Abstract
Adult C57BL/6 mice were injected with 100 micrograms of soluble, freshly deaggregated human serum albumin (HSA) to produce partial immunologic tolerance. Uninjected normal control (N) mice contain only approximately 100 B cells in their spleens with the capacity to (i) be activated in vitro into clonal proliferation by Escherichia coli lipopolysaccharide plus interleukins 2, 4, and 5, (ii) form IgG1 as well as IgM antibody, and (iii) display specificity for HSA when only IgG1 is allowed to score in an enzyme-linked immunosorbent assay (ELISA). Such N mice generate approximately 50,000 clonable anti-HSA IgG1 antibody-forming cell precursors in their spleens after T-dependent immunization with HSA absorbed onto alum and given with Bordetella pertussis adjuvant. Mice preinjected with soluble HSA (TOL) generate far fewer anti-HSA IgG1 antibody-forming cell precursors, termed anti-HSA memory cells. Splenocytes were transferred from N or TOL mice into lethally irradiated syngeneic recipients together with syngeneic bone marrow. Whereas N splenocytes generated plentiful memory cells within 2 weeks in antigenically challenged recipients, TOL splenocytes did not. Work with Ly-5 congenic mice ruled out memory cell generation from either the host or the bone marrow inoculum within this limited time. N T cells plus TOL B cells showed consistently lowered memory cell generation. TOL T cells plus N B cells showed an even greater lowering of adoptive memory cell generation. Thus the lowered response capacity of TOL mice resided in the T- and B-cell compartments. Attempts to show a suppressor component within the T-cell population were inconclusive, but a profound defect in capacity to respond to HSA in vitro was exhibited by the CD4+ T cells of TOL mice. B lymphocytes were harvested from T-dependently immunized mice 5 days after challenge, incubated with soluble HSA for 18 hr, and then adoptively transferred together with N T cells. The recently activated B cells were not rendered tolerant by this manipulation. The results argue for a major T-cell component in the process whereby soluble protein antigens ablate affinity maturation and memory cell generation.
Full text
PDF

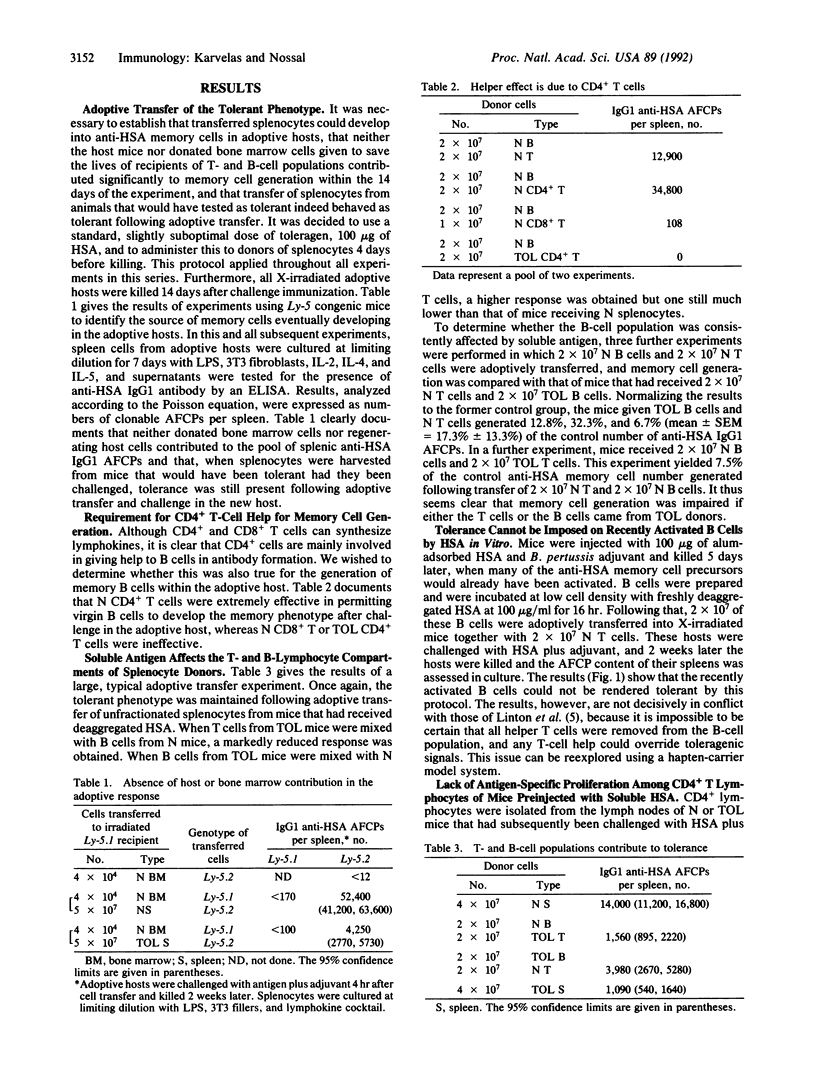
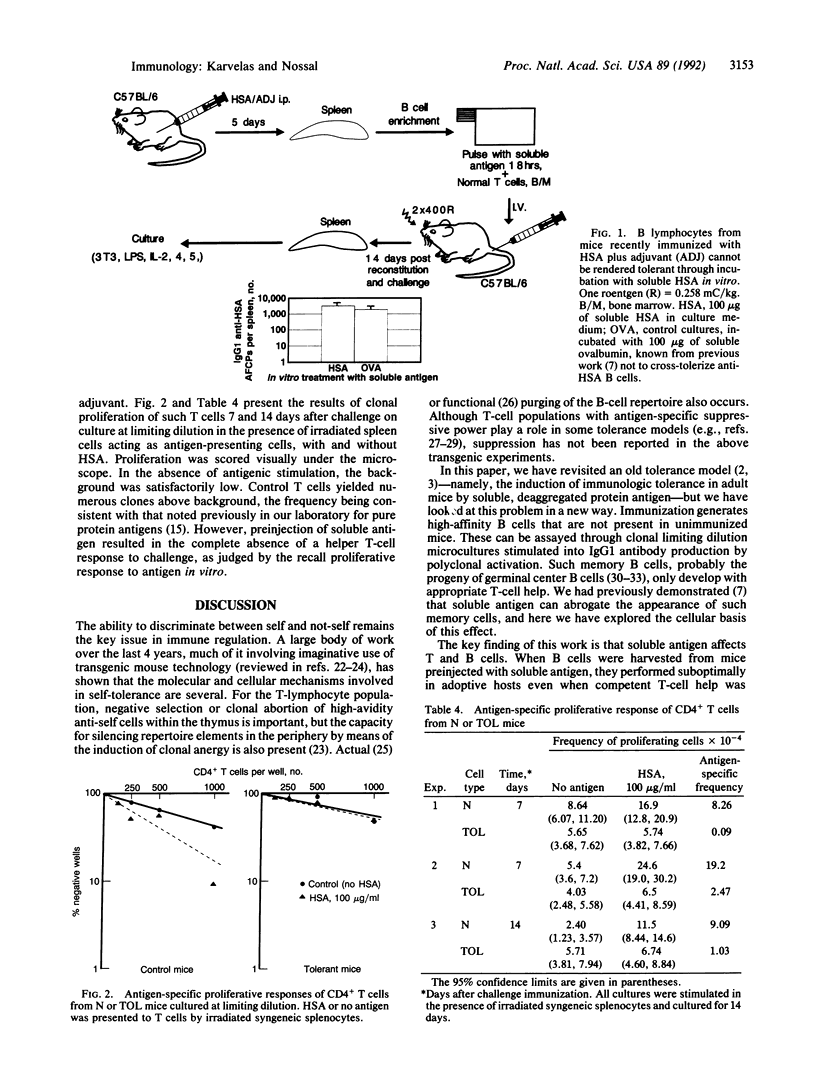

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Miller J. F., Johnson P. T cell-dependent suppression of an anti-hapten antibody response. Transplant Rev. 1975;26:130–169. doi: 10.1111/j.1600-065x.1975.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. I. Protein-over loading paralysis. Immunology. 1962 Jan;5:161–168. [PMC free article] [PubMed] [Google Scholar]
- Dorsch S., Roser B. Suppressor cells in transplantation tolerance. I. Analysis of the suppressor status of neonatally and adoptively tolerized rats. Transplantation. 1982 May;33(5):518–524. [PubMed] [Google Scholar]
- Ferrick D. A., Ohashi P. S., Wallace V. A., Schilham M., Mak T. W. Transgenic mice as an in vivo model for self-reactivity. Immunol Rev. 1990 Dec;118:257–283. doi: 10.1111/j.1600-065x.1990.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Gibson J., Basten A., Walker K. Z., Loblay R. H. A role for suppressor T cells in induction of self-tolerance. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5150–5154. doi: 10.1073/pnas.82.15.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Good M. F., Nossal G. J. Functional clonal deletion and suppression as complementary mechanisms operative in adult hapten-induced cytotoxic T cell tolerance. J Immunol. 1983 Dec;131(6):2662–2669. [PubMed] [Google Scholar]
- Goodnow C. C., Adelstein S., Basten A. The need for central and peripheral tolerance in the B cell repertoire. Science. 1990 Jun 15;248(4961):1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Kelso A., Troutt A. B., Maraskovsky E., Gough N. M., Morris L., Pech M. H., Thomson J. A. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol Rev. 1991 Oct;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Linton P. J., Rudie A., Klinman N. R. Tolerance susceptibility of newly generating memory B cells. J Immunol. 1991 Jun 15;146(12):4099–4104. [PubMed] [Google Scholar]
- MacLennan I. C., Liu Y. J., Oldfield S., Zhang J., Lane P. J. The evolution of B-cell clones. Curr Top Microbiol Immunol. 1990;159:37–63. doi: 10.1007/978-3-642-75244-5_3. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G. Combinations of interleukins 2, 4 and 5 regulate the secretion of murine immunoglobulin isotypes. Eur J Immunol. 1989 Nov;19(11):2025–2030. doi: 10.1002/eji.1830191109. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J., Lalor P. A. Molecular characterization of single memory B cells. Nature. 1991 Apr 11;350(6318):502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Morahan G., Allison J., Hoffmann M. A transgenic approach to the study of peripheral T-cell tolerance. Immunol Rev. 1991 Aug;122:103–116. doi: 10.1111/j.1600-065x.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Karvelas M. Soluble antigen abrogates the appearance of anti-protein IgG1-forming cell precursors during primary immunization. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1615–1619. doi: 10.1073/pnas.87.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Molecular and cellular aspects of immunologic tolerance. Eur J Biochem. 1991 Dec 18;202(3):729–737. doi: 10.1111/j.1432-1033.1991.tb16427.x. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Riedel C. Sudden appearance of anti-protein IgG1-forming cell precursors early during primary immunization. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4679–4683. doi: 10.1073/pnas.86.12.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992 Jan 10;68(1):1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A high-efficiency cloning system for single hapten-specific B lymphocytes that is suitable for assay of putative growth and differentiation factors. Proc Natl Acad Sci U S A. 1985 May;82(10):3395–3399. doi: 10.1073/pnas.82.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O., Chiller J. M., Habicht G. S. Effect of immunological unresponsiveness on different cell populations. Transplant Rev. 1972;8:3–25. doi: 10.1111/j.1600-065x.1972.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]



