Abstract
The problems that a patient experiences after the creation of a temporary or permanent stoma can result from many factors, but a carefully constructed stoma located in an ideal location is typically associated with appropriate function and an acceptable quality of life. The construction of the stoma can be confounded by many concomitant conditions that increase the distance that the bowel must traverse or shorten the bowel's capacity to reach. Stomas can be further troubled by a variety of problems that potentially arise early in the recovery period or months later. Surgeons must be familiar with these obstacles and complications to avoid their occurrence and minimize their impact.
Keywords: ostomy, retraction, peristomal hernia, technique, prolapse
Introduction
Despite common perception, the difficult stoma may be one of the most challenging problems in colorectal surgery. A poorly constructed stoma can impact a patient's outcome, and result in difficult management issues. Moreover, complications such as hernia, prolapse, retraction, and stenosis can occur despite the best circumstances. The surgeon must accordingly understand how to avoid and manage these issues.
Stoma Siting
Appropriate stoma siting can reduce the likelihood of problems with leakage and other issues causing undue hardship. Selecting a good site enhances the probability of patient independence in stoma care and the resumption of normal activities, and preoperative marking allows the abdomen to be assessed in lying, sitting, and standing positions. Several factors should be considered when choosing a site and should include the following:
Multiple sites; fecal and urinary stomas should be located at differing horizontal levels
Patient considerations (e.g., age, dexterity, diagnosis, occupation, prior radiation, vision)
Physical factors (e.g., abdominal creases/folds/scars/valleys, belt/waist line, bony structures, large/protruding abdomen, pendulous breasts)
Positioning issues (e.g., contracture, mobility, posture)
The selected site should be ideally located at the superior aspect of the infraumbilical fat mound, surrounded by 5 to 7 cm of relatively flat contour, visible to the patient, and positioned below the belt line (Fig. 1). A transrectus location has been traditionally recommended, but it remains unclear whether a stoma placed lateral to the rectus abdominis muscle is associated with a lesser or greater risk of peristomal herniation or stoma prolapse.1 More than one quadrant should be marked and the sites prioritized if the exact operation is uncertain or confounding variables are present.
Fig. 1.
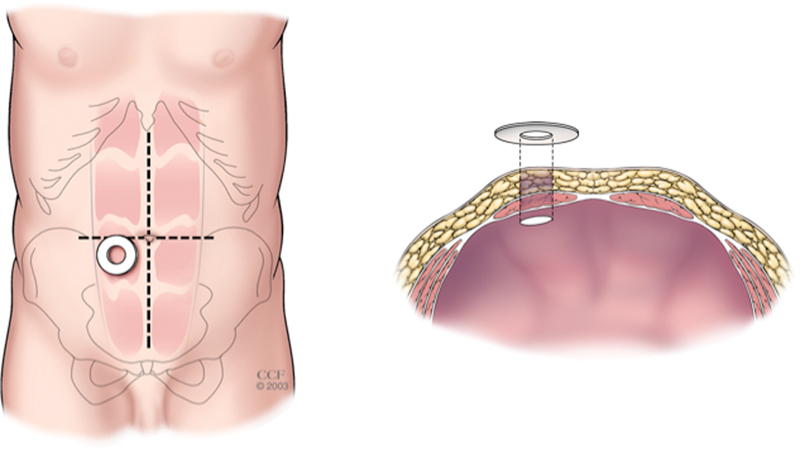
Appropriate siting for the stoma on the infraumbilical fat mound overlying the rectus abdominis muscle. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Stoma Creation
The stoma aperture is constructed at the previously marked site and many of the critical steps are similar whether the procedure is performed using an open or laparoscopic approach to create a colostomy or an enterostomy (e.g., ileostomy, jejunostomy). A slightly elliptical incision is made at the skin level with the long axis oriented in a caudad–cephalad direction. Regardless the diameter of the bowel, the stoma aperture created at the skin level should rarely exceed 2.5 to 3.5 cm in greatest dimension because even bowel that has been chronically dilated will eventually return to this size. The skin ellipse is excised leaving the underlying subcutaneous tissue intact to avoid future stoma retraction. All subsequent incisions through the abdominal wall are made in a single direction maintaining the same longitudinal orientation while assuring that the track of the final aperture is perpendicular to the abdominal wall.
The subcutaneous tissue and its fascia layers are incised until the fascia of the anterior rectus sheath is visualized. The anterior fascia is incised and the rectus abdominis muscle is separated parallel to its fibers to expose the posterior sheath while avoiding the epigastric vessels and dividing any interfering tendinous inscription. The posterior sheath is carefully incised to preclude injury to the contents of the underlying peritoneal cavity. The aperture in the abdominal wall should be generally sized to accommodate two fingers. Smaller openings can be associated with obstruction during the early period, and larger incisions risk later problems with a peristomal hernia.
Colostomies should typically protrude 1.5 to 2.5 cm and stomas of the small bowel should evert 2.5 to 3.5 cm. Stomas that do not evert at least 1 cm above the skin surface 48 hours after surgery have a 35% chance of causing problems.2 This eversion is best achieved using absorbable sutures placed around the circumference of the bowel. The sutures are passed through the full thickness of the bowel edge, the serosa of the bowel adjacent to the skin level, and through the dermis of the skin. Passage of the suture through the epidermis may lead to mucosal implants that can produce mucous and lead to undermining of the appliance faceplate. The sutures are tagged until the major axes have been addressed. The sutures are simultaneously retracted to evert the bowel and tied to oppose the mucosa and skin. Any areas of exposed serosa or mesentery at the mucocutaneous junction should be closed to prevent the development of serositis and resultant stenosis. If the surgeon must choose between a flush stoma in an ideal location and a protruding stoma at a poor site, the former is generally preferred.
Challenging Issues
Obesity
Creating a stoma in an obese patient presents several challenges that must be considered and overcome. The obesity can be predominantly localized to the subcutaneous tissue. In this setting, it can be difficult to pass the stoma through the thick abdominal wall, or the distance the bowel needs to traverse can dramatically increase when a panniculus settles to a more dependent position with ambulation. Alternatively, the obesity manifests itself within the abdomen with the thickening of the mesentery and largeness of the omentum that create problems during stoma construction. In either situation, it helps to use an upper quadrant of the abdomen as a stoma site because the subcutaneous tissue is thinner and anchored to the costal margins, and the distance between vascular origins and the proposed stoma site is usually shortened (Fig. 2).
Fig. 2.
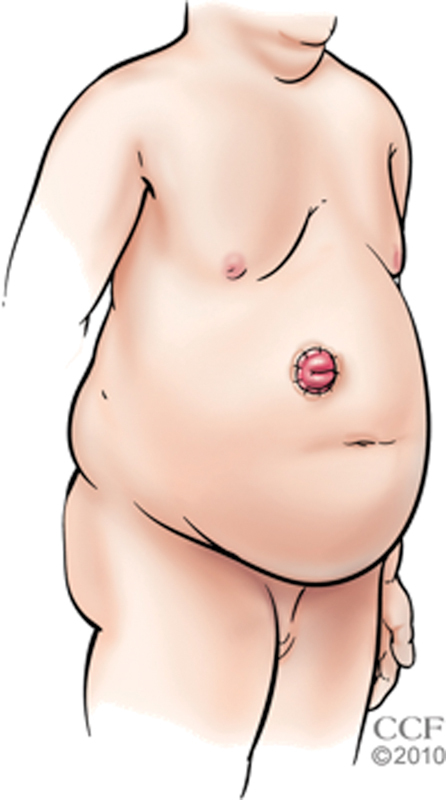
Upper quadrant siting of the stoma in an obese patient. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Shortened Mesentery
A shortened mesentery can be encountered in patients with central obesity as well as patients with a history of desmoid tumors, inflammatory bowel disease, previous laparotomies, or prior external beam radiotherapy. The shortened mesentery is often a result of fibrosis or inflammation and can be further complicated by fragility. Regardless the etiology, the shortened mesentery causes challenges with reach in patients requiring a stoma.
Stoma Types
Colostomy
When creating an end descending colostomy in the challenging patient, it is often necessary to completely mobilize the left colon, splenic flexure, and distal transverse colon from their attachments to the retroperitoneum and omentum. The inferior mesenteric artery is divided near its origin and the inferior mesenteric vein is ligated near the tail of the pancreas(Fig. 3). The left colic vessels are transected while preserving the bifurcation of their ascending and descending branches, and a window of avascular mesentery bordered by the left ascending vessels and the left middle colic vessels can be excised to increase length.
Fig. 3.
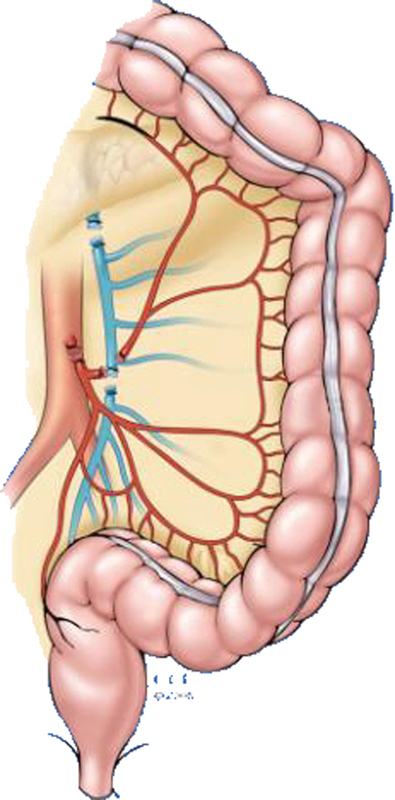
Division of the inferior mesenteric vessels and left colic vessels to add mobility and length for a descending colostomy. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
The bulk of mesentery that needs to traverse the abdominal wall can be lessened by dividing the mesentery inside the marginal artery for a long distance and amputating any large appendices epiploicae. Pulsatile bleeding from the marginal artery as it is divided at the site selected for transecting the colon assures adequate perfusion of the intended stoma. If pulsatile bleeding is not seen, the artery is divided in a more proximal location until arterial bleeding is witnessed, and the bowel is transected at that level. The amount of bowel that needs to be “tailored” in this fashion can be estimated by measuring the thickness of the abdominal wall at the level of the stoma aperture and adding a short (3–5 cm) distance for the eversion of the colostomy.
If mesenteric fat inhibits eversion of the exteriorized bowel, the bulk can be further reduced by touching the peritoneum of the fat in several locations with electrocautery to vaporize the moisture within the fat without damaging the vascular supply. Patients with short bowel syndrome or ostomates previously managed with external beam radiotherapy may benefit from 2.5 to 3.5 cm of colostomy protrusion because their stools tend to be more liquid in consistency.
Bowel that is distended can be often decompressed using a large bore needle to aspirate any gas, or the contents can be milked to a more proximal segment and temporarily secured at that location using an atraumatic clamp or cloth tie to keep the distal bowel empty. Efforts to evacuate problematic stool are generally avoided because they entail opening the bowel and risk spillage associated with increased rates of surgical site infection. The exception is when the stool is large and inspissated and unlikely to spontaneously pass through a normal-sized colostomy. In this case, on-table lavage is often warranted. Resection is indicated when the dilated bowel demonstrates ischemia or serosal splitting secondary to the impacted stool or associated distention.
If potential ischemia is a concern or the mesentery is prohibitively shortened, an end loop colostomy should be created using staged delivery of the bowel, as described later. The closed end of bowel is left in an intraperitoneal location or placed in the subcutaneous space, and the stoma is matured by transversely opening the antimesenteric aspect of the bowel several centimeters proximal to the transecting staple line. Extreme mesenteric shortening may preclude the creation of an end loop colostomy in rare individuals, and, in this setting, a loop ileostomy is typically employed to divert the fecal stream and vent the distal bowel assuming that an incompetent ileocecal valve is present.
Ileostomy
An end ileostomy for fecal or urinary diversion in the difficult scenario is managed using many of the same principles employed for a colostomy, but it is usually easier to construct an ileostomy because of less bulk and more mobility associated with this type of stoma.
A stoma site in the upper abdomen is generally preferred and the midgut mesentery is fully mobilized off the retroperitoneum. The ileocolic vessels can usually be divided without risking ischemia, and a window of avascular mesentery bordered by these vessels and an inner arcade of vessels that runs parallel to the mesenteric margin of the bowel wall can be excised to improve length(Fig. 4). Furthermore, the bowel can be “tailored” to a smaller bulk without compromising its blood supply by incising the mesentery between the inner and outer vessel arcades for a long distance. An adequate length (5–7 cm) of bowel must be delivered above the skin level to assure that adequate eversion of the ileostomy is possible.
Fig. 4.
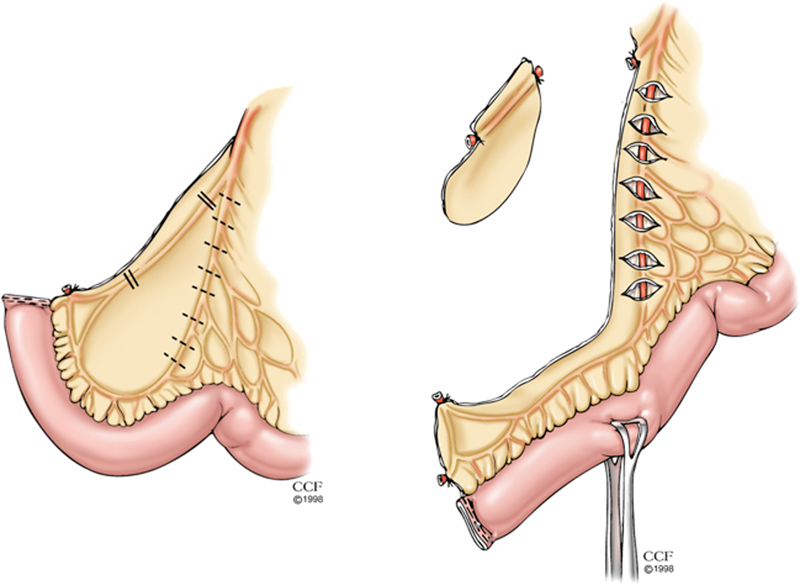
Division of the ileocolic vessels (left panel) and excision of the peritoneal window (right panel) to add mobility and length for an ileostomy. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Bowel that is distended can be decompressed by milking its contents into the stomach where the fluid and gas are evacuated through a nasogastric tube. An end loop ileostomy is constructed using staged delivery of the bowel, as described later, if ischemia is a concern or the mesentery is prohibitively shortened(Fig. 5).
Fig. 5.
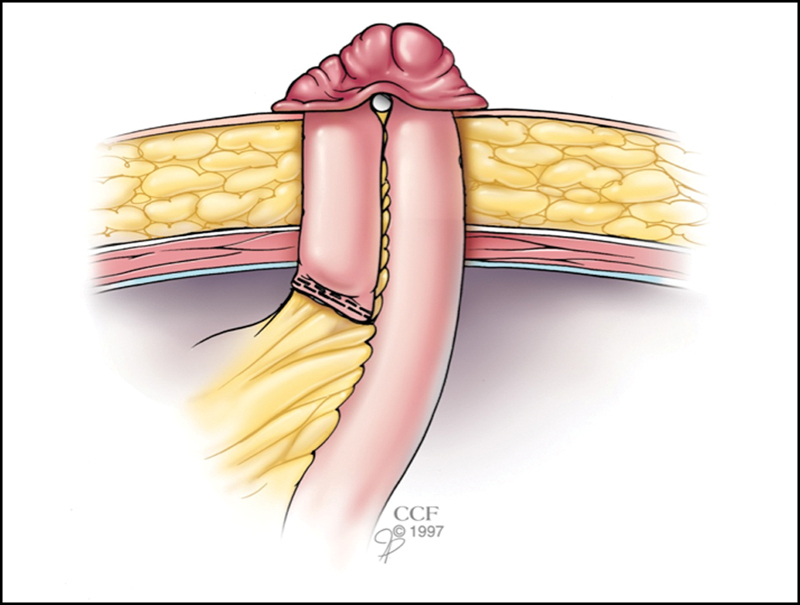
End loop ileostomy for a patient at risk for ischemia or demonstrating mesenteric shortening. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Loop Stoma
Loop stomas can be constructed from the colon, ileum, or jejunum and are usually intended to be temporary as they are generally protecting a more distal anastomosis. The aperture in the abdominal wall is usually larger than the opening associated with an end stoma, and this larger aperture is frequently complicated by a peristomal hernia or prolapse of the efferent stoma limb. These complications are accepted because they can be easily remedied when the stoma is reversed. Prior to passing a loop stoma to the skin level, the bowel should be marked to assure the surgeon can discriminate the afferent from the efferent limb. Likewise, once the bowel is delivered to its final location, care should be taken to avoid rotating the stoma during closure of the abdomen. Both these precautions are intended to prevent the surgeon from maturing the wrong limb of a loop stoma.
The mesenteric aspect of the bowel wall can be accidentally torn in some patients with a heavy mesentery or brittle bowel when a loop stoma is fashioned in the usual manner. Instead, the bowel can be supported by two stoma rods placed alongside one another to distribute the tension over a greater surface area. Alternatively, the mesentery can be braced at the fascia level rather than the skin level. In this approach, a long malleable tube (e.g., filiform, follower, vascular tunneling device) is passed through a skin incision remote from the stoma site down to the anterior fascia of the abdominal wall where it pierces an avascular portion of the stoma's mesentery before rising through the skin on the opposite side of the stoma(Fig. 6). This sterile tube is subsequently removed several days or weeks later when the bowel and its mesentery are relatively fixed in the subcutaneous tissue and at minimal risk for retraction.
Fig. 6.
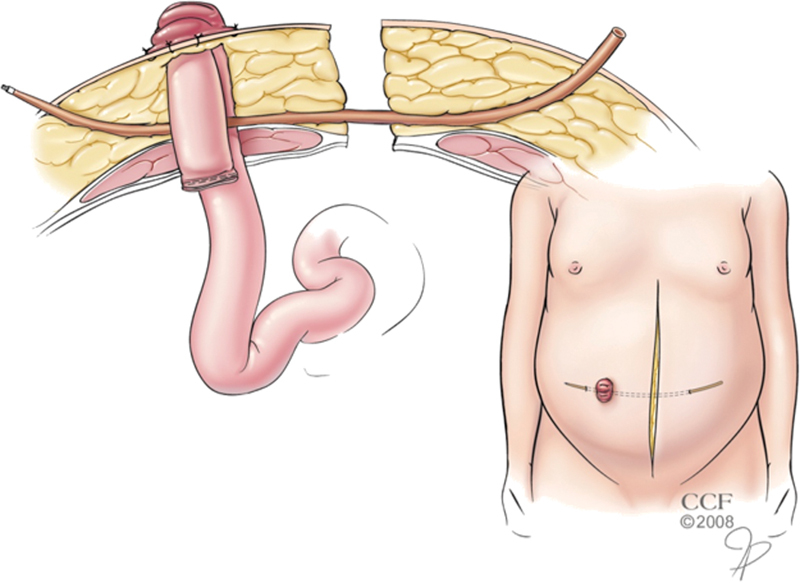
Support of the bowel mesentery with a follower tube in an obese patient with excessive tension. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Patients with a problematic colorectal or coloanal anastomosis requiring permanent fecal diversion may choose to undergo the creation of a diverting stoma without resection of the failed anastomosis due to concerns about operative morbidity.3 In such instances, a colostomy is preferred to an ileostomy because the colostomy is associated with lesser risk for dehydration and less mucus is produced by the shorter length of defunctionalized bowel. However, the surgeon must be cautious about the blood supply of the bowel distal to the colostomy, especially if the inferior mesenteric artery was sacrificed during the initial operation. In these cases, a loop colostomy with careful preservation of the marginal artery is recommended.3
Special Techniques
Bowel Passage
A few maneuvers can assist with passing the bowel through the abdominal wall. If the bowel is bulky or the abdominal wall is thick, an extra-small wound protector can be placed in the stoma aperture and moistened with normal saline(Fig. 7). The transected end of bowel is grasped using an atraumatic clamp passed through the abdominal wall and guided to a location above the skin level. It is important to assist the passage by feeding the bowel and mesentery into the wound protector as opposed to pulling the tissue and causing injury. The ring of the protector on the peritoneal cavity side is divided and amputated from the protector sheath. The sheath is then removed out the skin opening by pulling on the outer ring. Alternatively, the skin and subcutaneous tissue can be mobilized off the anterior sheath of the abdominal wall to a point lateral to the stoma aperture. The transected intestine is initially passed through the rectus muscle and fascia, and then through the subcutaneous tissue and skin in a staged manner(Fig. 8). Both the approaches allow the surgeon to minimize the defect created in the abdominal wall fascia and decrease the amount of resistance associated with delivery of the transected bowel above skin level. Enlarging the opening within the abdominal wall to accept a bulk of mesentery increases the risk for future problems resulting from a peristomal hernia, but is sometimes warranted.
Fig. 7.
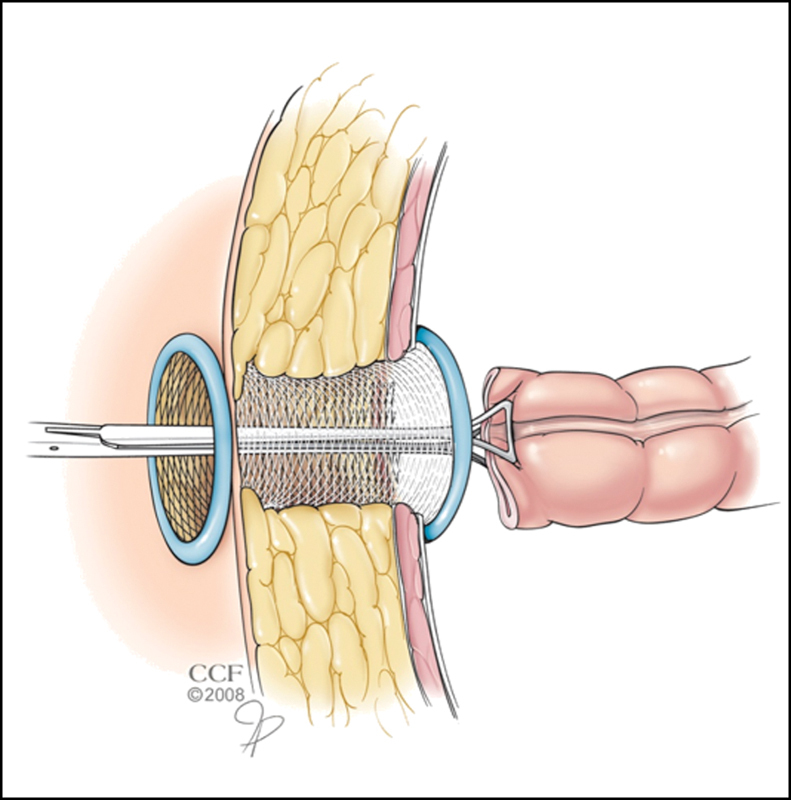
Passage of the transected colon through a thick abdominal wall facilitated by a wound protector. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Fig. 8.
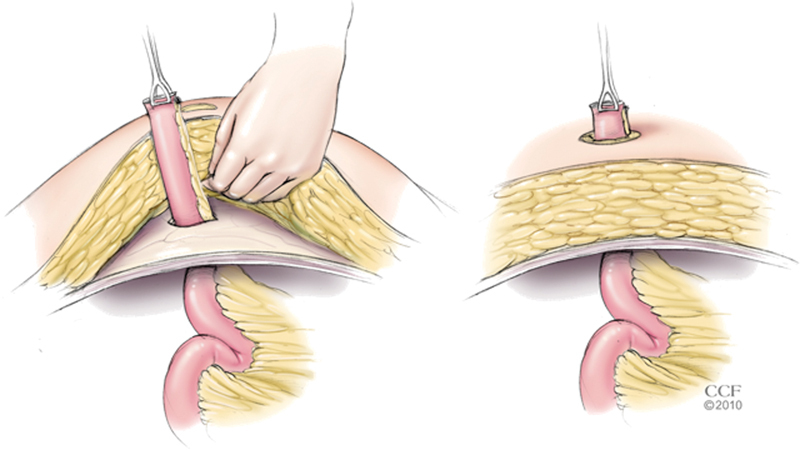
Passage of the transected ileum through a thick abdominal wall facilitated by staged delivery. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1998–2015. All Rights Reserved.)
Abdominal Wall Modification
Modification of the abdominal wall is rarely used when initially creating a stoma, but it sometimes avoids the need to enter the peritoneal cavity when revising a stoma because of stoma issues accompanying abdominal wall laxity after weight loss. The principal approaches employ an abdominoplasty or a peristomal flap. The abdominoplasty generally uses a low curvilinear transverse incision 2 to 3 cm above the pubis and anterior superior iliac spines. The skin and subcutaneous tissue are elevated off the underlying fascia to a point above the redundant skin or the existing stoma site, and the excess tissue of the flap is excised. Remaining subcutaneous tissue can removed to thin the flap, but care must be taken to avoid causing flap ischemia or necrosis. The umbilicus is repositioned, a new stoma opening is created, the bowel is passed through the aperture, closed suction drains are placed under the flap, and the flap is sutured to the lower wound edge. The other approach creates a flap to modify the abdominal wall near the stoma and typically involves thinning of the subcutaneous fat around the intended stoma site to provide a flat, smooth surface for adherence of the stoma appliance.
Complications
Ischemia
Ischemia is the most common cause of stoma-related problems in the early postoperative period and is linked to excessive tension on the mesentery or extreme trimming of the mesentery. Accordingly, any concerns about stoma ischemia in the operating room should be addressed prior to completion of the procedure. Despite best efforts, ischemia affects 1 to 10% of colostomies and 1 to 5% of ileostomies, and is more commonly seen in obese patients or patients undergoing emergency operations.4
Postoperative management of an ischemic stoma and prediction of its ultimate outcome can be based upon the relative length of affected bowel and the severity of ischemia. The length of ischemic bowel and its relationship to the fascia (i.e., suprafascial, subfascial) can differ, and this is judged by endoscopy. Alternatively, a transparent tube used for blood samples can be passed into the lumen of the stoma at the bedside and illuminated with a flashlight. Ischemia can also vary in severity and appearance depending upon whether the mucosa (red), submucosa (yellow), muscle layers (green), or full-thickness (black) of the bowel wall is affected.
Suprafascial ischemia can be nonoperatively managed in the acute setting, but the stoma is preferably revised during the early postoperative period if the deeper layers of the bowel wall are involved because of anticipated pouching problems in the shortterm and resultant stenosis in the longterm. If the patient is somewhat unstable or more than 1 week has transpired since surgery, stoma revision for deeper ischemia should be delayed until 6 months later when re-entry into the peritoneal cavity is less risky. For subfascial ischemia of the mucosa or submucosa, nonoperative management is commonly associated with no long-term sequelae. However, deeper bowel wall involvement in this setting must be urgently treated with stoma revision or else intra-abdominal fecal leakage will likely occur.
Peristomal Hernia
Peristomal hernias are a frequent complication more commonly associated with colostomies (18–40%) compared with ileostomies (9–22%).4 These hernias are often asymptomatic and can be managed with a nonoperative approach that employs a supportive belt. However, repair is warranted if incarceration, obstruction, or pouching problems develop.
Options for open repair of these hernias include stoma relocation and fascia repair with or without reinforcing onlay, sublay, or underlay mesh. Resiting is usually restricted to stomas that are poorly sited. Otherwise, suture repair alone or suture repair reinforced with mesh is used. A midline incision or a “tennis-racquet” incision may be used to approach the repair depending upon the surgeon's preference. The midline approach offers the ability to repair any associated midline hernia and potentially avoids the need to disconnect the mucocutaneous junction between the bowel and skin. Plus, postoperative separation of a midline surgical wound is less likely to expose onlay mesh when this technique is employed.
A systematic review of the literature showed that suture repair alone of the fascia resulted in a high (50%) recurrence rate whereas the recurrence rates for mesh repair were substantially lower at 8 to 15% depending on the length of follow-up.5 Morbidity and mortality did not significantly differ between the techniques used to repair a peristomal hernia.
Others have employed laparoscopic repair of these hernias as evidenced by a meta-analysis that included 469 patients undergoing this procedure.6 Postoperative complications included surgical site infection (3.8%), infected mesh (1.7%), and obstruction requiring reoperation (1.7%). The overall hernia recurrence rate was 17.4%, and the recurrence rates for a modified laparoscopic Sugarbaker approach versus a keyhole approach were 10.2 and 27.9%, respectively.
The high incidence of an initial peristomal hernia and proven success with mesh repairs have led many centers to explore the use of mesh placed at the time of laparotomy to prophylax against a peristomal hernia developing at a colostomy site. A recent systematic review of the literature revealed three randomized controlled trials including 64 patients who underwent laparotomy with mesh placement and 64 patients managed by conventional laparotomy closure.7 The two study groups were demographically well-matched. The incidence of peristomal hernia in the mesh group was 13% compared with 53% in the control group (p< 0.0001). Furthermore, no significant differences were observed in the incidence of seroma or wound infection following prophylactic mesh placement.
Prolapse
Stoma prolapse occurs in over 10% of patients with an end stoma whether it is a colostomy or ileostomy,8 9 but is more commonly seen with a loop stoma where the prolapse arises from the efferent or distal limb.4 In the acute setting, prompt resection without an attempt at reduction is indicated if the prolapse appears gangrenous.10 A nonischemic prolapse does not typically cause any functional abnormality for the patient. If the prolapse is uncomfortable, it can generally be reduced with gentle pressure sometimes aided by coating the mucosal surface of the exteriorized bowel with table sugar to create an osmotic gradient that reduces the edema of the prolapsed stoma.11 However, the prolapse promptly recurs in most cases and repair is often required.
Operative management of a chronic prolapse associated with an end stoma is best managed by the reduction of the prolapse, resection of a short segment of bowel, and repair of any associated hernia that will coexist in one-half of patients.12 Often the skin aperture is quite large and needs to be revised to accommodate the smaller diameter of the neostoma. Simple amputation of the prolapsed bowel and construction of a new stoma has been suggested by some surgeons12 and others have used multiple stapler firings to achieve a similar result,13 14 but these procedures are prone to recurrence of the prolapse or other problems if an underlying peristomal hernia is present.12
Prolapse associated with a temporary loop stoma is best addressed in an expectant manner until the stoma can be closed. If the loop stoma is intended to be permanent, the prolapse can be managed by stapled closure of the efferent limb and simple stoma revision if the distal out-of-circuit bowel is not obstructed.15 Repair of an associated hernia is concurrently performed when the patient does not have prohibitive comorbid conditions or short life expectancy.
Retraction
Early retraction is commonly caused by excess tension, but can also result from poor wound healing linked to glucocorticoid usage or malnutrition. Complete retraction with mucocutaneous separation is managed in a manner similar to ischemia of the deeper bowel layers. Retraction occurring months after surgery affects 2 to 13% of colostomies and 11 to 24% of ileostomies.4 One common cause is weight gain after stoma formation, while disease recurrence must be excluded in patients with Crohn's disease.
Retraction is initially treated with switching the patient to a more convex appliance to minimize the risk of leakage resulting from stoma function under the faceplate. In simple cases, the stoma can be locally revised with exteriorization of a bowel segment of sufficient length to assure adequate eversion. However, patients must be counseled about the potential need for a laparotomy as this approach is required in most cases, especially if the cause is obesity or recurrent Crohn's disease. Revision of the stoma in these instances employs the same basic tenets mentioned earlier.
In patients with an ileal conduit for urinary diversion, retraction of the stoma does not require revision of the conduit with reimplantation of the ureters. Instead, the existing conduit is merely augmented at its distal end with another segment of harvested small bowel that is matured with 3 to 4 cm of protrusion.
Stenosis
Stenosis or stricture of the stoma usually results from ischemia occurring at the time of stoma creation, but can be associated with prior infection or suggest recurrent Crohn's disease. The reported incidence of stenosis is 1 to 14% with colostomies and 2 to 17% with ileostomies4 with the majority of stenoses manifesting themselves in the first 5 years.8 9
Stenosis at the skin level can be definitively managed with simple local revision. Conversely, involvement of the suprafascial bowel is initially treated with gentle dilation, but multiple sessions are generally required and operative revision is eventually warranted in many cases. While attempts at local revision are tempting, laparotomy, resection of the stenotic bowel, and creation of a neostoma is often required.
Summary
A properly constructed stoma sited in an appropriate location will usually assure an ostomate a reasonable quality of life. Conditions that make stoma construction difficult can be overcome in most cases using creative approaches and proven techniques. Subsequent complications can be equally challenging, but a solution is generally available that can help the patient overcome the problem with the assistance of their surgeon.
References
- 1.Hardt J, Meerpohl J J, Metzendorf M I, Kienle P, Post S, Herrle F. Lateral pararectal versus transrectal stoma placement for prevention of parastomalherniation. Cochrane Database Syst Rev. 2013;11:CD009487. doi: 10.1002/14651858.CD009487.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Cottam J, Richards K, Hasted A, Blackman A. Results of a nationwide prospective audit of stoma complications within 3 weeks of surgery. Colorectal Dis. 2007;9(9):834–838. doi: 10.1111/j.1463-1318.2007.01213.x. [DOI] [PubMed] [Google Scholar]
- 3.Sabbagh C, Maggiori L, Panis Y. Management of failed low colorectal and coloanalanastomosis. J Vis Surg. 2013;150(3):181–187. doi: 10.1016/j.jviscsurg.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Shabbir J, Britton D C. Stoma complications: a literature overview. Colorectal Dis. 2010;12(10):958–964. doi: 10.1111/j.1463-1318.2009.02006.x. [DOI] [PubMed] [Google Scholar]
- 5.Al Shakarchi J, Williams J G. Systematic review of open techniques for parastomal hernia repair. Tech Coloproctol. 2014;18(5):427–432. doi: 10.1007/s10151-013-1110-z. [DOI] [PubMed] [Google Scholar]
- 6.DeAsis F J, Lapin B, Gitelis M E, Ujiki M B. Current state of laparoscopic parastomal hernia repair: A meta-analysis. World J Gastroenterol. 2015;21(28):8670–8677. doi: 10.3748/wjg.v21.i28.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabbir J, Chaudhary B N, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Colorectal Dis. 2012;14(8):931–936. doi: 10.1111/j.1463-1318.2011.02835.x. [DOI] [PubMed] [Google Scholar]
- 8.Leong A P, Londono-Schimmer E E, Phillips R K. Life-table analysis of stomal complications following ileostomy. Br J Surg. 1994;81(5):727–729. doi: 10.1002/bjs.1800810536. [DOI] [PubMed] [Google Scholar]
- 9.Londono-Schimmer E E, Leong A P, Phillips R K. Life table analysis of stomal complications following colostomy. Dis Colon Rectum. 1994;37(9):916–920. doi: 10.1007/BF02052598. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaos K, Michailidou E, Karakatsanis A, Margioulas A. Local treatment of an end colostomy prolapse 6 months after abdominoperineal resection. Am Surg. 2011;77(11):E218–E219. [PubMed] [Google Scholar]
- 11.Fligelstone L J, Wanendeya N, Palmer B V. Osmotic therapy for acute irreducible stoma prolapse. Br J Surg. 1997;84(3):390. doi: 10.1046/j.1365-2168.1997.02594.x. [DOI] [PubMed] [Google Scholar]
- 12.Allen-Mersh T G, Thomson J P. Surgical treatment of colostomy complications. Br J Surg. 1988;75(5):416–418. doi: 10.1002/bjs.1800750507. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson H J, Bhalerao S. Correction of end colostomy prolapse using a curved surgical stapler, performed under sedation. Tech Coloproctol. 2010;14(2):165–167. doi: 10.1007/s10151-010-0568-1. [DOI] [PubMed] [Google Scholar]
- 14.Masumori K, Maeda K, Hanai T. et al. Short-term outcomes of local correction of stoma prolapse with a stapler device. Tech Coloproctol. 2013;17(4):437–440. doi: 10.1007/s10151-012-0959-6. [DOI] [PubMed] [Google Scholar]
- 15.Masumori K, Maeda K, Koide Y. et al. Simple excision and closure of a distal limb of loop colostomy prolapse by stapler device. Tech Coloproctol. 2012;16(2):143–145. doi: 10.1007/s10151-011-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


