Abstract
Rectal cancer can recur locally in up to 10% of the patients who undergo definitive resection for their primary cancer. Surgical salvage is considered appropriate in the curative setting as well as select cases with palliative intent. Disease-free survival following salvage resection is dependent upon achieving an R0 resection margin. A clear understanding of applied surgical anatomy, appropriate preoperative planning, and a multidisciplinary approach to aggressive soft tissue, bony, and vascular resection with appropriate reconstruction is necessary. Technical tips, tricks, and pitfalls that may assist in managing these cancers are discussed and the roles of additional boost radiation and intraoperative radiation therapy in the management of such cancers are also discussed.
Keywords: pelvic exenteration, rectal cancer, cancer recurrence, colorectal surgery
Background
Colorectal cancer (CRC) is the third most common cancer in men and the second most frequent in females.1 Up to one-third of CRCs arise due to neoplasms in the rectum, where in the majority of the cases, treatment intent remains curative.1 Unfortunately, in up to 6 to 13% of such cases, disease recurrence in the pelvis and/or perineum can provide a treatment dilemma for the surgeon.2 Historically, local recurrence (LR) rates have been as high as 32% following primary curative resection of the rectum, and perhaps most famously the control group of the Swedish rectal cancer study had a 5-year LR rate of 27%.2 3 4 The uptake and popularization of total mesorectal excision (i.e., the standardized technique of extrafascial dissection of the mesorectum with the utmost respect to embryological planes) and improvements in preoperative radiation and chemotherapy uptake have reduced this disease burden for treating clinicians.4 5 6 7
Nearly half of the patients with recurrence of rectal cancer have disease that is confined to the pelvis and can be deemed technically resectable.2 8 For these patients, it is now established that radical surgery offers the best chance of long-term survival.9 10 11
This article addresses the preoperative selection of potentially operative candidates, the importance of preoperative assessment, and a multidisciplinary approach to management. It also focuses on technical tips and tricks associated with multivisceral resection based on the anatomical site of the recurrence. It discusses the potential role of boost radiation and the role of intraoperative radiation therapy (IORT) in the management of recurrent rectal cancer.
Timing and Risk Factors for Local Recurrence
The majority of LR occurs within 2 years of primary surgery, although it can also occur later. Heriot and colleagues reported that 43% of LRs were detected following 48 months from primary surgery,9 while a separate study from Sagar and colleagues showed that 40% of LR occurred after 36 months.10
Advanced primary tumors are more prone to LR, particularly if there is a threatened or involved circumferential resection margin (CRM), poor differentiation, lymphovascular invasion, venous or perineural invasion, obstruction, or tumor perforation.8 The authors also suggest that the timing of LR may be delayed if neoadjuvant chemoradiation therapy is used prior to the definitive primary resectional surgery.
Management of Recurrent Rectal Cancer
The management of rectal cancer is multidisciplinary. It requires specialized input from multiple teams. The aims are to improve quality of life (QOL) by symptom control, prolong survival, and provide a cure wherever possible, while minimizing associated morbidity. It is important to note that following multidisciplinary assessment, many patients with recurrent disease may not proceed to surgery and many factors contribute to the decision to operate or not.
The burden of metastatic disease, tumor size, and infiltration of adjacent structures, as well as the patient's performance status all influence the decision to proceed with major resectional surgery. Nonoperative treatment strategies such as external beam radiotherapy or palliative chemotherapy do not provide a potential for cure, with median survival in the vicinity of 5 months.2 12 Nonoperative therapies can play a role in the management of patients treated for palliative intent. Stoma formation, nephrostomy insertion, and ureteral stents can all selectively be employed particularly when radical surgery is deemed inappropriate.
Traditionally, peritoneal carcinomatosis, high sacral involvement, encasement of external iliac vessels, invasion of the sciatic notch, bilateral ureteral obstruction with bilateral hydronephrosis, and the presence of gross lower limb edema (in addition to unresectable distant metastases) were considered absolute contraindications to pelvic exenterative surgery.8 Many of these dogmas have been challenged as exenterative centers around the world extend the traditional boundaries of resection.
Extensive resections may come at the cost of functional compromise. However, patients with advanced pelvic malignancy without resection also may suffer severe pain from bony, muscular, or neural invasion.13 14 Young and colleagues assessed QOL scores in 148 patients who underwent exenterative surgery and compared these with patients who did not receive this treatment.15 The study showed that patients recovered relatively quickly from surgery, and at 3 months, on many of the QOL metrics, the Kaplan–Meier curves of time to deterioration appeared to cross over with patients from the nonoperative group continuing to experience a slow decline.15
The Exenteration Team: Many Hands Make Light Work
It is important to build an exenteration team. The cases can be technically challenging and having a team that regularly performs complex multivisceral resections can reduce operating times and stress level of operating theater staff and improve outcomes. Apart from having regular designated specialist urologists, plastic surgeons, orthopedic surgeons, and potentially vascular surgeons in select cases, it is important that an exenterative surgeon (colorectal surgeon with experience in cancer surgery and pelvic exenteration) takes the lead in pre- and intraoperative decision-making. Our approach is to have two specialist exenterative colorectal surgeons for most cases. We have found that such an approach reduces operative times and, while not quantified, reduces stress in the operating theater. Shared decision-making can also be helpful, particularly when attempting to preserve organs (e.g., part of bladder, prostate, or major nerves). In addition to these individuals, experienced medical and radiation oncologists, as well as radiologists, nuclear physicians, and pathologists help participate in tumor board meetings and discussions with input from enterostomal therapists, psychologists, dietitians, and specialist cancer care nurses.
To Reirradiate or Not?
Many patients who are referred for surgery having undergone rectal cancer surgery have undertaken standard long course chemoradiation (50.4Gy) previously. Our radiation oncologists quantify the amount of radiation received and the indication for the radiation. If the time period is greater than 2 years, many of our patients will receive repeat irradiation. The safety and efficacy profile for this regime has been articulated by Ng and colleagues.16 Guren and colleagues have recently completed a systematic review addressing the feasibility of redose irradiation.17 The study pooled 375 patients reirradiated for recurrent rectal cancer and reported an overall low acute toxicity rate. The authors recommended the use of hyperfractionated chemoradiotherapy to limit late toxicity.17
Local Recurrence—Where Is It?
Several classification systems have been described in the literature. The Mayo group has described a recurrence according to location (sacral, anterior, left or right) and according to the degree of fixation.18 Wanebo and colleagues have described it based on a more traditional tumor node metastases system, denoting “R” for recurrence.19 Moore and colleagues from Memorial Sloan Kettering Cancer Center describe LRs based on whether the recurrence is axial (involving anastomosis, perineum, perirectal soft tissues), anterior (involving genitourinary), posterior (sacrum or coccyx), or lateral (bony sidewall).20 Boyle and colleagues describes recurrences as either central, sacral, sidewall, or composite (when the sacrum or sidewall are affected by recurrence).21
This article describes the compartments according to Moore and colleagues; however, it is worth noting that each compartment can provide technical challenges. The first is the centralcompartment. While recurrences here are theoretically technically easier, vigilance is still required to ensure that an R0 resection is performed. For example, if a patient has a central recurrence following a standard low anterior resection, an extralevator abdominoperineal resection with accompanying coccygectomy is often required to ensure that an R0 is achieved. The second, the anteriorcompartment, may be technically more favorable however provides unique challenges. Often urological help is sought here, and consideration to both penile urethrectomy and composite pubic bone excision should be considered to increase R0 rates in high-risk cases. The posteriorcompartment is usually bony (although in many classifications relates to structures posterior to the uterovaginal axis). The sacrum can be removed in either prone or abdominolithotomy positions. In recent times, the level of dissection has extended higher and composite S1/L5 resections are deemed possible; however, expert specialist orthopedic or neurosurgical expertise is necessary here to avoid disastrous consequences and increased morbidity. Lateral recurrences have traditionally been the most difficult to achieve an R0 resection.
Prehabilitation Program and Perioperative Care
Patients who undergo advanced pelvic surgery are at an increased risk of major cardiac, respiratory, thrombotic, and wound complications. A dedicated prehabilitation program can reduce this risk.22 This involves early active cardiopulmonary assessment of patients and exercise programs as required. The program involves building up the patients exercise tolerance in a coordinated fashion to decrease the impact of the stressors of the operation. The program also helps compensate for fitness lost during the neoadjuvant treatment phase.22 Following primary irradiation or reirradiation, many patients enter a catabolic phase, and in the interval between reirradiation and surgery (often 10–12 weeks), dieticians in our department actively aim to improve nutrition through supplemental high-calorie and high-protein drinks. This allows patients to be in the best possible condition to withstand a major exenteration. Experienced anesthetic management with appropriate fluid monitoring, pressure area protection, and temperature control is augmented by an early recovery program, independent of the length of the surgery. Heriot and colleagues reported on the initial Australasian experience with extended radical resection for locally recurrent rectal cancers.9 The data included patients from Peter MacCallum Cancer Centre in Melbourne, Royal Prince Alfred in Sydney, and Christchurch Hospital (Christchurch, New Zealand), all dedicated exenterative centers. Of the 160 patients, 61% had an R0 resection margin, and only one perioperative death was observed. This mirrors our ongoing experience with extended resections.
Our Standard Approach
Patients are placed in the modified Lloyd–Davies position with both arms alwaystucked in by their side to allow access to the pelvis by both the primary surgeon and assistant. If a sacrectomy is intended, then two 2-L saline bags are placed beneath the lumbar region to elevate the distal sacrum. Following midline laparotomy and adhesiolysis, an assessment for peritoneal disease and liver metastases is made. Our preferred self-retaining retractor is the Balfour-Doyen as it allows for closer access to the patient. A third arm is used exclusively for the pelvic dissection. The initial dissection includes assessing loops of small bowel that may be adherent to the mass. A potential pitfall for surgeons is to try to bluntly free the small bowel off the mass or alternatively use sharp dissection to free the small bowel from the mass. There is a significant risk of rupturing the tumor if one adopts such a strategy. In such circumstances, small bowel loops are dissected and should be resected enbloc. This may involve resecting several separate loops attached to the tumor. The authors suggest taking an anterior to posterior approach whereby layers of attached small bowel are sequentially stapled and divided away from the tumor until the small bowel is completely free (Fig. 1).
Fig. 1.
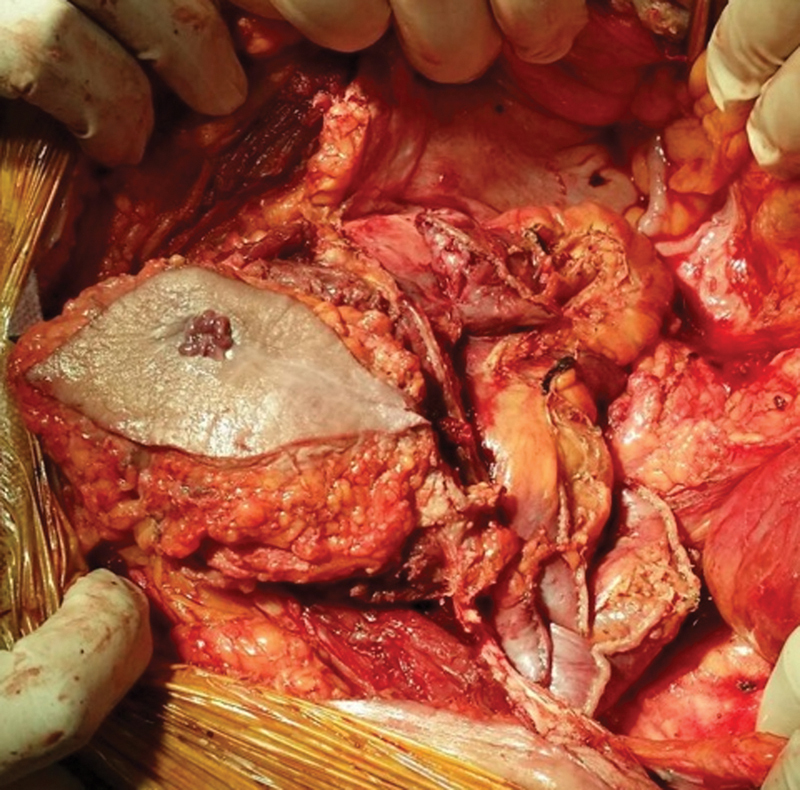
Anterior to posterior stapled division of the small bowel to gain access to the mass.
Following delivery of the small bowel package, the dissection is commenced with the left colon mobilized in its entirety for suitable access. Often the right colon must be mobilized as well. Ureters are identified bilaterally (ureteral catheters are placed at the commencement of the case unless cystectomy is planned) and slung with silicone vessel loops. The extrafascial plane (total mesorectal excision posterior plane) is followed posteriorly if no tumor is suspected. Mobilization of the right ureter continues down distally toward the vesicoureteral junction. If an anterior exenteration in a man is intended, then the bladder is mobilized following the retropubic space down toward the levator plate anteriorly. Urological input can be sought, although it is important that the colorectal surgeon dictates the plane of dissection and level of the urethral division. In the majority of the cases, the urethra can be divided by taking the dorsal venous complex from an anterior approach.
Central Recurrences
The extent of resection is dependent on the extent of the disease. Mirnezami and Sagar described a transperineal approach to isolated recurrences; however, in the majority of the cases, this is not possible.10 If urogenital structures are involved, they are best taken enbloc.8
If only the uterus and vagina are involved, these can be resected with a clear margin. A specialist gynecologic oncologist may be helpful for this portion. The purpose of enbloc hysterectomy in this situation is to ensure an R0 resection. Following a complete ureterolysis, the round ligament, broad ligament, and feeding gonadal vessels can be ligated separately. Using a swab on a holder in the vagina, an abdominal surgeon can accurately incise and open the vagina below the cervix, and an advanced bipolar or ultrasonic device can be used to break through the posterior wall as well as freeing the tributaries passing into the uterus. At this juncture, the vagina while open will be free from tumor, and the level of division of the rectum can be accurately determined. In cases where a posterior vaginectomy is required as part of an enbloc abdominoperineal resection, a dual surgeon approach, from above and below, is preferred. An example of this is shown in Fig. 2.
Fig. 2.
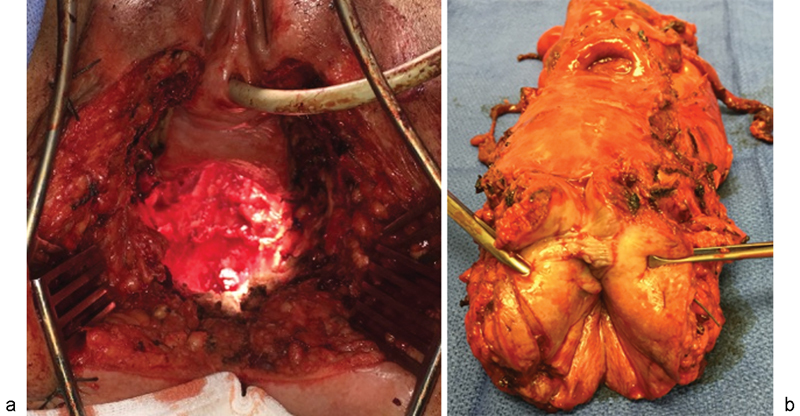
En bloc resection of the uterus and posterior vagina with abdominoperineal resection (APR) of the rectum. (a) Intraoperative view of the cavity created, with anterior vagina intact. (b) Specimen from en bloc resection of uterus and posterior vagina with APR.
While in most low pelvic recurrences, it is likely that an abdominoperineal resection is necessary (due to fibrosis and the prior low anastomosis), this is certainly not always the case. In some patients, reanastomosis is possible, or a low Hartmann's procedure is performed to avoid the morbidity of the perineal wound.9
Sparing Pelvic Organs
Bladder sparing exenterations are only considered selectively. Enbloc seminal vesiculectomies or prostatectomies are considered if the primary tumor is abutting or if minimally invading the prostate without involving other urogenital structures.23 Turner and colleagues have published on their experience of 10 enbloc prostatectomies compared with 20 enbloc cysto-prostatectomies from Christchurch (Christchurch, New Zealand).24 The series demonstrated that one-third of patients were continent of urine, and erectile function was severely affected. Concerns remain regarding urological function as well as the potential risk of urinary leaks and fistulae.
Where the tumor invades or abuts the dome of the bladder, a partial cystectomy can be performed using two-layered closure. It can be difficult to estimate the level of bladder involvement. If the preoperative evaluation highlights invasion near the trigone, one technique that can be adopted is to fill the bladder with water to facilitate resection and preserve as much bladder as possible. Alternatively, a cystotomy can be used to expose the ureteral orifices and, hence, accurately define the trigone.
In women, the central point of exenteration involves the uterus, cervix, or vaginal vault. For vaginal vault recurrences, our preference is to follow the ureteral rather than the vascular plane and remain posterior to the rectum. If a complete posterior vaginectomy is required, then a complete hysterectomy is performed to facilitate this. Careful discussion relating to fertility, menopausal status, and sexual function is performed prior to such resections.
A clear resection margin (both microscopic and macroscopic) remains the goal due to its significant impact on both local, distant relapse and survival.9 Not surprisingly, margin involvement (R1 or above) is associated with impaired survival. Involved macroscopic margins (R2) are associated with the worst outcomes. Lateral recurrences also conferred a significantly worse overall survival.9
Anterior Recurrences: Extending the Boundaries
Anterior recurrences can provide unique challenges. Dissection can be facilitated by an accompanying urologist involved in preoperative planning. It is important that the exenterative surgeon determines where the transection of the anterior structures occurs. Our unit routinely performs restaging (CT, MRI, PET scan) for advanced recurrent rectal cancers following preoperative chemoradiation (or reirradiation) therapy.
The central point of axis to the anterior compartment at the pelvic floor is the urethra.25 The anterior compartment is bounded by the symphysis pubis, and the superior and inferior pubic rami. Locally advanced rectal cancers (as well as soft tissue tumors of the bladder, prostate, vulva, vagina, cervix) can abut this margin. It has been our experience that there is a risk of a positive anterior margin particularly if transection of the urethra is pursued through an abdominal approach. In addition, ligation of the dorsal venous complex can be challenging in an irradiated field, with an accompanying risk of significant blood loss.
Our approach for select anterior recurrent tumors is to access the urethra through a perineal approach. An example of this is provided in Fig. 3, highlighting the correct plane of urethral division.
Fig. 3.
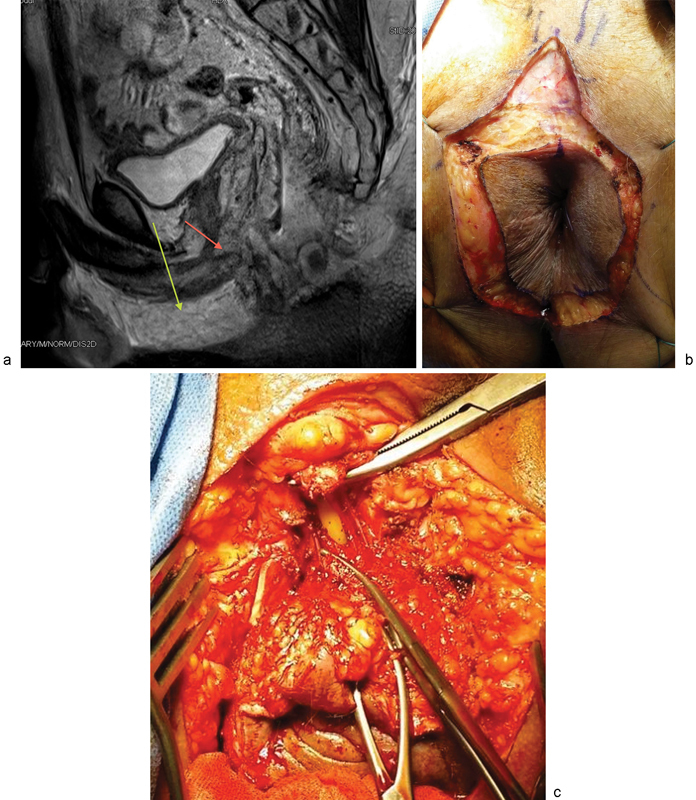
(a) Sagittal MRI showing both the incorrect line of urethral division (red arrow) and the correct line of urethral division (green arrow). (b) A “tear drop” incision for a perineal approach to urethral division. (c) Perineal urethrectomy to expose the inferior pubic rami.
The author's approach has been to operate in the abdominolithotomy position, with the patient in steep Trendelenburg. A separate dorsal skin extension slit is performed to allow access to the bulbospongiosus muscle following subcutaneous dissection. The muscles are then lifted off the periosteum of the inferior pubic rami. We suture ligate the base of the penis with 0-Vicryl (polyglactin) suture. The urethra is then visualized with transection of the urinary catheter. The urinary catheter is clamped on both sides to ensure that spillage does not occur. This allows easy anterior access to meet the abdominal team's dissection.
Solomon and colleagues describe a novel and more extended resection of the pubic bone for tumor invading the inferior pubic rami.25 Access to the bone is as described above, but the dissection is continued to the mobilized anterior wall plane on the superior aspect of pubis. This allows for the abdominal wall, penis, and scrotum to be completely suspended. Once vascular ligation of the tributaries of the internal iliac has been achieved, the superior and inferior pubic rami can be transected from the ischial bone using an oscillating saw. One may choose to perform either a complete composite pubic bone excision or a partial pubic bone excision including the inferior pubic rami and the lower half of the pubic symphysis with preservation of the superior pubic rami.25 This strategy is important in the surgical salvage of difficult anterior-based tumors. It is important that exenterative surgeons are familiar with such techniques.
Posterior Compartment and Extended Bony Resections
While many authors champion a prone approach for sacrectomy, our approach is to perform the sacrectomy utilizing an abdominolithotomy approach, particularly if the level of sacral resection is at or below S3. This is particularly useful when two exenterative surgeons are present at the procedure. Careful planning with appropriate positioning of the patient is critical to the procedure. The patient is positioned in a modified Lloyd–Davies position with both arms tucked and the perineum well beyond “the break” in the operating table. The pelvis is pushed anteriorly by two large saline bags under the lumbar region.26 We believe that the abdominolithotomy approach affords greater access to the lateral compartment, better control of the major vessels, and exposure of the lumbosacral trunk in the pelvis, with dissection of the sciatic nerve more laterally. It also allows for accurate placement of the vertical rectus abdominis myocutaneous (VRAM) flap.
The operation begins with an abdominal approach. Appropriate iliac vasculature is isolated depending on the intended extent of resection. Use of an open bipolar device such as a LigaSure Impact (LigaSure (Stryker), Tempe, AZ) can greatly facilitate dissection in the pelvis.
The anterior transection margin is defined by the extent of soft tissue (bladder, vagina, prostate, and urethral involvement in the pelvis) and bony (pubic) involvement. When the transabdominal phase of dissection is nearing completion, the perineal phase of dissection begins by the second exenterative surgeon. An elliptical excision is made around the anal margin; however, where sacrectomy is required, an additional posterior cut is performed to make it easier to access the sacrum. Dissection of the ischioanal fat pads is performed circumferentially, and the dissection continued posterior to the coccyx and sacrum. The gluteus maximus is freed from the sacrum, and posterior dissection is continued up until the S2 vertebral spine. The sacrum is completely exposed with the ligamentous (sacrococcygeal ligament) and muscular structures dissected from the bone.
The abdominal surgeon will use electrocautery to open up the presacral fascia and muscles (piriformis) to expose the anterior sacrum. A Kirschner (K) wire is driven into the sacrum at the level of dissection, ensuring that the perineal surgeon has a malleable retractor posterior to the sacrum at the appropriate level to ensure that the posterior skin is not breached. An extended length osteotome and hammer are utilized to perform the sacrectomy from a medial to lateral fashion.
Above the level of S3, a prone approach is preferable for the bony resection. The angle of the sacrum makes a sacrectomy in lithotomy position difficult above this level. Extended sacropelvic resections are feasible for select cases in experienced centers. Vascular exposure and control is essential in these cases. The lower aorta, vena cava, as well as distal iliac arteries and veins are exposed. The technique involves ligating the internal iliac artery branches distal to the posterior division of the superior gluteal artery branch. This preserves blood flow to the gluteal muscles and soft tissue of the perineum. The internal iliac vein and its multiple tributaries are sequentially divided.
An ileal conduit and colostomy are created during the abdominal phase of the operation. The level of the lumbosacral transection is identified and unicortical anterior osteotomies are performed. A titanium screw is placed at the level of the osteotomy site. This allows for accurate posterior osteotomies with aid of intraoperative fluoroscopy.
The second part of the procedure involves placing the patient in the prone position. A posterior midline incision is made along the sacrum and the gluteus maximus muscles are freed from their attachments. The sacrospinous and sacrotuberous ligaments are divided to access the pelvic cavity posteriorly. The piriformis muscles are divided while protecting the sciatic and pudendal nerves. Laminectomy, dural sac ligation (with neurosurgical or orthopedic assistance), and sacral resection are then performed.26
High spinal resections can result in spinal instability requiring reconstruction. Colibaseanu and colleagues describe using spinopelvic stabilization with posterior pelvic fusion from the lower lumbar spine to the remaining pelvis, as higher resections can sever spinopelvic continuity.27 Reconstruction is performed using dual fibula grafts and instrumental stabilization from the lower lumbar region to the remaining pelvis. Twenty-eight of 30 patients had R0 resections in Colibaseanu and colleagues' initial series, with no operative mortality.27 Early postoperative morbidity was high (40% Clavien grade III/IV). Half of patients had chronic pain and more than 20% had neuropathic bladder symptoms. Almost half required some form of ambulatory assistance. Overall, 2- and 5-year survival was 86 and 46%, respectively, albeit limited by short median follow up (2.7 years).27 The series demonstrates that lumbosacral resections are possible, however, with significant short-term morbidity and with some functional compromise.
Pelvic Sidewall Recurrences
LR may extend to involve the pelvic sidewalls. Such a recurrence is challenging and in our practice represents the most common reason for consideration of intraoperative radiation. Recurrent tumors can extend to involve the soft tissue of the sidewall, ureters, iliac vessels, sciatic nerve, piriformis muscle, and pelvic bones. Due to the restrictions of the bony pelvis, this type of recurrence still confers the worst prognosis and represents the most common site for a positive margin.9
An understanding of the anatomy in this region is critical as this plane is outside the expertise of most colorectal surgeons. The medial border of the external iliac vein provides entry onto the medial aspect of psoas major muscle. This, in turn, is the medial border of the obturator internus muscle, which represents the key dissection point for a lateral pelvic sidewall dissection. Bilateral ureteral stents should be placed preoperatively and the ureter medialized to the vesicoureteral junction. The internal iliac tributaries are then sequentially identified and ligated. The obturator internus can be partially removed if involved, potentially sacrificing the obturator nerve. Preservation of the obturator nerve is possible if the disease is isolated and the intent is to clear recurrent lateral pelvic sidewall lymph nodes. The internal iliac artery and vein can be ligated as required with tributaries taken as required to remove the sidewall mass and lymph nodes. Such a dissection should allow complete visualization of the lumbosacral trunk, with further dissection caudally allowing visualization and dissection, as needed, of the piriformis and splanchnic nerve roots.
A pitfall for the surgeon is not having control of the external and internal iliac vessels. We advocate use of vessel loops and having vascular clamps available as required for vessel ligation. While the majority of the dissection is performed sharply, we have found a peanut swab and selective use of energy devices (both advanced bipolar technology and ultrasonic energy) to be useful. Experienced assistance is important. Fig. 4 highlights a lateral pelvic wall dissection with or without intraoperative radiation. The figures also demonstrate femoral nerve exposure, internal iliac resection, and external iliac resection with reconstruction.
Fig. 4.
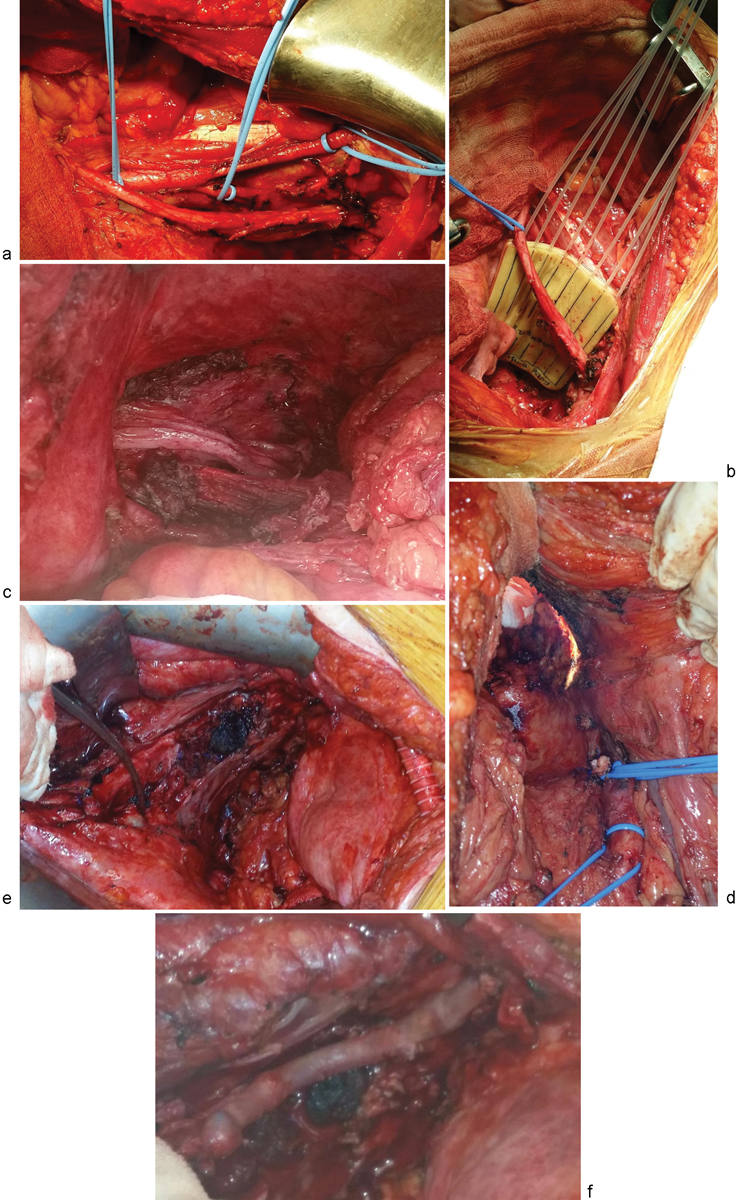
Extending the boundaries laterally. (a) Standard lateral pelvic sidewall dissection. (b) Lateral pelvic sidewall R0 resection, followed by IORT. (c) Resection of the iliacus and psoas major, and preservation of the femoral nerve. (d) Resection of the internal iliac artery. (e) En bloc vascular resection of the left common iliac artery and vein. (f) Vascular reconstruction with right femoral to left femoral artery crossover, and saphenous vein graft for the left common iliac vein.
There are emerging reports from Japan that demonstrate that a laparoscopic approach to this dissection is feasible in patients with low body mass index.28 The ureter should be isolated and medialized to the vesicoureteral junction. The external iliac vessels, once exposed, will allow exposure of the obturator internus muscle medially. This dissection can continue down to the levatorani musculature. The tissue medial to the medial umbilical ligament should be dissected allowing for selective dissection and ligation of the obturator vessels. The junction of the internal iliac artery and vein can be medialized to expose the lumbosacral trunk below, and further dissection will gain anterior exposure to the piriformis muscle. The infrapiriformis vessels and subsequent anterior branches of the internal iliac vessels can be ligated sequentially. The internal iliac vessels can be taken with a vascular stapler or suture ligated with a nonabsorbable monofilament suture. The pelvic splanchnic branches can be sacrificed, and the piriformis muscle can be partially or completely divided.
The St. Marks group has described their extended lateral pelvic sidewall excision experience of six R0 resections for sidewall recurrences.29 The authors describe two phases of dissection: the extrapelvic phase and the abdominal phase. The patient is initially placed prone, and dissection is continued down to the gluteal muscles. The gluteal muscle is reflected laterally and inferiorly, exposing the lumbodorsal fascia and sacrospinous ligament. This allows for exposure of the piriformis muscle that comes through the notch and lies over the sciatic nerve. The piriformis muscle is divided at its lateral extent where it becomes tendinous. If the tumor extends into the muscle, the gluteal muscle may need to be sacrificed. The sciatic notch is exposed, and the sciatic nerve, and superior and inferior gluteal arteries are identified. The periosteum and bony dissection is performed beyond the anterior sacrum in the midline. The sacrum and sacrotuberous ligaments are identified and divided. The divided ischial spine and ligament are then medialized. The authors describe taking the inferior and superior gluteal arteries through this approach with accompanying muscles. The sciatic nerve is sacrificed by infiltrating local anesthetic and dividing it with a scalpel, provided that the ipsilateral femoral nerve is preserved. The mass of tissue is mobilized so that it can be moved through the sciatic notch. Following this, the abdominal phase begins with the abdominal surgeon sacrificing the internal iliac on the ipsilateral side. The level of vascular division is dependent on whether or not the superior gluteal artery is sacrificed.29
Intraoperative Radiation Therapy
IORT delivers boost radiation to the tumor bed following surgical resection of the tumor. The majority of retrospective case series describe intraoperative electron therapy, but several centers are able to deliver high-dose-rate IORT.30 The advantage of this technique is that customized flexible applicators can be used for precise delivery of a single large fraction of radiation (typically 5 Gy). Penetration is for a short distance only, so it may be used in cases where the surgical margins are close or involved. Due to its limited availability, IORT is not widely used. IORT is an adjunct, not a substitute to an aggressive surgical resection. An R2 resection with IORT is not considered an acceptable curative strategy; however, where there is a close surgical resection margin (usually bony sidewall), IORT can offer additional treatment of 5mm. The advantages are minimal toxicity, sparing of health tissue, and short treatment times (30 minutes), with the potential to reduce recurrence.30 The ureter and nerves can be protected by the utilization of lead shields (Fig. 4b).
A systematic review assessing the benefits of IORT demonstrated significant heterogeneity among studies, but improved 5-year local control, and disease-free and overall survival in patients undergoing IORT, without differences in morbidity.31
Reconstruction Options
The perineal defects created in pelvic exenterations are often large. Many techniques have been described, including the use of abdominal-based myocutaneouspedicled flaps, buttock flaps, or pedicled groin flaps. We prefer VRAM flap reconstructions because they allow for healthy vascular tissue to fill the dead space created. Our plastic surgeon will harvest the flap preferentially from the right side (unless a prior ileostomy or ileal conduit has been performed). This flap can also be used to recreate the posterior wall of the vagina. We do not routinely use prophylactic mesh to buttress the muscle defect created from the muscle harvest. If an extended resection is performed laparoscopically, then most often an inferior gluteal artery myocutaneous flap is used. Postoperative care requires frequent side-to-side turning and regular flap observations. Sitting should be avoided, and ambulation can often be commenced around one week after the operation.
Adjuncts to Care: Sexual Function, Urinary Function, and Ostomy Placement
Ostomy placement, sexual function, and urinary function are all affected by radical extended pelvic resections, and specialized centers must have services to address these needs.
The ostomy should be sited at least 3cm from the costal margin, away from the iliac crest, and within the rectus muscle. Examination in both standing and sitting positions will allow for optimal ostomy sitting and avoidance of creases. In the event of dual ostomies, the urostomy is placed higher than the colostomy so that the ostomy belts do not clash.
When a VRAM flap is used, fixed anatomical markers (such as the nipple line) are used as a compass point, given the uncertainty of the final skin position.
Our center has a dedicated specialized sexual health and erectile dysfunction clinic. While it is unusual to have spontaneous erections following anterior exenterations, with appropriate help (i.e., PDE5 inhibitors, or injectable agents) these symptoms may improve.32
Conclusion
The management of recurrent rectal cancer is challenging. A multidisciplinary approach is necessary with specialist input in early decision-making, preoperative planning, intraoperative dissection, and postoperative care. Reirradiation and intraoperative radiation can be used selectively to improve outcomes, with acceptable morbidity. Understanding potential areas of concern for positive margins and extending the traditional planes can lead to good oncologic outcomes.
References
- 1.Cancer in Australia, an overview, 2006 AIHW cat.; 2007
- 2.Lopez-Kostner F, Fazio V W, Vignali A, Rybicki L A, Lavery I C. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44(2):173–178. doi: 10.1007/BF02234289. [DOI] [PubMed] [Google Scholar]
- 3.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336(14):980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Marijnen C A, Colenbrander A C. et al. Local recurrence in patients with rectal cancer diagnosed between 1988 and 1992: a population-based study in the west Netherlands. Eur J SurgOncol. 1998;24(6):528–535. doi: 10.1016/s0748-7983(98)93500-4. [DOI] [PubMed] [Google Scholar]
- 5.MacFarlane J K, Ryall R D, Heald R J. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 6.Heald R J, Husband E M, Ryall R D. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 7.Heald R J, Ryall R. Recurrent cancer after restorative resection of the rectum. Br Med J (Clin Res Ed) 1982;284(6318):826–827. doi: 10.1136/bmj.284.6318.826-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagar P M, Pemberton J H. Surgical management of locally recurrent rectal cancer. Br J Surg. 1996;83(3):293–304. doi: 10.1002/bjs.1800830305. [DOI] [PubMed] [Google Scholar]
- 9.Heriot A G, Byrne C M, Lee P. et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51(3):284–291. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 10.Mirnezami A H, Sagar P M. Surgery for recurrent rectal cancer: technical notes and management of complications. Tech Coloproctol. 2010;14(3):209–216. doi: 10.1007/s10151-010-0585-0. [DOI] [PubMed] [Google Scholar]
- 11.Madoff R D. Extended resections for advanced rectal cancer. Br J Surg. 2006;93(11):1311–1312. doi: 10.1002/bjs.5637. [DOI] [PubMed] [Google Scholar]
- 12.Moriya Y. Treatment strategy for locally recurrent rectal cancer. Jpn J ClinOncol. 2006;36(3):127–131. doi: 10.1093/jjco/hyi247. [DOI] [PubMed] [Google Scholar]
- 13.Temple L K, Bacik J, Savatta S G. et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48(7):1353–1365. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 14.Austin K K, Young J M, Solomon M J. Quality of life of survivors after pelvic exenteration for rectal cancer. Dis Colon Rectum. 2010;53(8):1121–1126. doi: 10.1007/DCR.0b013e3181e10c46. [DOI] [PubMed] [Google Scholar]
- 15.Young J M, Badgery-Parker T, Masya L M. et al. Quality of life and other patient-reported outcomes following exenteration for pelvic malignancy. Br J Surg. 2014;101(3):277–287. doi: 10.1002/bjs.9392. [DOI] [PubMed] [Google Scholar]
- 16.Ng M K, Leong T, Heriot A G, Ngan S Y. Once-daily reirradiation for rectal cancer in patients who have received previous pelvic radiotherapy. J Med Imaging Radiat Oncol. 2013;57(4):512–518. doi: 10.1111/1754-9485.12057. [DOI] [PubMed] [Google Scholar]
- 17.Guren M G, Undseth C, Rekstad B L. et al. Reirradiation of locally recurrent rectal cancer: a systematic review. RadiotherOncol. 2014;113(2):151–157. doi: 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Dozois R R, Devine R M. et al. Curative reoperations for locally recurrent rectal cancer. Dis Colon Rectum. 1996;39(7):730–736. doi: 10.1007/BF02054435. [DOI] [PubMed] [Google Scholar]
- 19.Wanebo H J, Antoniuk P, Koness R J. et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42(11):1438–1448. doi: 10.1007/BF02235044. [DOI] [PubMed] [Google Scholar]
- 20.Moore H G, Shoup M, Riedel E. et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47(10):1599–1606. doi: 10.1007/s10350-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 21.Boyle K M, Sagar P M, Chalmers A G, Sebag-Montefiore D, Cairns A, Eardley I. Surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2005;48(5):929–937. doi: 10.1007/s10350-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 22.West M A, Loughney L, Lythgoe D. et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114(2):244–251. doi: 10.1093/bja/aeu318. [DOI] [PubMed] [Google Scholar]
- 23.Davis P WR, Carne P, Bell S, Warrier S K. Bladder-sparing exenteration in locally advanced rectal cancer. ANZ J Surg. 2013;83(1):26. [Google Scholar]
- 24.Turner G A, Harris C A, Eglinton T W. et al. Cystoprostatectomy versus prostatectomy alone for locally advanced or recurrent pelvic cancer. ANZ J Surg. 2016;86(1–2):54–58. doi: 10.1111/ans.12808. [DOI] [PubMed] [Google Scholar]
- 25.Solomon M JA, Austin K K, Masya L, Lee P. Pubic bone excision and perineal urethrectomy for radical anterior compartment excision during pelvic exenteration. Dis Colon Rectum. 2015;58(11):1114–1119. doi: 10.1097/DCR.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 26.Solomon M J, Tan K K, Bromilow R G, Al-mozany N, Lee P J. Sacrectomy via the abdominal approach during pelvic exenteration. Dis Colon Rectum. 2014;57(2):272–277. doi: 10.1097/DCR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 27.Colibaseanu D T, Dozois E J, Mathis K L. et al. Extended sacropelvic resection for locally recurrent rectal cancer: can it be done safely and with good oncologic outcomes? Dis Colon Rectum. 2014;57(1):47–55. doi: 10.1097/DCR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 28.Akiyoshi T, Nagata J, Nagasaki T. et al. Laparoscopic salvage lateral pelvic lymph node dissection for locally recurrent rectal cancer. Colorectal Dis. 2015;17(10):O213–O216. doi: 10.1111/codi.13088. [DOI] [PubMed] [Google Scholar]
- 29.Shaikh I, Aston W, Hellawell G. et al. Extended lateral pelvic sidewall excision (ELSiE): an approach to optimize complete resection rates in locally advanced or recurrent anorectal cancer involving the pelvic sidewall. Tech Coloproctol. 2014;18(12):1161–1168. doi: 10.1007/s10151-014-1234-9. [DOI] [PubMed] [Google Scholar]
- 30.Tan J, Heriot A G, Mackay J. et al. Prospective single-arm study of intraoperative radiotherapy for locally advanced or recurrent rectal cancer. J Med Imaging Radiat Oncol. 2013;57(5):617–625. doi: 10.1111/1754-9485.12059. [DOI] [PubMed] [Google Scholar]
- 31.Mirnezami R, Chang G J, Das P. et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. SurgOncol. 2013;22(1):22–35. doi: 10.1016/j.suronc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoun F, Peltier A, van Velthoven R. Penile rehabilitation after pelvic cancer surgery. Scientific World Journal. 2015;2015:876046. doi: 10.1155/2015/876046. [DOI] [PMC free article] [PubMed] [Google Scholar]


