. Aquatic Microbial Ecology 2011 [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH, editors. Culture of marine invertebrate animals. New York: Plenum Press; 1975. pp. 26–60. [Google Scholar]
- Hasle GR. Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae. 2002;1:137–146. [Google Scholar]
- Hickey BM, Thomson RE, Yih H, Leblond PH. Velocity and temperature-fluctuations in a buoyancy-driven current off Vancouver Island. Journal of Geophysical Research-Oceans. 1991;96:10507–10538. [Google Scholar]
- Holbrook JR, Muench RD, DGK, Wright C. PME, editor. Circulation in the Strait of Juan de Fuca: Recent observations in the Eastern Basin in Laboratory. Seattle, WA: NOAA; 1980. Technical Report ERL. [Google Scholar]
- Holtermann KE, Bates SS, Trainer VL, Odell A, Armbrust EV. Mass sexual reproduction in the toxigenic diatoms Pseudo-nitzschia australis and P. pungens (Bacillariophyceae) on the Washington Coast, USA. J Phycol. 2010;46:41–52. [Google Scholar]
- Horner RA, Garrison DL, Plumley FG. Harmful algal blooms and red tide problems on the US west coast. Limnol Oceanogr. 1997;42:1076–1088. [Google Scholar]
- Hubbard KA, Rocap G, Armbrust EV. Inter- and intraspecific community structure within the diatom genus Pseudo-nitzschia (Bacillariophyceae) J Phycol. 2008;44:637–649. doi: 10.1111/j.1529-8817.2008.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarska I, Reid C, Martin JL, Moniz MBJ. Morphological, biological, and molecular characteristics of the diatom Pseudo-nitzschia delicatissima from the Canadian Maritimes. Botany-Botanique. 2008;86:763–772. [Google Scholar]
- Klein C, Claquin P, Bouchart V, Le Roy B, Veron B. Dynamics of Pseudo-nitzschia spp. and domoic acid production in a macrotidal ecosystem of the Eastern English Channel (Normandy, France) Harmful Algae. 2010;9:218–226. [Google Scholar]
- Lefebvre KA, Robertson A. Domoic acid and human exposure risks: A review. Toxicon. 2010;56:218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Lelong A, Hégaret H, Soudant P, Bates SS. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia. 2012;51:168–216. [Google Scholar]
- Levene H. In: Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Olkin I, et al., editors. Stanford University Press; Stanford, CA: 1960. pp. 278–292. [Google Scholar]
- Lim HC, Leaw CP, Su SNP, Teng ST, Usup G, Mohammad-Noor N, Lundholm N, Kotaki Y, Lim PT. Morphology and molecular characterization of Pseudo-nitzschia (Bacillariophyceae) from Malaysian Borneo, including the new species Pseudo-nitzschia circumpora sp. nov. J Phycol. 2012;48:1232–1247. doi: 10.1111/j.1529-8817.2012.01213.x. [DOI] [PubMed] [Google Scholar]
- Lim HC, Teng ST, Leaw CP, Lim PT. Three novel species in the Pseudo-nitzschia pseudodelicatissima complex: P. batesiana sp. nov., P. lundholmiae sp. nov., and P. fukuyoi sp. nov. (Bacillariophyceae) from the Strait of Malacca, Malaysia. J Phycol. 2013;49:902–916. doi: 10.1111/jpy.12101. [DOI] [PubMed] [Google Scholar]
- Loureiro S, Jauzein C, Garcés E, Collos Y, Camp J, Vaqué D. The significance of organic nutrients in the nutrition of Pseudo-nitzschia delicatissima (Bacillariophyceae) J Plankton Res. 2009;31:399–410. [Google Scholar]
- Lundholm N, Bates SS, Baugh KA, Bill BD, Connell LB, Léger C, Trainer VL. Cryptic and pseudo-cryptic diversity in diatoms with descriptions of Pseudo-nitzschia hasleana sp nov and P. fryxelliana sp nov. J Phycol. 2012;48:436–454. doi: 10.1111/j.1529-8817.2012.01132.x. [DOI] [PubMed] [Google Scholar]
- Lundholm N, Clarke A, Ellegaard M. A 100-year record of changing Pseudo-nitzschia species in a sill-fjord in Denmark related to nitrogen loading and temperature. Harmful Algae. 2010;9:449–457. [Google Scholar]
- Lundholm N, Hansen PJ, Kotaki Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar Ecol Prog Ser. 2004;273:1–15. [Google Scholar]
- Lundholm N, Moestrup O, Kotaki Y, Hoef-Emden K, Scholin C, Miller P. Inter- and intraspecific variation of the Pseudo-nitzschia delicatissima complex (Bacillariophyceae) illustrated by rRNA probes, morphological data and phylogenetic analyses. J Phycol. 2006;42:464–481. [Google Scholar]
- Magurran AE. Measuring biological diversity. Oxford, UK: Blackwell Publishing; 2004. [Google Scholar]
- McCune B, Grace JB. Analysis of Ecological Communities. Gleneden Beach, Oregon: MjM Software; 2002. [Google Scholar]
- Moore SK, Mantua NJ, Newton JA, Kawase M, Warner MJ, Kellogg JR. A descriptive analysis of temporal and spatial patterns of variability in Puget Sound oceanographic properties. Estuarine Coastal Shelf Sci. 2008;80:545–554. [Google Scholar]
- Olson MB, Lessard EJ. The influence of the Pseudo-nitzschia toxin, domoic acid, on microzooplankton grazing and growth: A field and laboratory assessment. Harmful Algae. 2010;9:540–547. [Google Scholar]
- Orive E, Perez-Aicua L, David H, Garcia-Etxebarria K, Laza-Martinez A, Seoane S, Miguel I. The genus Pseudo-nitzschia (Bacillariophyceae) in a temperate estuary with description of two new species: Pseudo-nitzschia plurisecta sp. nov. and Pseudo-nitzschia abrensis sp. nov. J Phycol. 2013;49:1192–1206. doi: 10.1111/jpy.12130. [DOI] [PubMed] [Google Scholar]
- Orsini L, Procaccini G, Sarno D, Montresor M. Multiple rDNA ITS-types within the diatom Pseudo-nitzschia delicatissima (Bacillariophyceae) and their relative abundances across a spring bloom in the Gulf of Naples. Mar Ecol Prog Ser. 2004;271:87–98. [Google Scholar]
- Penna A, Casabianca S, Perini F, Bastianini M, Riccardi E, Pigozzi S, Scardi M. Toxic Pseudo-nitzschia spp. in the northwestern Adriatic Sea: characterization of species composition by genetic and molecular quantitative analyses. J Plankton Res. 2013;35:352–366. [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd ECD, Remis RS. An Outbreak of Toxic Encephalopathy Caused by Eating Mussels Contaminated with Domoic Acid. N Engl J Med. 1990;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- Quijano-Scheggia SI, Garces E, Lundholm N, Moestrup O, Andree K, Campi J. Morphology, physiology, molecular phylogeny and sexual compatibility of the cryptic Pseudo-nitzschia delicatissima complex (Bacillariophyta), including the description of P. arenysensis sp nov. Phycologia. 2009;48:492–509. [Google Scholar]
- Ribalet F, Marchetti A, Hubbard KA, Brown K, Durkin CA, Morales R, Robert M, Swalwell JE, Tortell PD, Armbrust EV. Unveiling a phytoplankton hotspot at a narrow boundary between coastal and offshore waters. PNAS. 2010;107:16571–16576. doi: 10.1073/pnas.1005638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rines JEB, Donaghay PL, Dekshenieks MM, Sullivan JM, Twardowski MS. Thin layers and camouflage: hidden Pseudo-nitzschia spp. (Bacillariophyceae) populations in a fjord in the San Juan Islands, Washington, USA. Mar Ecol Prog Ser. 2002;225:123–137. [Google Scholar]
- Rynearson TA, Newton JA, Armbrust EV. Spring bloom development, genetic variation, and population succession in the planktonic diatom Ditylum brightwellii. Limnol Oceanogr. 2006;51:1249–1261. [Google Scholar]
- Sazhin AF, Artigas LF, Nejstgaard JC, Frischer ME. The colonization of two Phaeocystis species (Prymnesiophyceae) by pennate diatoms and other protists: a significant contribution to colony biomass. Biogeochemistry. 2007;83:137–145. [Google Scholar]
- Shapiro SS, Wilk MB, Chen HJ. A comparative study of various tests for normality. J Am Stat Assoc. 1968;63:1343–1372. [Google Scholar]
- Schoemann V, Becquevort S, Stefels J, Rousseau V, Lancelot C. Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. J Sea Res. 2005;53:43–66. [Google Scholar]
- Stehr CM, Connell L, Baugh KA, Bill BD, Adams NG, Trainer VL. Morphological, toxicological, and genetic differences among Pseudo-nitzschia (Bacillariophyceae) species in inland embayments and outer coastal waters of Washington state, USA. J Phycol. 2002;38:55–65. [Google Scholar]
- Sutherland DA, MacCready P, Banas NS, Smedstad LF. A Model Study of the Salish Sea Estuarine Circulation. J Phys Oceanogr. 2011;41:1125–1143. [Google Scholar]
- Tatters AO, Fu FX, Hutchins DA. High CO2 and Silicate Limitation Synergistically Increase the Toxicity of Pseudo-nitzshia fraudulenta. Plos One. 2012;7:e32116. doi: 10.1371/journal.pone.0032116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terseleer N, Gypens N, Lancelot C. Factors controlling the production of domoic acid by Pseudo-nitzschia (Bacillariophyceae): A model study. Harmful Algae. 2013;24:45–53. [Google Scholar]
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, Trick CG. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae. 2012;14:271–300. [Google Scholar]
- Trainer VL, Cochlan WP, Erickson A, Bill BD, Cox FH, Borchert JA, Lefebvre KA. Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae. 2007;6:449–459. [Google Scholar]
- United Nations Educational S, and Cultural Organization (UNESCO) Protocols for the Joint Global Ocean Flux Study (JGOFS) core measurements. 1994. [Google Scholar]
- Zhang Y, Lu S, Zhang C, Gao Y. Distribution and germination of viable diatom resting stage cells in sediments of the East China Sea. Acta Oceanol Sin. 2010;29:121–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.