Abstract
Management of enterocutaneous fistula represents one of the most protracted and difficult problems in colorectal surgery with substantial morbidity and mortality rates. This article summarizes the current classification systems and successful management protocols, provides an in-depth review of fluid resuscitation, sepsis control, nutrition management, medication management of output quantity, wound care, nonoperative intervention measures, operative timeline, and considerations, and discusses special considerations such as inflammatory bowel disease and enteroatmospheric fistula.
Keywords: enterocutaneous fistula, enteroatmospheric fistula, spontaneous closure, mortality, timeline
An enterocutaneous fistula (ECF) is an aberrant connection between the intra-abdominal gastrointestinal (GI) tract and skin/wound. Because of differences in management and significant preponderance of small intestinal and colonic fistulae, fistulae originating in the rectum, upper GI tract, or pancreas will not be discussed in this article.
There are several ways in which ECF has been classified, including by output, etiology, and source.1 2 3 Most often, a high-output ECF is characterized as one with >500 mL/24 hours, low output <200 mL/24 hours, and a moderate output fistula between 200 and 500 mL/24 hours. While the great majority of ECFs are iatrogenic (75–85%), between 15 and 25% occur spontaneously.3 Common causes of iatrogenic ECF are trauma; operations for malignancy, associated with extensive adhesiolysis, or in the setting of inflammatory bowel disease (IBD); and trauma.1 With respect to postoperative small bowel fistulae, about half are from an anastomotic leak, with the other half occurring from inadvertent injury to the small bowel during dissection.3 Spontaneous fistulae occur from IBD (most common), malignancy, appendicitis, diverticulitis, radiation, tuberculosis/actinomycosis, and ischemia.3 Organ of origin is another classification used for ECF and is useful as well in the consideration of management options: type I (abdominal, esophageal, gastroduodenal), type II (small bowel), type III (large bowel), and type IV (enteroatmospheric, regardless of origin).2
Closure rates without operative intervention in the era of advanced wound care and parenteral nutrition (PN) vary considerably in reports 19 to 92%,4 5 with most studies demonstrating closure rates in the 20 to 30% range.5 6 7 8 9 10 11 With historical wound care measures, 90% of spontaneous closure occurred in the first month after sepsis resolution, with an additional 10% closing in the second month, and none closing spontaneously after 2 months.10 With vacuum assisted closure (VAC) and other negative pressure wound therapies (NPWT) therapy, there are case reports of fistulae closure well into the second and third month.12 Table 1 cites favorable and unfavorable prognostic factors for spontaneous fistula closure.3 4 6 8 13 14
Table 1. Favorable and unfavorable factors predictive of nonoperative fistula closure.
| Favorable | Unfavorable |
|---|---|
| Surgical etiology | Ileal, jejunal, nonsurgical etiology |
| Appendicitis, diverticulitis | IBD, cancer, radiation |
| Transferrin > 200 mg/dL | Transferrin < 200 mg/dL |
| No obstruction, bowel in continuity, no infection, no inflamed intestine | Distal obstruction, bowel discontinuity, adjacent infection, adjacent active inflammation |
| Length > 2 cm, end fistula | Length < 2 cm, lateral fistula, multiple fistulas |
| Output < 200 mL/24 h | Output > 500 mL/24 h |
| No sepsis, balanced electrolytes | Sepsis, electrolyte disturbances |
| Initial referral to tertiary care center and subspecialty care | Delay getting to tertiary care center and subspecialty care |
Mortality rates in different series for patients with ECF are also markedly variable in distribution (5.5–33%),4 7 15 with most deaths attributable to sepsis, malnutrition, and fluid/electrolyte disturbances.4 6 7 15 16 Factors that are predictive of high mortality are infectious and noninfectious complications, high-output fistula,1 8 15 17 and age.18 Cost of fistula care is significant and typically more than $500,000.19
Approach to Enterocutaneous Fistula
A common acronym used to describe ECF care protocol is “SNAP,” which stands for management of skin and sepsis, nutrition, definition of fistula anatomy, and proposing a procedure to address the fistula.20 21 Many authors have suggested various stepwise systems and protocols for treating ECF.13 14 22 23 24 25 In addition, it is important that the patient is treated in a center with significant experience in treating ECFs and that multidisciplinary approach is used.9 11 This later measure results in 50% decrease in mortality.26 The different components of ECF cares are listed as follows in the order of immediacy to the patient.
Initial Resuscitation and Electrolyte Repletion
Fluid and electrolyte losses should be replaced with crystalloids. Patients with high-output ECFs will often require a urinary catheter until well characterized.20 Those patients with severe dehydration and electrolyte disturbances will require serum testing of renal function and electrolytes regularly to ensure the supplementation is progressing appropriately. Daily assessment of fluid status is mandatory, as well as monitoring of all intake and output sources.
Electrolytes that often require significant supplementation are sodium, potassium, and magnesium. Hypomagnesemia may result in nausea, apathy, and neuromuscular hyperexcitability.20 High-output fistula losses from the small intestine should be replaced with normal saline with 10 mEq/L potassium chloride.23 One method that can be used to deduce the composition of the best replacement fluid composition is to measure electrolyte concentration in fistula effluent and to match the electrolyte composition closely to the replacement fluid. Adequate intravenous access is mandatory to care for patients with dehydration and high-output fistulae. In addition to standard crystalloid fluid, the frequent and multiple electrolyte supplementation, as well as antibiotics, if infection is present, will often be needed. Central venous access may be necessary and may require careful monitoring and care because of frequent infectious complications.
Treatment of Sepsis
Sepsis is responsible for 77% of mortality associated with ECF.8 Computed tomography of the abdomen and pelvis along with percutaneous drainage with radiographic guidance is essential to evaluate and treat sources of infection. Computed tomography has an accuracy of more than 97% when enhancing contrast media are used appropriately.20 Other radiologic studies, such as ultrasonography and MRI, can also be used as adjuncts. Radiologically guided drainage provides the fastest and safest route to evacuate and control significant infection. In addition, much information can be gained about fistula anatomy and the enteric source from a fistulogram, and percutaneous drainage may decompress a complex fistula and convert it to a simple one. In cases of peritonitis and without the ability to obtain source control with more conservative means, prompt fluid resuscitation, antibiotic administration, and operative control of infection are essential.
Antibiotic management should follow the Surviving Sepsis guidelines,27 and empiric coverage should not exceed 4 to 7 days.19 27 Furthermore, there is no role for antibiotic coverage in a patient with ECF whose sepsis is fully controlled with percutaneous drainage.19 Operative sepsis control should focus on infection drainage and exteriorization of the source in the small or large intestine, and no anastomoses should be created in a critically ill patient or in the setting of significant purulence or fecal contamination. Resection of healthy bowel that may be involved in an inflammatory process should be avoided. Support of organ system functions and utilization of the intensive unit care are often necessary.
Throughout the process of ECF and sepsis management, multidisciplinary care is important in the care of the fistula patient. Resolution of sepsis is mandatory in order for ECF to close spontaneously. In the state on increased catabolism, malnutrition is a predictable outcome,20 as is immunosuppression. Sepsis can also present in a more subtle, subclinical fashion. For instance, even in the absence of overt sepsis, 50% of patients with ECFs harbor intra-abdominal abscesses, most of which are amenable to percutaneous drainage.
Nutrition
Nutrition is one of three necessities upon which the life and successful treatment of a patient with ECF hinges. The other two tenets are fluid resuscitation and sepsis control. Fazio et al showed that mortality is 0% when serum albumin is > 3.5 mg/dL.16 For most patients, a combination of EN and PN will be employed, at least initially. Fistula closure rates are twice as high in those receiving adequate supplemental nutrition as opposed to those who are not.28 The goal of successful nutrition management is achieving an anabolic state with weight gain, improvement in albumin, prealbumin, and transferrin, and successful management of micronutrient needs for optimal healing.
Wound Care and Fistula Effluent Control
Establishing and maintaining effective control of fistula drainage that enables wound healing and skin protection requires a responsible and experienced multidisciplinary team of nurses and physicians. Every individual patient and fistula requires a unique approach that requires ongoing reassessment as the wound changes with time. The goals of fistula drainage and wound care are prevention of skin loss, minimization of pain and social isolation, effective control of drainage, and facilitation of wound closure.
Definition of Fistula Anatomy
Defining fistula anatomy is essential to further planning of operative repair, an optimal nutrition strategy, and patient counseling. Often, a combination of studies is necessary to fully appreciate the fistula anatomy. Computed tomography, fistulography, a small bowel follow-through study, and contrast enemas are all useful modalities for defining anatomy depending on the location of the fistula.11 Magnetic resonance enterography is another adjunct, particularly useful in patients with IBD.24 These studies should only be undertaken at least 7 to 10 days after fluid and electrolyte resuscitation, infection control, and appropriate wound treatment cares.13
Support of Patient and Family
Treatments and chronic care of the fistula are psychologically draining for patients and families. Positive attitude, short-term goal-oriented discussions, and regularly planned social activities/diversions are essential. Patients find themselves isolated, living in fear of appliance leak, physically hungry, angry, and depressed.29 Positive coping mechanisms involve teaching both the patient and his/her family members dressing changes to achieve greater independence, setting goals for daily physical activity, and involving family members at every step of the way.29
Definitive Operative Intervention
Definitive operative intervention should have the goal of creating no new enterotomy, giving the best chance of cure from ECF, and re-establishing bowel continuity whenever possible. Secondary goals should be minimization of wound cares and further operative procedures, as well as maximal bowel length preservation. The time interval in which operative intervention is associated with a significantly higher mortality has been outlined by Fazio et al, counting from the day of previous surgery: return to the operating room within 10 days resulted in 13% mortality, operating between 11 and 42 days was associated with 21% mortality, and after 42 days mortality returns to 11% in average patients.16
The absolute minimal waiting interval after original surgery to return to the operating room is 6 weeks. However, the correct calculation for an individual patient is not as simple as a rigid time interval. Besides waiting out the period of postoperative obliterative peritonitis, taking into account the density of adhesions with the most recent surgery, operative history, and the degree of sepsis treated as well as ensuring optimization of nutritional status are all important factors in considering the optimal timing for operative treatment.
Nutrition
Sources of malnutrition in a patient with ECF are three and can overlap for an individual patient: inadequate calorie intake, catabolism related to ongoing sepsis, and ongoing losses from the GI tract.25 Up to 75 g of protein can be lost from enteric secretions daily.25 Basal energy needs may be estimated using the Harris-Benedict equation. However, a patient with ECF will require 1 to 2.5 times the basal energy of a healthy adult.25 A historic sentinel publication in 1964 reported a significant difference for survival with ECF in the setting of adequate nutritional support. Patients who consumed at least 1,500 kcal/day had 3.6-fold lower mortality than those whose caloric intake did not achieve 1,500 kcal/day.30 Table 2 details energy needs for caloric intake, vitamins, and elements.13 19 22 25 31
Table 2. Nutrition needs of patient with ECF.
| Calorie requirementa (kcal/kg/d) | Protein requirement (g/kg/d) | Vitamin C | Other vitamins | Elements (zinc, copper, selenium) | |
|---|---|---|---|---|---|
| Low-output fistula | 20–30 | 1–1.5 | 5–10 times normal | At least normal | At least normal |
| High-output fistula | 25–35b | 1.5–2.5c | 10 times normal | 2 times normal | 2 times trace elements |
Lipids should constitute 20 to 30% of all calories (Berry and Fischer).3
Depending on amount of output, the actual calorie requirement may be as high as 1.5 to 2 times that of healthy adult (Polk and Schwab;Dudrick et al).22 25
Depending on fistula losses (up to 75 g of protein), this number may be higher.
Monitoring for adequate nutrition intake can also be complicated. One measure of tracking success weekly is checking albumin, prealbumin, weights, and transferrin levels in a stable patient with ECF at least weekly while an inpatient. Albumin, prealbumin, and transferrin are all acute reactants, and their levels will be inaccurate in the setting of acute physiologic distress and sepsis. Prealbumin and albumin levels provide indirect assessment of visceral protein stores, while transferrin is a principle plasma iron transport protein. In addition, anthropometric assessment based on triceps skin fold thickness (approximates body fat reserves) and midarm muscle circumference (approximates muscle mass) can be utilized in the office as low-cost, noninvasive measures of progress.32 Both albumin and transferrin levels have been shown to predict spontaneous closure rates15 16 and mortality.33 34
To adjust the initial caloric intake goal and follow patient's progress, serum albumin, prealbumin, transferrin, CRP, patient weight, and anthropometrics can be followed over time. In addition, a nitrogen balance calculation with correction for enteric losses can be used to ensure that positive nitrogen balance is achieved and maintained. To do the calculation and follow the patient sequentially, regular 24-hour urine tests should be sent for urea nitrogen levels. It is important to note that calculation of nitrogen balance is only meaningful once the patient has had resolution of sepsis. Nitrogen balance can be calculated according to the following equation22:
NB = [Protein intake (g)/6.25] − [24-hour urine urea nitrogen + 4 g + (2 g × liters output from enteric sources and wound)]
Positive nitrogen balance signifies anabolic state. Negative balance implies inadequate calorie intake, excessive GI losses, or unresolved sepsis.25
Caloric intake can be in the form of EN or PN. As long as at least 20% of caloric intake is enteric, mucosal integrity, hormonal signaling, and immune functions of the gut tend to be preserved.13 In order for EN to be at least moderately successful (whether or not PN is also supplemented), the patient needs at least 4 feet of healthy intestine from the ligament of Treitz to the external fistula opening. While enteralfeeding in the situation of high-output small bowel fistula is challenging, it is often possible with careful treatment. A sentinel study that enrolled 335 patients with high-output ECF demonstrated 85% of enrollees ultimately tolerating exclusively enteric regimen, with a 40% spontaneous fistula closure rate and 19% mortality rate.17 Similarly, Rahbour et al reported initial 33% PN use during resuscitation and only 11.9% use of PN at discharge.11
After initial fluid and electrolyte resuscitation and percutaneous or operative drainage of infection, PN is started expeditiously in high-output fistula patients and an enteric diet is started in low-output fistula patients. The goal enteric output in 24 hours is < 1.5 L.24 High-calorie supplemental drinks are strongly recommended for those who can tolerate EN. In patients requiring supplementation of their orally ingested diet, nasogastric or nasojejunal tubes may be used to help deliver EN versus a percutaneous endoscopic gastric tube or percutaneous endoscopic gastrojejunostomy tube potentially in patients incapable of taking adequate oral intake on a medium to longer term basis.
When fistula output is on the high side, attempting oral nutrition should be made with the following modifications24: (1) limit intake of low sodium fluid to 500 mL/day,(2) provide patient with oral solution high in sodium (at least 90–120 mmol/L sodium content),(3) small volume of fluid intake with solid meals, and (4) proton pump inhibitor (PPI) therapy, antimotility drugs, and octreotide (see section “Medical ManagementFistula Output”). While no randomized study of ECF outcomes comparing EN and PN has been conducted, due to complications associated with PN, and physiological and system cost advantages of EN, PN is generally recommended as a supplement or a bridge to EN. Contraindications to PN are liver dysfunction/failure and difficulty with vascular access, or infection of the vascular access device. PN is necessary in the majority of high-output fistula patients, at least initially, as it decreases GI secretions by 30 to 50%, thereby aiding with ECF closure.22 PN can be started much earlier than EN and can be used to assist with fluid and electrolyte resuscitation. In a rare patient with high-output fistula or intestinal failure due to diffuse disease, PN may be the only option. Additionally, the cost of PN is high: based on nutrition therapy alone, PN is four times as expensive as EN.35 In addition, 40% of all peripherally placed vascular devices develop some degree of DVT and up to 80% of patients present at some point with a blood-borne infection.19
In some cases, EN can be contraindicated, including when insufficient bowel length is present (<75 cm), in cases of intestinal discontinuity, if fistula output increases significantly with start of EN and leads to electrolyte disturbances, if there is symptomatic intolerance of EN, and when feeding access is unable to be established/maintained.22 It is recommended to try EN if fistula output is < 1.5 L. Polymeric formula is tried first, and, if not tolerated or fistula output increases significantly, semielemental formula can be introduced. Semielemental nutrition has been demonstrated to significantly reduce volume of fistula output.22 If semielemental feeds are not tolerated, an elemental feeding regimen should be attempted.36 While expensive and not shown to improve mortality or closure rates in EC fistula patients, immunomodulated nutrition formulas are also available.
Finally, fistuloclysis can deliver EN to a distal opening in cases where distal enteric tract is devoid of obstruction. Details of fistuloclysis technique are well described elsewhere.35 While fistuloclysis is a cost-effective therapy and is typically successful, it can be time-consuming. Most patients will experience abdominal discomfort/diarrhea in the beginning after its initiation.35 In a small cohort of patients, fistuloclysis was effective in feeding 11 out of 12 patients.35 The setup for fistuloclysis requires an ostomy appliance, a gastrostomy or another enterostomy tube, and an adapter that allows the enterostomy tube to be fixed to the ostomy appliance, as it passes through the wall of the stoma bag, and tube feed bag and tubing.35
Medical Management of Fistula Output
One of the main issues with skin integrity and initiation of EN occurs in fistulae with output exceeding 1 L/day. Nasogastric drainage tubes are to be avoided because they do not improve outcomes and contribute significantly to sinusitis, sore throat, decreased mobility, and a decrease in quality of life.9 PPIs or H2 channel blockers decrease acidity and amount of gastric secretions and are recommended as part of standard treating regimen in a high-output fistula.4 9 13 In addition, sucralfate can be used for its gastric acid neutralizing and its constipating effects.13 None of the acid reduction therapies have been shown to increase rate of fistula closure.
Antidiarrheals (loperamide, diphenoxylate/atropine, codeine, and tincture of opium) have been used widely to decrease fistula output. In a British intestinal failure center, high doses of loperamide (up to 40 mg/day) and codeine (up to 240 mg/day) are being used to control many otherwise refractory high-output fistulas with success.9 24
Somatostatin is a natural antisecretory hormone with a half-life of 1 to 2 minutes produced in the pancreas and the GI tract. Octreotide is a somatostatin analogue with a half-life of 113 minutes.37 Physiologic studies have demonstrated that octreotide treatment (three times daily) reduces the volume of pancreatic secretions.37 Despite that, pancreatic enzyme concentration in those secretions begin to rise 4 hours postinjection.38 Small cohort study suggested that octreotide effect diminishes with repeat administrations, likely due to receptor downregulation. Highest output fistulas seem to be affected the most by octreotide (twice the effect than on low-output ECF).39 Somatostatin is renally cleared, and while it presents no physiologic problem for normal patients and those with liver disease, renal disease may impair its metabolism.40
Nine randomized or prospective studies of somatostatin analogues/somatostatin versus placebo have been published between 1992 and 2009.41 42 43 44 45 46 47 48 49 Two recent meta-analyses summarize their results: somatostatin analogues and somatostatin do not improve mortality, but they seem to decrease fistula output, allow faster spontaneous closure, and decrease hospital stay.50 51 The longest follow-up interval in studies is 90 days.41 Somatostatin analogues versus control resulted in greater success of spontaneous fistula closure (relative risk [RR] 1.36) and shorter time interval to closure.51 Somatostatin versus control resulted in even greater success of spontaneous fistula closure (RR 2.79) and demonstrated shorter time interval to closure.51 Out of the six trials that recorded fistula output, three noted no difference in amount of fistula secretions,41 44 48 while the other three reported 45 to 50% decrease in fistula output (two studies used somatostatin hormone, one study used lanreotide).43 45 47 Data on length of hospital stay are also based on a single study.49 In the end, intestinal failure units typically utilize a trial of somatostatin analogues for 3 days in an effort to decrease output in a fistula that produces >1L/day.9 24 If successful within 72 hours, the treatment is then utilized over a longer period of time.
Wound Management
Wound care is a priority in a malnourished patient. It is quintessential for patient's quality of life and ability to manage the physical and mental stresses of living with an ECF. Enteric output, especially succus from the proximal small intestine, will erode skin in less than 3 hours.52 Low-output fistulas can be treated with a wet to dry dressing or simply a dry gauze. Moderate output fistulas can be managed with an ostomy appliance with appropriate skin protection around the fistula in the form of adhesive ring, paste, powder, or hydrophilic dressing.53 The real challenge is management of high-output fistulas. Several collection device types exist, such as ostomy appliances, wound managers, pouching systems that can be connected to wall suction, and NPWT. The VAC is a type of NPWT that is not specifically approved for ECF, with increasing application but currently controversial utility for ECF. The choice and fit of the particular system is instrumental in wound healing and requires the expertise of a wound and ostomy nurse.
There are no level 1 data on the use of VAC. Several case series report both positive and negative outcomes. Wainstein et alhave reported a large series of 92 patients with postoperative high-output ECFs.54 All fistulas were dressed with a vacuumcompaction system with high negative pressure capability. The settings used were negative pressure at –350 to –600 mmHg,54 whereas KCI recommends up to –125 mmHg.55 Spontaneous closure rate was 46%, and control of output was obtained in 98% of patients (40% had output entirely suppressed after 1 to 7 days of treatment, 57% had output decreased to <500 mL/day).54 However, 41% experienced no improvement and required surgical correction.54 Medeiros et al reported another 74 patients with postoperative fistula managed with a Foley catheter, connected to a NPWT.56 Of those 74 patients, 92% had spontaneous fistula closure by 15 days.56 Both of these studies used modern ECF management algorithms. It is not possible to determine from these two studies whether there was a cause–effect relationship between NPWT and fistula closure/output reduction. Multiple small case reports exist to document benefit of VAC therapy (n = 1–5).12 57 58 59 Two case series report VAC therapy causing ECF.60 61 The cost of VAC system and the associated nursing care is substantial. Overall, there is no clear evidence that negative pressure wound management system leads to an improved fistula closure rate, and, in some cases it, may cause harm. On the other hand, it is undeniably a tremendous quality-of-life advancement for patients with ECFhaving open wounds, high-output fistulas, or difficult to pouch fistulas, where NPWT acts to protect surrounding skin and effectively gather effluent.
Nonoperative Therapies
Fibrin Sealant
An ideal fistula for treatment wound be long, narrow, low output, devoid of distal obstruction and IBD. From isolated case series, it appears that fibrin sealant therapy may expedite fistula closure, but often requires several treatments. Avalos-González et al reported on a series of 23 patients who underwent fibrin sealant fistula closure, demonstrating fistula closure at 12.5 days in the treatment group versus 32.5 days in the control group.62 Another study reported a series of 15 patients who underwent an average of 2.5 fibrin sealant procedures, which resulted in an 86.6% healing rate at 16 days.63 In contrast, Lippert et al reported a series of patients with heterogeneous fistulae (21 were small intestine and colorectal) that were treated with endoscopic therapies and fibrin sealant. These authors observed a 55.7% closure rate overall and 37% closure rate with fibrin sealant alone.64 Overall, fibrin sealant therapy should be used in selected cases favorable in configuration.
Endoscopic Clips
Endoscopic clip technology is available for acute fistulas and perforations and is not well suited to chronic ECF. With advent of through-the-scope clips, repair of fresh injury has become more favorable in the setting of controlled sepsis and a small-sized fistula.65 This technology has little application in the cure of chronic ECF.
Fistula Plug
Overall, only case series are available for fistula plugs in the treatment of ECF. However, fistula plugs have been more commonly used as an adjunct in the treatment of enteroatmospheric fistula (EAF). In particular, one case series of 6 patients using the Biodesign ECF plug (Cook Medical, Indianapolis, IN) described fistula closure in all patients followed by recurrence in two patients at 9 and 12 months.66
Timeline and Principles of Definitive Surgical Repair
Definitive repair of the ECF should be planned if no spontaneous closure occurs by 12 weeks after sepsis control, nutritional optimization, and establishing wound cares. Timeline to definitive repair is not firmly established but may be delayed in cases where nutrition is maintained and multiple surgeries have been previously performed. Fazio et al demonstrated that mortality doubles if an operation is attempted between 10 and 42 days after initial procedure resulting in ECF formation.16 23 Historically, operation was recommended in 12 weeks after inciting event.28 52 However, high volume ECF tertiary care centers are currently reoperating on patients at 6 to 12 months.9 11 20 24 67 Waiting longer than 12 months in those patients who have large wounds and potential hernias may cause loss of domain and complicate hernia repair,28 but may anecdotally be helpful in patients with previous sepsis or multiple surgeries.
Prerequisites to definitive fistula operative intervention include optimization of nutrition, eradication of infection, addressing psychological morbidity, and clinical evidence of softening scars and abdominal wall on exam. These cases should be scheduled for at least 6 to 8 hours to allow for complete adhesiolysis from the ligament of Treitz to the rectum.14 20 52 Avoidance and proper repair of any enterotomy is essential since 36% of recurrent fistulas result from inadvertent injury to the bowel.68 Operative success for definitive ECF resolution ranges from 80 to 95%.9 11 15 25 67 68 Failure is increased with the presence of infectious and noninfectious complications.8
Additionally, following surgery, ECF recurrence is 14 to 34%.11 15 67 68 Recurrence rates are minimized (18%) when the involved bowel is fully mobilized and resected. Oversewing or wedge resection/bowel repair results in higher rates of recurrence at 33%.67 Similarly, Runströmet al reported that ECF failure rate is lower when no anastomosis is constructed and, instead, a stoma is chosen (recurrence rate 14 vs. 34% with anastomosis).68 More broadly, one has to balance the risk of ECF recurrence with the morbidity of another operation if anastomosis is avoided.
Enteroatmospheric Fistula
EAF represents a fistula with an external opening in an open wound or directly exposed bowel (Fig. 1). There is a higher propensity for EAF if the abdomen has been left open for more than 8 days and in the absence of EN.28 Most patients with EAF are critically ill and require significant resuscitation. In these cases, the first priority should be source control, which is often difficult in the absence of fistula tract and the ability to manipulate edematous bowel safely.
Fig. 1.
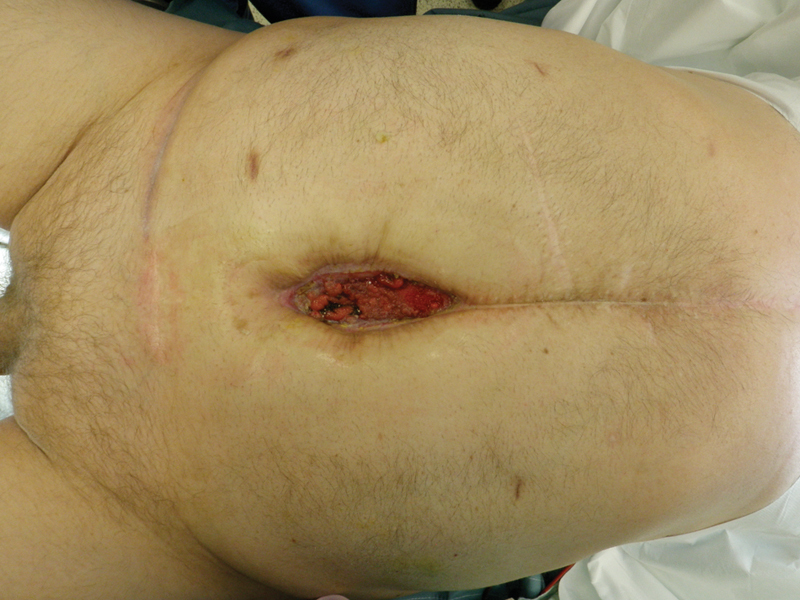
Enteroatmospheric fistula.
Management options include the creation of “floating stoma” by sewing exposed bowel mucosa in circumferential fashion to a plastic sheet that is used as an interface to attach the stoma appliance and placed on the surface of exposed bowel.69 Over time, a floating stoma with a deep EAF will gradually become more superficial.69
Overall, there are no significant differences in nutrition optimization or treatment algorithms for an EAF except for wound care. The use of NPWT such as VAC, applied so that black sponge is never in contact with bowel serosa or mucosa, may also substantially facilitate wound care in this population group and shrink the wound. Definitive operative intervention is not advised until at least 3 months after resolution of sepsis, malnutrition, and other injuries.24 Fistuloclysis in combination with NPWT may also be quite useful in select patients.
Inflammatory Bowel Disease
While all of the previously discussed fistula care protocols hold true for IBD, several additional considerations stand out for IBD patients. First, IBD patients tend to have less tolerance for EN. In a series of 15 IBD patients with ECF or high-output stoma managed at home with an average of 75 days of PN, Evans et al reported that 80% were managed successfully, with 53% undergoing definitive surgery and 27% achieving spontaneous closure thus not requiring surgery.70
ECF treated with infliximab in patients with IBD may result in an increase in fistula closure (up to 55%), compared with 13% closure with placebo,71 though only a minority of fistulas in this study were ECF. Another study with infliximab for ileal and ileocolonic ECF demonstrated 38% spontaneous fistula closure and 51% partial response.72 However, there was also a 50% recurrence rate in this cohort.72 Amiot et al reported on 47 patients with ECFs, including 35% postoperative ECFs after ileocolonic anastomotic leak, 33% complex in nature, and 23% high output.73 Treatment options included infliximab in 78%, adalimumab in 10%, and both drugs in sequence in 13%. Complete closure was achieved in 33% but 50% of these ECFs after closure recurred in the follow-up period. Successful fistula closure was associated with simple fistula and absence of stricture.73 Poritz et al showed in a small study of 26 patients (23% with ECF) that despite 61% of patients having complete or partial response to medical therapy, 54% still need definitive surgery.74
Conclusion
ECF remains a complex problem that is optimally management using a careful and interdisciplinary approach. In addition to primary management of sepsis, conservative treatment remains the treatment mainstay, including the combination of wound management, nutritional support with EN or PN sometimes in combination, and social support. Surgical treatment with resection should be carefully planned and is used in cases that fail conservative treatment.
References
- 1.Sitges-Serra A, Jaurrieta E, Sitges-Creus A. Management of postoperative enterocutaneous fistulas: the roles of parenteral nutrition and surgery. Br J Surg. 1982;69(3):147–150. doi: 10.1002/bjs.1800690310. [DOI] [PubMed] [Google Scholar]
- 2.Schein M, Decker G A. Postoperative external alimentary tract fistulas. Am J Surg. 1991;161(4):435–438. doi: 10.1016/0002-9610(91)91107-t. [DOI] [PubMed] [Google Scholar]
- 3.Berry S M, Fischer J E. Classification and pathophysiology of enterocutaneous fistulas. SurgClin North Am. 1996;76(5):1009–1018. doi: 10.1016/s0039-6109(05)70495-3. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd D A, Gabe S M, Windsor A C. Nutrition and management of enterocutaneous fistula. Br J Surg. 2006;93(9):1045–1055. doi: 10.1002/bjs.5396. [DOI] [PubMed] [Google Scholar]
- 5.Haffejee A A. Surgical management of high output enterocutaneous fistulae: a 24-year experience. CurrOpinClinNutrMetab Care. 2004;7(3):309–316. doi: 10.1097/00075197-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Martinez J L Luque-de-Leon E Mier J Blanco-Benavides R Robledo F Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes World J Surg 2008323436–443., discussion 444 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl) 2003;116(2):171–175. [PubMed] [Google Scholar]
- 8.Campos A C, Andrade D F, Campos G M, Matias J E, Coelho J C. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am CollSurg. 1999;188(5):483–490. doi: 10.1016/s1072-7515(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 9.Hollington P, Mawdsley J, Lim W, Gabe S M, Forbes A, Windsor A J. An 11-year experience of enterocutaneous fistula. Br J Surg. 2004;91(12):1646–1651. doi: 10.1002/bjs.4788. [DOI] [PubMed] [Google Scholar]
- 10.Reber H A, Roberts C, Way L W, Dunphy J E. Management of external gastrointestinal fistulas. Ann Surg. 1978;188(4):460–467. doi: 10.1097/00000658-197810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahbour G, Gabe S M, Ullah M R. et al. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: development of a validated scoring system. Colorectal Dis. 2013;15(9):1162–1170. doi: 10.1111/codi.12363. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann D, Drye C, Heller L, Wong M S, Levin S L. Abdominal wall defect and enterocutaneous fistula treatment with the vacuum-assisted closure (V.A.C.) system. PlastReconstrSurg. 2001;108(7):2066–2068. doi: 10.1097/00006534-200112000-00036. [DOI] [PubMed] [Google Scholar]
- 13.Evenson A R, Fischer J E. Current management of enterocutaneous fistula. J GastrointestSurg. 2006;10(3):455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Schecter W P. Management of enterocutaneous fistulas. SurgClin North Am. 2011;91(3):481–491. doi: 10.1016/j.suc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Martinez J L Luque-de-León E Ballinas-Oseguera G Mendez J D Juárez-Oropeza M A Román-Ramos R Factors predictive of recurrence and mortality after surgical repair of enterocutaneous fistula J GastrointestSurg 2012161156–163., discussion 163–164 [DOI] [PubMed] [Google Scholar]
- 16.Fazio V W, Coutsoftides T, Steiger E. Factors influencing the outcome of treatment of small bowel cutaneous fistula. World J Surg. 1983;7(4):481–488. doi: 10.1007/BF01655937. [DOI] [PubMed] [Google Scholar]
- 17.Lévy E, Frileux P, Cugnenc P H, Honiger J, Ollivier J M, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76(7):676–679. doi: 10.1002/bjs.1800760708. [DOI] [PubMed] [Google Scholar]
- 18.Mawdsley J E, Hollington P, Bassett P, Windsor A J, Forbes A, Gabe S M. An analysis of predictive factors for healing and mortality in patients with enterocutaneous fistulas. Aliment PharmacolTher. 2008;28(9):1111–1121. doi: 10.1111/j.1365-2036.2008.03819.x. [DOI] [PubMed] [Google Scholar]
- 19.Bleier J I, Hedrick T. Metabolic support of the enterocutaneous fistula patient. Clin Colon Rectal Surg. 2010;23(3):142–148. doi: 10.1055/s-0030-1262981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushal M, Carlson G L. Management of enterocutaneous fistulas. Clin Colon Rectal Surg. 2004;17(2):79–88. doi: 10.1055/s-2004-828654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samad S, Anele C, Akhtar M, Doughan S. Implementing a pro-forma for multidisciplinary management of an enterocutaneous fistula: a case study. Ostomy Wound Manage. 2015;61(6):46–52. [PubMed] [Google Scholar]
- 22.Polk T M, Schwab C W. Metabolic and nutritional support of the enterocutaneous fistula patient: a three-phase approach. World J Surg. 2012;36(3):524–533. doi: 10.1007/s00268-011-1315-0. [DOI] [PubMed] [Google Scholar]
- 23.Schecter W P, Hirshberg A, Chang D S. et al. Enteric fistulas: principles of management. J Am CollSurg. 2009;209(4):484–491. doi: 10.1016/j.jamcollsurg.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Datta V, Engledow A, Chan S, Forbes A, Cohen C R, Windsor A. The management of enterocutaneous fistula in a regional unit in the United Kingdom: a prospective study. Dis Colon Rectum. 2010;53(2):192–199. doi: 10.1007/DCR.0b013e3181b4c34a. [DOI] [PubMed] [Google Scholar]
- 25.Dudrick S J, Maharaj A R, McKelvey A A. Artificial nutritional support in patients with gastrointestinal fistulas. World J Surg. 1999;23(6):570–576. doi: 10.1007/pl00012349. [DOI] [PubMed] [Google Scholar]
- 26.Irving M, White R, Tresadern J. Three years' experience with an intestinal failure unit. Ann R CollSurgEngl. 1985;67(1):2–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Dellinger R P, Levy M M, Rhodes A. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 28.Davis K G, Johnson E K. Controversies in the care of the enterocutaneous fistula. SurgClin North Am. 2013;93(1):231–250. doi: 10.1016/j.suc.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Härle K, Lindgren M, Hallböök O. Experience of living with an enterocutaneous fistula. J ClinNurs. 2015;24(15–16):2175–2183. doi: 10.1111/jocn.12857. [DOI] [PubMed] [Google Scholar]
- 30.Chapman R, Foran R, Dunphy J E. Management of intestinal fistulas. Am J Surg. 1964;108:157–164. doi: 10.1016/0002-9610(64)90005-4. [DOI] [PubMed] [Google Scholar]
- 31.Makhdoom Z A, Komar M J, Still C D. Nutrition and enterocutaneous fistulas. J ClinGastroenterol. 2000;31(3):195–204. doi: 10.1097/00004836-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Singh R. Evaluation of nutritional status by different parameters and to predict spontaneous closure, morbidity, and mortality in patients with enterocutaneous fistulas: a study of 92 cases. The Internet J Nutrition Wellness. 2007;6(1):1–6. [Google Scholar]
- 33.Lu C Y, Wu D C, Wu I C. et al. Serum albumin level in the management of postoperative enteric fistula for gastrointestinal cancer patients. J Invest Surg. 2008;21(1):25–32. doi: 10.1080/08941930701833959. [DOI] [PubMed] [Google Scholar]
- 34.Lubana P S, Aggarwal G, Aggarwal H, Jain D K. Serum transferrin levels – a predictive marker of spontaneous closure and mortality in patients with enterocutaneous fistulae. Arab J Gastroenterol. 2010;11(4):212–214. [Google Scholar]
- 35.Teubner A, Morrison K, Ravishankar H R, Anderson I D, Scott N A, Carlson G L. Fistuloclysis can successfully replace parenteral feeding in the nutritional support of patients with enterocutaneous fistula. Br J Surg. 2004;91(5):625–631. doi: 10.1002/bjs.4520. [DOI] [PubMed] [Google Scholar]
- 36.Deitel M. Elemental diet and enterocutaneous fistula. World J Surg. 1983;7(4):451–454. doi: 10.1007/BF01655933. [DOI] [PubMed] [Google Scholar]
- 37.Hesse U, Ysebaert D, de Hemptinne B. Role of somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut. 2001;49 04:iv11–iv21. doi: 10.1136/gut.49.suppl_4.iv11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins S A, Nott D M, Baxter J N. Fluctuations in the secretion of pancreatic enzymes between consecutive doses of octreotide: implications for the management of fistulae. Eur J GastroenterolHepatol. 1995;7(3):255–258. [PubMed] [Google Scholar]
- 39.Paran H Neufeld D Kaplan O Klausner J Freund U Octreotide for treatment of postoperative alimentary tract fistulas World J Surg 1995193430–433., discussion 433–434 [DOI] [PubMed] [Google Scholar]
- 40.Sheppard M, Shapiro B, Pimstone B, Kronheim S, Berelowitz M, Gregory M. Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J ClinEndocrinolMetab. 1979;48(1):50–53. doi: 10.1210/jcem-48-1-50. [DOI] [PubMed] [Google Scholar]
- 41.Jamil M, Ahmed U, Sobia H. Role of somatostatin analogues in the management of enterocutaneous fistulae. J Coll Physicians Surg Pak. 2004;14(4):237–240. [PubMed] [Google Scholar]
- 42.Hernández-Aranda J C, Gallo-Chico B, Flores-Ramírez L A, Avalos-Huante R, Magos-Vázquez F J, Ramírez-Barba E J. [Treatment of enterocutaneous fistula with or without octreotide and parenteral nutrition] NutrHosp. 1996;11(4):226–229. [PubMed] [Google Scholar]
- 43.Torres A J Landa J I Moreno-Azcoita M et al. Somatostatin in the management of gastrointestinal fistulas. A multicenter trial Arch Surg 1992127197–99., discussion 100 [DOI] [PubMed] [Google Scholar]
- 44.Scott N A, Finnegan S, Irving M H. Octreotide and postoperative enterocutaneous fistulae: a controlled prospective study. ActaGastroenterolBelg. 1993;56(3–4):266–270. [PubMed] [Google Scholar]
- 45.Gayral F, Campion J P, Regimbeau J M. et al. Randomized, placebo-controlled, double-blind study of the efficacy of lanreotide 30 mg PR in the treatment of pancreatic and enterocutaneous fistulae. Ann Surg. 2009;250(6):872–877. doi: 10.1097/sla.0b013e3181b2489f. [DOI] [PubMed] [Google Scholar]
- 46.Isenmann R, Schielke D J, Morl F K. et al. Adjuvant therapy with somatostatin iv in postoperative fistulae of the pancreas, gall bladder and small intestine. A multicentre randomised study. Aktuelle Chirurgie. 1994;29:96–99. [Google Scholar]
- 47.Spiliotis J, Vagenas K, Panagopoulos K, Kalfarentzos F. Treatment of enterocutaneous fistulas with TPN and somatostatin, compared with patients who received TPN only. Br J ClinPract. 1990;44(12):616–618. [PubMed] [Google Scholar]
- 48.Sancho J J, di Costanzo J, Nubiola P. et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg. 1995;82(5):638–641. doi: 10.1002/bjs.1800820521. [DOI] [PubMed] [Google Scholar]
- 49.Leandros E, Antonakis P T, Albanopoulos K, Dervenis C, Konstadoulakis M M. Somatostatin versus octreotide in the treatment of patients with gastrointestinal and pancreatic fistulas. Can J Gastroenterol. 2004;18(5):303–306. doi: 10.1155/2004/901570. [DOI] [PubMed] [Google Scholar]
- 50.Coughlin S, Roth L, Lurati G, Faulhaber M. Somatostatin analogues for the treatment of enterocutaneous fistulas: a systematic review and meta-analysis. World J Surg. 2012;36(5):1016–1029. doi: 10.1007/s00268-012-1494-3. [DOI] [PubMed] [Google Scholar]
- 51.Rahbour G, Siddiqui M R, Ullah M R, Gabe S M, Warusavitarne J, Vaizey C J. A meta-analysis of outcomes following use of somatostatin and its analogues for the management of enterocutaneous fistulas. Ann Surg. 2012;256(6):946–954. doi: 10.1097/SLA.0b013e318260aa26. [DOI] [PubMed] [Google Scholar]
- 52.Galie K L, Whitlow C B. Postoperative enterocutaneous fistula: when to reoperate and how to succeed. Clin Colon Rectal Surg. 2006;19(4):237–246. doi: 10.1055/s-2006-956446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brindle C T, Blankenship J. Management of complex abdominal wounds with small bowel fistulae: isolation techniques and exudate control to improve outcomes. J Wound Ostomy Continence Nurs. 2009;36(4):396–403. doi: 10.1097/WON.0b013e3181aaf2b0. [DOI] [PubMed] [Google Scholar]
- 54.Wainstein D E, Fernandez E, Gonzalez D, Chara O, Berkowski D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32(3):430–435. doi: 10.1007/s00268-007-9235-8. [DOI] [PubMed] [Google Scholar]
- 55.KCI Website Available at: www.kci1.com. Accessed August 15, 2015
- 56.Medeiros A C, Aires-Neto T, Marchini J S, Brandão-Neto J, Valença D M, Egito E S. Treatment of postoperative enterocutaneous fistulas by high-pressure vacuum with a normal oral diet. Dig Surg. 2004;21(5–6):401–405. doi: 10.1159/000082317. [DOI] [PubMed] [Google Scholar]
- 57.Goverman J Yelon J A Platz J J Singson R C Turcinovic M The “Fistula VAC,” a technique for management of enterocutaneous fistulae arising within the open abdomen: report of 5 cases J Trauma 2006602428–431., discussion 431 [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-López M, Carrasco Campos J, Sánchez Pérez B, González Sánchez A, Fernández Aguilar J L, Bondía Navarro J A. [Negative pressure therapy in wounds with enteric fistulas] Cir Esp. 2009;86(1):29–32. doi: 10.1016/j.ciresp.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Cro C, George K J, Donnelly J, Irwin S T, Gardiner K R. Vacuum assisted closure system in the management of enterocutaneous fistulae. Postgrad Med J. 2002;78(920):364–365. doi: 10.1136/pmj.78.920.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao M, Burke D, Finan P J, Sagar P M. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colorectal Dis. 2007;9(3):266–268. doi: 10.1111/j.1463-1318.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- 61.Fischer J E. A cautionary note: the use of vacuum-assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am J Surg. 2008;196(1):1–2. doi: 10.1016/j.amjsurg.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Avalos-González J, Portilla-deBuen E, Leal-Cortés C A. et al. Reduction of the closure time of postoperative enterocutaneous fistulas with fibrin sealant. World J Gastroenterol. 2010;16(22):2793–2800. doi: 10.3748/wjg.v16.i22.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rábago L R, Ventosa N, Castro J L, Marco J, Herrera N, Gea F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy. 2002;34(8):632–638. doi: 10.1055/s-2002-33237. [DOI] [PubMed] [Google Scholar]
- 64.Lippert E, Klebl F H, Schweller F. et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26(3):303–311. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 65.Winder J S, Pauli E M. Comprehensive management of full-thickness luminal defects: the next frontier of gastrointestinal endoscopy. World J GastrointestEndosc. 2015;7(8):758–768. doi: 10.4253/wjge.v7.i8.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyon J W, Hodde J P, Hucks D, Changkuon D I. First experience with the use of a collagen fistula plug to treat enterocutaneous fistulas. J VascIntervRadiol. 2013;24(10):1559–1565. doi: 10.1016/j.jvir.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 67.Lynch A C, Delaney C P, Senagore A J, Connor J T, Remzi F H, Fazio V W. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240(5):825–831. doi: 10.1097/01.sla.0000143895.17811.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Runström B, Hallböök O, Nyström P O, Sjödahl R, Olaison G. Outcome of 132 consecutive reconstructive operations for intestinal fistula—staged operation without primary anastomosis improved outcome in retrospective analysis. Scand J Surg. 2013;102(3):152–157. doi: 10.1177/1457496913490452. [DOI] [PubMed] [Google Scholar]
- 69.Subramaniam M H, Liscum K R, Hirshberg A. The floating stoma: a new technique for controlling exposed fistulae in abdominal trauma. J Trauma. 2002;53(2):386–388. doi: 10.1097/00005373-200208000-00037. [DOI] [PubMed] [Google Scholar]
- 70.Evans J P, Steinhart A H, Cohen Z, McLeod R S. Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease. J GastrointestSurg. 2003;7(4):562–566. doi: 10.1016/S1091-255X(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 71.Present D H, Rutgeerts P, Targan S. et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340(18):1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 72.Bor R, Farkas K, Bálint A. et al. Efficacy of combined anti-TNF-alpha and surgical therapy in perianal and enterocutaneousfistulizing Crohn's disease—clinical observations from a tertiary Eastern European center. Scand J Gastroenterol. 2015;50(2):182–187. doi: 10.3109/00365521.2014.936033. [DOI] [PubMed] [Google Scholar]
- 73.Amiot A, Setakhr V, Seksik P. et al. Long-term outcome of enterocutaneous fistula in patients with Crohn's disease treated with anti-TNF therapy: a cohort study from the GETAID. Am J Gastroenterol. 2014;109(9):1443–1449. doi: 10.1038/ajg.2014.183. [DOI] [PubMed] [Google Scholar]
- 74.Poritz L S, Rowe W A, Koltun W A. Remicade does not abolish the need for surgery in fistulizing Crohn's disease. Dis Colon Rectum. 2002;45(6):771–775. doi: 10.1007/s10350-004-6296-8. [DOI] [PubMed] [Google Scholar]


