Abstract
Primary intracranial germ cell tumors are rare, representing less than 5% of all central nervous system tumors. Overall, the majority of germ cell tumors are germinomas and approximately one-third are non-germinomatous germ cell tumors (NGGCT), which include teratoma, embryonal carcinoma, yolk sac tumor (endodermal sinus tumor), choriocarcinoma, or mixed malignant germ cell tumor. Germ cell tumors may secrete detectable levels of proteins into the blood and/or cerebrospinal fluid, and these proteins can be used for diagnostic purposes or to monitor tumor recurrence. Germinomas have long been known to be highly curable with radiation therapy alone. However, many late effects of whole brain or craniospinal irradiation have been well documented. Strategies have been developed to reduce the dose and volume of radiation therapy, often in combination with chemotherapy. In contrast, patients with NGGCT have a poorer prognosis, with about 60% cured with multimodality chemoradiation. There are no standard approaches for relapsed germ cell tumors. Options may be limited by prior treatment. Radiation therapy has been utilized alone or in combination with chemotherapy or high-dose chemotherapy and transplant. We discuss two cases and review options for frameless radiosurgery or fractionated radiotherapy.
Keywords: Stereotactic Radiosurgery, frameless stereotactic radiotherapy, radiation oncology, gamma knife, linac, head immobilization, cns germ cell tumor, re-irradiation
Introduction and background
Primary intracranial germ cell tumors (IGT) are rare, representing less than 5% of all central nervous system tumors in Western series [1-2] but may be more common in East Asia [3-4]. These tumors most commonly occur in the suprasellar cistern and pineal gland and have a male predominance. Overall, the majority of germ cell tumors are germinomas and approximately one-third are non-germinomatous germ cell tumors (NGGCT), which include teratoma, embryonal carcinoma, yolk sac tumor (endodermal sinus tumor), choriocarcinoma, or mixed malignant germ cell tumor. Embryonal or endodermal sinus tumors are more common in adolescence and young adulthood [3]. Germ cell tumors may secrete detectable levels of proteins into the blood and/or cerebrospinal fluid (CSF), and beta-human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) are used for diagnostic purposes and monitor tumor recurrence. Pure germinomas may have elevated HCG [5]. Elevated serum or CSF HCG > 50 mIU/mL and/or elevated AFP are generally considered consistent with NGGCT and biopsy is not required.
Germinomas have long been known to be highly curable with radiation therapy (RT) alone. However, the late effects of whole brain or craniospinal irradiation (CSI) have been well documented, with adverse impacts on hearing, endocrine regulation, neurocognitive function, and risk of secondary malignancies [6-8]. To mitigate these risks, strategies have been developed to reduce the dose and volume of radiation therapy, often in combination with chemotherapy. In contrast, only about 20-45% of patients with NGGCT can be cured following radiation therapy alone, though results are improved to about 60% with multimodality chemoradiation [1].
The focus of this paper is to discuss treatment options for locally relapsed IGT without dissemination and to investigate patient and/or tumor characteristics that may affect the choice of re-irradiation modalities, such as stereotactic radiosurgery (SRS), hypofractionated fractionated stereotactic radiotherapy (FSRT), or full dose re-irradiation with external beam RT.
Case reports
Case 1
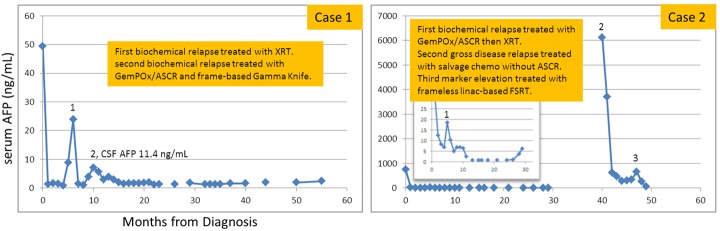
A 16-year-old Hispanic male without prior health problems presented with gradual memory loss and severe headache; an MRI brain with gadolinium revealed an enhancing 3.5 x 3.4 x 3.7 cm pineal gland tumor (Figure 1). His serum AFP was 49.3 ng/mL and CSF AFP was 33.9 ng/mL (Figure 2). Both serum and CSF HCG were negative. An MRI spine and CSF cytology were negative. He had hydrocephalus and an intratumoral hemorrhage following a ventriculostomy and ventriculoperitoneal (VP) shunt placement (Table 1). His neurological status deteriorated and he became unresponsive. Because of his intratumoral bleed and performance status, he was treated with systemic chemotherapy as per the Children’s Oncology Group (COG) Trial ACSN0122 with alternating carboplatin/etoposide and ifosfamide/etoposide. Following his first cycle of chemotherapy, he began to neurologically recover and his tumor markers normalized after two cycles of chemotherapy. After six cycles of chemotherapy, his serum and CSF tumor markers remained undetectable with a residual 1.3 x 2.1 x 1.3 cm enhancing pineal gland mass. About six weeks post-chemotherapy and before RT, his serum AFP rose to 8.9 ng/mL (institutional high normal: 7.3 ng/mL). MRI of the spine was negative. Although concerned about relapse, we began whole ventricular irradiation (WVI) and intensity-modulated radiation therapy (IMRT) with an intended dose of 30.6 Gy (Figure 3). Two weeks after starting WVI, his serum AFP increased to 23.9 ng/mL, and five days later was 15.3 ng/mL. With this AFP elevation, we changed his WVI to 36 Gy and subsequently completed an IMRT boost to the pineal gland to a cumulative total dose of 54 Gy. After peaking at 23.9 ng/mL early during RT, his serum and CSF AFP became undetectable one-month post-RT. His MRI brain showed a continued mild decrease in the size of enhancing residual tissue. Unfortunately, three months after RT, his CSF AFP was elevated at 11.4 ng/mL (serum 5.4 ng/mL), and MRI of the brain showed an interval increase in the size of enhancing tissue of the pineal gland. He was enrolled in a clinical trial of gemcitabine, paclitaxel, and oxaliplatin (GemPOx), and his CSF AFP became undetectable. After three cycles of GemPOx, he proceeded to consolidation chemotherapy with carboplatin, etoposide, and thiotepa, followed by autologous hematopoietic stem cell rescue (ASCR). He tolerated the transplant well and was discharged on Day 20. He was subsequently referred for stereotactic radiosurgery at an adult hospital where he received treatment on Day 97 (Table 2). He was treated with Gamma Knife (Elekta, Stockholm, Sweden) SRS to 18 Gy in one fraction (Figure 3, Table 3). With 34 months of follow-up post-SRS, his tumor markers remain normal with a stable MRI of the brain.
Figure 1. Serial sagittal T1-weighted MRI brain scans with gadolinium.

WVI = whole ventricular irradiation; CSI = craniospinal irradiation; FSRT = fractionated stereotactic radiotherapy; RT = radiotherapy
Figure 2. Serial values for serum AFP.
Table 1. Clinical Characteristics at Presentation, First and Second Recurrences.
M = male; sAFP = serum AFP; cAFP = CSF AFP; VP = ventriculoperitoneal; carbo = carboplatin; ifos = ifosfamide; VP-16 = etoposide; chemo = chemotherapy; WVI = whole ventricular irradiation; CR = complete response; RT = radiation therapy; ETV = endoscopic third ventriculostomy; PR = partial response; GemPOx = gemcitabine, paclitaxel, oxaliplatin; ASCR = autologous hematopoietic stem cell rescue; CSI = craniospinal irradiation; RT = radiotherapy
| Case | Age / Sex | Histology | Extent of Disease | Tumor Markers | Surgery | Chemo | Chemo Response | Progression | Treatment & Response | Second Recurrence |
| 1 | 16M | - | Pineal | sAFP 49.3 ng/mL, cAFP 33.9 ng/mL | Tumor bleed and VP shunt | Carbo/VP-16, Ifos/VP-16 | CR post-6th cycle | 1 month post-chemo, sAFP 8.9 ng/mL | WVI 36 Gy, plus boost to 54 Gy, CR | 3 months post-RT, cAFP 11.4 ng/mL |
| 2 | 17M | Yolk sac 80% and germinoma 20% | Pineal | sAFP 755 ng/mL, cAFP 350 ng/mL | ETV and biopsy | Carbo/VP-16, Ifos/VP-16 | PR post-6th cycle | 2 months post-chemo, sAFP 17.8 ng/mL | GemPOx with ASCR, PR, sAFP 5.2 ng/mL, then CSI 36 Gy, plus boost to 54 Gy, CR | 29 months post-RT, sAFP 6120 ng/mL, and cAFP 3000 ng/mL |
Figure 3. Comparison of initial and re-irradiation treatment plans (axial, coronal, and sagittal images) for Case 1.
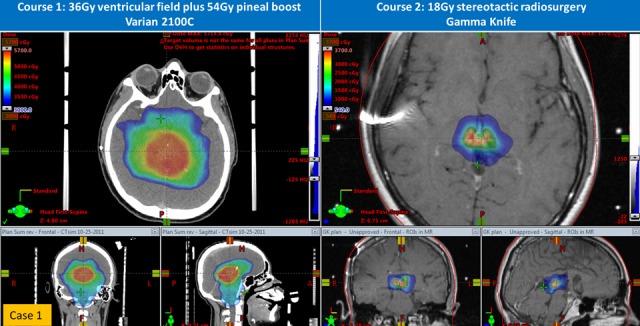
Table 2. Clinical Characteristics at Second and Third Recurrences.
FU = Follow up; GemPOx = gemcitabine, paclitaxel, oxaliplatin; ASCR = autologous hematopoietic stem cell rescue; GK SRS = Gamma Knife stereotactic radiosurgery; CR = complete response; ifos = ifosfamide; VP-16 = etoposide; sAFP = serum AFP; PR = partial response; chemo = chemotherapy; FSRT = fractionated stereotactic radiotherapy
| Case | Second Recurrence / Extent of Disease | Subsequent Treatment | Response | Third Recurrence | Subsequent Treatment | Response | FU Post-progression |
| 1 | Tumor marker elevation | GemPOx with ASCR, GK SRS | CR | - | - | - | Alive, 34 months |
| 2 | Pineal gross disease and tumor marker elevation | Cisplatin/Ifos/VP-16, BCNU/VP-16/Cisplatin | PR | 1 month post-chemo, sAFP 490 ng/mL | FSRT followed by oral VP-16 & thalidomide | PR, sAFP 19.8 ng/mL | Alive, 3 months |
Table 3. Comparison of Different Radiosurgery Techniques for Case 1 and Case 2.
*dose to PTV overlapping brainstem
**Indices derived for total PTV (includes volume overlapping brainstem)
RT1 = first course of radiotherapy; RT2 = second course of radiotherapy; IMRT = intensity-modulated radiation therapy; SRS = stereotactic radiosurgery
| Case | Technique & Interval Between RT1 & RT2 | Immobilization | PTV Volume & Prescription Dose | Shots / Beams | Dose Statistics | Conformity | Gradient | |
| 1 | Gamma Knife RT to SRS: 9.5 months | Head frame | 2.4 cm3, 18 Gy to 50% isodose line | 14 shots | 18 Gy margin, 36 Gy max | 1.46 | 2.94 | |
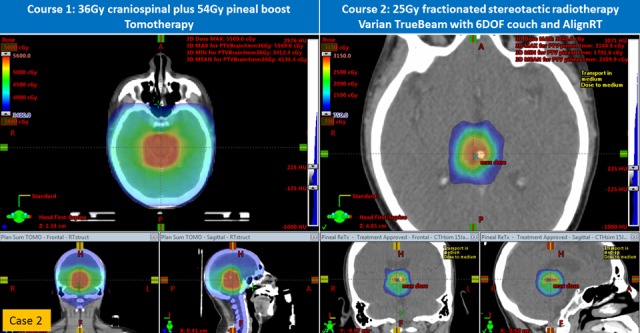
| 2 | Dose painting IMRT RT to FSRT: 36 months | Frameless vacuum-assisted mouthpiece with surface imaging | 4.3 cm3, 25 Gy to 79.4% with limit of 20 Gy to brainstem | 8 beam non-coplanar IMRT | 26.4 Gy mean, 31.5 Gy max | 19.1 Gy* mean, 22.8 Gy* max | 0.59** | 0.91** |
Case 2
A 17-year-old Hispanic male without prior health problems presented with headaches and multiple episodes of vomiting. He had an MRI of the brain, which showed a pineal gland tumor with hydrocephalus (Figure 1). Upon transfer to our institution, he had an endoscopic third ventriculostomy and biopsy, which revealed a mixed malignant germ cell tumor (80% yolk sac and 20% germinoma). His serum AFP was 755 ng/mL, a normal serum HCG, and the CSF AFP was 350 ng/mL (CSF HCG: 13). An MRI of the brain revealed a 1.5 x 1.3 x 1.3 cm T1-enhancing pineal region mass. An MRI of the spine and CSF cytology were negative. He was also treated as per COG ACNS0122 with six cycles of chemotherapy. His serum AFP reached a nadir of 7 ng/mL and CSF AFP was 17.4 ng/mL; serum and CSF HCG were negative. MRI of the brain showed only a small residual enhancement in the region of the pineal gland. About six weeks after chemotherapy and before his planned RT, his serum AFP rose to 17.8 ng/mL and the CSF AFP rose to 26.5 ng/mL (see inset graph on Figure 2). Instead of proceeding to RT as in Case 1, our patient was enrolled on the GemPOx clinical trial; after four cycles, his serum and CSF AFP decreased to 4.6 and 5.2 ng/mL, respectively, (Table 1). With post-chemotherapy serum and AFP stable at 5.2 and 6.8 ng/mL, respectively, he proceeded to consolidation chemotherapy with ASCR. After he recovered from the transplant, he started RT with 36 Gy CSI with TomoTherapy® (Accuray, Inc., Sunnyvale, CA) followed by an IMRT boost to the pineal gland for a cumulative dose of 54 Gy (Figure 4). One month post-RT, his serum and CSF AFP became undetectable. He was then followed for 16.5 months, after which he was lost to follow-up. He returned almost one year later with morning headaches and an MRI of the brain showed a large partially hemorrhagic, enhancing pineal region mass measuring 3.6 x 3.1 x 3.4 cm (Figure 1). He had a markedly elevated serum AFP, and CSF AFP was over 3,000 ng/mL (Figure 2). MRI of the spine was negative for leptomeningeal metastases with negative CSF cytology. He was salvaged with systemic chemotherapy (cisplatin, ifosfamide, etoposide for five cycles with one intervening cycle of BCNU, etoposide, and cisplatin). Initially, his serum AFP rapidly declined with chemotherapy but plateaued with a mean of 268 ng/mL. Because of prior treatment, his hematopoietic cell recovery was prolonged. After the sixth cycle of chemotherapy, his serum AFP rose to 490 ng/mL. At this point, he was considered for re-irradiation with SRS. MRI of the brain demonstrated a residual enhancing mass measuring 1.7 x 1.7 x 1.4 cm intimately associated with the thalamus, tectum, and midbrain. With a history of prior RT and involvement of brainstem and thalamus, we decided to offer fractionated stereotactic radiotherapy rather than single fraction SRS. The patient underwent CT simulation with a vacuum-assisted mouthpiece head immobilization system with 1.5 mm slice spacing and intravenous contrast. A gadolinium-enhanced MRI of the brain with 1 mm spacing was obtained and rigidly registered with the simulation CT scan. The gross target volume (GTV) was defined by a team of radiation oncologists, a neuroradiologist, and a neurosurgeon. A 1 mm margin was added to create the planning target volume. A dose of 25 Gy in five fractions was prescribed with a constraint of 20 Gy to the brainstem (Table 3). Dose-painting IMRT (Figure 4) was planned with the Eclipse treatment planning system, version 13.6 (Varian, Palo Alto, CA), and delivered on a Varian TrueBeam with a PerfectPitch™ 6-DOF (degrees of freedom) couch (Varian, Palo Alto, CA) with kVue couch top (Qfix, Avondale, PA). Cone beam CT (CBCT) daily image guidance was used for alignment to the calcified portion of the residual tumor. Intrafraction real-time optical surface monitoring system (OSMS) was performed with surface imaging using AlignRT (VisionRT, London, UK). The patient tolerated FSRT well with Grade 2 fatigue. At the start of the FSRT, his serum AFP was 656 ng/mL, peaked at 832 ng/mL, and decreased by 40% two weeks after FSRT. Oral etoposide and thalidomide were then added, and 2.5 months post-treatment, the serum AFP fell to 6.9 ng/mL.
Figure 4. Comparison of initial and re-irradiation treatment plans (axial, coronal, and sagittal images) for Case 2.
Review
These two cases contribute insight to the series demonstrating that recurrent germ cell tumors can be sensitive to chemotherapy and re-irradiation [9-12]. In the series described by Zissiadis, et al. [9], one patient with NGGCT recurred after subtotal resection, chemotherapy, and CSI. That patient, who subsequently received high-dose chemotherapy with ASCR and 15 Gy SRS, was alive at 32 months post-salvage therapy. Modak, et al. [10] described 21 relapsed IGT patient treated with high-dose chemotherapy and ASCR. There were five survivors among the twelve patients with NGGCT, and of those survivors, two had RT and one was treated focally, but not with SRS. Hasegawa, et al. [11] successfully salvaged a patient with chemotherapy and Gamma Knife SRS. In contrast, chemotherapy alone is not likely to be effective up front [1, 13-15] or at relapse [16], and avoiding RT with high-dose chemotherapy and ASCR is uncertain [10, 12, 17].
Consideration for re-irradiation must take into account size and location of tumor recurrence, prior treatments, the time interval from prior radiation therapy, the proximity of organs-at-risk (OAR), and the need for anesthesia. Not all patients are candidates for single fraction radiosurgery. In cases where re-irradiation has been performed with curative intent for medulloblastoma or ependymoma, brainstem toxicity has been an issue [18-22]. Less toxicity has been described with FSRT or conventional fractionation [23-24], and thus, these may be safer techniques. Effective palliation in children with recurrent or metastatic tumors with frameless SRS or FSRT can be achieved with attention to cumulative doses to critical structures [25]. Similarly, palliation in adults with brainstem metastases with the CyberKnife SRS/FSRT has been described with limited acute brainstem toxicity [26].
Our second case highlights some of the potential advantages of frameless radiosurgery, which include increased patient comfort, ability to fractionate treatment, greater time for the multidisciplinary team review of imaging, contours, and dosimetry, and shorter daily treatment appointments. For children, frame placement may be a higher risk due to their thinner and softer skulls and need for sedation, so frameless FSRT can be more a more acceptable option. When re-irradiation is planned, a diagnostic MRI should be obtained within two weeks of the simulation scan [27].
There are three main types of frameless immobilization: thermoplastic mask, open thermoplastic mask with or without bite block, and upper jaw fixation devices (bite block or vacuum-assisted mouthpieces) [28]. These devices are commonly used in conjunction with custom cushions conformed to the head or head and shoulder. Thermoplastic masks and vacuum-assisted mouthpiece systems seem to have similar accuracy and precision [29-31], although masks tend to be less rigid. In addition, some investigators have found the mask to be more comfortable [29]. However, in our experience over the past decade, children by far chose the mouthpiece system over a closed thermoplastic mask, which was described as ”scary” and “too tight.” We have used the vacuum-assisted mouthpiece with high accuracy in infants or edentulous patients [32].
Treatments can be planned with cylindrical collimators, dynamic conformal arcs, 3D conformal beams, IMRT (step-and-shoot or sliding window, coplanar or non-coplanar), volumetric modulated arc therapy (VMAT), or proton beams [9, 25, 33-38]. With the Extend frameless immobilization system (Elekta, Stockholm, Sweden) [39], fractionated Gamma Knife radiosurgery is possible [40] and is further supported by CBCT in the Icon system (Elekta, Stockholm, Sweden). Case 2 was treated with IMRT in order to reduce the dose to the adjacent brainstem, with IMRT being the best way to achieve dose-painting for a simultaneous integrated boost.
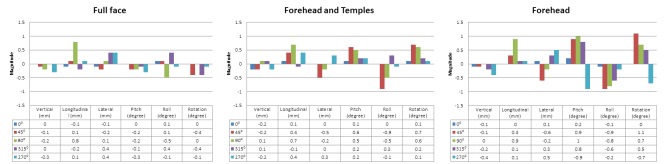
Treatment delivery can be accomplished on a variety of different platforms with different equipment, including linear accelerators, Gamma Knife, CyberKnife, TomoTherapy, or protons (Table 4). Some consider frameless immobilization systems to be less precise, even though patients can shift within frames and most frame-based systems ignore rotational shifts. To address this concern, orthogonal or stereoscopic kilovoltage (kV) or CBCT imaging guidance can permit shifts to correct for setup or immobilization inaccuracies. The time required for image guidance (acquisition, review, and adjustment) in the second case was a mean of 9 minutes (range: 4-13) and was reasonable and comparable to other investigators [34, 41]. Our workflow was similar to that described by Li, et al. [29]. In some centers, as a proxy for intrafraction motion, post-treatment imaging is often performed. More recently, real-time intrafraction monitoring can be performed with surrogate markers or the body surface and can interrupt treatment when movement exceeds a predefined tolerance (1-2 mm and 1°) [29, 41-42]. At our institution, we conducted a phantom study, which demonstrated the variability of the OSMS when the region of interest was decreased in size and as the couch angle changed (Figure 5). Based on these results, we utilized an intermediate patch monitoring the forehead and temples which were not obscured by the mouthpiece system. Over five treatments, the patient had very small intrafraction shifts (Table 5) while immobilized for a mean of 29 minutes (range: 21-42 min) with a mean treatment time of 19 minutes (range: 12-27 min). Mayo, et al. noted that their treatment times with noncoplanar VMAT were about 20 minutes and shorter than the 45-60 minutes required for frame-based treatment [34].
Table 4. Literature Review of Frameless Radiosurgery (Selected Series).
Ref = references; Pre-RT = pre-radiation therapy; HD = high definition; TPS = treatment planning system; IR = Infrared camera system with 4-6 reflectors or emitters mounted on bite-block tray; OSMS = optical surface monitoring system (AlignRT); kV = kilovoltage imaging; CBCT = cone beam CT; DOF = degrees of freedom; HD = high definition; MLC = multileaf collimator; SRS = stereotactic radiosurgery; VMAT = volumetric modulated arc therapy; FSRT = fractionated stereotactic radiotherapy; OBI = on-board imaging; GK = Gamma Knife; DCA = dynamic conformal arc; FFF = flattening fillter free; N = number; mets = metastases; OBI = on-board imager; AVM = arteriovenous malformation; CI = conformity index; HI = homogeneity index; GTV = gross target volume; PTV = planning target volume; IMRT = intensity modulate radiation therapy
| First Author [Ref], Institution, Publication year | Equipment | Image Guidance, Robotic Couch, Intrafraction Motion | Pre-RT Scans, Immobilization Devices | Patients | Technique, TPS | Notes, Results, or Conclusions |
| Mancosu [43] Milan-Rozzano 2016 | Varian Edge 120HD MLC | kV/CBCT 6-DOF couch OSMS | CT MRI | Phantom | - | Study of Edge linac with OSMS and CBCT. Tested ability of OSMS vs. CBCT ability to detect facial movements at isocenter, ability to recognize shifts, at different couch angles, and accuracy of OSMS when a camera is blocked. Submillimeter accuracy with rotational inaccuracy of 0.3 degrees. |
| Wen [44] Henry Ford 2015 | Varian Edge 120HD MLC | kV/CBCT 6-DOF couch OSMS | - | Commissioning | FFF VMAT Cones | Report of commissioning of Edge radiosurgery system. Deviation between OSMS and CBCT was -0.4, 0.1, and 0 mm in vertical, longitudinal, and lateral dimensions. Beam data and mechanical parameters similar to TrueBeam, with advanced imaging package, 6-DOF couch, and intracranial SRS accessory package. |
| Seravalli [45] MAASTRO 2015 | Elekta Synergy 10 mm MLC | kV/CBCT Pre- & post- CBCT | CT 1.2 mm MRI 1.2 mm Mask (BlueBAG) | N = 52 Brain mets | SRS Coplanar VMAT (Pinnacle) | Process of treatment. End-to-end test. GTV-PTV margin of 2.4 - 3.1 mm. Used Quantec constraints. |
| Li [29] MSK 2015 | Varian Trilogy kV/CBCT | OSMS | Bite block (PinPoint) vs Open Mask (Freedom) | N = 25 Bite block N = 8 Mask | FSRT Coplanar beams (iPlan) | Process of care diagram. Deliberate forced moves (15 volunteers) on ref Table 1. Study of volunteer comfort ref Table 2. |
| McTyre [40] Wake Forest 2015 | Gamma Knife Perfexion | No OBI | CT MRI Bite block (Extend) | N = 34 | Fractionated GK (GammaPlan) | Meningioma, schwannoma, metastases. GTV was treated without margin. 16-32 Gy to 50% isodose line over 4-5 fractions. Optic apparatus constrained to 4 Gy tangential to tumor. Daily repositioning errors < 1.2 mm. |
| Nanda [25] Emory 2014 | Novalis Tx HD MLC | kV/CBCT IR 6-DOF | CT 0.625 mm MRI | N = 5 Pediatric | SRS/FSRT Non-coplanar DCA IMRT 12 beams | GTV-PTV 1 mm 4/5 patients required anesthesia |
| Pan [41] UCSD 2012 | TrueBeam Trilogy | OSMS | CT 1.25 mm MRI 1.25 mm Open Mask (CIVCO) | N = 44 Adults | SRS/FSRT Multiple beams Cones or VMAT (Eclipse) | GTV-PTV 1 mm. Beam hold 1-2 mm and 1°. Treatment times – CBCT mean 11 min. Median shifts 1 mm, 2 mm, 1 mm vertical, longitudinal, lateral. Treatment time 15 min (shorter for TrueBeam). Compared local control to other series. |
| Schlesinger [39] UVA 2012 | Gamma Knife Perfexion | No OBI | CT MRI Bite block (Extend) | N = first 10 | Fractionated GK (GammaPlan) | Interfraction and intrafraction performance of Extend. Mean radial setup difference was 0.64 mm, SD 0.24 mm. Mean intrafractional positional difference was 0.47 mm. Cannot account for rotations. |
| Lu [35] BIDMC 2012 | Proton | Orthogonal kV Three 2 mm gold fiducial spheres | CT Frameless | N = 1 AVM | Proton | Description of novel technique with implanted fiducials to localize AVM identified on angiography and to transfer location information to CT for proton SRS planning. |
| Tryggestad [30] JHU 2011 | Elekta Synergy S | Pre- & post- CBCT | Mask - 4 types Nonrandom study Retrospective | N = 121 | FSRT/external RT | Demonstrated masks (ref. Figure 1). Best was type-S head and shoulder mask with head and shoulder cushion with mouthpiece. Can achieve intrafraction motion of 1 mm or less, and interfraction variability of less than 3 mm. |
| Ramakrishna [31] DFCI 2010 | Novalis | Stereoscopic kV (ExacTrac) IR | Frame (Radionics) Mask (BrainLAB) | N = 102 SRS N = 7 FSRT | SRS | End-to-end overall accuracy of Novalis Body ExacTrac is 0.7 mm ± 0.3 mm. Approximately 22% of mask-immobilized patients displayed intrafraction displacement of 1-2 mm. |
| Peng [49] UF Gainesville 2010 | Elekta Synergy Varian Trilogy | CT 2 mm Mask IR CBCT | N = 15 IR N = 18 Mask | - | Comparison of IR tracking system setup with CBCT. Setup with IR resulted in setup errors of 1.2 mm determined by CBCT, versus mask and laser setup errors of 3.2 mm. FSRT should not rely on IR alone. | |
| Mayo [34] U Mass 2010 | Varian Trilogy 5 mm MLC | kV/CBCT | CT 1.25 mm MRI 1.25 mm Mask (Alpha Cradle) | N = 12 Adults Brain mets | SRS Non-coplanar VMAT (Eclipse) | GTV-PTV 1-2 mm margin. Dosimetric details compared to CyberKnife, TomoTherapy, & IMRT. Reported on CI, gradient, & HI. Phantom end-to-end testing. Compared dose rate vs. survival in cell line (ref Figure 9). |
| Keshavarzi [50] UCSD 2009 | Varian Trilogy | IR | CT 1.25 mm MRI 1.5 mm Mask (AccuForm) | N = 12 Pediatric | SRS/FSRT MLC IMRT Cones (Eclipse) | GTV-PTV margin 1-3 mm |
Figure 5. Phantom study demonstrating increased variability of OSMS-reported 6-DOF couch shifts as the region of interest size decreases at five couch angles.
OSMS = Optical Surface Monitoring System; DOF = degrees of freedom
Table 5. Six Degrees of Freedom Couch Shifts Based on Daily Image Guidance with CBCT and OSMS.
*Representative real-time delta shifts across non-coplanar treatment couch angles
OSMS = optical surface monitoring system; CBCT = cone beam CT; FSRT = fractionated stereotactic radiotherapy
| Mean Translational Shifts (mm) | Mean Rotational Shifts (°) | |||||
| Vertical | Longitudinal | Lateral | Pitch | Roll | Rotation | |
| Localization CBCT | 3.1 (1.7 to 5.4) | 0.34 (-0.4 to 1.3) | 1.0 (0.5 to 1.5) | 0.4 (0.1 to 0.7) | 0.1 (0 to 0.2) | -0.1 (-0.3 to 0.2) |
| Setup OSMS | 5.4 | -0.40 | 0.96 | 0.07 | -0.08 | 0.18 |
| Verification CBCT | 0 | 0.15 | -0.3 | 0.05 | 0.05 | 0 |
| Intrafraction OSMS* | 0.30 | -0.29 | 0.02 | 0 | -0.01 | 0.07 |
| Post-FSRT CBCT | -0.2 | 0.85 | -0.25 | 0.2 | 0.05 | 0.15 |
Some common features of the latest equipment for radiosurgery include: higher mechanical precision, higher dose rate, smaller collimators, image guidance, intrafraction motion detection, and robotic 6DOF couches. Several investigators have performed end-to-end accuracy tests [43-44] and have found the equipment to be highly accurate and suitable for frameless SRS, with GTV-PTV margins of 1-2 mm [31, 34, 41]. By comparison, an end-to-end test with older equipment utilizing 10 mm MLC leaves without 6-DOF couch advocated a GTV-PTV margin of 2.8 mm [45].
Conclusions
Overall, intracranial germ cell tumors are rare. There are no standard approaches for patients with recurrent germ cell tumors. Curative options are limited by prior treatment. For patients with pure germinomas treated initially with either radiation or chemotherapy [36, 46], high salvage rates are achieved. However, for patients with prior chemoradiation or those with relapsed NGGCT, sustained responses to commonly used salvage chemotherapy regimens are difficult to achieve. To date, cure rates of about 50% have been achieved using a salvage paradigm with an initial intensive chemotherapy to achieve minimal residual tumor, followed by high-dose chemotherapy with ASCR. However, compared to germinomas, relapsed NGGCT patients have a worse prognosis with two-thirds progressing within 18 months of treatment.
When re-irradiating recurrent IGT, the cumulative dose to the optic apparatus or brainstem will often be an issue since these tumors tend to occur in the suprasellar cistern or pineal gland. Data from re-irradiation of pediatric posterior fossa tumors or radiosurgery of lesions near critical structures can inform us about radiobiological dose constraints and guide treatment planning [47-48]. Fractionated treatments may have a lower risk of toxicity.
Frameless immobilization is the best choice for multiple repeated treatments. With our current technology and policies and procedures, we can safely and accurately deliver either SRS or FSRT. With short follow-up, decrement in the tumor markers in our second patient indicated a partial response, although further follow-up is needed to assess response and toxicity.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Current concepts and future strategies in the management of intracranial germinoma. Kortmann RD. Expert Rev Anticancer Ther. 2014;14:105–119. doi: 10.1586/14737140.2014.856268. [DOI] [PubMed] [Google Scholar]
- 2.Pediatric central nervous system germ cell tumors: a review. Echevarría ME, Fangusaro J, Goldman S. Oncologist . 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 3. Intracranial germ-cell tumors: natural history and pathogenesis. Jennings MT, Gelman R, Hochberg F. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 4.Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE, Dolecek TA, Kruchko C, Engelhard HH, Villano JL. Neuro Oncol. 2012;14:1194–1200. doi: 10.1093/neuonc/nos155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combined chemotherapy and radiation therapy for CNS germ cell tumors--the Japanese experience. Matsutani M; Japanese Pediatric Brain Tumor Study Group. J Neurooncol. 2001;54:311–316. doi: 10.1023/a:1012743707883. [DOI] [PubMed] [Google Scholar]
- 6.Factors affecting functional outcomes in long-term survivors of intracranial germinomas: a 20-year experience in a single institution. Jinguji S, Yoshimura J, Nishiyama K, Aoki H, Nagasaki K, Natsumeda M, Yoneoka Y, Fukuda M, Fujii Y. J Neurosurg Pediatr. 2013;11:454–463. doi: 10.3171/2012.12.PEDS12336. [DOI] [PubMed] [Google Scholar]
- 7.Dose-effect relationships for adverse events after cranial radiation therapy in long-term childhood cancer survivors. van Dijk IW, Cardous-Ubbink MC, van der Pal HJ, Heinen RC, van Leeuwen FE, Oldenburger F, van Os RM, Ronckers CM, Schouten-van Meeteren AY, Caron HN, Koning CC, Kremer LC. Int J Radiat Oncol Biol Phys. 2013;85:768–775. doi: 10.1016/j.ijrobp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, Krull KR, Robison LL, Packer RJ. J Natl Cancer Inst. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stereotactic radiotherapy for pediatric intracranial germ cell tumors. Zissiadis Y, Dutton S, Kieran M, Goumnerova L, Scott RM, Kooy HM, Tarbell NJ. Int J Radiat Oncol Biol Phys. 2001;51:108–112. doi: 10.1016/s0360-3016(01)01569-3. [DOI] [PubMed] [Google Scholar]
- 10.Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. Modak S, Gardner S, Dunkel IJ, Balmaceda C, Rosenblum MK, Miller DC, Halpern S, Finlay JL. J Clin Oncol. 2004;22:1934–1943. doi: 10.1200/JCO.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 11.Stereotactic radiosurgery for CNS nongerminomatous germ cell tumors. Report of four cases. Hasegawa T, Kondziolka D, Hadjipanayis CG, Flickinger JC, Lunsford LD. Pediatr Neurosurg. 2003;38:329–333. doi: 10.1159/000070417. [DOI] [PubMed] [Google Scholar]
- 12.Successful salvage using combined radiation and ABMT for patients with recurrent CNS NGGCT following failed initial transplant. Malone K, Croke J, Malone C, Malone S. BMJ Case Rep. 2012;2012:0. doi: 10.1136/bcr-2012-006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL. J Clin Oncol . 2004;22:846–853. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: failure of a primary chemotherapy approach. Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL. Pediatr Blood Cancer. 2004;43:126–133. doi: 10.1002/pbc.20026. [DOI] [PubMed] [Google Scholar]
- 15.Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. da Silva NS, Cappellano AM, Diez B, Cavalheiro S, Gardner S, Wisoff J, Kellie S, Parker R, Garvin J, Finlay J. Pediatr Blood Cancer. 2010;54:377–383. doi: 10.1002/pbc.22381. [DOI] [PubMed] [Google Scholar]
- 16.Focal and craniospinal irradiation for patients with intracranial germinoma and patterns of failure. Nguyen QN, Chang EL, Allen PK, Maor MH, Ater JL, Mahajan A, Wolff JE, Weinberg JS, Woo SY. Cancer . 2006;107:2228–2236. doi: 10.1002/cncr.22246. [DOI] [PubMed] [Google Scholar]
- 17.The role of myeloablative chemotherapy with autologous hematopoietic cell rescue in central nervous system germ cell tumors. Bouffet E. Pediatr Blood Cancer. 2010;54:644–646. doi: 10.1002/pbc.22376. [DOI] [PubMed] [Google Scholar]
- 18.A retrospective study of surgery and reirradiation for recurrent ependymoma. Merchant TE, Boop FA, Kun LE, Sanford RA. Int J Radiat Oncol Biol Phys. 2008;71:87–97. doi: 10.1016/j.ijrobp.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE. Cancer. 2014;120:3731–3737. doi: 10.1002/cncr.28907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stereotactic radiosurgery for recurrent ependymoma. Stafford SL, Pollock BE, Foote RL, Gorman DA, Nelson DF, Schomberg PJ. Cancer. 2000;88:870–875. [PubMed] [Google Scholar]
- 21.The role of Gamma Knife Radiosurgery in the management of unresectable gross disease or gross residual disease after surgery in ependymoma. Lo SS, Abdulrahman R, Desrosiers PM, Fakiris AJ, Witt TC, Worth RM, Dittmer PH, Desrosiers CM, Frost S, Timmerman RD. J Neurooncol. 2006;79:51–56. doi: 10.1007/s11060-005-9112-y. [DOI] [PubMed] [Google Scholar]
- 22.Stereotactic radiosurgery for patients with recurrent intracranial ependymomas. Stauder MC, Ni Laack N, Ahmed KA, Link MJ, Schomberg PJ, Pollock BE. J Neurooncol. 2012;108:507–512. doi: 10.1007/s11060-012-0851-2. [DOI] [PubMed] [Google Scholar]
- 23.Reirradiation for recurrent medulloblastoma. Bakst RL, Dunkel IJ, Gilheeney S, Khakoo Y, Becher O, Souweidane MM, Wolden SL. Cancer. 2011;117:4977–4982. doi: 10.1002/cncr.26148. [DOI] [PubMed] [Google Scholar]
- 24.Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Bouffet E, Hawkins CE, Ballourah W, Taylor MD, Bartels UK, Schoenhoff N, Tsangaris E, Huang A, Kulkarni A, Mabbot DJ, Laperriere N, Tabori U. Int J Radiat Oncol Biol Phys. 2012;83:1541–1548. doi: 10.1016/j.ijrobp.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 25.The feasibility of frameless stereotactic radiosurgery in the management of pediatric central nervous system tumors. Nanda R, Dhabbaan A, Janss A, Shu HK, Esiashvili N. J Neurooncol. 2014;117:329–335. doi: 10.1007/s11060-014-1392-7. [DOI] [PubMed] [Google Scholar]
- 26.CyberKnife radiosurgery for brainstem metastases: Management and outcomes and a review of the literature. Liu SH, Murovic J, Wallach J, Cui G, Soltys SG, Gibbs IC, Chang SD. J Clin Neurosci . 2016;25:105–110. doi: 10.1016/j.jocn.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Interval from imaging to treatment delivery in the radiation surgery age: How long is too long? Seymour ZA, Fogh SE, Westcott SK, Braunstein S, Larson DA, Barani IJ, Nakamura J, Sneed PK. Int J Radiat Oncol Biol Phys. 2015;93:126–132. doi: 10.1016/j.ijrobp.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Intracranial stereotactic positioning systems: Report of the American Association of Physicists in Medicine Radiation Therapy Committee Task Group no. 68. Lightstone AW, Benedict SH, Bova FJ, Solberg TD, Stern RL; American Association of Physicists in Medicine Radiation Therapy Committee. Med Phys. 2005;32:2380–2398. doi: 10.1118/1.1945347. [DOI] [PubMed] [Google Scholar]
- 29.Clinical experience with two frameless stereotactic radiosurgery (fSRS) systems using optical surface imaging for motion monitoring. Li G, Ballangrud A, Chan M, Ma R, Beal K, Yamada Y, Chan T, Lee J, Parhar P, Mechalakos J, Hunt M. http://jacmp.org/index.php/jacmp/article/view/5416. J Appl Clin Med Phys. 2015;16:149–162. doi: 10.1120/jacmp.v16i4.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inter- and intrafraction patient positioning uncertainties for intracranial radiotherapy: a study of four frameless, thermoplastic mask-based immobilization strategies using daily cone-beam CT. Tryggestad E, Christian M, Ford E, Kut C, Le Y, Sanguineti G, Song DY, Kleinberg L. Int J Radiat Oncol Biol Phys. 2011;80:281–290. doi: 10.1016/j.ijrobp.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 31.A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zygmanszki P, Hacker F. Radiother Oncol. 2010;95:109–115. doi: 10.1016/j.radonc.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Adaptation of vacuum-assisted mouthpiece head immobilization system for precision infant brain radiation therapy. Wong K, Cheng J, Bowlin K, Olch A. Pract Radiat Oncol. 2016:0. doi: 10.1016/j.prro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Intensity-modulated radiation therapy with dose painting: A brain-sparing technique for intracranial germ cell tumors. Yang JC, Terezakis SA, Dunkel IJ, Gilheeney SW, Wolden SL. Pediatr Blood Cancer. 2016;63:646–651. doi: 10.1002/pbc.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald TJ, Moser R. Int J Radiat Oncol Biol Phys. 2010;78:1457–1466. doi: 10.1016/j.ijrobp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Frameless angiogram-based stereotactic radiosurgery for treatment of arteriovenous malformations. Lu XQ, Mahadevan A, Mathiowitz G, Lin PJ, Thomas A, Kasper EM, Floyd SR, Holupka E, La Rosa S, Wang F, Stevenson MA. Int J Radiat Oncol Biol Phys. 2012;84:274–282. doi: 10.1016/j.ijrobp.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 36.Salvage treatment for recurrent intracranial germinoma after reduced-volume radiotherapy: a single-institution experience and review of the literature. Hu YW, Huang PI, Wong TT, Ho DM, Chang KP, Guo WY, Chang FC, Shiau CY, Liang ML, Lee YY, Chen HH, Yen SH, Chen YW. Int J Radiat Oncol Biol Phys. 2012;84:639–647. doi: 10.1016/j.ijrobp.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 37.Intracranial application of IMRT based radiosurgery to treat multiple or large irregular lesions and verification of infra-red frameless localization system. Lawson JD, Wang JZ, Nath SK, Rice R, Pawlicki T, Mundt AJ, Murphy K. J Neurooncol. 2010;97:59–66. doi: 10.1007/s11060-009-9987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Does intensity-modulated stereotactic radiotherapy achieve superior target conformity than conventional stereotactic radiotherapy in different intracranial tumours? Sharma SD, Jalali R, Phurailatpam RD, Gupta T. Clin Oncol (R Coll Radiol) 2009;21:408–416. doi: 10.1016/j.clon.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Interfraction and intrafraction performance of the Gamma Knife Extend system for patient positioning and immobilization. Schlesinger D, Xu Z, Taylor F, Yen CP, Sheehan J. http://thejns.org/doi/pdf/10.3171/2012.6.GKS12989. J Neurosurg . 2012;117:217–224. doi: 10.3171/2012.6.GKS12989. [DOI] [PubMed] [Google Scholar]
- 40.Emerging indications for fractionated Gamma Knife radiosurgery. McTyre E, Helis CA, Farris M, Wilkins L, Sloan D, Hinson WH, Bourland JD, Dezarn WA, Munley MT, Watabe K, Xing F, Laxton AW, Tatter SB, Chan MD. Neurosurgery . 2016;March 9, 2016:0. doi: 10.1227/NEU.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frameless, real-time, surface imaging-guided radiosurgery: clinical outcomes for brain metastases. Pan H, Cerviño LI, Pawlicki T, Jiang SB, Alksne J, Detorie N, Russell M, Carter BS, Murphy KT, Mundt AJ, Chen C, Lawson JD. Neurosurgery. 2012;71:844–851. doi: 10.1227/NEU.0b013e3182647ad5. [DOI] [PubMed] [Google Scholar]
- 42.Motion monitoring for cranial frameless stereotactic radiosurgery using video-based three-dimensional optical surface imaging. Li G, Ballangrud A, Kuo LC, Kang H, Kirov A, Lovelock M, Yamada Y, Mechalakos J, Amols H. Med Phys. 2011;38:3981–3994. doi: 10.1118/1.3596526. [DOI] [PubMed] [Google Scholar]
- 43.Accuracy evaluation of the optical surface monitoring system on EDGE linear accelerator in a phantom study. Mancosu P, Fogliata A, Stravato A, Tomatis S, Cozzi L, Scorsetti M. Med Dosim. 2016:0. doi: 10.1016/j.meddos.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Characteristics of a novel treatment system for linear accelerator-based stereotactic radiosurgery. Wen N, Li H, Song K, Chin-Snyder K, Qin Y, Kim J, Bellon M, Gulam M, Gardner S, Doemer A, Devpura S, Gordon J, Chetty I, Siddiqui F, Ajlouni M, Pompa R, Hammoud Z, Simoff M, Kalkanis S, Movsas B, Siddiqui MS. http://www.jacmp.org/index.php/jacmp/article/view/5313. J Appl Clin Med Phys. 2015;16:125–148. doi: 10.1120/jacmp.v16i4.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A comprehensive evaluation of treatment accuracy, including end-to-end tests and clinical data, applied to intracranial stereotactic radiotherapy. Seravalli E, van Haaren PMA, van der Toorn PP, Hurkmans CW. Radiother Oncol. 2015;116:131–138. doi: 10.1016/j.radonc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Radiation therapy for relapsed CNS germinoma after primary chemotherapy. Merchant TE, Davis BJ, Sheldon JM, Leibel SA. http://jco.ascopubs.org/content/16/1/204.short. J Clin Oncol. 1998;16:204–209. doi: 10.1200/JCO.1998.16.1.204. [DOI] [PubMed] [Google Scholar]
- 47.Radiation tolerance limits of the brainstem. Sharma MS, Kondziolka D, Khan A, Kano H, Niranjan A, Flickinger JC, Lunsford LD. Neurosurgery. 2008;63:728–732. doi: 10.1227/01.NEU.0000325726.72815.22. [DOI] [PubMed] [Google Scholar]
- 48.Dose-volume effects on brainstem dose tolerance in radiosurgery. Xue J, Goldman HW, Grimm J, LaCouture T, Chen Y, Hughes L, Yorke E. http://thejns.org/doi/abs/10.3171/2012.7.GKS12962. J Neurosurg. 2012;117:189–196. doi: 10.3171/2012.7.GKS12962. [DOI] [PubMed] [Google Scholar]
- 49.Quality assessment of frameless fractionated stereotactic radiotherapy using cone beam computed tomography. Peng LC, Kahler D, Samant S, Li J, Amdur R, Palta JR, Liu C. Int J Radiat Oncol Biol Phys. 2010;78:1586–1593. doi: 10.1016/j.ijrobp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Initial clinical experience with frameless optically guided stereotactic radiosurgery/radiotherapy in pediatric patients. Keshavarzi S, Meltzer H, Ben-Haim S, Newman CB, Lawson JD, Levy ML, Murphy K. Childs Nerv Syst. 2009;25:837–844. doi: 10.1007/s00381-009-0840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]