Abstract
The optimal management of the perineal defect following abdominoperineal excision for anorectal malignancy remains a source of debate. The repopularization of extralevator resection means colorectal surgeons are confronted with larger perineal wounds. There are several surgical options available—primary perineal closure and drainage, omentoplasty, biological or synthetic mesh placement, musculocutaneous flap repair, and negative wound pressure therapy. These options are discussed along with the potential benefits and complications of each. There remains no consensus on which management strategy is superior; thus, each case must be tailored for each individual patient. Surgical expertise and availability of a multidisciplinary team approach are important considerations.
Keywords: abdominoperineal excision, extralevator, rectal cancer, perineal wound, reconstruction
Perineal wounds are a major cause of morbidity after abdominoperineal excision (APE) for cancer or inflammatory bowel disease (IBD). With widespread adoption of neoadjuvant chemoradiotherapy (CRT)for locally advanced low rectal cancer, allied to the evolution of extralevator abdominoperineal excision (ELAPE), major challenges are faced in management of the perineal defect. This article will focus predominantly on the management of the perineal wound in patients undergoing operative (or reoperative) intervention for anorectal malignancy.
The perineal wound remains an ongoing challenge for both colorectal and reconstructive surgeons following radical resection of either primary or recurrent anorectal malignancy. Perineal wound morbidity results in prolonged postoperative hospital admissions, hospital readmission, and homecare nursing costs, and represents a major socioeconomic burden in terms of healthcare economic cost.1 Perineal wound morbidity is of multifactorial etiology, with wound complications reported in 25 to 60% of the cases.2 3 4 Specific complications may be immediate or long term and include pain, delayed or nonhealing of the wound, hemorrhage, infection (of the skin, muscle graft, or pelvic abscess), perineal fistula or sinus,5 6 7 and, in the longterm, development of a perineal hernia.8
Evolution in Surgical Technique
Despite advances in reconstructive surgery for low rectal cancer (low anterior resection and intersphinctericproctectomy), outcomes following APE remain suboptimal, related specifically to a positive circumferential resection margin (CRM) rates and intraoperative tumor perforation (IOTP). The CRM positivity rate is higher for APE compared with low anterior resection9; similarly, up to 33% of the specimens have been reported to show evidence of tumor perforation, often attributable to poor surgical technique.10 11 Thus, surgical technique has been modified. In 1908, Sir Ernest Miles described the technique of excising the levator musculature in an attempt to achieve an R0 resection.12 With the evolution of total mesorectal excision (TME) for rectal cancer, surgeons have become accustomed to dissecting in onto the low rectum at the level of the upper anal canal, which, when performed at APE, leads to “coning in” and results in a waist created in the specimen. Some data suggest that positive CRM and IOTP rates are lower with ELAPE, while other studies disagree.13 14 An increase in perineal wound complications was noted with more radical surgery (38 vs. 20%). ELAPE is a specialized procedure, with specific indications, required to manage aggressive low rectal cancers, and one that colorectal surgeons must have in their armamentarium. It creates a large, noncollapsible dead space with the fixed bony structures of the pelvis as the borders, rendering closure of the perineal defect difficult.
Neoadjuvant CRT is now the standard of care for T Stage 3–4 and/or radiologically node positive primary mid and low rectal cancer, and CRT remains the gold standard for management of localized anal squamous cell carcinoma. Radiotherapy has a series of pathophysiological effects that contribute to the development of perineal wound complications. It has been shown that preoperative radiotherapy increases the risk of developing a perineal wound complication up to 10-fold after APE with primary closure,4 15 16 with Bullard et al reporting a perineal wound complication rate of 41% in their series. This complication ensues as a result of the presence of the large perineal defect,17 18 19 the ensuing poor vascularity of surrounding irradiated tissues,20 primary closure of irradiated skin, and concomitant localized bacterial contamination due to resection of the colorectum.21 22 A fibrotic reaction following radiotherapy, coupled with capillary obliteration, reduces oxygen supply to tissues, resulting in an alteration of the cellular immune response and a decrease in fibroblastic collagen production.23 24 Radiotherapy has previously been shown to be the only significant risk factor on univariate analysis for a nonhealed wound.25
More advanced disease may require multivisceral resection of the genital tract, sacrum, or pelvic exenteration, resulting in a larger wound cavity. This larger cavity is likely to accumulate fluid and the attempted primary wound closure may be under tension—both of which increase the risk of developing a wound complication.
Surgical Options
Primary Perineal Closure, Peritoneal Closure, and Presacral Drain Placement
In 1975, Irvin and Goligher published a trial in which they published three management strategies for the perineal wound in 106 patients undergoing proctectomy for cancer or IBD21: (1) leaving the perineal wound open to heal by secondary intention, (2) primary closure of the perineum with closure of the peritoneum and placement of presacral drains, and (3) primary closure of the perineum and placement of presacral drains without closure of the peritoneum. Primary closure of the perineal skin, with or without closure of the pelvic peritoneum, had better outcomes than those who healed by secondary intention. However, less than half of those with primary perineal closure had long-term healing. For many years, the policy of primary perineal wound closure with closure of the peritoneum and placement of drains was the standard of care. A decade later, Tompkins and Warshaw published their technique for the management of the perineal wound by primary closure of the perineal tissues with approximation of the levator muscles, leaving the pelvic inlet open and placing pelvic drains in the pelvis.26 The results were very impressive with nearly all patients healed. The authors hypothesized that their improved results were “attributable to the elimination of the closed pelvic space.”
Epiploplasty/Omentoplasty
This technique refers to filling the noncollapsible pelvic cavity with omentum (Fig. 1).27 Varying results have been reported with perineal wound complication rates approaching 20%.28 29 This technique is limited by omental length, volume, and mobility, and may be insufficient in thin patients. However, bisecting the omentum may allow it to reach the deep pelvis based on its rich blood supply. The omental flap does not address the factor that many believe to be more pertinent—the perineal tissue, which is likely to have been irradiated and, thus, be poorly vascularized and still prone to wound complications.30 31
Fig. 1.
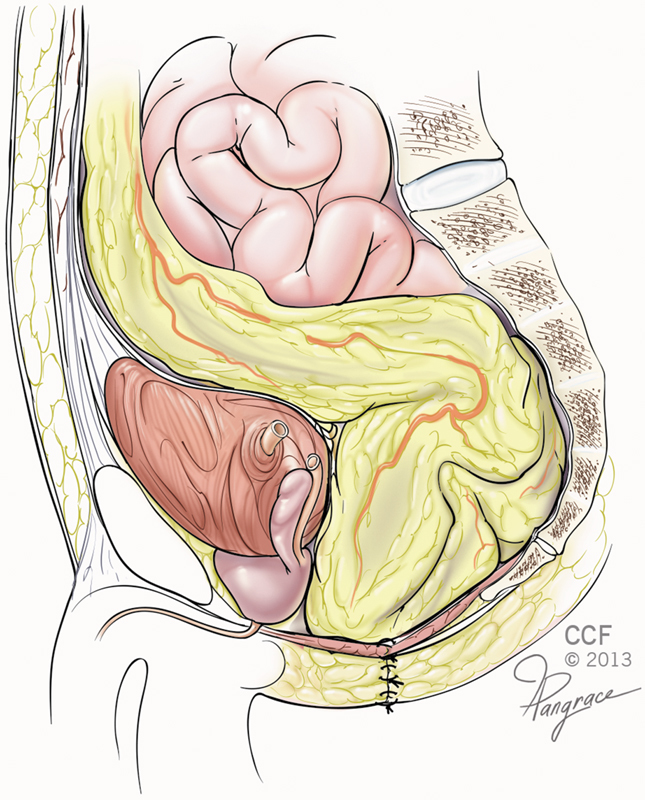
An omentoplasty—the omentum is mobilized on a vascular pedicle and positioned into the pelvic cavity following resection of the rectum and formation of the end colostomy. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2009–2015. All Rights Reserved.)
A systematic review found that omentoplasty was more commonly performed in patients who had undergone neoadjuvant therapy and added a median of 20 minutes of operating time.32 The options for the vascular supply for the omental graft are the left gastroepiploic vessel, the right gastroepiploic vessel, and the second-order vessels adjacent to the duodenum. The omental graft can be placed in either a retrocolic or paracolic position. Patients with primary closure with omentoplasty versus primary closure alone had improved healing (66.8 vs. 50.1%), shorter time to complete healing (23 vs. 79 days), lower overall wound complications (14.4 vs. 18.5%), and fewer perineal sinuses (4.5 vs. 9.2%). The authors concluded that omentoplasty with buttressing of the perineal wound following APE reduces perineal wound morbidity with minimal additional operative time or flap-associated morbidity. These conclusions were similar to those of a previous systematic review that indicated that there is likely a benefit with omentoplasty, but the evidence was inconclusive due to a lack of randomized studies.33 Although not associated with significant complications, omentoplasty has previously been shown to prolong postoperative ileus in rectal cancer surgery patients.34
Perineal Mesh Placement—Synthetic
Cui and colleagues have published the largest series reporting on synthetic mesh placement to reconstruct the perineal defect following APE.35 Sixty patients were assigned to two groups: the first group underwent primary closure of the perineum and the second group underwent closure using GORE-TEX Dual Mesh (W. L. Gore &Associates, Flagstaff, AZ). Significant reductions in the time confined to bed, the recovery of bowel function, the length of time fasting, and the need for drainage were reported in the mesh group. There was a 10% incidence of bowel obstruction in the primary closure group, with no obstructions in the mesh group. These results led the authors to conclude that the use of this mesh leads to a quicker postoperative recovery and a decreased incidence of postoperative bowel obstruction compared with primary closure. Given the small patient cohort, this is of questionable significance. However, a review on synthetic mesh placement after APE performed by Marshall and colleagues36 reports that the use of such meshes has been limited in this context due to the potential for infection in the contaminated field and the development of small bowel adhesions and fistulae formation.
Perineal Mesh Placement—Biological
Porcine Dermal Collagen
The first report of using mesh comprising porcine dermal collagen for perineal defect closure following APE was presented in 2005.37 A subsequent series of 11 patients with rectal cancer noted a fairly low complication rate, with only 1patient requiring mesh removal for pelvic sepsis.38 Porcine collagen mesh is immunologically inert39 and can often be left in situ should the field become contaminated.40 Over half the patients had pain lasting a median of 5 weeks. Chronic pain might be due to the radicality of the surgery coupled with the fact that implantable meshes are reported to cause pain in as many as 30% of the patients after laparoscopic ventral hernia repair.41
A retrospective analysis of 57 patients with T3 or T4 rectal cancer, more than 80% of who underwent neoadjuvant CRT, found that perineal defects closed with Permacol mesh (Covidien-Medtronic, Minneapolis, MN) compared with reconstruction with gluteus maximus flaps had fewer infections and complications, shorter length of stay, and lower incidence of perineal hernia.42 All wounds had healed completely at 3 months, with a single patient in each group developing a perineal sinus.
Human Acellular Dermal Matrix
Closure of the perineal defect can be performed with human acellular dermal matrix (HADM), a dermal biomaterial in which the cellular elements have been removed.43 The graft provides a biologic scaffold that encourages native cellular ingrowth and tissue remodeling44 45 and has been shown to be effective in both noncontaminated and contaminated wounds.46 Han and colleagues report that 11 of 12 consecutive rectal cancer patients who underwent a cylindrical APE with HADM perineal reconstruction had complete wound healing at two weeks postoperatively.43 Patients were permitted to mobilize fully from the second postoperative day onward. Only 25% of the cohort received neoadjuvant radiation treatment. A third experienced chronic pain, requiring the use of opioids for two months postoperatively.
Surgisis Biodesign Acellular Porcine Small Intestinal Submucosa (Cook Medical, West Lafayette, IN)
The first reported use of this mesh was on a single patient in 2009.47 A subsequent series in 2012 of 10 patients with this mesh compared with 5 patients undergoing vertical rectus abdominis muscle (VRAM) reconstruction showed a significantly higher cost in the latter group (£11,075 vs. £6,513, or $17,377 vs. $10,218)48 (Table 1).
Table 1. Outcomes of biological mesh and VRAM flap for perineal wound closure.
| Mesh | VRAM | p-Value | |
|---|---|---|---|
| Number of patients | 10 | 5 | |
| Operative time, min (median/range) | 259 (165–340) | 405 (390–435) | 0.0013 |
| Postoperative stay, days(median/range) | 10 (8–20) | 20 (7–74) | 0.067 |
| Early complications (<30 d) | 7 (70%) | 4 (80%) | 0.37 |
| Late complications (>30 d) | 3 (30%) | 1 (20%) | Not reported |
Abbreviation: VRAM, vertical rectus abdominis muscle.
Source: Adapted from Peacock et al.48
A review of complications, pain, operating time, quality of life, and cost associated with biologic meshes in perineal reconstruction following ELAPE supports the use of biologic mesh as outcomes are comparable to myocutaneous flaps.36 Biologic mesh repair offers a significant cost-saving compared with a muscle flap as well as a reduction in the length of postoperative hospital stay. Long-term follow-up after biologic mesh reconstruction demonstrates no incidence of mesh explant.49
Flap Reconstruction
Flap reconstruction of the perineal defect following APE for anorectal malignancy has been wellstudied, with comprehensive reviews on flap repair after APE,50 51 flap and mesh repair after ELAPE,52 53 and the various techniques of perineal reconstruction.54 The common theme across these reviews is that there remains a paucity of high quality data and prospective trials to compare results across all groups.
Vertical Rectus Abdominis Muscle Flap
Technique
A review by Nisar and Scott summarizes the techniques of myocutaneous flap reconstruction after APE.50 For the VRAM studies, all flaps were raised on the basis of the inferior epigastric artery pedicle (Fig. 2). The flap was transposed, rotated, and used to fill the pelvic–perineal defect (Fig. 3). The skin paddle orientation differed between studies, as some used an oblique one55 56 while others used a vertical one. Other variations include the use of bilateral flaps57 and the placement of a nonabsorbable abdominal wall mesh. To evaluate the effectiveness of the VRAM flap, studies that directly compared VRAM reconstruction with primary repair have been chosen here.
Fig. 2.
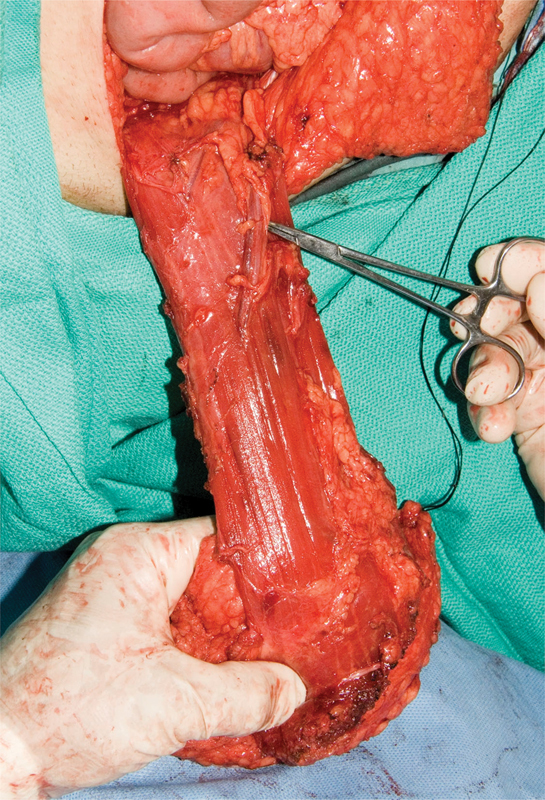
A vertical rectus abdominis muscle flap mobilized on the inferior epigastric artery pedicle.
Fig. 3.
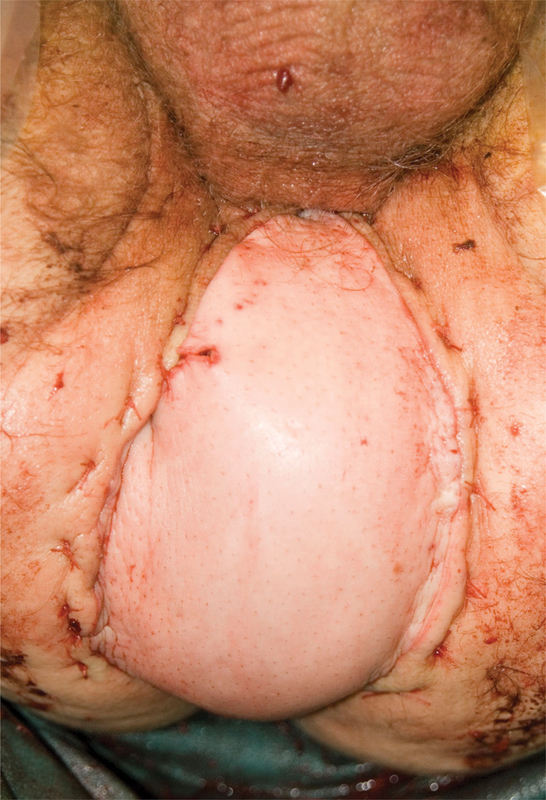
A vertical rectus abdominis muscle flap sutured in situ to close the perineal defect.
In 1999, Radice and colleagues compared primary closure, primary closure with omentoplasty, and immediate VRAM closure,58 and found a reduction in the wound complication rate following the introduction of selective VRAM use from 38 to 26%, with no increase in operating time or length of stay (Table 2). Patients undergoing omentoplasty and VRAM closure underwent more extensive resections and were more likely to have undergone CRT.
Table 2. Outcomes with early VRAMuse58 .
| Primary closure | Primary closure with omentoplasty | VRAM | |
|---|---|---|---|
| Number of patients | 20 | 24 | 13 |
| 30-d complication rate | 8 (40%) | 9 (37%) | 3 (23%) |
| Readmission for wound | 4 (20%) | 7 (29%) | 2 (15%) |
| Reoperation on wound | 5 (25%) | 7 (29%) | 0 (0%) |
Abbreviation: VRAM, vertical rectus abdominis muscle.
Another retrospective study of VRAM reconstruction versus primary closure in patients with anorectal malignancy who had undergone radiation treatment showed a significant reduction in perineal wound complications in the VRAM group (15.8 vs. 44.1%, p = 0.03), no evidence of donor site morbidity, and no difference in nonperineal complications.3 59 Butler and colleagues found a 4-fold reduction in major perineal wound dehiscence and a 10-fold reduction in perineal abscess formation in their series of 35 patients undergoing VRAM repair, without morbidity associated with the donor site.60
Outcomes of a cohort of 95 patients with persistent or recurrent anal cancer, 43 of which underwent VRAM flap repair and 52 of which underwent omentoplasty, showed a significantly reduced incidence of perineal complications in the VRAM group (26.8 vs. 48.9%, p = 0.0336) and a reduced time to healing (18.7 days vs. 117 days, p = 0.0019).61 There was no difference in abdominal incisional hernias, with no perinealherniae observed in the VRAM group and eight in the omentoplasty group at a mean follow-up period of just over 40 months. There was one evisceration in the VRAM group. The VRAM cohort had a higher rate of T3 and T4 tumors than the omentoplasty group (67.4 vs. 38.4%) and a higher surgical margin positivity rate (26.1 vs. 11.5%). There was no difference in 5-year overall and disease-free survival. Overall, the authors concluded that in an anal cancer cohort, the VRAM flap reconstruction was beneficial.
Gracilis Flap
The gracilis flap has been described for closure of the perineal defect since 1983,62 and there is a lack of high-quality evidence regarding its outcomes.63 64 65 66 Gracilis flap repair has been compared with primary closure as well as VRAM flap. The wound outcomes favored the flap groups and are outlined in Table 3.
Table 3. Wound complication rates comparing gracilis flap repair to primary closure.
There was no difference between patients undergoing gracilis flap closure and primary closure in terms of the postoperative leukocyte count, temperature, number of blood transfusions, length of bed rest, and length of inpatient hospital stay, leading to the conclusion that gracilis flap reconstruction does not impinge on patients' postoperative recovery and should be considered in patients who have undergone pelvic radiation (Fig. 4).
Fig. 4.
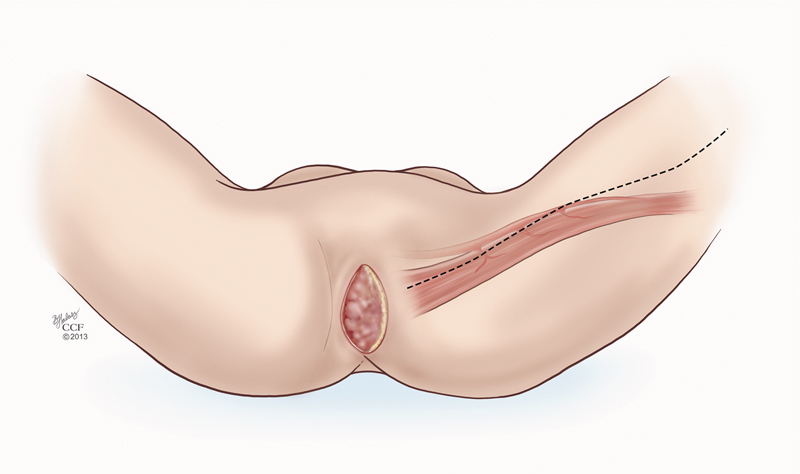
The line of incision to mobilize the gracilis muscle for a flap repair of the perineal defect. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2009–2015. All Rights Reserved.)
Gluteus Maximus Flap
The first report of the gluteus maximus flap for patients undergoing APE in 1990 described16 patients with cancer or IBD, most of whom underwent delayed reconstruction.19 Of the nine patients with wound complications, over half required additional surgery as a result. Other series report rates of perineal wound complication between 0 and 41.5%, the majority of which represent minor wound issues, and an overall long-term healing rate over 91%.66 67 68 An advantage of this flap is the lack of major donor-site morbidity. Evaluation of postoperative functional outcomes demonstrates reductions in the ability to sit as well as the ability to stand for prolonged periods of time due to reduced gluteus maximus function.69 Thus, preoperative counseling in terms of potential postoperative functional limitations is imperative in these patients.
Inferior Gluteal Artery Perforator Flap
The inferior gluteal artery perforator flap is a muscle-sparing fasciocutaneous flap usually designed in a V–Y fashion with the lower border placed in the buttock crease and the lateral extent medial to the greater trochanter. A report of 40 patients with low rectal cancer, most of whom underwent neoadjuvant CRT, demonstrated a 10% rate of major wound complications requiring reoperation.70 While operating times are longer than with other flap techniques, advantages of this flap include the avoidance of using of irradiated tissue and compatibility with both open and laparoscopic approaches for the resection.
Local Flaps
A small series of perineal reconstruction with local flaps found that neoadjuvant pelvic radiation was a risk factor for major wound dehiscence (p< 0.05).71 In the setting of prior pelvic radiation, nonirradiated vascular pedicle flaps are a better alternative than local flaps.
Dynamic Graciloplasty with Malone Appendicostomy
In the very small subset of highly motivated patients who refuse permanent colostomy but require APE for rectal cancer, dynamic graciloplasty with Malone appendicostomy is an option.72 A small series of 10 rectal cancer patients who underwent this operation reported that 1 patient required intraabdominal reoperation and 9 patients required at least one local revision, predominantly for coloperineal anastomotic strictures or mucosal prolapse. Functional results were good and quality of life scores remained stable over time. This is a rarely performed procedure requiring a high level of surgical expertise and a highly selected patient cohort.
Negative Pressure Wound Therapy
The success of negative pressure wound therapy for the perineal defect after APE has been described in several case reports.73 74 75 A retrospective cohort study found that patients with the incisional negative pressure wound therapy (iNPWT) device placed over a primarily closed perineal wound for 5 days were less likely to have a perineal surgical site infection (SSI) than those who had only a gauze dressing (15 vs. 41%, p = 0.04).76 The iNPWT group had a longer inpatient length of stay (11 vs. 8 days, p = 0.03) and the median length of stay for patients was the same regardless of the development of a perineal SSI (10 days). iNPWT was an independent predictor of not developing an SSI (OR [odds ratio] 0.11, p = 0.01), and smoking was a risk factor for SSI development (OR 4.67, p = 0.02).
Conclusion
The perineal wound following APE or ELAPE can be managed in a variety of ways, from primary closure to mesh repair to muscle flap reconstruction. Given the lack of robust level 1 evidence, it is difficult to make recommendations on which method to employ.
It would appear that there is no longer a role for primary closure alone and the placement of abdominal drains. If primary closure is undertaken, it should be accompanied by either an omentoplasty or application of negative pressure wound therapy. There are little data concerning the use of both omentoplasty and negative pressure wound therapy.74 A systematic review by Killeen and colleagues32 makes a compelling case for omentoplasty, but as with all systematic reviews/analyses of available evidence, heterogeneity of included studies makes coming to a convincing conclusion difficult. Indeed, it would be interesting to compare and combine these strategies in a trial setting—omentoplasty alone, negative pressure wound therapy alone, and a combination of both. Omentoplasty is certainly cost-effective and the limited data suggest that 5 days of negative pressure therapy resulting in a greatly reduced SSI rate is also a very cost-effective measure. Both add very little time to the operation, do not require a donor site and its potential morbidity, and do not require postoperative functional limitation in terms of sitting and walking.
The increasing use of ELAPE, either as standard of care for low rectal cancer or for managing more aggressive, locally advanced tumors, means that larger perineal wounds are more often created, presenting a considerable therapeutic challenge. Muscle flaps negate the risk of infection of prosthetic material, are readily available, and have certainly been shown to improve outcomes in comparison to primary closure. While there is no consensus on which flap is superior and when to use a particular type of flap, there is consensus in the literature that neoadjuvant radiation treatment is an indication for the use of a muscle graft. There is no doubt that flaps increase the overall operative time and are subject to potentially serious complications: increased postoperative immobility, flap necrosis, and impaired long-term function. The available data would seem to favor mesh repair over flap reconstruction. Meshes, albeit expensive, are readily available, easily implanted, and the reported outcomes are equivalent in terms of wound complication and superior in terms of operative times, postoperative length of stay, and, importantly, within the current economic climate, cost-effectiveness.
Currently, no definitive recommendations can be made regarding the most appropriate management strategy for the perineal defect after APE. There is a dearth of prospective, randomized trials in this area. It is the authors' view that omentoplasty and mesh repair is an acceptable approach to addressing the perineal defect but that muscle flaps offer similar outcomes in specialized centers. In managing complex, locally advanced carcinomas of the anorectum, the surgeon must have access to all therapeutic strategies in their armamentarium to ensure the best possible outcomes for individual patients.
Acknowledgments
The authors thank Dr. Stocchi, Department of Colorectal Surgery, Cleveland Clinic, Cleveland, OH, for his operative photographs and Dr. Remzi, Department of Colorectal Surgery, and Ms. Halasz and Mr. Pangrace, Center for Medical Art and Photography, Cleveland Clinic, Cleveland, OH, for the use of their surgical illustrations.
References
- 1.Wiatrek R L, Thomas J S, Papaconstantinou H T. Perineal wound complications after abdominoperineal resection. Clin Colon Rectal Surg. 2008;21(1):76–85. doi: 10.1055/s-2008-1055325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchel E W, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominismusculocutaneous flaps. Ann Plast Surg. 2004;52(1):22–26. doi: 10.1097/01.sap.0000099820.10065.2a. [DOI] [PubMed] [Google Scholar]
- 3.Chessin D B, Hartley J, Cohen A M. et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: a cohort study. Ann Surg Oncol. 2005;12(2):104–110. doi: 10.1245/ASO.2005.03.100. [DOI] [PubMed] [Google Scholar]
- 4.Bullard K M, Trudel J L, Baxter N N, Rothenberger D A. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48(3):438–443. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 5.Rosen L, Veidenheimer M C, Coller J A, Corman M L. Mortality, morbidity, and patterns of recurrence after abdominoperineal resection for cancer of the rectum. Dis Colon Rectum. 1982;25(3):202–208. doi: 10.1007/BF02553101. [DOI] [PubMed] [Google Scholar]
- 6.Pollard C W, Nivatvongs S, Rojanasakul A, Ilstrup D M. Carcinoma of the rectum. Profiles of intraoperative and early postoperative complications. Dis Colon Rectum. 1994;37(9):866–874. doi: 10.1007/BF02052590. [DOI] [PubMed] [Google Scholar]
- 7.Rothenberger D A, Wong W D. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg. 1992;16(3):478–485. doi: 10.1007/BF02104451. [DOI] [PubMed] [Google Scholar]
- 8.So J B, Palmer M T, Shellito P C. Postoperative perineal hernia. Dis Colon Rectum. 1997;40(8):954–957. doi: 10.1007/BF02051204. [DOI] [PubMed] [Google Scholar]
- 9.Wibe A Syse A Andersen E Tretli S Myrvold H E Søreide O; Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection Dis Colon Rectum 200447148–58. [DOI] [PubMed] [Google Scholar]
- 10.Heald R J, Smedh R K, Kald A, Sexton R, Moran B J. Abdominoperineal excision of the rectum—an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997;40(7):747–751. doi: 10.1007/BF02055425. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen M T Wibe A Syse A Haffner J Wiig J N; Norwegian Rectal Cancer Group; Norwegian Gastrointestinal Cancer Group. Inadvertent perforation during rectal cancer resection in Norway Br J Surg 2004912210–216. [DOI] [PubMed] [Google Scholar]
- 12.Miles W E. A method of performing abdominoperineal excision of carcinoma of the rectum and of the terminal portion of the pelvic colon. Lancet. 1908;2:1812–1813. [Google Scholar]
- 13.West N P Anderin C Smith K J Holm T Quirke P; European Extralevator Abdominoperineal Excision Study Group. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer Br J Surg 2010974588–599. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Sun T, Xie H, Zhang Y, Zeng H, Fu W. Extralevatorabdominoperineal excision for low rectal cancer: a systematic review and meta-analysis of the short-term outcome. Colorectal Dis. 2015;17(6):474–481. doi: 10.1111/codi.12921. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick M A, Vieten D, Pettitt E, Dixon A R, Roe A M. Short course preoperative radiotherapy is the single most important risk factor for perineal wound complications after abdominoperineal excision of the rectum. Colorectal Dis. 2006;8(9):756–761. doi: 10.1111/j.1463-1318.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 16.Nissan A Guillem J G Paty P B et al. Abdominoperineal resection for rectal cancer at a specialty center Dis Colon Rectum 200144127–35., discussion 35–36 [DOI] [PubMed] [Google Scholar]
- 17.Hilsabeck J R. The presacral space as a collector of fluid accumulations following rectal anastomosis: tolerance of rectal anastomosis to closed suction pelvic drainage. Dis Colon Rectum. 1982;25(7):680–684. doi: 10.1007/BF02629540. [DOI] [PubMed] [Google Scholar]
- 18.Miller L B, Steele G, Cady B, Wolfort F G, Bothe A Jr. Resection of tumors in irradiated fields with subsequent immediate reconstruction. Arch Surg. 1987;122(4):461–466. doi: 10.1001/archsurg.1987.01400160087014. [DOI] [PubMed] [Google Scholar]
- 19.Baird W L, Hester T R, Nahai F, Bostwick J III. Management of perineal wounds following abdominoperineal resection with inferior gluteal flaps. Arch Surg. 1990;125(11):1486–1489. doi: 10.1001/archsurg.1990.01410230080014. [DOI] [PubMed] [Google Scholar]
- 20.Farid H, O'Connell T X. Methods to decrease the morbidity of abdominoperineal resection. Am Surg. 1995;61(12):1061–1064. [PubMed] [Google Scholar]
- 21.Irvin T T, Goligher J C. A controlled clinical trial of three different methods of perineal wound management following excision of the rectum. Br J Surg. 1975;62(4):287–291. doi: 10.1002/bjs.1800620409. [DOI] [PubMed] [Google Scholar]
- 22.Borel Rinkes I H, Wiggers T. Gracilis muscle flap in the treatment of persistent, infected pelvic necrosis. Eur J Surg. 1999;165(4):390–391. doi: 10.1080/110241599750006956. [DOI] [PubMed] [Google Scholar]
- 23.Gabka C J, Benhaim P, Mathes S J. et al. An experimental model to determine the effect of irradiated tissue on neutrophil function. Plast Reconstr Surg. 1995;96(7):1676–1688. doi: 10.1097/00006534-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Delanian S, Martin M, Bravard A, Luccioni C, Lefaix J L. Abnormal phenotype of cultured fibroblasts in human skin with chronic radiotherapy damage. Radiother Oncol. 1998;47(3):255–261. doi: 10.1016/s0167-8140(97)00195-3. [DOI] [PubMed] [Google Scholar]
- 25.Artioukh D Y, Smith R A, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2007;9(4):362–367. doi: 10.1111/j.1463-1318.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 26.Tompkins R G, Warshaw A L. Improved management of the perineal wound after proctectomy. Ann Surg. 1985;202(6):760–765. doi: 10.1097/00000658-198512000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins D, Hogan A M, O'Shea D, Winter D C. The omentum: anatomical, metabolic, and surgical aspects. J Gastrointest Surg. 2009;13(6):1138–1146. doi: 10.1007/s11605-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 28.Rice M L, Hay A M, Hurlow R H. Omentoplasty in abdominoperineal resection of the rectum. Aust N Z J Surg. 1992;62(2):147–149. doi: 10.1111/j.1445-2197.1992.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 29.Page C P, Carlton P K Jr, Becker D W. Closure of the pelvic and perineal wounds after removal of the rectum and anus. Dis Colon Rectum. 1980;23(1):2–9. doi: 10.1007/BF02587192. [DOI] [PubMed] [Google Scholar]
- 30.Liebermann-Meffert D The greater omentum.Anatomy, embryology, and surgical applications Surg Clin North Am 2000801275–293., xii [DOI] [PubMed] [Google Scholar]
- 31.Topor B, Acland R D, Kolodko V, Galandiuk S. Omental transposition for low pelvic anastomoses. Am J Surg. 2001;182(5):460–464. doi: 10.1016/s0002-9610(01)00764-4. [DOI] [PubMed] [Google Scholar]
- 32.Killeen S, Devaney A, Mannion M, Martin S T, Winter D C. Omental pedicle flaps following proctectomy: a systematic review. Colorectal Dis. 2013;15(11):e634–e645. doi: 10.1111/codi.12394. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson P J. Omentoplasty in abdominoperineal resection: a review of the literature using a systematic approach. Dis Colon Rectum. 2006;49(9):1354–1361. doi: 10.1007/s10350-006-0643-x. [DOI] [PubMed] [Google Scholar]
- 34.Klaver Y L, Nienhuijs S W, Nieuwenhuijzen G A, Rutten H J, de Hingh I H. Omentoplasty in rectal cancer surgery prolongs post-operative ileus. Int J Colorectal Dis. 2008;23(2):165–169. doi: 10.1007/s00384-007-0392-x. [DOI] [PubMed] [Google Scholar]
- 35.Cui J, Ma J P, Xiang J. et al. Prospective study of reconstructing pelvic floor with GORE-TEX Dual Mesh in abdominoperineal resection. Chin Med J (Engl) 2009;122(18):2138–2141. [PubMed] [Google Scholar]
- 36.Marshall M J, Smart N J, Daniels I R. Biologic meshes in perineal reconstruction following extra-levatorabdominoperineal excision (elAPE) Colorectal Dis. 2012;14 03:12–18. doi: 10.1111/codi.12044. [DOI] [PubMed] [Google Scholar]
- 37.Murphy E MA Croxford M A Daniel M Moran B J Gould D M A novel pelvic floor closure technique using porcine dermal collagen after abdomino-perineal excision of the rectum 2nd ECCP/EACP Joint Meeting, Bologna, Italy; September 15–17, 2005
- 38.Wille-Jørgensen P, Pilsgaard B, Møller P. Reconstruction of the pelvic floor with a biological mesh after abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2009;24(3):323–325. doi: 10.1007/s00384-008-0607-9. [DOI] [PubMed] [Google Scholar]
- 39.Hammond T M, Chin-Aleong J, Navsaria H, Williams N S. Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg. 2008;95(4):438–446. doi: 10.1002/bjs.5883. [DOI] [PubMed] [Google Scholar]
- 40.Parker D M, Armstrong P J, Frizzi J D, North J H Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63(4):255–258. doi: 10.1016/j.cursur.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Eriksen J R, Poornoroozy P, Jørgensen L N, Jacobsen B, Friis-Andersen H U, Rosenberg J. Pain, quality of life and recovery after laparoscopic ventral hernia repair. Hernia. 2009;13(1):13–21. doi: 10.1007/s10029-008-0414-9. [DOI] [PubMed] [Google Scholar]
- 42.Christensen H K, Nerstrøm P, Tei T, Laurberg S. Perineal repair after extralevatorabdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2011;54(6):711–717. doi: 10.1007/DCR.0b013e3182163c89. [DOI] [PubMed] [Google Scholar]
- 43.Han J G, Wang Z J, Gao Z G, Xu H M, Yang Z H, Jin M L. Pelvic floor reconstruction using human acellular dermal matrix after cylindrical abdominoperineal resection. Dis Colon Rectum. 2010;53(2):219–223. doi: 10.1007/DCR.0b013e3181b715b5. [DOI] [PubMed] [Google Scholar]
- 44.Wong A K, Schonmeyr B, Singh P, Carlson D L, Li S, Mehrara B J. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg. 2008;121(4):1144–1152. doi: 10.1097/01.prs.0000302505.43942.07. [DOI] [PubMed] [Google Scholar]
- 45.Menon N G, Rodriguez E D, Byrnes C K, Girotto J A, Goldberg N H, Silverman R P. Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in the rabbit model. Ann Plast Surg. 2003;50(5):523–527. doi: 10.1097/01.SAP.0000044252.76804.6B. [DOI] [PubMed] [Google Scholar]
- 46.Patton J H Jr Berry S Kralovich K A Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions Am J Surg 20071933360–363., discussion 363 [DOI] [PubMed] [Google Scholar]
- 47.Boereboom C L, Watson N FS, Sivakumar R, Hurst N G, Speake W J. Biological tissue graft for pelvic floor reconstruction after cylindrical abdominoperineal excision of the rectum and anal canal. Tech Coloproctol. 2009;13(3):257–258. doi: 10.1007/s10151-009-0512-4. [DOI] [PubMed] [Google Scholar]
- 48.Peacock O, Pandya H, Sharp T. et al. Biological mesh reconstruction of perineal wounds following enhanced abdominoperineal excision of rectum (APER) Int J Colorectal Dis. 2012;27(4):475–482. doi: 10.1007/s00384-011-1325-2. [DOI] [PubMed] [Google Scholar]
- 49.Peacock O, Simpson J A, Tou S I. et al. Outcomes after biological mesh reconstruction of the pelvic floor following extra-levatorabdominoperineal excision of rectum (APER) Tech Coloproctol. 2014;18(6):571–577. doi: 10.1007/s10151-013-1107-7. [DOI] [PubMed] [Google Scholar]
- 50.Nisar P J, Scott H J. Myocutaneous flap reconstruction of the pelvis after abdominoperineal excision. Colorectal Dis. 2009;11(8):806–816. doi: 10.1111/j.1463-1318.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 51.Howell A M, Jarral O A, Faiz O, Ziprin P, Darzi A, Zacharakis E. How should perineal wounds be closed following abdominoperineal resection in patients post radiotherapy—primary closure or flap repair? Best evidence topic (BET) Int J Surg. 2013;11(7):514–517. doi: 10.1016/j.ijsu.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Foster J D, Pathak S, Smart N J. et al. Reconstruction of the perineum following extralevatorabdominoperineal excision for carcinoma of the lower rectum: a systematic review. Colorectal Dis. 2012;14(9):1052–1059. doi: 10.1111/j.1463-1318.2012.03169.x. [DOI] [PubMed] [Google Scholar]
- 53.Butt H Z, Salem M K, Vijaynagar B, Chaudhri S, Singh B. Perineal reconstruction after extra-levatorabdominoperineal excision (eLAPE): a systematic review. Int J Colorectal Dis. 2013;28(11):1459–1468. doi: 10.1007/s00384-013-1660-6. [DOI] [PubMed] [Google Scholar]
- 54.Frasson M, Flor-Lorente B, Carreño O. Reconstruction techniques after extralevatorabdominoperineal rectal excision or pelvic exenteration: meshes, plasties and flaps. Cir Esp. 2014;92 01:48–57. doi: 10.1016/S0009-739X(14)70008-9. [DOI] [PubMed] [Google Scholar]
- 55.Bakx R, van Lanschot J J, Zoetmulder F A. Inferiorly based rectus abdominismyocutaneous flaps in surgical oncology: indications, technique, and experience in 37 patients. J Surg Oncol. 2004;85(2):93–97. doi: 10.1002/jso.20014. [DOI] [PubMed] [Google Scholar]
- 56.Bell S W, Dehni N, Chaouat M, Lifante J C, Parc R, Tiret E. Primary rectus abdominismyocutaneous flap for repair of perineal and vaginal defects after extended abdominoperineal resection. Br J Surg. 2005;92(4):482–486. doi: 10.1002/bjs.4857. [DOI] [PubMed] [Google Scholar]
- 57.McAllister E, Wells K, Chaet M, Norman J, Cruse W. Perineal reconstruction after surgical extirpation of pelvic malignancies using the transpelvic transverse rectus abdominal myocutaneous flap. Ann Surg Oncol. 1994;1(2):164–168. doi: 10.1007/BF02303561. [DOI] [PubMed] [Google Scholar]
- 58.Radice E, Nelson H, Mercill S, Farouk R, Petty P, Gunderson L. Primary myocutaneous flap closure following resection of locally advanced pelvic malignancies. Br J Surg. 1999;86(3):349–354. doi: 10.1046/j.1365-2168.1999.01044.x. [DOI] [PubMed] [Google Scholar]
- 59.Tei T M, Stolzenburg T, Buntzen S, Laurberg S, Kjeldsen H. Use of transpelvic rectus abdominismusculocutaneous flap for anal cancer salvage surgery. Br J Surg. 2003;90(5):575–580. doi: 10.1002/bjs.4073. [DOI] [PubMed] [Google Scholar]
- 60.Butler C E, Gündeslioglu A O, Rodriguez-Bigas M A. Outcomes of immediate vertical rectus abdominismyocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206(4):694–703. doi: 10.1016/j.jamcollsurg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Lefevre J H, Parc Y, Kernéis S. et al. Abdomino-perineal resection for anal cancer: impact of a vertical rectus abdominismyocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250(5):707–711. doi: 10.1097/SLA.0b013e3181bce334. [DOI] [PubMed] [Google Scholar]
- 62.Palmer J A, Vernon C P, Cummings B J, Moffat F L. Gracilismyocutaneous flap for reconstructing perineal defects resulting from radiation and radical surgery. Can J Surg. 1983;26(6):510–512. [PubMed] [Google Scholar]
- 63.Chan S, Miller M, Ng R. et al. Use of myocutaneous flaps for perineal closure following abdominoperineal excision of the rectum for adenocarcinoma. Colorectal Dis. 2010;12(6):555–560. doi: 10.1111/j.1463-1318.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- 64.Shibata D, Hyland W, Busse P. et al. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6(1):33–37. doi: 10.1007/s10434-999-0033-4. [DOI] [PubMed] [Google Scholar]
- 65.Persichetti P, Cogliandro A, Marangi G F. et al. Pelvic and perineal reconstruction following abdominoperineal resection: the role of gracilis flap. Ann Plast Surg. 2007;59(2):168–172. doi: 10.1097/01.sap.0000252693.53692.e0. [DOI] [PubMed] [Google Scholar]
- 66.Gould W L, Montero N, Cukic J, Hagerty R C, Hester T R. The “split” gluteus maximusmusculocutaneous flap. Plast Reconstr Surg. 1994;93(2):330–336. doi: 10.1097/00006534-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 67.Holm T, Ljung A, Häggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94(2):232–238. doi: 10.1002/bjs.5489. [DOI] [PubMed] [Google Scholar]
- 68.Anderin C, Martling A, Lagergren J, Ljung A, Holm T. Short-term outcome after gluteus maximusmyocutaneous flap reconstruction of the pelvic floor following extra-levatorabdominoperineal excision of the rectum. Colorectal Dis. 2012;14(9):1060–1064. doi: 10.1111/j.1463-1318.2011.02848.x. [DOI] [PubMed] [Google Scholar]
- 69.Haapamäki M M, Pihlgren V, Lundberg O, Sandzén B, Rutegård J. Physical performance and quality of life after extended abdominoperineal excision of rectum and reconstruction of the pelvic floor with gluteus maximus flap. Dis Colon Rectum. 2011;54(1):101–106. doi: 10.1007/DCR.0b013e3181fce26e. [DOI] [PubMed] [Google Scholar]
- 70.Hainsworth A, Al Akash M, Roblin P, Mohanna P, Ross D, George M L. Perineal reconstruction after abdominoperineal excision using inferior gluteal artery perforator flaps. Br J Surg. 2012;99(4):584–588. doi: 10.1002/bjs.7822. [DOI] [PubMed] [Google Scholar]
- 71.Orkin B A. Perineal reconstruction with local flaps: technique and results. Tech Coloproctol. 2013;17(6):663–670. doi: 10.1007/s10151-013-0978-y. [DOI] [PubMed] [Google Scholar]
- 72.Abbes Orabi N, Vanwymersch T, Paterson H M. et al. Total perineal reconstruction after abdominoperineal excision for rectal cancer: long-term results of dynamic graciloplasty with Malone appendicostomy. Colorectal Dis. 2011;13(4):406–413. doi: 10.1111/j.1463-1318.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 73.Jethwa P, Lake S P. Using topical negative pressure therapy to resolve wound failure following perineal resection. J Wound Care. 2005;14(4):166–167. doi: 10.12968/jowc.2005.14.4.26766. [DOI] [PubMed] [Google Scholar]
- 74.Cresti S, Ouaïssi M, Sielezneff I. et al. Advantage of vacuum assisted closure on healing of wound associated with omentoplasty after abdominoperineal excision: a case report. World J Surg Oncol. 2008;6:136. doi: 10.1186/1477-7819-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.GarcíaManzanares M E, Hidalgo Dóniga C, De Arriba Amado S. et al. V.A.C. therapy application on perineum wound after a Miles operation or Miles resection [in Spanish] Rev Enferm. 2009;32(12):30–34. [PubMed] [Google Scholar]
- 76.Chadi S A, Kidane B, Britto K, Brackstone M, Ott M C. Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon Rectum. 2014;57(8):999–1006. doi: 10.1097/DCR.0000000000000161. [DOI] [PubMed] [Google Scholar]


