Abstract
Radiotherapy not only plays a pivotal role in the cancer care pathways of many patients with pelvic malignancies, but can also lead to significant injury of normal tissue in the radiation field (pelvic radiation disease) that is sometimes as challenging to treat as the neoplasms themselves. Acute symptoms are usually self-limited and respond to medical therapy. Chronic symptoms often require operative intervention that is made hazardous by hostile surgical planes and unforgiving tissues. Management of these challenging patients is best guided by the utmost caution and humility.
Keywords: radiation proctitis, radiation enteritis or enteropathy, radiation-induced bowel damage
Radiotherapy plays a pivotal role in the cancer care pathways of many patients with pelvic malignancies such as urologic, gynecologic, and anorectal cancers.1 Nearly 70% of cancer patients will undergo radiotherapy for these malignancies to prolong disease-free survival and reduce local recurrence.2 This modality can also lead to significant injury of normal tissue in the radiation field that is sometimes as challenging to treat as the neoplasms themselves.3 Some of the most devastating injuries occur to the small intestine, historically termed radiation enteritis or enteropathy and now undergoing a reclassification of its nomenclature to either pelvic radiation disease or radiation-induced small bowel disease.2 4
Historical Perspective and Classification
Radiation-induced intestinal damage was first described by Walsh in 1897 as “a direct inflammation of the gastrointestinal (GI) mucous membranes,” observed in a coworker suffering from bowel dysfunction after X-ray exposure and whose symptoms improved after he was kept from further exposure.5 In the 1920s, Warren and Whipple conducted the first in vivo study on dogs to elucidate intestinal radiation damage.6 Today, radiation-induced intestinal disease is classified into two groups: acute/early changes and chronic/late changes. These classifications vary in their temporal relationship to treatment, pathologic sequelae, and, most critically, treatment strategies.
Incidence and Prevalence
As many as 300,000 patients worldwide undergo pelvic irradiation per year.2 Studies estimate that 50 to 70% of patients will develop acute damage, while 5 to 11% exhibit signs of chronic radiation toxicity.7 Presence of acute symptoms does not necessarily predict later development of chronic damage. Of those with sequelae of chronic radiation disease, 30% of patients will require operative intervention.8 As the number of affected patients increases with the use of radiation therapy in pelvic cancers, physicians must be adept in its early diagnosis and continue to improve strategies in operative and nonoperative management.9
Pathology and Pathogenesis
Ionizing radiation exerts its effect in two ways: through direct damage to target macromolecules (DNA) and through indirect damage via oxygen free radicals. Target macromolecules absorb energy and become ionized or transformed into free radicals. Both direct and indirect pathways of injury result in free radical formation, causing damage of cellular structures and compromising cell function, leading to apoptosis.10 DNA transcription and replication are often impaired, and cells with a high rate of replication are particularly affected. Cells of the mucosa are first affected, then those of the submucosa, muscularis, and then serosa.10
The GI tract is the second most radiosensitive organ in the abdomen and pelvis and often proves to be the major limiting factor determining tolerance of radiation therapy.3 Because most radiation treatments are directed into the pelvis, the rectum is at particular risk for injury, as are organs that are mobile and able to move into the pelvis. The cecum and the sigmoid colon are common sites of involvement because of their fixed positions at the pelvic brim and are likely to receive higher doses of ionizing radiation.11 A mobile distal ileum may easily slide into the pelvis, especially in patients with a wide pelvis. Adhesive disease tethering a redundant transverse colon to the pelvis may put this bowel segment at risk as well. The hepatic and splenic flexures are almost never affected because of their fixed location outside of the pelvis.
Acute Changes
The acute effects of ionizing radiation on normal bowel occur within hours of initiation of treatment and cause breakdown of the rapidly dividing crypt cells and the intestinal mucosal barrier, and atrophy and blunting of villi.12 Fluid and nutrient absorption is impaired and mucosal ulcerations develop, leading to diarrhea, tenesmus, bloody and mucous discharge, hematochezia, and incontinence. If ulcerations persist, bacterial translocation can occur and lead to systemic sepsis. More than half of patients undergoing pelvic radiation therapy will develop varying symptoms in the acute setting.13 Once therapy is halted, intestinal crypts regenerate and mucosal healing ensues, but symptoms can last up to 6 months. Acute symptoms are typically self-limiting, improve shortly after cessation of treatment, and are responsive to normal medical treatments for enteritis. Symptoms are usually dose-dependent and respond favorably to reduction in dose. Cessation of treatment is rarely required to control symptoms, and the presence of acute toxicity does not necessarily prognosticate the development of symptomatic chronic radiation injury.11
Chronic Changes
Effects of chronic radiation injury appear 6 to 12 months after treatment. The toxic effects of radiation cause the endothelium of supporting vasculature to become irregular, progressively leading to fibrosis of capillaries that support the intestine. This creates an obliterative endarteritis that leads to ischemia and fibrosis of the bowel (Fig. 1).14 These pathologic changes manifest clinically as partial or complete bowel obstructions due to strictures, impaired motility, fistulae, GI bleeding, impaired nutrition absorption, and sometimes perforation with sepsis.14 15 For the surgeon, it is management of these late complications that is often the more formidable challenge than those in the acute setting. These chronic changes are typically not reversible and do not correlate to the occurrence of acute symptoms.
Fig. 1.
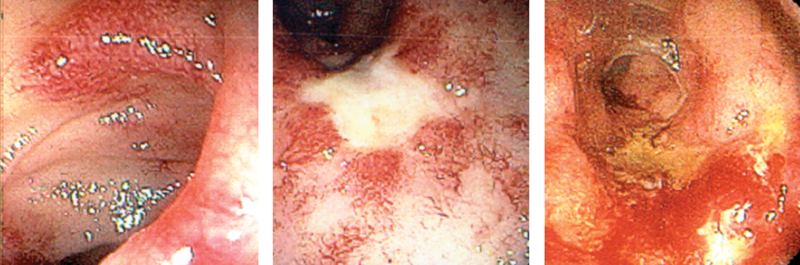
Endoscopic findings of radiation injury. (Reprinted with permission from Bo Shen, MD, Cleveland Clinic Foundation, 2015. All rights reserved.)
Management of Radiation-Induced Small Bowel Injury
Diagnosis and Evaluation
Since surgical intervention is rarely necessary for those with acute radiation toxicity, the focus of this section will center on the surgical management of chronic radiation disease. These patients present with some of the most challenging problems in abdominal surgery, and inadequate understanding of the medical history or poor surgical planning often results in untoward surgical outcomes. Important factors include duration of radiotherapy treatment, dosing regimen, target fields, and complications during treatment, as well as the primary neoplasm and prior surgical history. Recurrent neoplastic disease must be ruled out as an etiology of symptoms.2 Patients are often nutritionally compromised, and assessment of the degree of malnutrition helps to determine the strategy for preoperative optimization should surgical intervention be required.12
In most cases, small bowel radiation disease can be diagnosed and characterized using contrasted small bowel imaging. Small bowel contrast (enteroclysis) studies were traditionally used to detect strictures with upstream dilation, fistulae, and other elements of mechanical obstruction. These studies may be poorly tolerated and give nonspecific results, with narrowed segments not always clinically contributory to symptoms. Ischemia presents as “thumbprinting” on these studies, and separation of bowel loops may represent bowel wall edema and fibrosis.3 16
Computed tomography (CT) enterography has largely replaced enteroclysis as the preferred imaging modality for chronic radiation injury due as it is well-tolerated, delineates strictures and fistulae, and distinguishes radiation injury from recurrent or persistent malignancy.3 Integrated positron emission tomography-CT is also helpful in distinguishing posttreatment changes from recurrent malignancy.
Magnetic resonance enterography has emerged as a useful tool to evaluate the small bowel for radiation injury.17 Benefits of this modality include avoidance of radiation exposure and nephrotoxic contrast, as well as high-resolution soft tissue imaging.18
Capsule endoscopy is a liability in the setting of chronic radiation injury, as the capsule may become lodged in a stenotic segment of bowel, completing an obstruction. It is critical to identify location, extent, and severity of clinically relevant radiation-induced lesions. Failure to do so equates with poor surgical planning and will result in poor surgical outcomes.
Medical Management
Although some patients with chronic radiation disease will ultimately need surgical intervention, those with mild symptoms may be managed nonoperatively. Patients with mild obstructive symptoms may benefit from taking a soft, low residue diet or fortified liquid diets.19 20 Parenteral nutrition may be needed for those who do not tolerate enteral feedings to palliate symptoms or to boost nutrition for impending surgery. Survival rates of patients maintained on long-term total parenteral nutrition (TPN) are more than 50% at 5 years, as these patients generally succumb to complications of either radiation injury or the underlying malignancy. Antidiarrheal therapy must be tailored to its suspected etiology. Diarrhea due to bacterial overgrowth may respond to antibiotics. Patients with functional short bowel syndrome may benefit from motility agents such as loperamide. Cholestyramine should be given if bile acid malabsorption is suspected.21 22 Early trials evaluating probiotics have shown a protective role and improvement in postradiation diarrhea with use of some probiotic mixtures.23 The probiotic VSL#3 has been investigated in a double-blind, placebo-controlled trial and led to fewer bowel motions and less diarrhea.24 Several case reports have supported a role for hyperbaric oxygen therapy to enhance recovery, but its expense and limited availability make its routine use challenging.25
Surgical Management and Principles
More than 30% of patients with chronic radiation injury will require surgical intervention.8 Prior to any surgical intervention, patients and surgeons must agree that the goals of surgery are not to cure the disease, but to palliate the complications of the disease. Radiation damage is cumulative over time and is likely to recur in more than half of patients following surgery, often in a location differed from the original site of pathology.
Surgery is usually performed to relieve symptoms of partial or complete obstruction due to tissue fibrosis and subsequent formation of small bowel strictures. Other indications include fistula, perforation, or bleeding. Patients face high rates of postoperative morbidity (30%) and mortality (5%).8 26 Those patients determined to have an unacceptable risk/benefit ratio can be considered for decompressive gastrostomy to accompany TPN or a proximal diverting small bowel ostomy.
Preparations for Surgery
A detailed discussion and documentation of risks and benefits with informed consent is a necessity, with explicit explanation of the expectations and the palliative goals of surgery. Once the decision is made to proceed with surgical intervention, every effort must be made to correct modifiable factors such as anemia and electrolyte derangement in patients with chronic losses from fistulae or diarrhea, and malnutrition due to anorexia, functional obstruction, and mechanical obstruction. Those with severe malnutrition (weight loss >15% ideal body weight or serum albumin less than 2.5 g/dL) may require a preoperative course of TPN.8 Although the data are limited regarding duration of preoperative therapy,3 27 a 7 to 10 day course is reasonable with continuation postoperatively until enteral nutrition is reliably tolerated.28
Enterostomal nurse consultation and marking in all four quadrants should also be performed. Arrangement for placement of ureteral stents should be strongly considered. Appropriate intravenous antibiotics and subcutaneous heparin therapy should be given prior to incision.
Intraoperative Strategies
Patients should be placed in a modified lithotomy position, allowing for access to the perineum. Intraoperative endoscopy should be available. The abdomen is usually accessed through a midline incision with sharp dissection to avoid thermal injury to bowel that may be densely adherent to the anterior abdominal wall. Entering the abdomen away from the most affected area allows one to “circle the enemy” by avoiding the area with the worst disease when trying to gain entry. An infraumbilical transverse incision to avoid radiated abdominal wall tissues can also be used and has been associated with fewer wound complications.29
Once the abdomen is entered and severity of disease is assessed, it is important to develop an exit strategy in case the disease is too formidable or patient factors preclude completion of the surgery. To facilitate this, dissection should begin from proximal (where radiation-induced damage is likely less severe) to distal. Thus, proximal fecal diversion can be performed if multiple enterotomies are made or if patient factors require aborting further dissection.
There is debate regarding whether to completely mobilize and examine the entire small bowel or to address only the segments of diseased bowel. Proponents of a limited dissection argue that more adhesiolysis increases the risk for inadvertent enterotomy and development of postoperative enterocutaneous fistulae. Radiated bowel is inherently delicate, friable, and unforgiving, and an injury (especially one that is missed) is a disastrous complication in this patient population. Proponents of a complete mobilization from duodenojejunal flexure to ileocecal valve argue that the origin of clinical symptoms is not always known, and fistulae and abscesses not clearly apparent on preoperative imaging may be discovered. Operative strategy should be tailored to each individual patient.
Radiated bowel exhibits a pale yellow or grayish hue, often with inflamed, friable mesentery that bleeds easily and must be handled gently. Strictures of varying lengths may be accompanied with chronic upstream dilation. The controversy over whether to resect or bypass the affected bowel segment centers on the relative risks of anastomotic leak and mortality.8 26 30 Benefits of resection over bypass include the risk for early recurrence of symptoms, ongoing pain, GI bleeding, and risk for development of malignancy in the bypassed segment.29 31 32 Bowel that may have been included in the field of radiation (terminal ileum, cecum, and right colon) should not be used for an anastomosis to mitigate the leak risk.
In some situations, both resection and bypass would produce a clinically undesirable result. For example, a case involving a long segment of bowel with multiple, clinically significant strictures may result in short bowel syndrome if resected or may be at risk for bacterial overgrowth and persistent symptoms if bypassed. A small series has described the use of strictureplasty in these segments as a “last-chance” maneuver to avoid these outcomes and showed promising results with no anastomotic leaks and successful avoidance of TPN dependence.33
Technical Strategies
The standard principles of creating bowel anastomoses apply, and emphasis is placed on using healthy, nonradiated tissue with good blood supply. Some sections of bowel, such as the terminal ileum, cecum, and right colon, may have sustained radiation injury but appear grossly normal and should not be used for anastomosis if possible. Dense interloop bowel adhesions may be handled with hydrodissection, which uses injectable saline to better delineate tissue planes, thus minimizing iatrogenic serosal injuries or enterotomies. Mesenteric tissue is notoriously friable, foreshortened, and fibrotic, and control of the mesentery with conventional clamps and ties, or commercial vessel sealing devices is not sufficient. A helpful technique for better control is division of mesentery between Kocher clamps, with interlocking heavy #1 chromic suture to control blood vessels along the entire length of the cut mesenteric edge (Fig. 2).
Fig. 2.
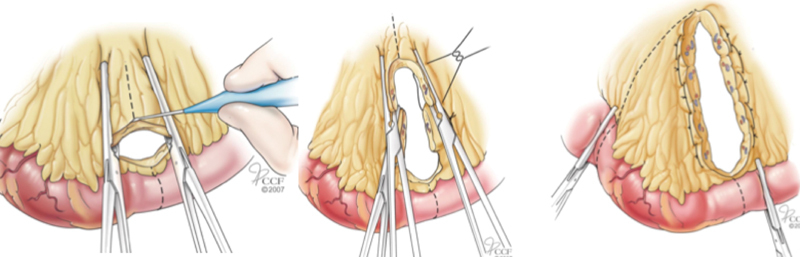
Control and division of mesenteric vessels of radiation-injured intestine. (Reprinted with permission from Cleveland Clinic Center for Medical Art and Photography, ©1996–2010. All rights reserved.)
Management of Large Bowel Injury
Diagnosis
Endoscopic evaluation is critical to assess large bowel radiation disease with the remainder of the evaluation similar to that of small bowel disease.12 Erosions, rectal strictures, ulcerations, and fistulae are common sequelae of radiation therapy of the distal GI tract and can result in refractory pain, tenesmus, rectal bleeding, and change in bowel habits. One of the most common symptoms of radiation proctitis is rectal bleeding that usually develops one year after treatment and commonly resolves spontaneously within 18 months of treatment.
Nonsurgical Therapy
Rectal bleeding from proctitis is often encountered and can be treated in several ways. Topical treatments such as anti-inflammatory enemas and suppositories are usually considered first-line therapy, but studies evaluating their protective role have shown mixed results. Two double-blind, placebo-controlled trials have shown improved stool consistency and reduced frequency in patients using sucralfate enemas,34 35 as well as a reduction in severity of rectal bleeding after 4 weeks of treatment.36 Others have suggested that sucralfate enemas do not reduce the rate of rectal bleeding and may even be associated with worsened bowel function.37 The use of steroid enemas is common but without strong evidence supporting their benefit.
Topical formalin treatment has been used for proctitis for more than three decades.38 Its ease of use and tolerance is attractive, but studies show that 30% will experience recurrent bleeding.39 This procedure requires at least moderate sedation. Rigid proctoscopy is used to identify bleeding surfaces and a 4% topical formalin solution is applied to the surface for several minutes or until bleeding stops. Care must be taken to protect the perianal skin from exposure to the caustic formalin solution. The rectum is then copiously irrigated with saline after treatment. Unfavorable side effects of this therapy include proctitis, stricture, and chronic anal pain.
Endoscopic therapy with cryoablation40 and argon plasma coagulation (Fig. 3) are safe and effective modalities to control bleeding from rectal telangiectasia in chronic radiation disease,41 although only low-level evidence supports their use. Care must be taken to avoid aggressive treatment in severely damaged tissue or areas of prior biopsy, as fistulae may occur, for instance, the development of a rectourethral fistula after radiation therapy for prostate cancer.42 43 44
Fig. 3.
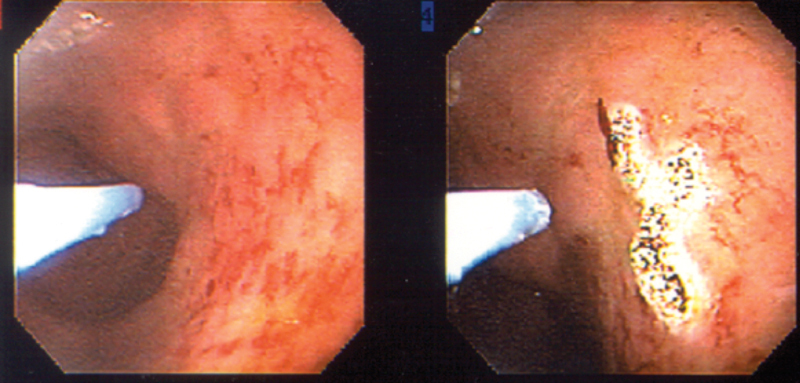
Argon plasma coagulation to control rectal bleeding from radiation proctitis. (Reprinted with permission from Bo Shen, MD, Cleveland Clinic Foundation, 2015. All rights reserved.)
Surgical Therapy
Surgical measures may be necessary in patients who develop fistulae, sepsis, perforation, recalcitrant bleeding, or recurrent carcinoma. These situations are especially challenging given the complex nature of pathology in the setting of an already decompensated patient. The surgeon must consider the patient's primary disease, nutritional status, overall life expectancy, and etiology of symptoms when formulating a surgical plan.
Other Considerations
Most radiation-induced large bowel disease is limited to the rectosigmoid colon, which is often amenable to resection with primary anastomosis as long as tissues are healthy and anal sphincters are intact. Using healthy tissue for anastomosis is ideal but may not be possible; reasonable outcomes have been reported if only one end of the intestine is healthy.45 Handsewn anastomosis is favored as it avoids staple line-related ischemia in already compromised bowel.46 Although some studies report acceptable healing of undiverted coloproctostomy after radiation damage, creation of a covering stoma should be strongly considered.
Fecal diversion in patients with chronic pelvic radiation disease can relieve acute symptoms associated with anal ulcers, sepsis and nonhealing wounds associated with fistulae, and defecatory obstruction caused by rectal strictures. Using nonradiated bowel is ideal as stomas created with diseased bowel are prone to stricture, bleeding, and necrosis. Compared with a diverting colostomy, diverting ileostomy is generally easier to create and close, and avoids mobilization and interruption of colonic blood supply that may stymie future reconstruction.
A difficult situation for both patient and surgeon is the management of fistulae caused by radiation damage. Rectovaginal and rectourethral fistulae are most commonly seen, but vesicovaginal, ileovaginal, and ileovesical fistulae may also occur. Preoperative diagnosis with appropriate imaging is critical to determine origin of the fistula. Many methods of repair have been described, including various rotational tissue and endorectal advancement flap closures.47 48 49 50 51 When tissues are not ideal for local repair, a transabdominal approach with mobilization of the rectum below the level of the fistula, resection of diseased bowel, and anastomosis using healthy proximal colon may be the only option. Interposition of normal tissue such as omentum between connecting organs is recommended, and a covering stoma should always be employed. When disease is extensive and poor tissue quality is present, patients are at additional risk for anastomotic leak or recurrent fistulae. In these situations, a coloanal pull-through with delayed anastomosis (Turnbull–Cutait procedure) is a helpful alternative (Fig. 4). Proctectomy with mucosectomy is performed, followed by pulling a mobile, healthy colon conduit through the anal canal, the end of which remains exteriorized. Stay sutures are placed in the anal canal. Amputation and completion of the anastomosis by placing the stay sutures through the free edge is performed 7 days later, after allowing the natural adhesive properties of the tissue to secure the anastomosis and reducing the risk for anastomotic complications.52 53 54
Fig. 4.
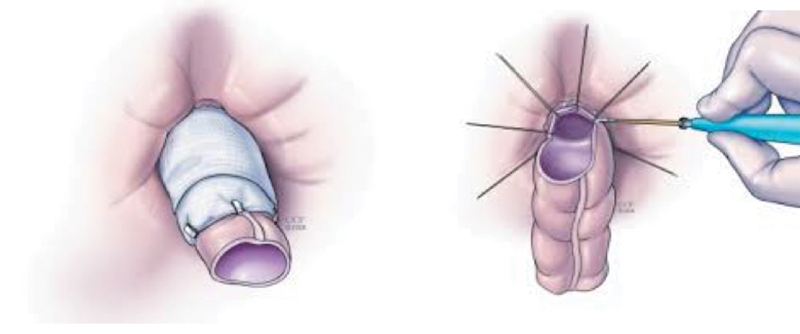
Turnbull–Cutait abdominoperineal pull-through with delayed coloanal anastomosis. Externalization of colonic conduit (left), followed by amputation and delayed anastomosis 7 days later (right). (Reprinted with permission from Cleveland Clinic Center for Medical Art and Photography, ©1996–2010. All rights reserved.)
Conclusion
Radiation-induced bowel disease is an important problem because of its negative impact on quality of life, compromise of radiation tolerance, and associated additional economic burden to the already costly process of cancer care. Surgery is justified in a select group of patients with symptoms of chronic disease unresponsive to nonsurgical treatments and should be cautiously undertaken only after creation of a comprehensive and individualized perioperative plan.
References
- 1.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13(22):3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreyev H J. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19(10):790–799. doi: 10.1016/j.clon.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Theis V S, Sripadam R, Ramani V, Lal S. Chronic radiation enteritis. Clin Oncol (R Coll Radiol) 2010;22(1):70–83. doi: 10.1016/j.clon.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Andreyev H J, Wotherspoon A, Denham J W, Hauer-Jensen M. “Pelvic radiation disease”: new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol. 2011;46(4):389–397. doi: 10.3109/00365521.2010.545832. [DOI] [PubMed] [Google Scholar]
- 5.Walsh D. Deep tissue traumatism from roentgen ray exposure. BMJ. 1897;2(1909):272–273. doi: 10.1136/bmj.2.1909.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren S L, Whipple G H. Roentgen Ray intoxication: IV. Intestinal lesions and acute intoxication produced by radiation in a variety of animals. J Exp Med. 1923;38(6):741–752. doi: 10.1084/jem.38.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerer T, Böcker U, Wenz F, Singer M V. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? a short review. Z Gastroenterol. 2008;46(5):441–448. doi: 10.1055/s-2008-1027150. [DOI] [PubMed] [Google Scholar]
- 8.Regimbeau J M Panis Y Gouzi J L Fagniez P L; French University Association for Surgical Research. Operative and long term results after surgery for chronic radiation enteritis Am J Surg 20011823237–242. [DOI] [PubMed] [Google Scholar]
- 9.Waddell B E, Rodriguez-Bigas M A, Lee R J, Weber T K, Petrelli N J. Prevention of chronic radiation enteritis. J Am Coll Surg. 1999;189(6):611–624. doi: 10.1016/s1072-7515(99)00199-4. [DOI] [PubMed] [Google Scholar]
- 10.Delaney J P, Bonsack M E, Felemovicius I. Lumenal route for intestinal radioprotection. Am J Surg. 1993;166(5):492–501. doi: 10.1016/s0002-9610(05)81143-2. [DOI] [PubMed] [Google Scholar]
- 11.Shadad A K, Sullivan F J, Martin J D, Egan L J. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19(2):185–198. doi: 10.3748/wjg.v19.i2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theis V S, Sripadam R, Ramani V, Lal S. Chronic radiation enteritis. Clin Oncol (R Coll Radiol) 2010;22(1):70–83. doi: 10.1016/j.clon.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Curtis N J, Bryant T, Raj S, Bateman A R, Mirnezami A H. Acute radiation enteritis causing small bowel obstruction. Ann R Coll Surg Engl. 2011;93(7):e129–e130. doi: 10.1308/147870811X602122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarnold J, Brotons M C. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Kountouras J, Zavos C. Recent advances in the management of radiation colitis. World J Gastroenterol. 2008;14(48):7289–7301. doi: 10.3748/wjg.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreyev H J, Vlavianos P, Blake P, Dearnaley D, Norman A R, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist? Int J Radiat Oncol Biol Phys. 2005;62(5):1464–1471. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 17.Cronin C G, Lohan D G, Browne A M, Alhajeri A N, Roche C, Murphy J M. MR enterography in the evaluation of small bowel dilation. Clin Radiol. 2009;64(10):1026–1034. doi: 10.1016/j.crad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Algin O, Turkbey B, Ozmen E, Algin E. Magnetic resonance enterography findings of chronic radiation enteritis. Cancer Imaging. 2011;11:189–194. doi: 10.1102/1470-7330.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekhon S. Chronic radiation enteritis: women's food tolerances after radiation treatment for gynecologic cancer. J Am Diet Assoc. 2000;100(8):941–943. doi: 10.1016/S0002-8223(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 20.McGough C, Baldwin C, Frost G, Andreyev H J. Role of nutritional intervention in patients treated with radiotherapy for pelvic malignancy. Br J Cancer. 2004;90(12):2278–2287. doi: 10.1038/sj.bjc.6601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludgate S M, Merrick M V. The pathogenesis of post-irradiation chronic diarrhoea: measurement of SeHCAT and B12 absorption for differential diagnosis determines treatment. Clin Radiol. 1985;36(3):275–278. doi: 10.1016/s0009-9260(85)80059-3. [DOI] [PubMed] [Google Scholar]
- 22.Danielsson A, Nyhlin H, Persson H, Stendahl U, Stenling R, Suhr O. Chronic diarrhoea after radiotherapy for gynaecological cancer: occurrence and aetiology. Gut. 1991;32(10):1180–1187. doi: 10.1136/gut.32.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol. 2001;13(4):391–396. doi: 10.1097/00042737-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Delia P, Sansotta G, Donato V. et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13(6):912–915. doi: 10.3748/wjg.v13.i6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fok T C, Jan A, Peel S A, Evans A W, Clokie C M, Sándor G K. Hyperbaric oxygen results in increased vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(4):417–422. doi: 10.1016/j.tripleo.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Galland R B, Spencer J. Surgical management of radiation enteritis. Surgery. 1986;99(2):133–139. [PubMed] [Google Scholar]
- 27.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group . Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325(8):525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 28.Braga M Ljungqvist O Soeters P Fearon K Weimann A Bozzetti F; ESPEN. ESPEN guidelines on parenteral nutrition: surgery Clin Nutr 2009284378–386. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Gong J, Li Y, Li N, Li J. A retrospective study of surgical treatment of chronic radiation enteritis. J Surg Oncol. 2012;105(7):632–636. doi: 10.1002/jso.22099. [DOI] [PubMed] [Google Scholar]
- 30.Wobbes T, Verschueren R C, Lubbers E J, Jansen W, Paping R H. Surgical aspects of radiation enteritis of the small bowel. Dis Colon Rectum. 1984;27(2):89–92. doi: 10.1007/BF02553982. [DOI] [PubMed] [Google Scholar]
- 31.Tamai O, Nozato E, Miyazato H. et al. Radiation-associated rectal cancer: report of four cases. Dig Surg. 1999;16(3):238–243. doi: 10.1159/000018715. [DOI] [PubMed] [Google Scholar]
- 32.Martel P, Deslandes M, Dugue L, Sezeur A, Gallot D, Malafosse M. Radiation injuries of the small intestine. Surgical treatment [in French] Ann Chir. 1996;50(4):312–317. [PubMed] [Google Scholar]
- 33.Dietz D W, Remzi F H, Fazio V W. Strictureplasty for obstructing small-bowel lesions in diffuse radiation enteritis—successful outcome in five patients. Dis Colon Rectum. 2001;44(12):1772–1777. doi: 10.1007/BF02234454. [DOI] [PubMed] [Google Scholar]
- 34.Henriksson R, Franzén L, Littbrand B. Effects of sucralfate on acute and late bowel discomfort following radiotherapy of pelvic cancer. J Clin Oncol. 1992;10(6):969–975. doi: 10.1200/JCO.1992.10.6.969. [DOI] [PubMed] [Google Scholar]
- 35.Valls A, Pestchen I, Prats C. et al. Multicenter double-blind clinical trial comparing sucralfate vs placebo in the prevention of diarrhea secondary to pelvic irradiation [in Spanish] Med Clin (Barc) 1999;113(18):681–684. [PubMed] [Google Scholar]
- 36.Kochhar R, Sriram P V, Sharma S C, Goel R C, Patel F. Natural history of late radiation proctosigmoiditis treated with topical sucralfate suspension. Dig Dis Sci. 1999;44(5):973–978. doi: 10.1023/a:1026612731210. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien P C, Franklin C I, Poulsen M G, Joseph D J, Spry N S, Denham J W. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial—Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54(2):442–449. doi: 10.1016/s0360-3016(02)02931-0. [DOI] [PubMed] [Google Scholar]
- 38.Ma T H, Yuan Z X, Zhong Q H. et al. Formalin irrigation for hemorrhagic chronic radiation proctitis. World J Gastroenterol. 2015;21(12):3593–3598. doi: 10.3748/wjg.v21.i12.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chattopadhyay G, Ray D, Chakravartty S, Mandal S. Formalin instillation for uncontrolled radiation induced haemorrhagic proctitis. Trop Gastroenterol. 2010;31(4):291–294. [PubMed] [Google Scholar]
- 40.Rustagi T, Mashimo H. Endoscopic management of chronic radiation proctitis. World J Gastroenterol. 2011;17(41):4554–4562. doi: 10.3748/wjg.v17.i41.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Serna Higuera C, Martín Arribas M, Rodríguez Gómez S, Pérez Villoria A, Martínez Moreno J, Betancourt González A. Efficacy and safety of argon plasma coagulation for the treatment of hemorrhagic radiation proctitis. Rev Esp Enferm Dig. 2004;96(11):758–764. doi: 10.4321/s1130-01082004001100003. [DOI] [PubMed] [Google Scholar]
- 42.Gelblum D Y, Potters L. Rectal complications associated with transperineal interstitial brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48(1):119–124. doi: 10.1016/s0360-3016(00)00632-5. [DOI] [PubMed] [Google Scholar]
- 43.Theodorescu D, Gillenwater J Y, Koutrouvelis P G. Prostatourethral-rectal fistula after prostate brachytherapy. Cancer. 2000;89(10):2085–2091. doi: 10.1002/1097-0142(20001115)89:10<2085::aid-cncr8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 44.Shakespeare D, Mitchell D M, Carey B M. et al. Recto-urethral fistula following brachytherapy for localized prostate cancer. Colorectal Dis. 2007;9(4):328–331. doi: 10.1111/j.1463-1318.2006.01119.x. [DOI] [PubMed] [Google Scholar]
- 45.Thompson S K, Chang E Y, Jobe B A. Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26(3):131–136. doi: 10.1002/micr.20197. [DOI] [PubMed] [Google Scholar]
- 46.Hauer-Jensen M, Wang J, Denham J W. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13(3):357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 47.de Parades V, Dahmani Z, Blanchard P, Zeitoun J D, Sultan S, Atienza P. Endorectal advancement flap with muscular plication: a modified technique for rectovaginal fistula repair. Colorectal Dis. 2011;13(8):921–925. doi: 10.1111/j.1463-1318.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 48.Sonoda T, Hull T, Piedmonte M R, Fazio V W. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45(12):1622–1628. doi: 10.1007/s10350-004-7249-y. [DOI] [PubMed] [Google Scholar]
- 49.Kniery K, Johnson E K, Steele S R. How I do it: Martius flap for rectovaginal fistulas. J Gastrointest Surg. 2015;19(3):570–574. doi: 10.1007/s11605-014-2719-6. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz D Bashankaev B Speranza J Wexner S D Graciloplasty for rectourethral, rectovaginal and rectovesical fistulas: technique overview, pitfalls and complications Tech Coloproctol 2008123277–281., discussion 281–282 [DOI] [PubMed] [Google Scholar]
- 51.Jarrar A, Church J. Advancement flap repair: a good option for complex anorectal fistulas. Dis Colon Rectum. 2011;54(12):1537–1541. doi: 10.1097/DCR.0b013e31822d7ddd. [DOI] [PubMed] [Google Scholar]
- 52.Remzi F H, El Gazzaz G, Kiran R P, Kirat H T, Fazio V W. Outcomes following Turnbull-Cutait abdominoperineal pull-through compared with coloanal anastomosis. Br J Surg. 2009;96(4):424–429. doi: 10.1002/bjs.6458. [DOI] [PubMed] [Google Scholar]
- 53.Turnbull R B Jr. Pull-through resection of the rectum, with delayed anastomosis, for cancer or Hirschsprung's disease. Surgery. 1966;59(3):498–502. [PubMed] [Google Scholar]
- 54.Kirwan W O, Turnbull R B. The Turnbull-Cutait pullthrough procedure for certain cancers of the rectum and Hirschsprung disease. Int Adv Surg Oncol. 1981;4:173–187. [PubMed] [Google Scholar]


