Abstract
Previous efforts to target the mouse genome for the addition, subtraction, or substitution of biologically informative sequences required complex vector design and a series of arduous steps only a handful of labs could master. The facile and inexpensive clustered regularly interspaced short palindromic repeats (CRISPR) method has now superseded traditional means of genome modification such that virtually any lab can quickly assemble reagents for developing new mouse models for cardiovascular research. Here we briefly review the history of CRISPR in prokaryotes, highlighting major discoveries leading to its formulation for genome modification in the animal kingdom. Core components of CRISPR technology are reviewed and updated. Practical pointers for two-component and three-component CRISPR editing are summarized with a number of applications in mice including frameshift mutations, deletion of enhancers and non-coding genes, nucleotide substitution of protein-coding and gene regulatory sequences, incorporation of loxP sites for conditional gene inactivation, and epitope tag integration. Genotyping strategies are presented and topics of genetic mosaicism and inadvertent targeting discussed. Finally, clinical applications and ethical considerations are addressed as the biomedical community eagerly embraces this astonishing innovation in genome editing to tackle previously intractable questions.
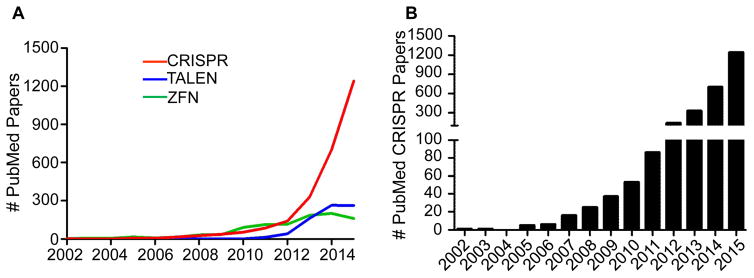
The 2007 Nobel Prize in Physiology or Medicine was awarded for pioneering work in embryonic stem cells (ESC), which developed techniques to modify the mouse genome leading to the creation of thousands of knockout mouse strains, including hundreds that exhibit cardiovascular phenotypes (ftp://ftp.informatics.jax.org/pub/reports/MGI_Knockout_Full.html). Traditionally, the generation of knockin or knockout mouse models for cardiovascular study required intricate design and construction of a targeting vector; electroporation of the vector into a limited number of ESC lines; selection and enrichment of targeted ESC clones; screening of clones with validated probes by Southern blotting; and expansion of correctly targeted clones for injection into the blastocyst of a mouse embryo.1 If ensuing chimeric mice were able to pass the targeted allele through the germline, and if phenotypic haploinsufficiency leading to premature debilitating disease and/or death did not occur, heterozygous offspring could be intercrossed to create mice homozygous for the targeted allele. The time and effort involved in creating a mouse in this manner is considerable (typically more than one year) with no guarantee of success. The advent of nuclease-directed genome editing (NDGE) in animals with zinc finger nucleases 2 and transcription activator-like effector nucleases 3 simplified the labor-intensive task of generating genetically-modified mice while reducing the time to completion. These technologies also augmented the range of animals that could successfully undergo genome modifications.4 However, the more recent CRISPR system has rapidly ascended as the méthode de choix for genome editing (Figure 1A) because of its incredible simplicity and high efficiency. Indeed, more than half of the entire literature pertaining to CRISPR through 2015 was reported in that year (Figure 1B).
Figure 1. Explosive rise in CRISPR-related publications.
(A) Comparative publication track record for the three major methods of NDGE over the last 14 years. (B) Annual number of CRISPR publications based on PubMed search. Following a relatively inactive period (2002–2006), a first wave of papers was published based on the prokaryote adaptive immune function of CRISPR (2007–2011) followed by a second wave relating to CRISPR genome editing (2012-present). Data for both graphs obtained on December 30, 2015.
Although many reviews have been published on the subject of CRISPR (see for example 5–7) the field has moved at such a ferocious pace, with many pearls of knowledge only recently shared and debated through a discussion group started in early 2013 (https://groups.google.com/forum/!forum/crispr), that an update is timely, particularly in cardiovascular biology where only a few papers have been published using CRISPR genome editing in mice. Here, we briefly summarize the historical roots of CRISPR and the key elements of the technology as they apply to the generation of new mouse models. We provide an overview of two-component and three-component CRISPR, highlighting the strengths and weaknesses of each with specific applications. We address important issues related to the design and testing of crucial components of CRISPR as well as genetic mosaicism, robust genotyping, and inadvertent (off) targeting, all of which must be carefully considered for unambiguous evaluation of CRISPR-derived mice. Finally, we provide some clinical perspective for future applications and challenges that must be overcome for CRISPR to have tangible impact in the treatment of cardiovascular disease.
Origins of CRISPR and its Transition to the Animal Kingdom
In 1987, a peculiar arrangement of homologous direct repeats having dyad symmetry and unknown function was discovered in Escherichia coli.8 Similarly arrayed sequences were subsequently found in archaea and other species of bacteria.9 These repeat sequences were named Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR).10 No CRISPR sequences were identified in eukaryotes or viruses, suggesting a unique function of CRISPR to species of prokaryotes.10 The first clue as to the function of CRISPR sequences emerged with the discovery of similarly-sized CRISPR spacers between CRISPRs as well as flanking CRISPR-associated (Cas) protein-coding genes, implying inter-related functional gene loci.10 An important breakthrough occurred in 2005 when the CRISPR spacers were found to be of bacteriophage and plasmid origin.11, 12 These findings sparked the prescient hypothesis that CRISPRs endow bacteria with genetic immunity against foreign DNA.12 Then, in 2007, studies proved CRISPR and adjacent Cas operons confer acquired resistance in bacteria to invading viruses.13 The mechanism underlying such adaptive immunity occurs via endonuclease-mediated cleavage of pre-CRISPR transcripts by Cas proteins and subsequent processing into smaller CRISPR RNA (crRNA) that are complementary to viral DNA leading to silencing of the alien invasion.14 Extended CRISPR sequences, reflecting sequential infections of bacteria, imparted greater resistance to bacteriophage assault.12, 13 Thus, many prokaryotes acquire immunity that is hard-wired into the host genome. Importantly, in the type II CRISPR-Cas system, the procurement of CRISPR spacers (or proto-spacers) from the initial invasion of a bacteriophage or plasmid requires a proto-spacer adjacent motif (PAM) immediately 3′ of the CRISPR spacer sequence in invading DNA.15 This orientation with respect to CRISPR spacer and PAM is also critical for CRISPR-mediated NDGE in the mouse genome. Although the CRISPR acronym is more aptly applied to prokaryotic immunity, it has become engrained in the lexicon of eukaryotic biologists and the public as a powerful means of altering genomes. For a more in depth study of CRISPR in prokaryotic biology, the reader is referred to a number of outstanding reviews from pioneers in the field.16–18
Two key breakthroughs precipitated the ‘CRISPR Craze.’19 First, a transactivating crRNA (tracrRNA) was established as essential for crRNA processing in Streptococcus pyogenes. 20 The second important discovery occurred one year later when crRNA, tracrRNA, and Cas9 were combined in vitro and shown to cleave the complementary and non-complementary strands of target plasmid DNA three base pairs upstream of a PAM.21, 22 Importantly, the crRNA and tracrRNA could be combined into one hybrid transcript (so called single guide RNA; sgRNA) and still direct Cas9 cutting of target DNA.21 Over the ensuing months, an avalanche of papers demonstrated NDGE in human and mouse cell lines 23–25 as well as various animal models 26–34 using the main components comprising CRISPR genome editing, each of which is considered next.
Components of CRISPR Genome Editing
Component 1: Cas9 Endonuclease
The most common endonuclease used in CRISPR genome editing is the Class II effector protein, Cas9, from Streptococcus pyogenes (Spycas9).35 The Spycas9 gene is transcribed as a ~4.2 kilobase transcript encoding a 1,368 amino acid protein of ~160 kDa. The transcript has been codon-optimized for efficient translation in mammals and engineered to carry nuclear localization signals for proper targeting to the nucleus as well as epitope tags for easy detection.23–25 The SpyCas9 cDNA is found in several plasmids, most notably pX330 from addgene (https://www.addgene.org/42230/).23 The pX330 plasmid serves as a template for in vitro transcription of Spycas9. However, it is much easier to purchase ready-made SpyCas9 protein (PNA Bio, http://pnabio.com) or, more frequently, Spycas9 mRNA (TriLink BioTechnologies, http://www.trilinkbiotech.com) for immediate microinjection in mouse zygotes. The longer 4.5 kb Spycas9 mRNA from TriLink includes a capped, 5′ synthetic untranslated region harboring a strong Kozak sequence for enhanced translation, an alpha globin 3′ untranslated region, and a 120 bp polyadenylated tail to augment stability (Tiffany Teng of TriLink BioTechnologies, personal communication).
It is possible to inject mouse zygotes directly with a plasmid containing both Spycas9 and sgRNA.36 However, this approach is complicated by the reduced chance of transmitting an edited allele through the germline due to embryonic cell divisions preceding Cas9/sgRNA transcription and Cas9 translation. Further, there are unpredictable effects of a randomly integrated plasmid in the mouse genome and unknown consequences of continuous expression of Cas9 protein over the life of the mouse. Nevertheless, a recent study showed that mice carrying a Cas9 transgene under control of the cardiac-specific Myh6 promoter exhibited no observable toxicity. These mice allow for rapid assessment of gene function in the adult heart following a single injection of adeno-associated virus carrying a sgRNA of interest, thus circumventing potential embryonic lethality that otherwise occurs with conventional gene targeting strategies.37 Additional mouse models will likely emerge that further limit expression of Cas9 to heart or blood vessels using drug-responsive, cell-specific promoters driving Cas9 directly or indirectly via Cre recombinase in the context of the Rosa26 floxed stop Cas9 transgenic mouse (https://www.jax.org/strain/024857).38
The SpyCas9 protein has a unique bilobed structure.39, 40 The α-helical target recognition lobe makes important contacts with the sgRNA bound to its target genomic DNA. The nuclease lobe contains the HNH and RuvC-like nuclease domains, each of which mediates an individual nick in the complementary (via HNH) or non-complementary (via RuvC-like) strand of the double helix, thus creating a blunt double-strand break (DSB). In addition, a PAM-interacting domain within the nuclease lobe binds PAM sequences (NGG or NAG) that are necessary for igniting SpyCas9 nuclease activity leading to target DNA cleavage 41 three base pairs upstream of the PAM sequence.42 The PAM-interacting domain may also function to facilitate unwinding of the double helix in a 3′ to 5′ direction from the PAM site to allow for strand invasion of the sgRNA, Watson-Crick base pairing between the sgRNA and its complementary target DNA sequence, and formation of a so-called R loop structure.40, 43 PAM sequences may be diversified through alterations in SpyCas9 44 or the utilization of other RNA-guided endonucleases.45–47 This increases the sequence space that may be efficiently targeted such that virtually every nucleotide in the mouse genome may undergo genome editing.
Additional derivations of SpyCas9 have been engineered that expand its functionality. For example, either of the nuclease domains may be mutated (D10A or H840A) to create a nickase (Cas9n), which increases the specificity of genome editing. 21, 48 We do not advocate the use of Cas9n for generating cardiovascular mouse models because of the inherent complexity in experimental design as well as the lower efficiency of SpyCas9n versus wild type SpyCas9 in mediating genome edits, 49 though editing genomes is highly locus-dependent and there may be instances where Cas9n is desirable.50 On the other hand, newer generations of SpyCas9 carrying mutations that do not alter the two nuclease domains have been developed that also reduce off-targeting events. These include “enhanced specificity” SpyCas9 (https://www.addgene.org/71814/)51 and a “high fidelity” SpyCas9 (https://www.addgene.org/72247/).52 It will be important to see whether these newer versions of SpyCas9 are offered as ready-made mRNA or protein and how well they perform in editing the mouse genome as compared to wild type SpyCas9.
Another modification of SpyCas9 involves mutation of both nuclease domains which effectively yields a ‘dead’ or ‘deactivated’ version of the enzyme (dCas9). This alteration in SpyCas9 preserves its sgRNA-directed binding to target DNA; however, dCas9 is unable to cut DNA rendering the protein a suitable platform for a number of applications, most notably activation or repression of genes through the conjugation of transcriptional activators or repressors.53 This approach offers a potentially powerful means of rescuing mouse phenotypes resulting from CRISPR-mediated NDGE of distal enhancer sequences by directly activating the endogenous promoter. Another application of dCas9 involves joining the protein with a fluorophore for purposes of tracking Cas9 binding across the genome.54 A recent study utilizing such an approach revealed Cas9 targeting both euchromatic and heterochromatic sequences through a diffusion-like mechanism suggesting most of the mouse genome may be targeted for editing regardless of epigenetic state.55 This finding is consistent with Cas9-mediated cleavage of methylated DNA.56 Recently, however, an in vitro study showed that nucleosomes represent a barrier for Cas9 binding and cleavage suggesting the state of chromatin around a gene of interest in the mouse zygote may be an important determinant of CRISPR-mediated NDGE.57 The latter point underscores the need to test each sgRNA in advance of experimentation (below).
Endonuclease activity of SpyCas9 requires a conformational change in its structure, which is mediated by the sgRNA/target DNA sequence.40 We know little, however, about endonuclease-independent activity of this protein in the mouse. Preliminary physiological data suggest no overt phenotypes arising from constitutive expression of Cas9 in mice 37, 38, and RNA-seq studies have been done in cultured cells transiently 58 or stably 59 expressing targeted Cas9 with little changes in gene expression beyond those expected. It must be stressed, however, that no studies have yet to interrogate the transcriptome or proteome of tissues in mice with chronic expression of Cas9. It is possible that Cas9 binds other proteins in the cytosol or nucleus effecting subtle perturbations in cell physiology. Further, as a bacterial protein Cas9 may elicit an immune response. Thus, until more thorough studies are carried out it is premature to conclude that constitutive expression of Cas9 is not without side effects. Efforts should therefore be made to minimize the window of time in which Cas9 is expressed in mice. This is best achieved by microinjecting Cas9 mRNA or protein into the mouse zygote in combination with the next component of CRISPR technology.
Component 2: Single Guide RNA
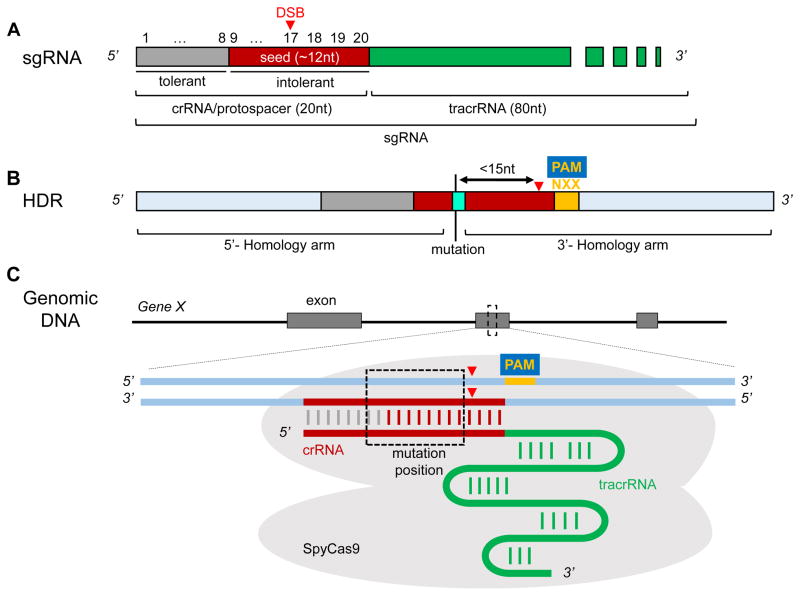
The Cas9 endonuclease is inactive until it is bound and ushered to a specific genomic address by the sgRNA. The sgRNA serves a dual function of both chaperone and activator of Cas9 endonuclease activity through the union of two separable sequences transcribed as a chimeric RNA.21 The chaperone (or guiding) function of the sgRNA is achieved by a user-defined 20 nucleotide CRISPR RNA (crRNA or proto-spacer sequence in recognition of similarly functioning sequences in bacteria and archaea) while the activator function is served by the invariant 80-nucleotide tracrRNA (Figure 2A). The tracrRNA, derived from the Streptococcus pyogenes genome, comprises a number of structural modules that serve critical roles for Cas9 binding and activation.60 The crRNA sequence is complementary to the target sequence to be edited in the mouse genome and although sequences longer than 20 nucleotides might intuitively confer greater specificity, they are cleaved to ~20 nucleotides during processing.48
Figure 2. Characteristics of sgRNA and HDR repair template.
(A) The single guide RNA is a chimeric molecule comprising a user-defined 20 nucleotide crRNA containing mismatch-tolerant (gray) and intolerant (red) nucleotides and an invariant 80 nucleotide tracrRNA (green). The red triangle here and below denotes the double-strand break (DSB) that occurs following Cas9 binding (C). (B) The HDR template is generally a single-strand oligonucleotide with homology arms of 50–70 nucleotides on each side. Note position of sequence edit (labeled mutation) at center of HDR template within 15 nucleotides of the DSB. The XX below PAM denotes silent nucleotide substitutions that prevent secondary NHEJ. Intolerant sequence changes in the crRNA are important when considering unintended edits and making substitutions for prevention of secondary NHEJ. (C) Schematic of sgRNA and bilobed SpyCas9 over a genomic target site. Note absence of PAM in sgRNA sequence.
The 20 nucleotide crRNA sequence must precede a PAM recognition site (NGG or NAG for SpyCas9) located immediately 3′ of the last nucleotide of the crRNA sequence (Figure 2A). Binding and cleavage of DNA by Cas9 requires the PAM sequence.21, 22 Importantly, the PAM sequence must not be included in the design of the 20 nucleotide crRNA sequence as it would compete for Cas9 binding or perturb normal structural changes that must occur for Cas9 to be activated (Rodolphe Barrangou, personal communication).60 The crRNA sequence encompasses a PAM proximal seed sequence of ~10–12 nucleotides that is relatively intolerant to mutations; 61 that is, nucleotide substitutions in the seed sequence tend to mitigate Cas9 binding and cleavage of the target DNA sequence. On the other hand, a PAM distal sequence of approximate equal length is more tolerant to mutations and thus changes here tend to have less impact on Cas9 binding and activity (Figure 2A). These sequence characteristics of the crRNA have been incorporated in many programs that predict potential off target sites in the mouse genome (below). As a final point, chemical modifications to the crRNA sequence that enhance stability have been shown to exhibit superior activity over un-modified crRNA sequences,62 providing a means of targeting Cas9 to potentially intractable genomic sites, though this has yet to be formally demonstrated in the mouse.
Numerous online tools are available for the identification of optimal crRNA sequences (reviewed in 63). The most commonly used algorithm is the CRISPR Design Tool from the Feng Zhang Lab (http://crispr.mit.edu,56). The algorithm is built upon a number of important sequence characteristics such as a PAM (NGG or NAG) following the crRNA sequence, absence of polyU at the 3′ end of the crRNA sequence, and as little sequence identity to other potential off target sites in genome as possible, especially in the seed region of the crRNA. The output display is a rank-ordered list of optimal crRNA sequences for a user to select based on the number of theoretical off target sites (higher scored crRNAs are best).
Essentially all of the front-end work of CRISPR-mediated NDGE involves preparation of the sgRNA. Once a crRNA sequence is selected, a pair of complementary oligonucleotides should be ordered for cloning into the pX330 vector. Following verification of the cloned crRNA sequence, the plasmid is tested for sgRNA activity and then processed for in vitro transcription, purification, and visual analysis for integrity with a BioAnalyzer. A distilled version of a detailed protocol for cloning the crRNA into the pX330 vector and in vitro transcription/purification of the sgRNA 64 is available online (Supplemental Method 1). Some commercial companies now offer custom synthesis of crRNA and tracrRNA sequences, which minimizes the hands-on workload; however, companies do not typically test synthetic crRNAs for Cas9-dependent cleavage activity.
Most (>80% in our experience) sgRNAs will direct a Cas9-induced double-strand break (DSB). However, a recent report indicated that nucleosomes may obstruct Cas9 binding and cleavage.57 Given the cost in time and money to engineer a genetically modified mouse, we advise careful attention to sgRNA design and activity testing before its synthesis and purification for microinjection. We typically design and test at least two sgRNAs for activity before injecting into a mouse zygote. There are at least two ways of testing the activity of a sgRNA. One method measures DSB cleavage directly at the intended genomic site using a nuclease such as Surveyor, which digests heteroduplex DNA following the annealing of wild type sequence with related sequence containing insertions or deletions (indels) that result during the repair of a DSB.65 Another approach assesses DSB cleavage indirectly through the reconstitution of a split EGxxFP reporter plasmid (http://www.addgene.org/50716/).36 Both methods involve transfecting cells with a plasmid carrying Cas9 and a sgRNA (e.g., pX330) or individual store-bought components. The Surveyor assay is slightly less labor intensive but more temperamental than the split reporter assay, which we routinely use in testing sgRNAs. An advantage of the split reporter is that the same highly transfectable cell line (e.g., HEK-293) may be used, regardless of the species’ genome to be edited. Typical results using the split reporter assay are presented online (Supplemental Figure I). In addition, we provide some key tips for testing sgRNAs using the split reporter assay (Supplemental Method 1).
Component 3: Homology Directed Repair Templates
A third component of CRISPR is essential for precision-guided genome editing and involves introduction of a donor DNA template that is incorporated into the host genome during homology directed repair (HDR). The donor DNA template may either be a single-strand oligonucleotide (SSO) or a double-strand DNA (DSD) molecule, both of which contain a centrally positioned sequence substitution or addition flanked by homology arms that overlap the Cas9-sgRNA targeted region of the genome, including the PAM sequence (Figure 2B). The centrally located sequence may represent nucleotide substitutions, short sequence inserts (e.g., restriction site, loxP site, small epitope tag), or longer sequence inserts (e.g., Cre recombinase, reporter gene). The function of the SSO or DSD template is to incorporate a subtle (e.g., single nucleotide substitution) or more substantial (e.g., transgene) edit to the targeted genomic site during repair of a DSB thereby generating a predictable and inheritable modification of the mouse genome.
The decision to use a SSO or DSD repair template is dependent upon the size of the sequence change to be merged into the mouse genome. Commercial synthesis of SSO is limited to a length of 200 nucleotides, though recent studies in mice have utilized longer SSO of nearly 1 kilobase.66 We suggest the use of SSO HDR templates for integrating single or multiple nucleotide substitutions, restriction sites, loxP sites, or small epitope tags such as 3xFLAG. It is recommended that SSO be purified, generally by polyacrylamide gel electrophoresis (e.g., PAGE Ultramers from Integrated DNA technologies, https://www.idtdna.com). The length of a SSO is dictated by the size of the centrally located sequence change. For example, if a disease-associated single nucleotide polymorphism (SNP) is to be integrated into the mouse genome, the SSO could be as short as 100 nucleotides, but more generally is 120–140 nucleotides in length with the SNP situated near the middle of the SSO and close to the PAM sequence. Sequence inserts such as loxP (34 nucleotides) or 3xFLAG (63 nucleotides) necessitate longer SSO, typically in the range of 165–185 nucleotides. We do not recommend using SSO of length greater than 185 nucleotides due to poor coupling efficiencies and the increased risk of sequence errors during synthesis (search loxP under the MIT CRISPR Discussion Forum for further details, https://groups.google.com/forum/!forum/crispr). Recently, improved integration of inserts up to 100 nucleotides was reported with the use of phosphorothioate-modified SSO with shorter homology arms.67
In addition to the length and central sequence to be recombined into the mouse genome, there are additional points to consider in the design of a SSO. First, we recommend the SSO be of the same strand as the sgRNA so as to avoid competitive hybridization with sgRNA and its complementary genomic sequence. Further consideration should be taken when using multiple SSO templates to avoid template hybridization such as using two SSO templates for loxp insertion. In the latter instance, the palindromic sequence of loxp, when represented by SSO templates on opposite strands, may lead to unintended SSO complementary binding thus compromising HDR efficiency and accuracy. Second, the HDR repair mechanism following a DSB competes with another repair mechanism known as non-homologous end joining (NHEJ) that can occur following incorporation of the SSO into the mouse genome. To avoid the latter undesirable circumstance, many investigators mutate the PAM sequence (e.g., NGG>NXX) within the SSO (Figure 2B). If the PAM is within a protein-coding region, then the change must preserve the amino acid sequence. Alternatively, silent mutations could be introduced in the SSO at wobble positions of codons along the PAM proximal seed region of the corresponding crRNA sequence. Mutations in the seed region of the SSO HDR template can also reduce secondary NHEJ by rendering the sgRNA ineffective in creating a DSB due to poor binding of the sgRNA to the newly modified genome. Irrespective of the SSO alteration, the goal is to minimize secondary genome editing via NHEJ and subsequent loss of the desired sequence edit.
Another important consideration in the design of a SSO (or DSD) is the position of the sequence substitution or insert relative to the DSB three base pairs upstream of the PAM. The most successful HDR templates will have the position of the substitution or inserted sequence no more than 10–15 nucleotides from the DSB (Figure 2B) as the efficiency of incorporating a sequence change or insert falls dramatically as the distance of the edit increases from the DSB.68, 69 We have noted recombination of a PAM mutation without the flanking desired edit, suggesting a short sequence length is sufficient for homologous recombination. Given the close association of Cas9 with the PAM sequence, we routinely place the sequence edits 5′ of the DSB. Newer endonucleases such as Cpf1 with broader PAM sequence recognition and unique cutting attributes, 46 increase targeted sequence space for genome editing thus potentially allowing more flexibility in getting desired edits as close to the DSB as possible.
A primary limitation of CRISPR genome editing is the unfavorable recombination of larger insertions requiring double-strand DNA (DSD) templates than those that can be incorporated with single-strand oligonucleotide (SSO) templates. Nevertheless, there are reports of DSD templates injected into the mouse zygote for the integration of reporter transgenes 70, 71 or a conditional allele.72 These studies utilized conventional homology arms of variable lengths (3–9 kb) in a circular DSD template; linearization of the DSD donor is not done so as to reduce random integration in the mouse genome. Recently, DSD templates with shorter homology arms (0.3 kb) were used in mouse ESC to generate a reporter mouse line.73 This finding is noteworthy because of the emergence of gBlocks and GeneArt strings technology where a DSD template (up to 3kb) can be synthesized and sequence confirmed for direct zygote injections without the need for labor-intensive PCR cloning of homology arms around the sequence of interest. These synthetic DSD donor templates have yet to be used on a large scale as of this date, 74 although we have noted some success when using gBlocks DNA in the mouse. While there has been reported success with DSD templates in vivo, use of SSO is more prevalent and efficient, and we therefore recommend SSO for most CRISPR editing applications in the mouse. Now that each component of CRISPR editing has been discussed, we turn to their combined use for versatile genome editing in mice.
Two-Component CRISPR
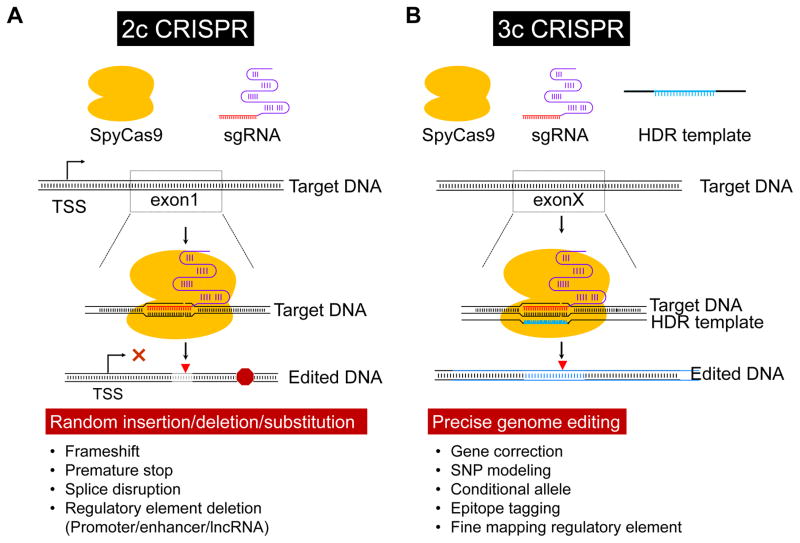
The most basic form of CRISPR-mediated NDGE in mice utilizes two components, Cas9 and sgRNA. In two-component CRISPR (2c CRISPR), a DSB is repaired through the error-prone NHEJ mechanism (Figure 3A). During NHEJ repair, host DNA sequences are most frequently deleted; however, inserted sequences and substitutions may also occur.75, 76 NHEJ is the dominant mode of DSB repair in the mouse zygote with upwards of 80% of pups showing some form of nucleotide change in targeted sequences.28 Importantly, the multiplex nature of 2c CRISPR 23 allows for multiple genes to be targeted simultaneously in the mouse.28 Further, unlike ESC-based knockouts, where only one allele generally recombines the mutation following electroporation, 2c CRISPR often results in bi-allelic targeting in the mouse zygote.28 Thus, multiplex 2c CRISPR affords the unprecedented opportunity to study multiple inactivated alleles in founder mice.28 Of course, upon breeding founders to the Filial 1 generation targeted alleles will segregate out and the same complex breeding scheme required in traditional compound knockouts will apply.
Figure 3. Versatility of CRISPR genome editing.
(A) In two component CRISPR (2c CRISPR), the SpyCas9 and sgRNA mediate unpredictable insertions, deletions, or substitutions close to the DSB (red triangle) resulting in an altered reading frame, sometimes with an attending premature termination codon (red octagon). Two sgRNAs can also lead to deletions of genomic DNA segments between two DSBs (not shown). (B) In three component CRISPR (3c CRISPR), introduction of an HDR repair template facilitates precision-guided genome editing over NHEJ resulting in the incorporation of subtle sequence edits or small sequence tags such as loxP sites or 3xFLAG (blue). See text for more details. TSS, transcription start site.
Targeting Protein-Coding Genes
Several outcomes are possible following NHEJ repair of a DSB within a protein-coding sequence. The most desirable result would be disruption of the reading frame and introduction of a premature termination codon (PTC) leading to nonsense mediated mRNA decay and loss of protein expression. This would most likely yield a true knockout, especially if the first coding exon is targeted with sgRNA and no internal promoters driving expression of an alternative isoform are present. Such an approach was used to target exon 1 of the Pcsk9 gene in mice using adenovirus delivery to the liver with results showing a decrease in plasma levels of both PCSK9 protein and cholesterol.77 A second consequence of NHEJ repair of a coding exon 1 is deletion of promoter elements leading to complete silencing of gene expression. A third scenario is indels near splice donor or acceptor sites leading to aberrant splicing with loss of critical amino acids or the generation of a frameshift mutant. A fourth effect of NHEJ repair is an in-frame mutation with loss or gain of one or more contiguous amino acid or, in the case of single nucleotide substitutions, introduction of a missense mutation. The latter genetic modifications are less likely to result in a true null phenotype, but could inform the role of key amino acids in the biology of a protein (e.g., stability, trafficking, activity). Finally, a single nucleotide substitution may also result in the introduction of a nonsense codon with loss of normal protein expression. These examples highlight the major limitation of 2c CRISPR, namely the unpredictability in how NHEJ repair will occur. Some labs, including our own, utilize more than one sgRNA to exon 1 of a protein-coding gene to increase the likelihood of a null allele.78 It should be noted, however, that with every additional sgRNA used in 2c CRISPR, there is an increased risk of unintended edits and an expanded number of different alleles, which can complicate analysis. Given the unpredictability in 2c CRISPR of coding exons, extensive sequence analysis around the targeted site and breeding of mice to homozygosity are essential to determine whether a null or partial null allele is generated.
A limitation of targeting coding genes with 2c CRISPR relates to pervasive transcription and the disproportionate number of 5′ antisense long non-coding RNA (lncRNA) genes that are adjacent to or overlap the first exon of many protein-coding genes (Supplemental Figure II). It may be necessary in such instances to target an alternate downstream exon coding for a critical functional domain so as to avoid confounding interpretation of data with the simultaneous inactivation of both coding and non-coding genes. Of course, targeting downstream exons with sgRNA requires that disruption of the exon will lead to loss in protein expression. As with any approach that removes or disrupts an internal exon, there is the chance for hypomorphic alleles (i.e., incomplete knockout of the gene). Thus, the choice of downstream exon to be targeted with 2c CRISPR must be weighed carefully.
In some instances, it may be prudent to use a pair of sgRNAs flanking a faulty coding exon to remove the exon and restore expression of the defective protein. A classic example is the dystrophin gene in the mdx mouse model of muscular dystrophy, where a nonsense codon in exon 23 results in a truncated dystrophin protein.79 Recently, 2c CRISPR was applied via adeno-associated virus to adult mdx mice with encouraging results: a significant number of skeletal, cardiac, and smooth muscle cells showed restored expression of dystrophin upon deletion of exon 23 and restoration of the reading frame.80, 81 Further, skeletal myopathy was partially rescued with this therapeutic intervention.80, 81 This highlights the power of 2c CRISPR-mediated NDGE in potentially curing a disease of the heart and blood vessels. On the other hand, 2c CRISPR may be used to model human diseases where truncation of a protein exists. For example, premature termination codons (PTC) in the ALPK3 gene were recently found in neonates who succumbed to precocious cardiomyopathy.82 It may prove informative to generate similar alleles of Alpk3 in mice to understand the nature of this new genetic form of human cardiomyopathy.
As with any gene inactivation study, it is important to know about the protein-coding gene of interest before designing sgRNA(s) for genome modifications. This can best be discerned with the UCSC Genome Browser (https://genome.ucsc.edu/; 83) and inspecting gene structure for the first coding exon as well as the presence of any downstream isoforms that may not be inactivated by a frameshift mutation. For example, some genes harbor multiple transcript variants arising from independent promoters thus requiring judicious design of a sgRNA to ensure all isoforms are targeted with 2c CRISPR.84 It is also advisable to evaluate multiple gene prediction tracks for less well-annotated transcripts as well as ENCODE-derived chromatin remodeling tracks (e.g., H3K4Ac27), DNase I hypersensitivity tracks, and ChIP-seq tracks on the UCSC Genome Browser to determine the presence of any functional regulatory regions that could be lost with 2c CRISPR deletion of protein-coding sequence. The latter is of importance given the presence of regulatory elements within protein-coding exons.85
Targeting Non-Coding Genes
In addition to targeting protein-coding genes, 2c CRISPR may be used for knocking out non-coding genes. For example, a 23-kb segment of the Rian lncRNA was deleted from the mouse using two pairs of sgRNAs flanking the region of interest.86 In addition to loss in expression of Rian, the deletion also resulted in reduced expression of adjacent genes suggesting Rian, a nuclear lncRNA, functions in cis to regulate gene expression. A limitation of the latter approach is the use of so many sgRNAs, which heightens the risk of unintended editing. Inasmuch as lncRNAs exhibit diverse functions in maintaining cellular homeostasis, 2c CRISPR will find increasing utility in deciphering the function of this class of non-coding RNA in the vascular system.87 In addition to lncRNA genes, microRNAs are also amenable to 2c CRISPR deletion, though none have yet to be edited in the mouse. Interestingly, microRNAs may be found within a host lncRNA so it will be important to try and distinguish the biological significance of each using carefully designed 2c CRISPR editing.
Targeting Non-Coding Regulatory Regions
Most sequence variants occur in noncoding sequence space and some are associated with human diseases.88 Further, increasing evidence indicates some non-coding sequence variants overlap with binding sites for transcription factors or microRNAs that regulate gene expression.89–91 Many gene regulatory sites are found in larger sequence blocks known as enhancers. Thus, genome editing of enhancers in mice will be a critical approach towards defining the functionality of sequence variants implicated in human disease. Historically, elucidating the function of regulatory sequences or enhancers via genetic inactivation in mice lagged far behind that of protein-coding genes. This stems from the large ratio of enhancers (> 1 million) to protein-coding genes (~20,000) and the inherent difficulty in pinpointing dominant-acting enhancers in vivo. On the other hand, many papers have shown the functional activity of cardiovascular-restricted enhancers in mice with reporter genes such as lacZ.92 Such studies have yielded insight into target gene regulation and the development of cell-specific Cre driver mice for gene inactivation studies, but they can be limited by copy number variations, random integration events in the genome, and the “out-of-genomic” context in which they are evaluated.93
Now, with 2c CRISPR, the deletion of enhancers in their native genomic milieu is as easy as inactivating a protein-coding sequence. Indeed, in the last year, several enhancers have been deleted in mice using 2c CRISPR including those associated with expression of Bcl11a,94 Blimp1,95 Mef2c,96 Stat5,97 Pcdh,98 Mmp13,99 Gata3,100 and Cebpa.101 In each case, deletion of the enhancer compromised expression of the associated target gene. In addition to enhancers, boundary elements such as locus control regions or insulators may be interrogated with 2c CRISPR. In this context, an insulator located within repeat sequences, and hence intractable for removal through conventional gene targeting, was deleted with 2c CRISPR; mice lacking the regulatory region exhibited reduced expression of the Tyr gene.102
The deletion of regulatory regions is best achieved with paired sgRNAs that flank the enhancer or boundary element (Supplemental Figure III). It should be emphasized that deleting regulatory regions in this manner has some limitations. For example, there is the risk of segmental inversion or (less likely) duplication.103 Further, deletions result in a ‘genomic cavity’ with excision of hundreds or, in the case of super-enhancers, 104 thousands of nucleotides. This presents a challenge when attempting to attribute differences in target gene expression to a dominant transcription factor binding site (TFBS). Further, deleting large segments of DNA may directly perturb expression of genes outside the immediate target gene of interest. However, when combined with chromosome conformation capture and RNA-seq data, the deletion of enhancers or boundary elements will often provide very strong evidence for a regulatory region enhancing or reducing the expression of a specific gene locus. The approach of deleting regulatory regions also provides a foundation for finer resolution studies in mice that carry more precise edits of key TFBS as well as specific SNPs associated with human disease.105
The trove of ENCODE data (e.g., histone modifications of chromatin, gene expression) on the UCSC Genome Browser could inform the potential function of an enhancer and seems a reasonable starting point for testing the in vivo function of a putative regulatory region (Supplemental Figure III). However, recent transgenic mouse work found that enhancer activity was more a function of transcription factor occupancy over an enhancer region than specific chromatin marks.106 Moreover, changes in gene expression following 2c CRISPR deletion of a putative distal regulatory domain in the human genome could not easily be predicted by the presence of a chromatin mark.107 These studies underscore our primitive knowledge of functional TFBS within enhancers and their activity in an in vivo setting. The multiplexed editing regulatory assay (MERA) was recently reported as a large scale in vitro 2c CRISPR screen for functional regulatory elements in the mouse genome.108 This assay also uncovered “unmarked regulatory elements” indicating the full complement of functional DNA sequences in the human and mouse genomes is larger than previously thought. Accordingly, there will be an increasing need for elucidating functional regulatory elements across the genome. One approach to prioritizing putative functional elements is through 2c CRISPR-mediated deletion of enhancer regions carrying variants linked to disease followed by the use of precision-guided CRISPR editing of the specific variant (below).
To summarize, 2c CRISPR is an easy and widely applied method of CRISPR-mediated NDGE in the mouse. All an investigator needs is a validated sgRNA (or pair when deleting an exon, microRNA, long noncoding RNA, or enhancer) and store-bought Cas9 mRNA or Cas9 protein for submission to an academic transgenic mouse core (a list of many such cores within the continental United States is available upon request). 2c CRISPR is highly efficient given that NHEJ occurs throughout much of the cell cycle, making it especially attractive for in vivo editing of post-mitotic cells such as cardiomyocytes. 2c CRISPR may be multiplexed allowing an investigator to target multiple gene loci, particularly gene paralogs in which inactivation of one may be compensated for by another. The primary weaknesses with 2c CRISPR are the unpredictable nature of editing and the elevated risk of off targeting when using multiple sgRNAs.
Three Component CRISPR
Three component CRISPR (3c CRISPR) combines a donor DNA template with Cas9 and sgRNA to promote HDR over NHEJ following a DSB (Figure 3B). The primary strength of 3c CRISPR is precision editing of the genome through user-defined substitutions or additions of sequences during synthesis or construction of the donor template. Given the high frequency of SpyCas9 PAM sequences in the mouse genome (one in every ~12 base pairs), and attainable edits within the HDR template recombining when placed within 1–15 base pairs from the DSB, it is theoretically possible to perform precision-guided 3c CRISPR editing at virtually every nucleotide in the mouse genome. Newer generation endonucleases 45–47 that recognize distinct PAM sequences from those of SpyCas9 further strengthen this notion.
3c CRISPR facilitates very subtle edits to the genome such as single nucleotide replacements. This approach has extraordinary power in modeling human disease as well as elucidating the function of specific amino acids or regulatory sequences. Similar to 2c CRISPR, 3c CRISPR may be multiplexed for the editing of more than one sequence.28 Further, bi-allelic targeting is often observed allowing for immediate investigative work in founder mice.70 The primary weakness of 3c CRISPR is its lower efficiency than NHEJ repair in mice,109 probably because HDR is confined to the replication phase of the cell cycle when sister chromatid pairs are made available for normal HDR to proceed. There is a report of enhanced HDR in mice following co-injection of an inhibitor of NHEJ (e.g., Scr7);110 however, our own experience and that of others111 is that use of such an inhibitor has little apparent benefit in elevating HDR in the mouse zygote. Recently, the application of a small molecule agonist of RAD51 (promotes homologous recombination) increased the efficiency of HDR in rabbits.111 There has yet to be a consensus as to the utility of this approach in the mouse. Other interventions to increase efficiency of 3c CRISPR in mice include electroporation of zygotes 112 and phosphorothioate modification of the ends of SSO.67 Since the injected mouse zygote undergoes rapid cell divisions, enhancement of 3c CRISPR in this context is less critical than, for example, the targeting of post-mitotic cardiomyocytes. Below, we summarize several applications of 3c CRISPR in mice.
Targeting Protein-Coding Sequences
A popular application of 3c CRISPR is the introduction of subtle edits into coding sequences that fix or model an experimental human disease. In fact, one of the first applications of 3c CRISPR in mice involved the correction of cataracts by repairing a disrupted reading frame in the Crygc gene.113 A similar remedial intervention was achieved in the mdx mouse model of muscular dystrophy where a PTC yielding a truncated Dmd gene was corrected with base pair changes engineered within the SSO repair template.114 With thousands of Mendelian traits now catalogued (http://www.omim.org/), including many associated with cardiovascular disease,115 3c CRISPR will have profound utility in the biological study of point mutations in protein-coding genes strongly linked to human diseases. In addition, exome sequencing will continue to disclose rare mutations associated with defects in the cardiovascular system that will be amenable for study in the mouse.82 Since the desired edit will generally involve only one nucleotide substitution, single-strand oligonucleotide donors of 120–140 nucleotides length will be sufficient as HDR templates. A challenge in correcting or modeling a human variant in protein-coding sequence is the constrained sequence space for designing an optimal sgRNA; if the desired edit is too far removed from the DSB, integration of the edit may not occur or will do so inefficiently. New generation sgRNAs and SSO repair templates with chemical or spatial modifications116 as well as alternative endonucleases with different PAM requirements (above) could circumvent these challenges.
There are many protein-coding genes in the mouse genome whose function is completely unknown. Rather than use 2c CRISPR to inactivate such a gene in an unpredictable manner, one could use 3c CRISPR with a SSO targeting the first coding exon and carrying a nucleotide substitution that would generate a PTC and nonsense mediated decay. One important consideration is the position of the precision-guided PTC relative to a downstream exon-exon junction. In general, nonsense mediated decay will occur if the PTC is at least 50–55 nucleotides upstream of an exon-exon junction.117 3c CRISPR has distinct advantages in this regard because not all PTC generated through 2c CRISPR will fulfill this important criterion. Moreover, it is much easier to genotype a precision-guided mutation than variable indels and substitutions stemming from 2c CRISPR (see Genotyping section).
In addition to modeling human diseases or exploring the function of uncharacterized protein-coding genes, 3c CRISPR will be increasingly used to interrogate the functional significance of individual amino acids. For example, definitive function of such post-translational modifications as phosphorylation, ubiquitinylation, sumoylation, and acetylation will necessitate the introduction of missense mutations to show loss in modification has biological consequences in the mouse. Critical amino acids involved in nucleocytoplasmic shuttling, secretion, and interactions with other proteins, DNA, or RNA will also be subject to 3c CRISPR in mice. Studies conducted in this manner may reveal what was true in cultured cells is untrue in a living animal.
In this context, CRISPR-mediated edits in cell culture models may be undermined by frequent contamination with other cell types or species of mycoplasma as well as genomic instability.118 Of course, some cell types such as ESC or human inducible pluripotent stem cells will be essential for experimental therapeutic gene editing. In addition, if a human protein-coding gene is not represented in the mouse genome, CRISPR editing would need to be carried out in a suitable human cell line. In general, however, we suggest CRISPR editing be done in the mouse over cultured cells. Not only will results be obtained in a more physiological setting, CRISPR editing of a diploid genome (mouse) is easier than a polyploid genome (many cell lines). Further, unintended edits in mice are less problematic than those in cell culture where off targeting is permanent. Published protocols are available in those instances where CRISPR editing of cultured cells is necessary,119, 120 though we advise using one of the newer generation Cas9 endonucleases exhibiting very low off target activity.51, 52
Targeting Non-Coding Sequences
3c CRISPR genome editing of lncRNA genes in mice is somewhat problematic. One challenge relates to the relatively low sequence conservation of lncRNAs as compared to protein-coding genes. For example, a human-specific lncRNA involved in smooth muscle cell 121 and endothelial 122 cell differentiation cannot be modeled in a mouse and thus would require 3c CRISPR editing in a human vascular cell line. On the other hand, Braveheart, a mouse-specific lncRNA involved in cardiomyocyte differentiation,123 represents an attractive lncRNA for CRISPR editing in the mouse. Given our primitive understanding of the functionality of transcribed non-coding sequences, the approach to 3c CRISPR may not be as intuitive as with protein-coding genes. One idea would be injecting an HDR template carrying a subtle edit at a splice donor/acceptor site to disrupt normal splicing. Alternatively, one could introduce a polyadenylation signal cassette in the first exon of the lncRNA thereby generating a truncated transcript due to premature termination of transcription. The latter would require a double-strand DNA HDR template.
Historically, evaluating loss of a regulatory region (e.g., enhancer) on target gene expression in mice utilized Cre/loxP technology leaving a ‘genomic cavity.’ More subtle approaches that generated mice in which the phasing of the DNA sequence was preserved through base pair substitutions showed the importance of regulatory elements in target gene expression; however, these studies are few in number and still had the issue of removing a selectable marker with a retained loxP site.124–126 On the surface, the confounding effects of Cre-mediated deletions or retention of a 34 bp loxP site may seem of trivial consequence. However, given the pervasive transcription of the mouse genome with many thousands of lncRNAs and the known effects of even small sequence additions to the genome following genetic modifications,127 it is best to make as subtle a nucleotide change as possible for optimal interpretations. Interestingly, out of a survey of 646 mutant mice in which subtle mutations were introduced, only 16 involved regulatory regions 128 and only one of these mutant mice carried the desired mutation without added or subtracted sequences.129 The latter was achieved with the labor intensive “hit and run” approach to genetic modification of the mouse genome, a technology that is rarely used today.128
Engineering mice with subtle edits to regulatory elements such as TFBS or microRNA binding sites requires advanced knowledge as to the functionality of the relevant sequence. At a minimum, traditional methods of analyzing regulatory sequences (e.g., mutagenesis in luciferase reporter, gel shift) should be performed before the generation of a CRISPR mouse. It may be wise to delete a larger segment of DNA (e.g., enhancer) first in the mouse with 2c CRISPR and then perform secondary experiments with 3c CRISPR that disrupt individual TFBS within the DNA segment. An obvious challenge exists with enhancers that have multiple TFBS; 100 ENCODE and SNP data will be a useful guide for prioritizing those elements of most interest. In addition, it is possible to engineer more than one edit in closely juxtaposed TFBS in the mouse. For example, we have recently generated a mouse derived from a SSO repair template in which two separable regulatory elements were mutated (unpublished). As of this writing, only one published study has reported the effects of subtle nucleotide substitutions in a TFBS using 3c CRISPR in mice. A serum response factor-binding CArG box located in the first intron of the Cnn1 gene was altered at three positions (CCT>GAC) and shown to nearly abolish expression of the mRNA and protein as well as in vivo serum response factor binding to the mutated element.130 This result is somewhat surprising given the large number of regulatory sequences over target gene loci in the mouse genome. 3c CRISPR studies that assess the role of single regulatory elements in the mouse offer definitive proof of function and will inform the nature of regulatory SNPs associated with human disease.
Generating Conditional Alleles in Mice
A limitation of CRISPR editing in mice is that all cells will carry the sequence change. On the one hand, if the edit is a heritable SNP associated with human disease, the mouse would more closely model the human condition. On the other hand, from an experimental perspective, elucidating the underlying cause of a phenotype will be challenging. Fortunately, it is possible to generate conditional alleles with loxP sites using 3c CRISPR and subsequently excise the intervening DNA with a suitable Cre mouse line.70 As with ESC-based gene targeting, it is crucial to carefully deliberate over where exactly the 34 base pair loxP sequences should be inserted using ENCODE and other experimental data.1 If possible, we find it best to ‘flox’ the promoter region and first coding exon to generate a true null allele following Cre-mediated excision. However, if an adjacent or overlapping antisense lncRNA is present near the first coding exon or an internal promoter exists, alternative plans will be necessary. We also recommend a floxed interval of DNA in the range of 1–2 kilobases in order to minimize the size of the Cre-mediated deletion and for ease in sequence validation of the edited mouse.
Several approaches exist for making a conditional mouse using 3c CRISPR. One method combines a sgRNA with Cas9n and a double-strand DNA donor template carrying both loxP sites.72 A second method involves the synthesis of two SSO of length no more than 180 nucleotides with the 34 base pair loxP sequence placed at the center of the SSO within 10 base pairs of the DSB (Supplemental Figure IV). This approach is essentially identical to one previously published.70 Because two loxP sequences must integrate on the same strand, the efficiency of generating a conditional allele with 3c CRISPR is low (see MIT Discussion Forum). The key to success is screening many pups (at least 40–50) with primers flanking each loxP site (Supplemental Figure IV). Both of the aforementioned methods have low success rates. A third approach, one we are currently evaluating, involves a two-step process wherein a mouse zygote is injected with sgRNA, Cas9, and one SSO containing one of the loxP sites. Pups are then PCR screened for proper integration of the loxP site (Supplemental Figure IV) and Sanger sequenced for loxP nucleotide integrity followed by breeding for germ line transmission of the first loxP site. Positive mice are then used for the expansion of zygotes and another round of microinjections using a second sgRNA and SSO carrying the other loxP sequence.
We wish to point out that while conditional mice can be generated using 3c CRISPR, we have noted a number of mutations and truncations of one or both loxP sequences following integration in the mouse. Whether these sequence changes stem from the initial commercial synthesis of the SSO or during HDR is unknown. Of note, several tolerable substitutions exist across the loxP sequence,131 and recent testing in our lab has confirmed Cre-mediated excision of a floxed segment of DNA in a mouse, despite the presence of a single base pair deletion in position 3 of the loxP sequence. A comprehensive summary of all tolerable changes to a loxP sequence would be a helpful resource for the mouse genetics community.
Finally, it is possible to limit gene inactivation to specific cardiovascular cell types without expending the time and effort in generating a floxed protein-coding or non-coding gene. For example, the Rosa26 floxed stop Cas9 transgenic mouse (https://www.jax.org/strain/024857)38 could be crossed with a cell-specific and inducible Cre-driver mouse resulting in restricted Cas9 availability to the cardiovascular cell of interest. Delivery of sgRNA and HDR template to the heart or vasculature may be achieved using emerging viral and non-viral modes of transfer.132, 133 The approach here, however, is complicated by the lack of Cas9 expression in all cells of interest due to inefficient Cre activity; persistent Cas9 expression in those cells where Cre excised the floxed stop cassette; ineffective delivery of sgRNA/HDR template to vascular cells with adeno-associated virus;134 and possible inflammatory response with use of adenoviral vectors.
Generating Epitope-Tagged Mice
A persistent problem in biomedical research is the ineffective or inconsistent performance of an antibody for the detection of a protein in cultured cells, a Western blot, or a tissue section. Indeed, the recent National Institutes of Health notice on Scientific Rigor and Transparency (NOT-OD-16-011) will require assurances that a specific antibody will perform in the anticipated manner. An exciting application of 3c CRISPR is integrating a synthetic peptide (e.g., 3xFLAG) to which a well-characterized antibody exists into an endogenous protein-coding gene. As with loxP sequences, these small peptides are small enough that they may be engineered within a SSO template as close to the DSB as possible. The most important considerations in epitope tagging an endogenous protein-coding gene are the nature of the tag and whether the tag should be placed at the 5′ or 3′ end of the gene. We find that the 3xFLAG and HA tags yield the most specific results, at least in cultured cells.
The generation of an epitope tagged protein with 3c CRISPR has a number of useful applications beyond simple expression analysis under normal and pathological conditions. For example, if the tagged protein is a transcription factor, in vivo chromatin immunoprecipitation-sequence analysis may be done in the heart or vasculature to assess genome-wide in vivo occupancy of a TFBS. This can be very profitable when the TFBS is well characterized.89 In addition, pulldown assays may be done to define interacting partners of the protein or to elucidate post-translational modifications. If the tagged protein undergoes nucleocytoplasmic shuttling or is secreted out of the cell, studying the biology of these and other processes may be done in an unambiguous fashion. Furthermore, tagged proteins can be directly purified from primary cells by immunoprecipitation and used for functional characterization (e.g., enzymatic activity).
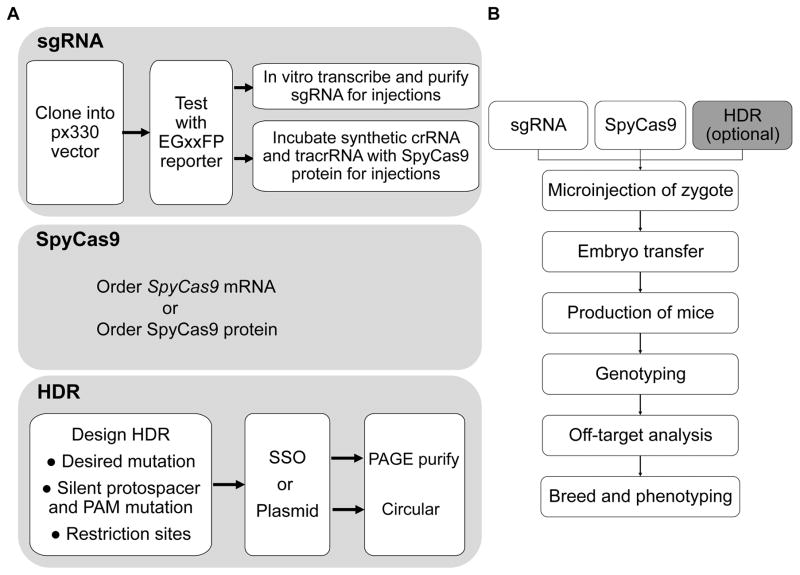
In summary, 3c CRISPR is the most versatile mode of genome editing with wide applications in the mouse. Though the efficiency (~10%) is much lower than that observed with 2c CRISPR (~50%), the strength of precision-guided genome editing afforded by 3c CRISPR makes it a very attractive approach for modeling human diseases and elucidating the function of coding and non-coding sequences in the mouse. Over the last two years, we have generated ten mouse models carrying a variety of edits in protein-coding and non-coding sequences as well as the incorporation of loxP sites and an epitope tag. The formula we follow for 3c CRISPR in mice is the one first described by the Jaenisch lab.28 Recently, a cloning-free method of preassembled Cas9 protein and commercially synthesized crRNA and tracrRNA along with SSO repair template was reported to exhibit superior genome editing rates.135 Time will tell whether this approach to CRISPR-mediated NDGE will supplant original methods of injecting Cas9 mRNA, sgRNA, and HDR donor template. A practical summary of the key steps to follow for 2c CRISPR and 3c CRISPR in mice (Figure 4) is provided in the supplement (Supplemental Method 2). The work associated with CRISPR genome editing does not end with the birth of live-born mice. There are three important issues to be carefully considered once founder mice are obtained and these are summarized next.
Figure 4. Work flow for CRISPR genome editing in mice.
(A) Each CRISPR component is indicated in a gray rectangle. The sgRNA requires careful design and cloning into the pX330 vector followed by testing in the EGxxFP split reporter to validate its activity (see Supplemental Method 1). Verification of activity is followed either by in vitro transcription of chimeric sgRNA with T7 polymerase and purification of the sgRNA for injection with SpyCas9 mRNA or, less frequently, SpyCas9 protein (present approach), or independent synthesis of the crRNA and tracrRNA and preincubation with SpyCas9 protein (emerging approach). (B) General workflow of operations in generating a CRISPR edited mouse line. See text for more details.
Genetic Mosaicism
Genetic mosaicism represents cell-cell variability in genotype wherein some cells exhibit no genome modification (wild type) and others show expected or unexpected genome modifications. In ESC-based genome modification, there are generally only two possible outcomes across a population of cells in a newborn (founder) mouse: cells that carry only wild type alleles or cells carrying an allele with a specific genomic modification derived from the engineered ESC. The relative distribution of each cell type is unpredictable because ESC carrying a genome modification are microinjected into the blastocyst of a mouse embryo (~3.5 days of development) and admix with existing (wild type) stem cells comprising the inner cell mass. Thus, it is not unusual in this scenario to have a modified allele in a founder mouse incapable of being transmitted through the germline to the Filial 1 generation. Moreover, even if the modification is passed on to the next generation, the resulting mouse is considered to be a “chimera” due to frequent differences in the strain of microinjected ESC versus the ESC of the inner cell mass in the recipient mouse. The latter often necessitates considerable back-cross breeding to generate a near homogenous strain of mouse for unambiguous assessment of the genome modification.
Genetic heterogeneity in CRISPR-derived mice is quite different and has its own advantages and challenges. On the one hand, chimerism is a non-issue because there is no admixing of different strains of ESC. Moreover, microinjections may be done in C57BL/6J zygotes, a common strain among knockout mouse models. In this case extensive back-crossing is avoided. Further, since editing occurs early in embryonic development, there is a high likelihood of an edited allele being transmitted to the next generation; indeed, we have yet to experience an edited allele not undergoing germline transmission. On the other hand, because of the competition between the unpredictable genome editing of 2c CRISPR and the more precision-guided editing of 3c CRISPR, as well as the prolonged editing that presumably occurs following zygotic division in either 2c CRISPR or 3c CRISPR, the number of different alleles even in a single founder mouse can be considerable (Supplemental Figure V).78, 136 In fact, there is diversity of alleles both within and between founder pups (Supplemental Figures IV and VIB). This presents a challenge in sorting through the different alleles across cells in tail snips or other tissues prepared for genotyping in order to clearly define whether the desirable genome modification exists. Fortunately, there are discriminating methods of genotyping CRISPR-derived mice and we turn to this subject next.
Genotyping of CRISPR Mice
Most methods of genotyping CRISPR mice are based on the judicious design of PCR primers and the validation of genome edits via Sanger sequencing of cloned PCR amplicons. Recently, a digital PCR-based assay was reported for high resolution determination of specific edits in various cell culture systems.50 In some cases, such as the integration of donor templates with long homology arms, Southern blotting may be needed to ensure proper genome editing. Below, we describe simple PCR-based assays, including one that often identifies single base pair edits introduced with 3c CRISPR.
Genotyping 2c CRISPR Mice
The genotyping of 2c CRISPR founder mice derived from one or more closely spaced sgRNAs across a coding exon requires PCR primers flanking the DSB(s) by ~200 nucleotides. Note that this approach will not detect very large deletions.137 However, indels in the range of 35–100 nucleotides can easily be resolved in a 2% agarose gel (Supplemental Figure VIIa–VIIb). The PCR products may then be excised from the gel, column purified (Qiagen), and cloned directly into a plasmid (TOPO PCR cloning kit) for Sanger sequence analysis. Since there likely will be multiple alleles due to mosaicism, we recommend sequencing at least 4–6 clones per founder mouse. Once a desired allele is defined, the founder mouse should be bred for germline transmission of the edited allele in accordance with Mendel’s first law of allelic segregation during gamete formation. Depending on the nature of the edit, a new and more discriminating PCR primer may be synthesized to differentiate wild type from heterozygous and homozygous mutant pups (i.e., making the 3′ most nucleotides of primer specific to the indel or substitution).
The genotyping of deleted exons, enhancers or other non-coding sequences with two sgRNAs is somewhat easier since the deleted sequence can be distinguished from the wild type sequence on a 1% agarose gel (Supplemental Figure VIIB). The PCR primers should be designed 100–200 nucleotides outside the two DSBs flanking the segment to be deleted. Once germ line transmission occurs, it may be necessary to design a third primer to the deleted sequence in order to differentiate the different genotypes arising from heterozygous interbreeding (Supplemental Figure VIIB).
Genotyping 3c CRISPR Mice
There are two general approaches to genotyping founder mice derived from 3c CRISPR genome editing. One involves the design of flanking primers outside a precision-guided insertion (e.g., loxP or 3xFLAG) followed by resolution on a 2% agarose gel (Supplemental Figures IV and VIa). However, this approach is inadequate for subtle genome edits such as point mutations. Although introducing a novel restriction site in the SSO repair template may be done for genotyping slight edits, this approach requires PCR amplification and restriction digestion. Thus, we developed a novel multiplex PCR assay to differentiate wild type from mutant genotypes in mice carrying minor substitutions in DNA, including single base pair changes (130 and unpublished). In this scenario, two forward primers are used: one forward primer of ~50 nucleotides contains a 5′ extension of ~30 nucleotides of sequence unrelated to anything in the mouse genome, followed by 20 nucleotides matching the wild type genome; and another forward primer that does not have an extended 5′ end but is identical to the 20 nucleotide core sequence save the desired substitution(s) located at the terminal 3′ end of the primer. The two forward primers are combined with a common reverse primer in a PCR reaction and the products are run on a 2–2.5% agarose gel (Supplemental Figure VIIC).130 This PCR approach requires some front-end work, including the design of both wild type and mutant templates in a plasmid for pre-testing of the primers and optimization of the PCR parameters (Supplemental Method 3). We recommend following this procedure as we have had much success in using this robust genotyping strategy, including detection of a SNP. It is also possible to design a forward primer that anneals specifically to an inserted sequence (e.g., 3xFLAG). We recently found this approach to be quite helpful in filtering down a high number of edited mice (Supplemental Figure VIB–VIC). As with 2c CRISPR, Sanger sequencing amplified DNA around the edited region should be performed, especially with inserts such as loxP and epitope tags where initial PCR genotyping may suggest successful integration, but unacceptable mutations may be present (see sequencing data in Supplemental Figure VID).
Inadvertent Targeting
A limitation of CRISPR genome editing with wild type Cas9 is inadvertent targeting of distal sites sharing sequence complementarity to the crRNA, especially in the seed sequence of the PAM proximal region (Figure 2A). This is of major concern when using primary or stable cell lines where such off targeting is indelible. For this and other reasons (above), we do not advocate the generation of CRISPR edited cell culture models unless it is absolutely necessary (e.g., human inducible pluripotent stem cells or mouse ESC, both of which exhibit low levels of inadvertent targeting138). Inadvertent targeting is less problematic in the mouse genome because of Mendel’s second law of independent assortment of alleles during gametogenesis, assuming the off target edit is not in linkage disequilibrium with the intended target. Nonetheless, there are a number of ways to mitigate CRISPR off targeting in the mouse. For example, select a crRNA with little to no homology to coding exons or functional non-coding sequences. If point mutations are planned using 3c CRISPR, then the reduced sequence landscape may limit options for choosing an ideal crRNA. In addition, shortening the crRNA sequence at the 5′ end from 20 to 17–18 nucleotides may reduce inadvertent targeting that otherwise would occur with full-length crRNA.139 In addition, several derivatives of Cas9 exist that exhibit low off targeting including Cas9n,48 esCas9,51 and Cas9-HF1.52 Cas9n is cumbersome and less efficient than its wild type counterpart; however, the latter two Cas9 nucleases are likely more efficient since mutations are in the PAM interacting domain and not the nuclease domains. Neither esCas9 nor Cas9-HF1 has yet been tested for generating CRISPR mice. It may also be desirable to inject Cas9 protein with sgRNA and HDR template to limit inadvertent targeting. Some cores inject lower concentrations of each CRISPR component; however, there is no consensus as to the efficacy of this approach in lowering inadvertent targeting.
Whichever measures are taken to minimize off-targeting at the front-end, because each sgRNA and its intended target site will be unique and have variable efficiencies in terms of chromatin accessibility, assessing inadvertent genome editing at the back-end will be essential and journal/grant reviewers will request that this issue be addressed in some manner. A minimum requirement is to identify related sequences in the mouse genome, particularly those occurring in exons or non-coding sequences with potential function, using a number of computational tools.7 Potential inadvertent target sequences (~10 based on computer analysis) are then PCR-cloned and subjected to Sanger sequencing to ascertain any indels/substitutions. A number of sophisticated methods exist for interrogating the genome for inadvertent edits (e.g., whole genome sequencing),7 but these are impractical for animal studies and are mainly used in cell culture models that demand a more thorough analysis for off-targeting events.
Clinical Perspectives for CRISPR Genome Editing
Genome editing has already made its way into the clinic,140 so the application of CRISPR editing to prevent or treat some aspects of cardiovascular disease may soon be realized. The most direct approach will be 2c CRISPR inactivation of a gene expressed in the liver whose product exacerbates a cardiovascular disease. One such therapeutic target is proprotein convertase subtilisin/kexin type 9 (PCSK9), a protease that cleaves the LDL receptor, thereby decreasing LDL receptor surface density and increasing plasma LDL levels, which can lead to atherosclerosis and myocardial infarction.141 As proof of principle, delivery of Cas9 and a sgRNA targeting PCSK9 to a humanized mouse liver chimera effectively reduced circulating levels of human PCSK9 protein without observable toxicity.142 Similar targeting of mouse Pcsk9 with adeno-associated virus carrying a smaller version of Cas9 and a sgRNA has been reported.45
An equally exciting approach will be using 3c CRISPR to correct individual mutations in genes that cause inherited cardiovascular diseases. For example, genetic disorders associated with life threatening arrhythmias such as the Long QT Syndrome could be treated by 3c CRISPR genome correction of such mutated genes as KCNQ1, KCNH2, and SCN5A.143 Inherited cardiomyopathies due to mutations in titin or myosin binding protein c may also be amenable to 3c CRISPR genome editing.115 In the clinical realm of hemostasis and thrombosis, 3c CRISPR could be used to treat patients with hemophilia A caused by mutations in the F8 gene encoding factor VIII or hemophilia B caused by mutations in F9 encoding Factor IX.144
CRISPR genome editing offers great advantages over other forms of genetic therapy: CRISPR is rapid, specific, and permanent. However, extensive technical, financial, and ethical challenges remain before CRISPR-based therapies enter the clinic. For example, CRISPR components must be effectively delivered to the correct organ and cell, perhaps using emerging nanotechnologies.133 There will also be a lot of genome editing work done in patient-derived inducible pluripotent stem cells that could be engineered to differentiate to correct cell types and then deployed on biocompatible grafts. Of course, CRISPR genome editing must be efficient and off target effects must be minimized with the latest generation of Cas9 endonucleases.51, 52 Given these constraints, the first cardiovascular diseases to be treated with CRISPR-Cas9 may be autosomal diseases caused by genes such as PCSK9 or F9 that are expressed in the liver.
Finally, enormous practical and ethical challenges must be overcome before CRISPR genome editing is used to correct heritable cardiovascular diseases in human embryos generated by in vitro fertilization. A highly publicized and controversial paper used 3c CRISPR in polyspermic human zygotes to edit the HBB locus that encodes beta-globin, a gene with multiple mutations underlying beta thalassemias.145 The team found that genome targeting was inefficient and not very specific. This paper sparked much debate over the ethics of genome editing in the human germline,146, 147 and an international meeting that included the National Academy of Sciences in the USA recommended a moratorium on genome editing of human embryos until the risks are assessed and society agrees on the appropriate use of CRISPR and other powerful genome editing techniques.148 Recently, however, regulators in the United Kingdom have given permission for scientists to edit genomes of human embryos.149
The path of CRISPR genome editing is paved well for exciting discoveries, innovative technologies, and new hopes for treating some forms of human disease. To date, only a small handful of CRISPR-mediated NDGE studies related to cardiovascular-related genes have been conducted in mice.37, 77, 114, 130, 142 However, the ‘CRISPR Craze’ will continue to inspire more and more investigators to engineer new mouse strains for a variety of scientific endeavors, from elucidating functional coding and non-coding sequences and modeling human cardiac and vascular disorders to assessing the safety and efficacy of novel CRISPR-based therapies80, 142 for the prevention and treatment of cardiovascular diseases.
Supplementary Material
Highlights.
CRISPR-Cas9 is a facile and highly efficient genome editing technology that essentially supplants traditional embryonic stem cell-based approaches to genome modification in the mouse.
The three components to CRISPR technology are the Cas9 endonuclease, a single (chimeric) guide RNA, and a homology directed repair template for precision-guided genome editing.
CRISPR technology offers an investigator a wide range of genome editing options, from deletions and frameshift mutations (using 2 component CRISPR) to nucleotide replacement editing and integration of small sequence tags (using 3 component CRISPR).
Robust genotyping assays can discriminate desired edits among multiple alleles stemming from genetic mosaicism while inadvertent (off) targeting is addressed with carefully planned front-end strategies in CRISPR design followed by back-end Sanger sequencing of possible off-target sites and proper back-crossing of mice to segregate out any unintended sequence edits.
CRISPR genome editing presents some exciting opportunities to combat cardiovascular diseases with a genetic basis.
Acknowledgments
We wish to thank the following CRISPR mouse cores for performing mouse zygote microinjections: University of Rochester School of Medicine and Dentistry; Cornell University supported in part by Empire State Stem Cell Fund Contract Number C024174); and the University of Nevada Reno. We also thank the MIT CRISPR Discussion Forum (https://groups.google.com/forum/!forum/crispr) to which we have both contributed and obtained technical information pertaining to CRISPR over the last two and one-half years.
Sources of Funding
CRISPR work is supported by National Institutes of Health grants HL-112793 and HL-117907 to JMM and HL-124042 and American Heart Association grant 14GRNT19020033 to CJL.
Abbreviations
- 2c CRISPR
2 component CRISPR
- 3c CRISPR
3 component CRISPR
- CRISPR
clustered regularly interspaced short palindromic repeats
- Cas9
CRISPR-associated protein 9
- crRNA
CRISPR RNA
- DSB
double-strand break
- DSD
double-strand DNA
- ESC
embryonic stem cell
- HDR
homology directed repair
- indel
insertion or deletion
- lncRNA
long non-coding RNA
- NDGE
nuclease-directed genome editing
- NHEJ
non-homologous end joining
- PAM
protospacer adjacent motif
- PTC
premature termination codon
- sgRNA
single guide RNA
- SNP
single nucleotide polymorphism
- SSO
single-strand oligonucleotide
- tracrRNA
transactivating crRNA
- TFBS
transcription factor binding site
Footnotes
Disclosure
None.
Bibliography
- 1.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: A user’s guide for genetically manipulating the mouse in cardiac research. Circ Res. 2012;111:761–777. doi: 10.1161/CIRCRESAHA.111.262717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood AJ, Lo TW, Zeitler B, et al. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 7.Bolukbasi MF, Gupta A, Wolfe SA. Creating and evaluating accurate CRISPR-Cas9 scalpels for genomic surgery. Nat Methods. 2015;13:41–50. doi: 10.1038/nmeth.3684. [DOI] [PubMed] [Google Scholar]
- 8.Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojica FJ, Diez-Villasenor C, Soria E, Juez G. Biological significance of a family of regularly spaced repeats in the genomes of archaea, bacteria and mitochondria. Mol Microbiol. 2000;36:244–246. doi: 10.1046/j.1365-2958.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 10.Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 11.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 12.Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 13.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 14.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 16.van der Oost J, Westra ER, Jackson RN, Wiedenheft B. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nature reviews Microbiology. 2014;12:479–492. doi: 10.1038/nrmicro3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marraffini LA. CRISPR-Cas immunity in prokaryotes. Nature. 2015;526:55–61. doi: 10.1038/nature15386. [DOI] [PubMed] [Google Scholar]
- 18.Sontheimer EJ, Barrangou R. The bacterial origins of the CRISPR genome-editing revolution. Hum Gene Ther. 2015;26:413–424. doi: 10.1089/hum.2015.091. [DOI] [PubMed] [Google Scholar]
- 19.Pennisi E. The CRISPR craze. Science. 2013;341:833–836. doi: 10.1126/science.341.6148.833. [DOI] [PubMed] [Google Scholar]
- 20.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, Zhang X, Zhang P, Huang X. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–723. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. Onestep generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O’Connor-Giles KM. Genome engineering of drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA. Heritable genome editing in c. Elegans via a CRISPR-Cas9 system. Nat Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Teng F, Li T, Zhou Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat Biotechnol. 2013;31:684–686. doi: 10.1038/nbt.2652. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Mol Cell Biol. 2014;6:97–99. doi: 10.1093/jmcb/mjt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu Y, Shen B, Cui Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K, Zhang F, Koonin EV. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell. 2015;60:385–397. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. doi: 10.1038/srep03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll KJ, Makarewich CA, McAnally J, Anderson DM, Zentilin L, Liu N, Giacca M, Bassel-Duby R, Olson EN. A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc Natl Acad Sci U S A. 2016;113:338–343. doi: 10.1073/pnas.1523918113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt RJ, Chen S, Zhou Y, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156:935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinek M, Jiang F, Taylor DW, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadan AH, Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 43.Jiang F, Doudna JA. The structural biology of CRISPR-Cas systems. Curr Opin Struct Biol. 2015;30:100–111. doi: 10.1016/j.sbi.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, Gonzales AP, Li Z, Peterson RT, Yeh JJ, Aryee MJ, Joung JK. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015 doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinstiver BP, Prew MS, Tsai SQ, Nguyen NT, Topkar VV, Zheng Z, Joung JK. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol. 2015 doi: 10.1038/nbt.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merkle FT, Neuhausser WM, Santos D, Valen E, Gagnon JA, Maas K, Sandoe J, Schier AF, Eggan K. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell reports. 2015;11:875–883. doi: 10.1016/j.celrep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, Karlin-Neumann GA, Conklin BR. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci Rep. 2016;6:23549. doi: 10.1038/srep23549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2015 doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016 doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Russa MF, Qi LS. The new state of the art: Cas9 for gene activation and repression. Mol Cell Biol. 2015;35:3800–3809. doi: 10.1128/MCB.00512-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A. 2015;112:3002–3007. doi: 10.1073/pnas.1420024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight SC, Xie L, Deng W, Guglielmi B, Witkowsky LB, Bosanac L, Zhang ET, El Beheiry M, Masson JB, Dahan M, Liu Z, Doudna JA, Tjian R. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 56.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horlbeck MA, Witkowsky LB, Guglielmi B, Replogle JM, Gilbert LA, Villalta JE, Torigoe SE, Tijan R, Weissman JS. Nucleosomes impede Cas9 access to DNA in vivo and in vitro. eLife. 2016;5 doi: 10.7554/eLife.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Essletzbichler P, Konopka T, Santoro F, Chen D, Gapp BV, Kralovics R, Brummelkamp TR, Nijman SM, Burckstummer T. Megabase-scale deletion using CRISPR/Cas9 to generate a fully haploid human cell line. Genome Res. 2014;24:2059–2065. doi: 10.1101/gr.177220.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang P, Lin M, Pedrosa E, Hrabovsky A, Zhang Z, Guo W, Lachman HM, Zheng D. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Molecular autism. 2015;6:55. doi: 10.1186/s13229-015-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briner AE, Donohoue PD, Gomaa AA, Selle K, Slorach EM, Nye CH, Haurwitz RE, Beisel CL, May AP, Barrangou R. Guide RNA functional modules direct Cas9 activity and orthogonality. Mol Cell. 2014;56:333–339. doi: 10.1016/j.molcel.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, Jaenisch R, Zhang F, Sharp PA. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol. 2014;32:670–676. doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendel A, Bak RO, Clark JT, et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham DB, Root DE. Resources for the design of CRISPR gene editing experiments. Genome Biol. 2015;16:260. doi: 10.1186/s13059-015-0823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC, Rebar EJ. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol. 2010;649:247–256. doi: 10.1007/978-1-60761-753-2_15. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimi K, Kunihiro Y, Kaneko T, Nagahora H, Voigt B, Mashimo T. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nature communications. 2016;7:10431. doi: 10.1038/ncomms10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renaud JB, Boix C, Charpentier M, et al. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell reports. 2016 doi: 10.1016/j.celrep.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Inui M, Miyado M, Igarashi M, Tamano M, Kubo A, Yamashita S, Asahara H, Fukami M, Takada S. Rapid generation of mouse models with defined point mutations by the CRISPR/Cas9 system. Sci Rep. 2014;4:5396. doi: 10.1038/srep05396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bialk P, Rivera-Torres N, Strouse B, Kmiec EB. Regulation of gene editing activity directed by single-stranded oligonucleotides and CRISPR/Cas9 systems. PLoS One. 2015;10:e0129308. doi: 10.1371/journal.pone.0129308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang B, Li K, Wang A, Reiser M, Saunders T, Lockey RF, Wang JW. Highly efficient CRISPR/HDR-mediated knock-in for mouse embryonic stem cells and zygotes. Biotechniques. 2015;59:201–208. doi: 10.2144/000114339. [DOI] [PubMed] [Google Scholar]
- 72.Lee AY, Lloyd KC. Conditional targeting of Ispd using paired Cas9 nickase and a single DNA template in mice. FEBS open bio. 2014;4:637–642. doi: 10.1016/j.fob.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrezselyova S, Kinsky S, Truban D, Sedlacek R, Burtscher I, Lickert H. Homology arms of targeting vectors for gene insertions and Crispr/Cas9 technology: Size does not matter; quality control of targeted clones does. Cell Mol Biol Lett. 2015 doi: 10.1515/cmble-2015-0047. [DOI] [PubMed] [Google Scholar]
- 74.Perry KJ, Henry JQ. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis. 2015;53:237–244. doi: 10.1002/dvg.22843. [DOI] [PubMed] [Google Scholar]
- 75.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canver MC, Bauer DE, Dass A, Yien YY, Chung J, Masuda T, Maeda T, Paw BH, Orkin SH. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J Biol Chem. 2014;289:21312–21324. doi: 10.1074/jbc.M114.564625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, Cowan CA, Rader DJ, Musunuru K. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014;115:488–492. doi: 10.1161/CIRCRESAHA.115.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliver D, Yuan S, McSwiggin H, Yan W. Pervasive genotypic mosaicism in founder mice derived from genome editing through pronuclear injection. PLoS One. 2015;10:e0129457. doi: 10.1371/journal.pone.0129457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 80.Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, Olson EN. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, Asokan A, Zhang F, Duan D, Gersbach CA. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403–407. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Almomani R, Verhagen JM, Herkert JC, et al. Biallelic truncating mutations in ALPK3 cause severe pediatric cardiomyopathy. J Am Coll Cardiol. 2016;67:515–525. doi: 10.1016/j.jacc.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 83.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Streb JW, Kitchen CM, Gelman IH, Miano JM. Multiple promoters direct expression of three AKAP12 isoforms with distinct tissue and subcellular distribution profiles. J Biol Chem. 2004;279:56014–56023. doi: 10.1074/jbc.M408828200. [DOI] [PubMed] [Google Scholar]
- 85.Neznanov N, Umezawa A, Oshima RG. A regulatory element within a coding exon modulates keratin 18 gene expression in transgenic mice. J Biol Chem. 1997;272:27549–27557. doi: 10.1074/jbc.272.44.27549. [DOI] [PubMed] [Google Scholar]
- 86.Han J, Zhang J, Chen L, Shen B, Zhou J, Hu B, Du Y, Tate PH, Huang X, Zhang W. Efficient in vivo deletion of a large imprinted lncRNA by CRISPR/Cas9. RNA Biol. 2014;11:829–835. doi: 10.4161/rna.29624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miano JM, Long X. The short and long of noncoding sequences in the control of vascular cell phenotypes. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-1936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benson CC, Zhou Q, Long X, Miano JM. Identifying functional single nucleotide polymorphisms in the human CArGome. Physiol Genomics. 2011;43:1038–1048. doi: 10.1152/physiolgenomics.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller CL, Haas U, Diaz R, et al. Coronary heart disease-associated variation in tcf21 disrupts a mir-224 binding site and mirna-mediated regulation. PLoS Genet. 2014;10:e1004263. doi: 10.1371/journal.pgen.1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson R, Richter N, Bogu GK, Bhinge A, Teng SW, Choo SH, Andrieux LO, de Benedictis C, Jauch R, Stanton LW. A genome-wide screen for genetic variants that modify the recruitment of REST to its target genes. PLoS Genet. 2012;8:e1002624. doi: 10.1371/journal.pgen.1002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pennacchio LA, Ahituv N, Moses AM, et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature. 2006;444:499–502. doi: 10.1038/nature05295. [DOI] [PubMed] [Google Scholar]
- 93.Long X, Miano JM. Remote control of gene expression. J Biol Chem. 2007;282:15941–15945. doi: 10.1074/jbc.R700010200. [DOI] [PubMed] [Google Scholar]
- 94.Canver MC, Smith EC, Sher F, et al. Bcl11a enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192–197. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S, Sengel C, Emerson MM, Cepko CL. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev Cell. 2014;30:513–527. doi: 10.1016/j.devcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu J, Verzi MP, Robinson AS, Tang PL, Hua LL, Xu SM, Kwok PY, Black BL. Endothelin signaling activates Mef2c expression in the neural crest through a MEF2C-dependent positive-feedback transcriptional pathway. Development. 2015;142:2775–2780. doi: 10.1242/dev.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Metser G, Shin HY, Wang C, Yoo KH, Oh S, Villarino AV, O’Shea JJ, Kang K, Hennighausen L. An autoregulatory enhancer controls mammary-specific STAT5 functions. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Shou J, Guo Y, Tang Y, Wu Y, Jia Z, Zhai Y, Chen Z, Xu Q, Wu Q. Efficient inversions and duplications of mammalian regulatory DNA elements and gene clusters by CRISPR/Cas9. J Mol Cell Biol. 2015;7:284–298. doi: 10.1093/jmcb/mjv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meyer MB, Benkusky NA, Onal M, Pike JW. Selective regulation of Mmp13 by 1,25(oh)2d3, PTH, and Osterix through distal enhancers. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohmura S, Mizuno S, Oishi H, Ku CJ, Hermann M, Hosoya T, Takahashi S, Engel JD. Lineage-affiliated transcription factors bind the Gata3 Tce1 enhancer to mediate lineage-specific programs. J Clin Invest. 2016 doi: 10.1172/JCI83894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avellino R, Havermans M, Erpelinck C, et al. An autonomous CEBPA enhancer specific for myeloid-lineage priming and neutrophilic differentiation. Blood. 2016 doi: 10.1182/blood-2016-01-695759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seruggia D, Fernandez A, Cantero M, Pelczar P, Montoliu L. Functional validation of mouse tyrosinase non-coding regulatory DNA elements by CRISPR-Cas9-mediated mutagenesis. Nucleic Acids Res. 2015;43:4855–4867. doi: 10.1093/nar/gkv375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kraft K, Geuer S, Will AJ, et al. Deletions, inversions, duplications: Engineering of structural variants using CRISPR/Cas in mice. Cell reports. 2015 doi: 10.1016/j.celrep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Rivera CM, Ishii H, Jin F, Selvaraj S, Lee AY, Dixon JR, Ren B. CRISPR reveals a distal super-enhancer required for sox2 expression in mouse embryonic stem cells. PLoS One. 2014;9:e114485. doi: 10.1371/journal.pone.0114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao L, Tak YG, Berman BP, Farnham PJ. Functional annotation of colon cancer risk SNPs. Nature communications. 2014;5:5114. doi: 10.1038/ncomms6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dogan N, Wu W, Morrissey CS, Chen KB, Stonestrom A, Long M, Keller CA, Cheng Y, Jain D, Visel A, Pennacchio LA, Weiss MJ, Blobel GA, Hardison RC. Occupancy by key transcription factors is a more accurate predictor of enhancer activity than histone modifications or chromatin accessibility. Epigenetics & chromatin. 2015;8:16. doi: 10.1186/s13072-015-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tak YG, Hung Y, Yao L, Grimmer MR, Do A, Bhakta MS, O’Geen H, Segal DJ, Farnham PJ. Effects on the transcriptome upon deletion of a distal element cannot be predicted by the size of the H3K27Ac peak in human cells. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkv1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rajagopal N, Srinivasan S, Kooshesh K, Guo Y, Edwards MD, Banerjee B, Syed T, Emons BJ, Gifford DK, Sherwood RI. High-throughput mapping of regulatory DNA. Nat Biotechnol. 2016;34:167–174. doi: 10.1038/nbt.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist’s practical guide to CRISPR applications. Genetics. 2015;199:1–15. doi: 10.1534/genetics.114.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song J, Yang D, Xu J, Zhu T, Chen YE, Zhang J. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nature communications. 2016;7:10548. doi: 10.1038/ncomms10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin W, Dion SL, Kutny PM, Zhang Y, Cheng AW, Jillette NL, Malhotra A, Geurts AM, Chen YG, Wang H. Efficient CRISPR/Cas9-mediated genome editing in mice by zygote electroporation of nuclease. Genetics. 2015;200:423–430. doi: 10.1534/genetics.115.176594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, Yan Z, Li D, Li J. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell stem cell. 2013;13:659–662. doi: 10.1016/j.stem.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184–1188. doi: 10.1126/science.1254445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McNally EM, Barefield DY, Puckelwartz MJ. The genetic landscape of cardiomyopathy and its role in heart failure. Cell metabolism. 2015;21:174–182. doi: 10.1016/j.cmet.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol. 2016 doi: 10.1038/nbt.3481. [DOI] [PubMed] [Google Scholar]
- 117.Maquat LE. Nonsense-mediated mRNA decay: Splicing, translation and mrnp dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 118.Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JR, Meredith J, Stacey GN, Thraves P, Vias M Cancer Research UK. Guidelines for the use of cell lines in biomedical research. Br J Cancer. 2014;111:1021–1046. doi: 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang L, Yang JL, Byrne S, Pan J, Church GM. CRISPR/Cas9-directed genome editing of cultured cells. Curr Protoc Mol Biol. 2014;107:31 31 31–17. doi: 10.1002/0471142727.mb3101s107. [DOI] [PubMed] [Google Scholar]
- 120.Abrahimi P, Chang WG, Kluger MS, Qyang Y, Tellides G, Saltzman WM, Pober JS. Efficient gene disruption in cultured primary human endothelial cells by CRISPR/Cas9. Circ Res. 2015;117:121–128. doi: 10.1161/CIRCRESAHA.117.306290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, Miano JM. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol. 2014;34:1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boulberdaa M, Scott E, Ballantyne M, et al. A role for the long non-coding RNA SENCR in commitment and function of endothelial cells. Mol Ther. 2016 doi: 10.1038/mt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klattenhoff CA, Scheuermann JC, Surface LE, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tavner F, Frampton J, Watson RJ. Targeting an E2F site in the mouse genome prevents promoter silencing in quiescent and post-mitotic cells. Oncogene. 2007;26:2727–2735. doi: 10.1038/sj.onc.1210087. [DOI] [PubMed] [Google Scholar]
- 125.Boulende Sab A, Bouchard MF, Beland M, Prud’homme B, Souchkova O, Viger RS, Pilon N. An ebox element in the proximal Gata4 promoter is required for Gata4 expression in vivo. PLoS One. 2011;6:e29038. doi: 10.1371/journal.pone.0029038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: In vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 127.Meier ID, Bernreuther C, Tilling T, Neidhardt J, Wong YW, Schulze C, Streichert T, Schachner M. Short DNA sequences inserted for gene targeting can accidentally interfere with off-target gene expression. FASEB J. 2010;24:1714–1724. doi: 10.1096/fj.09-140749. [DOI] [PubMed] [Google Scholar]
- 128.Menke DB. Engineering subtle targeted mutations into the mouse genome. Genesis. 2013;51:605–618. doi: 10.1002/dvg.22422. [DOI] [PubMed] [Google Scholar]
- 129.Studer M, Gavalas A, Marshall H, Ariza-McNaughton L, Rijli FM, Chambon P, Krumlauf R. Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development. 1998;125:1025–1036. doi: 10.1242/dev.125.6.1025. [DOI] [PubMed] [Google Scholar]
- 130.Han Y, Slivano OJ, Christie CK, Cheng AW, Miano JM. CRISPR-Cas9 genome editing of a single regulatory element nearly abolishes target gene expression in mice. Arterioscler Thromb Vasc Biol. 2015;35:312–315. doi: 10.1161/ATVBAHA.114.305017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Santoro SW, Schultz PG. Directed evolution of the site specificity of cre recombinase. Proc Natl Acad Sci U S A. 2002;99:4185–4190. doi: 10.1073/pnas.022039799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li L, He ZY, Wei XW, Gao GP, Wei YQ. Challenges in CRISPR/cas9 delivery: Potential roles of nonviral vectors. Hum Gene Ther. 2015;26:452–462. doi: 10.1089/hum.2015.069. [DOI] [PubMed] [Google Scholar]
- 133.Yin H, Song CQ, Dorkin JR, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016 doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Denby L, Nicklin SA, Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- 135.Aida T, Chiyo K, Usami T, Ishikubo H, Imahashi R, Wada Y, Tanaka KF, Sakuma T, Yamamoto T, Tanaka K. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol. 2015;16:87. doi: 10.1186/s13059-015-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yen ST, Zhang M, Deng JM, Usman SJ, Smith CN, Parker-Thornburg J, Swinton PG, Martin JF, Behringer RR. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev Biol. 2014;393:3–9. doi: 10.1016/j.ydbio.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parikh BA, Beckman DL, Patel SJ, White JM, Yokoyama WM. Detailed phenotypic and molecular analyses of genetically modified mice generated by CRISPR-Cas9-mediated editing. PLoS One. 2015;10:e0116484. doi: 10.1371/journal.pone.0116484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan EP, Li Y, del Velasco-Herrera MC, Yusa K, Bradley A. Off-target assessment of CRISPR-Cas9 guiding RNAs in human iPS and mouse ES cells. Genesis. 2015;53:225–236. doi: 10.1002/dvg.22835. [DOI] [PubMed] [Google Scholar]
- 139.Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 142.Wang X, Raghavan A, Chen T, Qiao L, Zhang Y, Ding Q, Musunuru K. CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.307227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moss AJ, Kass RS. Long QT syndrome: From channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S, Kim JH, Kim DW, Kim JS. Functional correction of large factor VIII gene chromosomal inversions in Hemophilia A patient-derived iPSCs using CRISPR-Cas9. Cell stem cell. 2015;17:213–220. doi: 10.1016/j.stem.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 145.Liang P, Xu Y, Zhang X, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & cell. 2015;6:363–372. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Church G. Perspective: Encourage the innovators. Nature. 2015;528:S7. doi: 10.1038/528S7a. [DOI] [PubMed] [Google Scholar]
- 147.Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature. 2015;519:410–411. doi: 10.1038/519410a. [DOI] [PubMed] [Google Scholar]
- 148.Olson S, editor. International summit on human gene editing: A global discussion. Washington (DC): 2016. [PubMed] [Google Scholar]
- 149.Callaway E. UK scientists gain licence to edit genes in human embryos. Nature. 2016;530:18. doi: 10.1038/nature.2016.19270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.