Abstract
Objective
The c-Jun NH2-terminal kinases (JNK) are regulated by a wide variety of cellular stresses and have been implicated in apoptotic signaling. Macrophages express two JNK isoforms, JNK1 and JNK2, which may have different effects on cell survival and atherosclerosis.
Approach and Results
To dissect the impact of macrophage JNK1 and JNK2 on early atherosclerosis, Ldlr−/− mice were reconstituted with wild type (WT), Jnk1−/− and Jnk2−/− hematopoietic cells and fed a high-cholesterol diet. Jnk1−/−→Ldlr−/− mice have larger atherosclerotic lesions with more macrophages and fewer apoptotic cells than mice transplanted with WT or Jnk2−/− cells. Moreover, genetic ablation of JNK to a single allele (Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/−) in marrow of Ldlr−/− recipients further increased atherosclerosis compared to Jnk1−/−→Ldlr−/− and WT→Ldlr−/− mice. In mouse macrophages, anisomycin-mediated JNK signaling antagonized Akt activity, and loss of Jnk1 gene obliterated this effect. Similarly, pharmacological inhibition of JNK1, but not JNK2, markedly reduced the antagonizing effect of JNK on Akt activity. Prolonged JNK signaling in the setting of ER stress gradually extinguished Akt and Bad activity in WT cells with markedly less effects in Jnk1−/− macrophages, which were also more resistant to apoptosis. Consequently, anisomycin increased and JNK1 inhibitors suppressed ER stress-mediated apoptosis in macrophages. We also found that genetic and pharmacologic inhibition of phosphatase and tensin homolog (PTEN) abolished the JNK-mediated effects on Akt activity, indicating that PTEN mediates crosstalk between these pathways.
Conclusions
Loss of Jnk1, but not Jnk2, in macrophages protects them from apoptosis increasing cell survival and this accelerates early atherosclerosis.
Keywords: Atherosclerosis, Macrophages, JNK signaling, apoptosis, Akt pathway
Introduction
Macrophages play central roles in the development of atherosclerosis through modulation of cholesterol homeostasis, the immune-inflammatory response, and plaque cellularity 1. Macrophage activation and survival are crucial determinants of atherosclerotic lesion development 2. In addition, macrophages contribute to the integration of immune and metabolic responses, and their dysfunction contributes to chronic metabolic disorders such as obesity, type 2 diabetes and cardiovascular disease 3.
The c-Jun NH2-terminal kinases (JNK) belong to the stress-activated protein kinase family, which are activated by a variety of environmental (radiation, osmotic and redox stress), and metabolic stresses, cytokines, and growth factors4,5. JNK plays an important role in inflammatory signaling, and its activation is crucial for programmed cell death 6. In mammals, the JNK protein kinases are encoded by three genes: Jnk1, Jnk2 and Jnk3, which transcribe several alternatively spliced isoforms 7. Jnk1 and Jnk2 genes are expressed ubiquitously, whereas the Jnk3 gene is restricted to the brain, cardiac smooth muscle, pancreatic islets and testis4. The targeted disruption of the Jnk1 or Jnk2 genes revealed that they compensate for each other’s activity and are functionally redundant 8, but each isoform also exhibits distinct roles 9. For example, activation of CD8+ T cells is impaired in Jnk1 knockout mice but enhanced in Jnk2 null mice 10. Loss of Jnk1, but not Jnk2, suppresses obesity and improves insulin sensitivity in mice 11. JNK1, but not JNK2, activation plays an important role in the pathogenesis of insulin resistance 12–14. Examination of cell types involved in metabolic functions of JNK illustrated contributions from many stromal cell types including neuronal cells, adipocytes and hepatocytes 14, 15. Several studies also demonstrated the involvement of macrophage JNK activity at varying degrees in obesity and insulin resistance 8, 12, 14. Ricci et al. 16 have shown that apoE null (apoE−/−) mice lacking Jnk2 (apoE−/−/Jnk2−/− mice) develop less atherosclerosis than apoE−/− or apoE−/−/Jnk1−/− mice. The impact of loss of Jnk2 on atherosclerosis was attributed to reduced scavenger receptor A expression and foam cell formation by macrophages 16. However, the role of macrophage JNK isoforms on apoptosis in the setting of atherosclerosis was not assessed and additional studies are needed to evaluate the role of individual macrophage JNK isoforms in atherogenesis5.
JNK signaling has been implicated in apoptosis in response to a variety of stress stimuli 4, 6. Though both JNK1 and JNK2 are involved in apoptotic signaling, only JNK1 is considered to be essential for apoptosis17. Murine embryonic fibroblasts (MEF) lacking Jnk1, but not Jnk2, have reduced c-Jun phosphorylation and UV-induced cell death 18. Loss of both Jnk1 and Jnk2 in MEF produces a defect in death signaling and protects them from apoptosis 19. Interestingly, the role of JNK in apoptosis depends on the activity of other cellular signaling pathways, including the pro-survival phosphatidylinositol-3-kinase (PI3K/Akt) 20, 21. Aikin and coauthors 22 were the first to report cross-talk between the PI3K/Akt and JNK pathways that protects islet cells from apoptosis. In addition, Sunayama and co-workers 23 have shown that JNK signaling antagonizes Akt activity in mammalian cells making them more susceptible to apoptosis. Similarly, JNK inhibition significantly suppresses pancreatic β-cell death 24 and decreases macrophage apoptosis 25. Interestingly, phosphatase and tensin homolog (PTEN) may play a key role in the cross-talk between the PI3K/Akt and JNK pathways and PTEN deficiency impairs negative feedback regulation of PI3K in cancer cells 26. However, the precise role of JNK signaling in apoptosis depends on cell type and the nature of the death stimulus 6, 17. It is unclear whether JNK antagonizes Akt activity in mouse macrophages, or whether this cross-talk is mediated via PTEN with consequent suppression of cell survival that affects atherogenesis.
Here we used genetic loss-of-function and pharmacologic inhibition approaches to investigate the impact of JNK1 and JNK2 on Akt signaling in mouse macrophages and atherogenesis. Our data demonstrates the critical role of JNK1 signaling in macrophage apoptosis and development of early atherosclerosis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
JNK deficiency in hematopoietic cells increases early stage atherosclerotic lesions
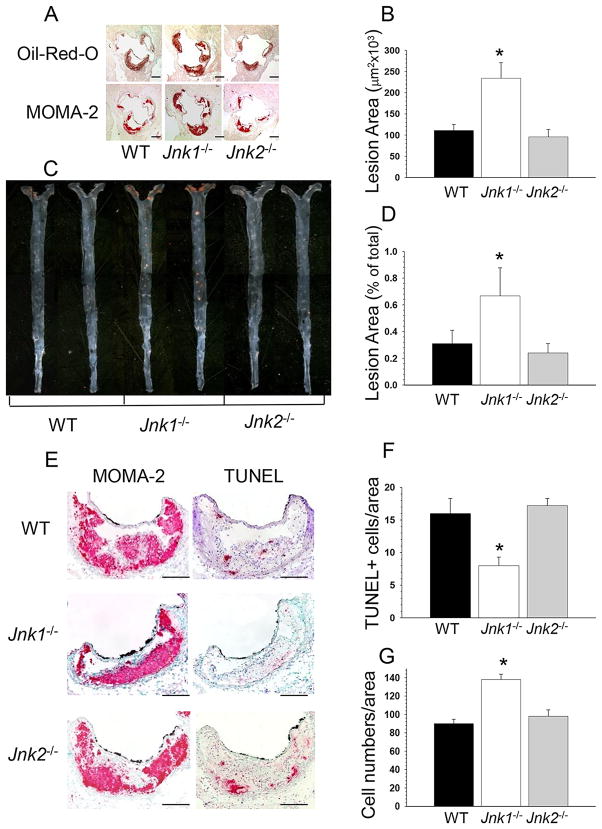
To examine the impact of hematopoietic cell Jnk1- and Jnk2-deficiency on atherosclerosis, 22-week-old male Ldlr−/− mice were lethally irradiated and transplanted with male wild type (WT; n=14), Jnk1−/− (n=11), or Jnk2−/− (n=13) bone marrow. After 4 weeks on a normal chow diet, mice were fed with the Western diet for another 8 weeks. No significant differences between the recipient groups were detected in body weight, serum total cholesterol and triglyceride levels on the chow and the Western diets (Table 1A). Size exclusion chromatography of serum revealed an accumulation of cholesterol in VLDL, LDL, IDL fractions in Ldlr−/− recipients with no differences between control and experimental groups in either experiment (data not shown). Mice reconstituted with WT, Jnk1−/− and Jnk2−/− marrow had similar levels of blood glucose (133.7±5.3, 139±6.8 and 137±6.7 mg/dl, respectively), erythrocytes (9.7±0.8, 9.9±1.1 and 9.7±0.9 ×106/μl), platelets (649±66, 679±73 and 613±61 ×103/μl) and white blood cells (7.8±06, 9.1±0.7 and 7.5±0.45 ×106/ml). In contrast, the extent of atherosclerotic lesions in aortic sinus of the Jnk1−/−→Ldlr−/− mice was markedly increased (Figure 1A,B) compared to mice reconstituted with WT or Jnk2−/− marrow cells (Figure 1B; 241.6±38.1 vs. 110.8±13.4 and 95.8±17.6 ×103μm2, respectively). Similarly, Jnk1−/−→Ldlr−/− mice had significantly increased size of atherosclerotic lesions in the distal aorta compared to WT→ Ldlr−/− and Jnk2−/−→ Ldlr−/− mice (Figure 1C,D; 0.67±0.22 vs. 0.31±0.10% and 0.24±0.07%, respectively),
Table 1.
Body weight (BW), total serum cholesterol (TC) and triglyceride (TG) levels in male Ldlr−/− mice reconstituted with WT, Jnk1−/−, Jnk2−/−, Jnk1+/−/Jnk2−/− and Jnk1−/−/Jnk2+/− hematopoietic cells on chow and high-fat diets
| Chow diet | High-fat diet | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Type of bone marrow reconstituted | BW (g) | TC (mg/dl) | TG (mg/dl) | BW (g) | TC (mg/dl) | TG (mg/dl) |
| A. | ||||||
| WT (n=14) | 29.3±0.6 | 242±11 | 125±4 | 32.3±0.7 | 1063±49 | 453±40 |
| Jnk1−/− (n=11) | 27.8±0.5 | 245±16 | 137±5 | 29.8±0.9 | 1030±74 | 472±79 |
| Jnk2−/− (n=13) | 28.0±0.8 | 213±13 | 135±6 | 29.9±0.4 | 1073±84 | 465±59 |
| p = | 0.21 | 0.20 | 0.15 | 0.75 | 0.44 | 0.90 |
|
| ||||||
| B. | ||||||
| WT (n=10) | 28.0±0.7 | 208±4 | 116±6 | 30.8±1.2 | 974±98 | 322±21 |
| Jnk1−/− (n=10) | 26.9±0.6 | 216±8 | 121±5 | 30.3±2.2 | 982±62 | 362±39 |
| Jnk1+/−/2−/−(n=13) | 27.3±0.9 | 218±9 | 118±3 | 29.7±1.1 | 955±52 | 334±13 |
| Jnk1−/−/2+/−(n=12) | 27.2±0.8 | 213±5 | 121±5 | 29.3±0.7 | 966±48 | 345±24 |
| p = | 0.66 | 0.75 | 0.12 | 0.33 | 0.99 | 0.72 |
Values are in mg/dl (Mean ± SEM). The number of recipient mice in each group is indicated by n. The differences are not statistically significant between the groups by One Way ANOVA.
Figure 1. Loss of Jnk1 in hematopoietic cells increases atherosclerosis.
(A,C) Detection of atherosclerotic lesions in the aortic sinus and aortas pinned out en face in WT→Ldlr−/−, Jnk1−/−→Ldlr−/− and Jnk2−/−→Ldlr−/− mice. Serial sections of the aortic sinus were stained with Oil-Red-O to detect neutral lipids or with the MOMA-2 antibody followed by biotinylated goat anti-rat IgG as the secondary antibody, avidin-biotin complex labeled with alkaline phosphatase and Fast Red TR/Naphthol AS-NX substrate to reveal macrophages. Aortas were pinned out and stained with Sudan IV. Scale bars, 200μm; a pin size, 10μm.
(B,D) The extent of atherosclerotic lesions in the proximal and distal aorta of Ldlr−/− mice reconstituted with WT(■), Jnk1−/−(□), or Jnk2−/−(■) bone marrow. Note, atherosclerotic lesions are bigger in Jnk1−/−→Ldlr−/− than in WT→Ldlr−/− and Jnk2−/−→Ldlr−/− mice. Graphs represent atherosclerotic lesion area (mean ± SEM) of the recipient Ldlr−/− mice (*p<0.05 compared to control group, WT→Ldlr−/− mice, by Kruskal-Wallis One Way Analysis of Variance on Ranks, Dunn’s Method).
(E) Detection of macrophages by staining with anti-MOMA-2 antibodies and apoptotic cells by TUNEL in serial sections of the aortic sinus. Scale bars, 50μm
(F,G) Percent of TUNEL+ cells (F) and DAPI-stained nucleus numbers in MOMA-2+ area (G) in atherosclerotic lesions of WT→Ldlr−/−, Jnk1−/−→Ldlr−/− and Jnk2−/−→Ldlr−/− mice (*p<0.05 compared to control group by One Way Analysis of Variance on Ranks).
Next, examination of the cellular composition of atherosclerotic lesions in the aortic sinus of recipients showed that the proportion of smooth muscle, T and B cells in atherosclerotic lesions did not differ significantly between the three groups (data not shown). The lesions predominantly consisted of macrophage-derived foam cells and Jnk1−/−→Ldlr−/− mice had significantly bigger lesion area stained with MOMA-2 versus WT→Ldlr−/− and Jnk2−/−→Ldlr−/− mice (Figure 1A; 167.1±29.4 vs. 82.4±10.3 and 76.4±4.6 ×103μm2, respectively). The analysis of serial aortic sections stained with MOMA-2 and terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL) revealed that Jnk1−/−→Ldlr−/− mice contained significantly fewer numbers of apoptotic cells in macrophage-rich areas of lesions than WT→Ldlr−/− and Jnk2−/−→Ldlr−/− mice (Figure 1E,F). Double staining of macrophages with MOMA-2 and cell nuclei with DAPI revealed increased (153%) numbers of nuclei per macrophage lesion area in Jnk1−/−→Ldlr−/− mice compared to lesions of WT→Ldlr−/− and Jnk2−/−→Ldlr−/− mice (Figure 1G). Together the data indicate that lack of Jnk1 in hematopoietic cells increases the burden of early atherosclerotic lesions in the absence of changes in plasma lipid or glucose levels. The dramatic increase of macrophage numbers together with reduced apoptosis in atherosclerotic lesions of Jnk1−/−→Ldlr−/− mice also suggested changes in viability of JNK1−/−macrophages in vivo.
Genetic ablation to a single JNK allele further increases atherosclerosis
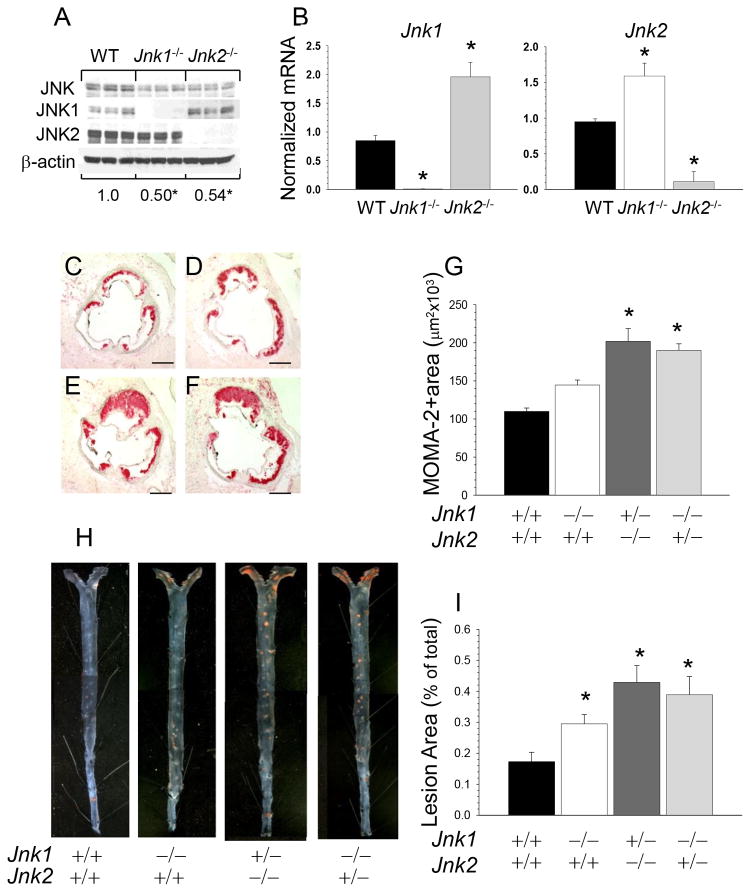
Peritoneal macrophages isolated from Jnk1−/−→Ldlr−/− and Jnk2−/−→Ldlr−/− mice exhibited a significant decrease in JNK protein content compared to WT cells (Figure 2A; 0.49±0.03 and 0.54±0.03 vs. 1.0±0.01, p<0.05 by One Way Analysis) and JNK kinase activity (Figure SI). They also had minimal residual expression of the knocked out isoform with compensatory increased expression of the other isoform (Figure 2B) indicating that maintaining total JNK activity is a vital for macrophages. Hence, to examine the impact of further genetic suppression of JNK signaling on atherosclerosis, we generated mice expressing a single allele of Jnk1 or Jnk2 in hematopoietic cells. Since the complete absence of both Jnk1 and Jnk2 causes early embryonic lethality, we intercrossed Jnk1+/−/Jnk2+/− mice and collected FLC. Then, seventeen-week-old male Ldlr−/− mice were lethally irradiated and reconstituted with male WT(n=10), Jnk1−/−(n=10), Jnk1+/−/Jnk2−/−(n=13) and Jnk1−/−/Jnk2+/−(n=12) FLC. Four weeks after transplantation, these mice were challenged with the Western diet for 8 weeks. Again, there were no differences between the recipient groups in body weight and plasma lipid levels either on the chow or the Western diets (Table 1B). Macrophages isolated from mice with a single JNK allele exhibited further decrease in JNK protein content compared to Jnk1−/− and WT cells (Figure SII). Remarkably, both Jnk1+/−/Jnk2−/−→Ldlr−/− and Jnk1−/−/Jnk2+/−→Ldlr−/− mice developed larger atherosclerotic lesions with increased macrophage MOMA-2-positive area in the proximal aorta (Figure 2E,F) than Jnk1−/−→Ldlr−/− and WT−/−→Ldlr−/− mice (Figure 2C,F,G; 183 and 172% vs. 131 and 100%, respectively). Similarly, the analysis of aorta en face demonstrated that these Jnk1+/−/Jnk2−/−→Ldlr−/− and Jnk1−/−/Jnk2+/−→Ldlr−/− mice had larger atherosclerotic lesions compared to Jnk1−/−→Ldlr−/− and WT−/−→Ldlr−/− mice (Figure 2H, I; 248 and 225% vs. 171 and 100%). Thus, genetic ablation of JNK to a single allele in hematopoietic cells resulted in further increases of atherosclerosis.
Figure 2. Genetic suppression of JNK signaling to a Jnk single allele further increases atherosclerosis.
(A) JNK protein contents in WT, Jnk1−/− and Jnk2−/− macrophages (n=3/group); Proteins were isolated and JNK protein contents were analyzed by western blot, the ratio of JNK/β-actin is presented compared to WT cells (*p<0.05 by One Way ANOVA analysis).
(B) Jnk1 or Jnk2 gene expression levels in peritoneal macrophages from mice reconstituted with WT(■), Jnk1−/−(□), or Jnk2−/−(■) FLC; mRNA levels were analyzed by real-time PCR. Graphs represent data (mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 by One Way ANOVA analysis).
(C–F) Detection of macrophages in the aortic sinus lesions of mice reconstituted with WT(C), Jnk1−/−(D), Jnk1+/−/Jnk2−/−(E) or Jnk1−/−/Jnk2+/−(F) FLC. Sections were stained with MOMA-2; Scale bars, 50μm.
(G) The extent of macrophage lesion area in the proximal aorta of mice reconstituted with WT(■), Jnk1−/−(□), Jnk1+/−/Jnk2−/−(■) or Jnk1−/−/Jnk2+/−(■) FLC (*p<0.05 by One way Analysis of Variance, multiple comparisons versus control group, Tukey Test).
(H) Atherosclerotic lesions in pinned out en face aorta of mice reconstituted with WT, Jnk1−/−, Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/− FLC; A pin size, 10μm.
(I) The extent of the atherosclerotic lesion area in Ldlr−/− mice reconstituted with WT, Jnk1, or Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/− FLC (*p<0.05 by Kruskal-Wallis One Way Analysis of Variance on Ranks, Dunn’s Method, versus control group, WT→Ldlr−/− mice).
JNK1 signaling antagonizes Akt activity in macrophages
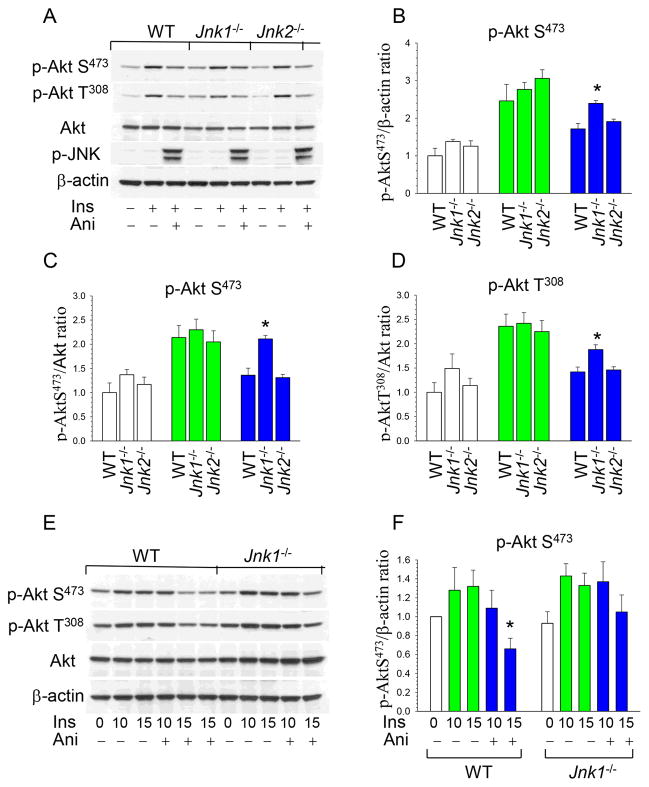
Next we investigated the mechanism(s) responsible for the increased macrophage numbers in atherosclerotic lesions of Jnk1−/−→Ldlr−/− mice by focusing on Akt signaling, which is crucial for cell survival 20. In macrophages Akt is constitutively activated, and inhibition of Akt signaling induces apoptosis 27, 28. In addition, a recent report demonstrated that JNK activity antagonizes Akt signaling in some types of cells 23. To examine whether JNK affects p-Akt in mouse macrophages, WT, Jnk1−/− and Jnk2−/− peritoneal macrophages were treated with insulin alone or together with anisomycin, a known activator of JNK signaling 23. Insulin significantly (2–3-fold) activated phosphorylation of both Akt sites (p-AktS473 and T308) in all types of cells (Figure 3A), whereas anisomycin suppressed Akt signaling activity in WT and Jnk2−/− macrophages, respectively, with no changes in total Akt or β-actin content (Figure 3A). Importantly, Jnk1−/− macrophages showed significantly less impact of JNK signaling on Akt activity than WT or Jnk2−/− cells (Figure 3B). The analysis of p-AktS473/Akt and p-Akt T308/Akt ratio in the same blot indicated a similar protective effect of Jnk1 deficiency compared to WT or Jnk2−/− cells (Figure 3C,D). Direct comparison of WT and Jnk1−/− macrophages treated with insulin and anisomycin demonstrated a statistically significant inhibitory effect of JNK signaling in the p-Akt/β-actin ratio of WT, but not Jnk1−/− macrophages (Figure 3E,F). Thus, JNK1 is the isoform primarily responsible for JNK-mediated inhibition of Akt signaling in macrophages.
Figure 3. JNK signaling antagonizes p-Akt activity and loss of JNK1 obliterated this effect.
(A) WT, Jnk1−/− and Jnk2−/− peritoneal macrophages were pre-incubated in serum-free media for 24 hours then untreated or treated with insulin (100nM) alone or together with anisomycin (10μg/ml) for 15min. Macrophage proteins were extracted, resolved by electrophoresis (50μg), and analyzed by Western blot.
(B–D) Ratio of p-AktS473/b-actin, p-AktS473/Akt and p-Akt T308/Akt in untreated (white color) or treated with insulin (green color) or insulin plus anisomycin (blue color). Graphs represent data (mean ± SEM) of three experiments (*p<0.05 by One Way Analysis of Variance on Rank compared to control WT cells treated with insulin together with anisomycin).
(E,F) WT and Jnk1−/− macrophages were treated with insulin alone (green color) or together with anisomycin (blue color) for 10 and 15 min. Graphs represent data (mean ± SEM) of three experiments (*p<0.05 by One Way Analysis of Variance on Rank compared to WT cells treated with insulin).
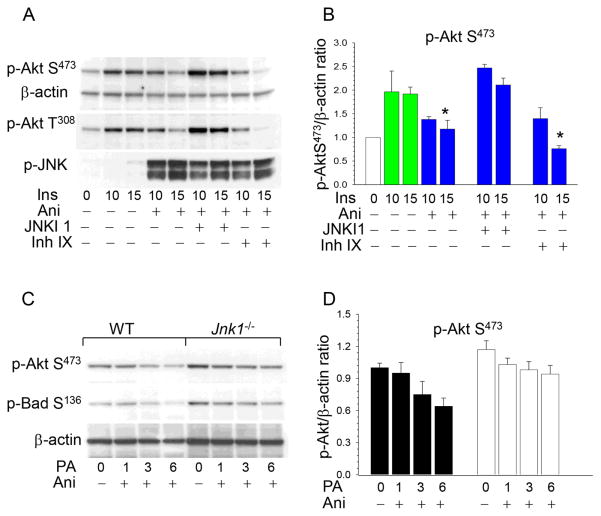
We also examined whether pharmacological inhibition of JNK can prevent the negative effects of JNK signaling on p-Akt. WT peritoneal macrophages were incubated with a mixture of insulin and anisomycin alone or in the presence of a JNK inhibitor. There was a 39% reduction of p-Akt levels in WT cells treated with anisomycin and a cell-permeable peptide inhibitor of JNK1, JNKI1, which preserved p-Akt levels in macrophages (Figure 4A,B). In contrast, treatment with a cell-permeable inhibitor IX, selective for JNK2 and JNK3 with little or no activity against JNK1, had no protective effects on Akt activity (Figure 4A,B). Taken together these data indicate that genetic ablation and pharmacological inhibition of JNK1, but not JNK2, eliminates the suppressive effects of JNK signaling on Akt activity.
Figure 4. The JNK1 inhibitor, JNKI1, preserves Akt signaling, and Jnk1−/− macrophages are more resistant to ER stress than WT cells.
(A,B) WT peritoneal macrophages were pre-incubated in serum-free media for 24 hours then treated with insulin alone (green color) or together with anisomycin (blue color) without or with the specific JNK inhibitor 1, JNKI1 (3μM) or specific JNK2 and JNK3 inhibitor, inhibitor IX (50nM) for indicated time. Macrophage proteins were extracted, resolved (60μg/well) and analyzed by Western blot with noted antibodies. Graphs represent data (mean ± SEM) of experiments with four mice/group (*p<0.05 compared to control WT cells treated with insulin for 15min by One Way Analysis of Variance on Ranks);
(C,D) WT(■) and Jnk1−/−(□)peritoneal macrophages were untreated or treated with 0.5mM PA-BSA and anisomycin (10μg/ml) for the indicated time. Graphs represent data (mean ± SEM) of three experiments.
JNK1 deficiency protects macrophages from apoptosis
JNK signaling has pro- or anti-apoptotic functions, depending on cell type, nature of the death stimulus, duration of its activation and the activity of other signaling pathways 17. Taking into consideration the critical role of Akt in cell survival 20, we suggested that sustained JNK activation (1–6 hours) may promote apoptosis by exhausting anti-apoptotic Akt signaling and by subsequently reducing Bad S136 phosphorylation, which normally serves to inhibit apoptosis in macrophages 27, 29. To test this hypothesis, we examined the impact of anisomycin on Akt signaling in WT and Jnk1−/− macrophages treated with palmitic acid (PA), a stress-mediated lipotoxic factor inducing ER stress and apoptosis30. The increased JNK signaling gradually suppressed p-Akt S473 in WT cells, whereas Jnk1−/− macrophages had higher p-Akt S473 levels and were more resistant to p-Akt suppression (Figure 4C,D). Similarly, the treatment progressively reduced p-Bad S136 levels in WT macrophages but there was less attenuation of p-Bad S136 in Jnk1−/− cells (Figure 4C). Thus, compared to WT cells, Jnk1−/− macrophages were able to preserve higher levels of Akt and Bad phosphorylation, which are important protective and anti-apoptotic factors under conditions of ER stress 31.
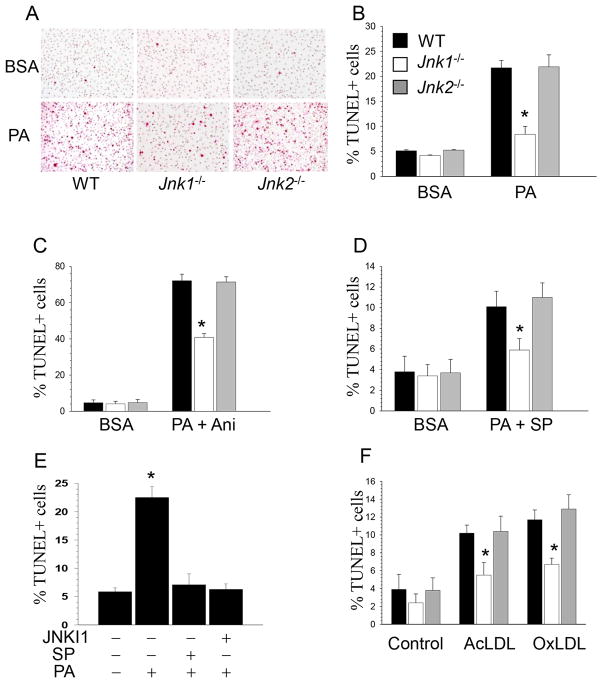
In addition, to define the role of JNK signaling in macrophage apoptosis, WT, Jnk1−/− and Jnk2−/− macrophages were treated with BSA or PA. Treatment with BSA generated only a few apoptotic TUNEL-positive (TUNEL+) cells with no differences between cell types, whereas PA increased TUNEL+ cells 4-fold in WT and Jnk2−/− macrophages but not in Jnk1−/− cells (Figure 5A,B). The addition of anisomycin markedly (3-fold) increased the percentage of TUNEL+ cells to a similar degree in WT and Jnk2−/− cells, whereas apoptosis was significantly reduced (57% of WT cells) in Jnk1−/− macrophages (Figure 5C). In contrast, the selective inhibitor JNKI1 significantly (2-fold) reduced apoptosis in all types of cells but Jnk1−/− macrophages had less apoptosis than WT and Jnk2−/− cells (Figure 5D). When WT macrophages were treated with the specific inhibitors of JNK, JNKI1 and SP600125, they demonstrated similar levels apoptosis (Figure 5E). Importantly, when cells were loaded with human oxidized or acetylated LDL in combination with an ACAT inhibitor, Jnk1−/− macrophages generated significantly less apoptosis than WT and Jnk2−/− cells (Figure 5F). In addition, macrophages expressing a single Akt isoform (Figure SIII), Akt1 (Akt2−/−/Akt3−/−) or Akt3 (Akt1−/−/Akt2−/−) PA-BSA treatment induced a stepwise increase in apoptosis that was especially high in Akt1−/−/Akt2−/− cells compared to WT cells. However, suppression of JNK signaling by the JNK inhibitor, SP600125, completely reversed the impact on cell survival with no differences between the groups (Figure SIV). Taken together our data indicate that JNK1 signaling regulates ER stress-mediated apoptosis in mouse macrophages and Jnk1−/− macrophages displayed clear resistance to apoptosis induced by different stimuli.
Figure 5. Jnk1−/− macrophages are protected from apoptosis and anisomycin increases, whereas JNK inhibition suppresses ER-mediated apoptosis.
(A) Detection of apoptosis in WT, Jnk1−/− and Jnk2−/− macrophages treated with BSA (control) and 0.5mM PA-BSA for 24 hours by TUNEL assay. Note TUNEL-positive cells (red), nuclei counterstained with Mayer hematoxylin;
(B) Percent of TUNEL+ WT, Jnk1−/− and Jnk2−/− macrophages treated with BSA or PA-BSA (*p<0.05 by One Way Analysis of Variance on Rank compared to WT cells treated with PA-BSA);
(C, D) Percent of TUNEL+ WT, Jnk1−/− and Jnk2−/− macrophages treated with BSA or PA-BSA together with anisomycin (10mg/ml) or the JNK inhibitor, JNKI1 (3μM) for 24 hours (*p<0.05 by One Way Analysis of Variance on Rank compared to WT cells treated with PA+Ani or PA+SP);
(E) Percent of TUNEL+ cells in WT macrophages untreated or treated with 0.5mM PA-BSA alone or together with JNK inhibitors, SP600125 (100nM) or JNKI1 (3μM) for 24 hours (*p<0.05 by One Way Analysis of Variance on Rank compared to untreated WT cells);
(F) Percent of TUNEL+ in WT, Jnk1−/− and Jnk2−/− macrophages untreated (control) or treated with human acetylated LDL (100μg/ml) in the presence of the ACAT inhibitor CP-113,818 (2μg/mL) or human oxidized LDL (100μg/ml) for 48 hours (*p<0.05 compared to control WT cells treated with Ac-LDL by One Way Analysis of Variance on Rank).
PTEN suppression impairs effects of JNK signaling on Akt activity
Recently Vivanco et al.26 have shown that JNK regulates p-Akt via PTEN, and Pten null mouse embryonic fibroblasts exhibit an impaired negative feedback loop. To test whether PTEN plays a critical role in regulating this pathway in mouse macrophages, WT and Pten−/− cells were treated with insulin alone or together with anisomycin. In contrast to WT cells, which showed increased p-Akt S473 in response to insulin and reduced p-Akt S473 after treatment with anisomycin, Pten−/− macrophages had markedly increased basal p-Akt, which was not suppressed in response to anisomycin (Figure 6A,B). Similarly, treatment with BpV(pic), a potent PTEN inhibitor, with an IC50 about 10–100 fold lower than for other tyrosine phosphatases 32, decreased the inhibitory effects of JNK on p-Akt (Figure 6C,D). Taken together these results indicate that both genetic ablation and pharmacologic inhibition of PTEN effectively eradicated JNK-mediated inhibition of Akt phosphorylation in mouse macrophages.
Figure 6. Genetic and pharmacologic inhibition of PTEN eradicates anisomycin-mediated suppression of p-Akt in macrophages.
(A,B) Akt signaling in WT and Pten−/− macrophages treated with insulin and anisomycin. Cells were pre-incubated with serum-free media for 16 hours, then untreated or treated with insulin alone (green color) or together with anisomycin (blue color) for the indicated time. Graphs represent data (Mean ± SEM) of three experiments (*p<0.05 between untreated and treated cells by One Way ANOVA analysis).
(C,D) PTEN inhibitor bpV(pig) preserves p-Akt signaling in WT peritoneal macrophages treated with anisomycin. Cells were pre-incubated in serum-free media for 24 hours and treated with insulin alone (green color) or with anisomycin (blue color) with or without bpV(pig) (0.1μM) for 15min. Graphs represent data (mean ± SEM) of two (*p<0.05 by One Way Analysis of Variance on Rank compared to cells treated with insulin).
Discussion
Numerous studies have linked macrophage or hematopoietic JNK1 activity to insulin resistance and abnormal glucose homeostasis in obesity12, 33–35. These studies targeting individual JNK isoforms have produced varying degrees of impact in different models, perhaps owing to interactions between isoforms and redundancies 8. In fact, a recent report using Jnk1-, Jnk2-combined deletion has shown that macrophage JNK promotes the establishment of obesity-induced insulin resistance and pancreatic islet dysfunction12. These findings suggest that macrophage JNK signaling may be crucial in other pathological conditions and warrants detailed studies of individual isoforms in cardiovascular disease models. Here, we examined the impact of Jnk1 or Jnk2 deficiency in hematopoietic cells on early stages of atherosclerosis using the Ldlr-deficiency model. Mice reconstituted with Jnk1−/− hematopoietic cells had significantly bigger atherosclerotic lesions compared to mice transplanted with WT or Jnk2−/− marrow with no differences in serum lipids. Genetic ablation to a single Jnk allele (either Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/−) in hematopoietic cells further increased atherosclerosis compared to Jnk1−/−→Ldlr−/− mice. We also found that JNK signaling antagonizes Akt activity in mouse macrophages acting mainly through JNK1. Therefore, Jnk1−/− macrophages had less suppression of p-Akt in response to sustained ER stress and were protected from apoptosis. Based on these data, we conclude that this resistance to apoptotic stimuli in Jnk1 null macrophages increases lesion burden at the early stages of atherogenesis.
JNK signaling is over-expressed and activated in atherosclerotic lesions of cholesterol-fed rabbits36. Considering the role of JNK in inflammatory and metabolic responses, it is plausible that this stress-mediated JNK activation may impact macrophage viability and atherosclerosis. In fact, Ricci et al. were the first to report the involvement of JNK2 in atherosclerosis16 showing that Jnk2−/−/apoE−/− mice developed less atherosclerosis compared to control apoE−/− and Jnk1−/−/apoE−/− mice. They analyzed a later stage of atherosclerosis with more severe lesions induced by a high-cholesterol (1.25%) diet for 14 weeks in total body JNK isoform deficiency in the apoE-deficienct model on a hybrid C57BL6/129SV background, whereas in the current study we explored early stage atherosclerosis using Ldlr−/− mice on C57BL/6 background reconstituted with hematopoietic cells null for JNK isoforms and fed with the Western diet (containing 21% milk fat and 0.15% cholesterol) for 8 weeks. The variation in genetic background of mice, stage-specific lesion burden, and Jnk deficiency in specific compartments are all important determinants of cholesterol absorption37 and susceptibility to atherosclerosis38, and they may underlie the apparent differences in our results.
In the current study, we observed a higher lesion burden in early atherosclerosis as a result of deficiency of Jnk1, but not Jnk2, in hematopoietic cells in the Ldlr null mice. Similar results were also obtained when combined deletion models (either Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/−) were used as donors to produce hematopoietic JNK-deficiency. These results may point to several possibilities. For example, it is possible that total JNK activity may be a more important determinant of the impact on macrophage apoptosis and atherogenesis than separate JNK isoforms. In the future, it would be highly informative to examine interactions between JNK isoforms in supporting total JNK activity in vivo. In this sense, our data are consistent with a recent report 39 indicating that loss of apoptosis signal-regulating kinase 1, which is upstream of JNK in certain contexts, in apoE null mice significantly reduced apoptosis and increased atherosclerosis by forming lesions enriched with macrophages. Due to the complexity of signaling upstream of JNK, multiple mechanisms may affect atherogenesis in a differential manner. For example, lack of mitogen-activated protein kinase phosphotase-1 protects apoE-null mice from atherosclerosis 40; whereas genetic deletion of Jnk1 reduces apoptosis in endothelial cells at atheroprone sites of the artery and thus diminishes atherosclerosis 41. Similarly, the administration of anisomycin via osmotic minipump increased apoptosis and decreased the macrophage content in atherosclerotic lesions of rabbits 42. In this scenario, prevention of macrophage death is likely a dominant feature of Jnk-deficiency, at least during early stages of atherosclerosis, supporting growth of vascular lesions enriched in macrophages. If this is the case, careful consideration of JNK’s role in atherosclerosis and how it could be best utilized for therapeutic intervention would be well warranted. It is however equally likely that Jnk1 deficiency and early preservation of macrophage death may yield favorable functional outcomes by ensuring plaque stability and preventing rupture, the predominant cause of morbidity and mortality due to atherosclerosis 43. In fact, this would be quite reminiscent of the role of certain endoplasmic reticulum stress responses that are also related to macrophage death 2. For example, CHOP-deficiency can prevent macrophage death and support the stability of vascular lesions and prevent rupture 44. Finally, it is possible that isolated examination of hematopoietic JNK activity only may have limitations and may not reflect the complete role of JNK in the pathogenesis of atherosclerosis. Future studies should dissect these possibilities in additional models.
Next, to identify the mechanism(s) responsible for the actions of JNK signaling in macrophages, we focused on the fact that Jnk1−/−→Ldlr−/− mice had a dramatic decrease in apoptosis and increased numbers of macrophages in their atherosclerotic lesions compared to lesions of WT→Ldlr−/− and Jnk2−/−→Ldlr−/− mice. These results suggested that Jnk1 deficiency changes the balance between survival and pro-apoptotic signaling in macrophages at least in the setting they are examined. Indeed, our in vitro studies demonstrated that JNK signaling directly antagonizes Akt activity in mouse macrophages. This effect occurs within a short time (3–15min) and may be beneficial for inflammatory and stress responses by diverting energy sources from the synthetic Akt pathway 3. In contrast, prolonged or sustained JNK activation suppresses Akt signaling and induces cell apoptosis 6. Interestingly, this antagonizing effect is mediated mainly through JNK1, but not JNK2, and genetic ablation or pharmacological inhibition of JNK1 completely obliterated this effect. These data are consistent with the previous reports indicating that JNK signaling acts as a negative feedback loop that attenuates insulin action and insulin-induced PI3K activation 7, 12, 23, 45–47. Together our data indicate that JNK1 signaling antagonizes and suppresses Akt activity in mouse macrophages.
It is important to note that bone marrow transplantation may change every component of hematopoietic system in mice including monocyte-macrophages, T- and B cells and platelets. Several studies have shown that JNK is required for effector T-cell function 48. JNK2 is important for T cell activation, apoptosis of immature thymocytes 49 and plays a role in control of CD8+ T cell expansion in vivo, while JNK1 is involved in survival of activated T cells during immune responses 50. Moreover, JNK1 is essential for platelet secretion and thrombus formation 51. Therefore, we cannot exclude that these changes may also affect atherogenesis.
It is known that sustained JNK signaling restrains Akt activity, the major pro-survival signaling pathway that opposes apoptosis 20, suggesting a potential mechanism for impaired macrophage viability. In our experiments, sustained JNK signaling under conditions of ER stress gradually extinguished Akt and Bad (S136) activity in WT cells, whereas Jnk1−/− macrophages were much less affected (Figure 4C,D). Compared to WT cells, Jnk1−/− macrophages were also protected from apoptosis initiated by different stimuli. Moreover, JNK1 inhibition distinctly decreased ER stress-mediated apoptosis in macrophages. These results are consistent with the concept that chronically activated JNK1 signaling is crucial in type 2 diabetes and obesity 8, 11, 47, 52. JNK-mediated phosphorylation of insulin receptor substrates 1 and 2 disrupts Akt signaling33, 46 possibly by releasing Bad for translocation to the mitochondria23 or association with Bcl-2/Bcl-xL and initiation of apoptosis. In addition, we examined whether macrophages utilize a natural brake of Akt signaling, PTEN, to suppress p-Akt. Given that PTEN has been reported to cooperate with JNK 53 to couple the PI3K/Akt and JNK signaling pathways 26, we examined whether PTEN mediates cross-talk between these pathways in mouse macrophages. Our results demonstrate that genetic and pharmacologic inhibition of PTEN virtually eradicates the JNK-mediated effect on p-Akt in macrophages. Thus, JNK signaling may also act via PTEN to antagonize Akt activity and suppress macrophage survival. Macrophage-derived foam cells are the predominant cell type of early atherosclerotic lesions, and loss of macrophages through increased apoptosis may reduce the size of early atherosclerotic lesions 54. Together these data demonstrate that Jnk1 deficiency significantly increases macrophage survival and this leads to cell accumulation in early stage atherosclerotic lesions. Importantly, JNK and PTEN signaling in macrophages can be altered pharmacologically with the use of their ligands or inhibitors, supporting these pathways as new potential therapeutic targets for the prevention of atherosclerosis and allowing for functional studies in a stage specific manner.
Supplementary Material
Highlights.
JNK1 signaling antagonizes pro-survival Akt activity in mouse macrophages
Jnk1 null macrophages were less affected by the stress factors and more protected from apoptosis than WT and Jnk2 null macrophages
Loss of Jnk1, but not Jnk2, in hematopoietic cells significantly increases early atherosclerosis
Genetic ablation of JNK to a single allele (Jnk1+/−/Jnk2−/− or Jnk1−/−/Jnk2+/−) in bone marrow recipients further increased atherosclerosis compared to mice reconstituted with WT or Jnk1 null bone marrow
Acknowledgments
Acknowledgements and Sources of funding
This work was supported in part by National Institutes of Health grants HL105375, HL116263, DK50435, DK52539, and DK59637 (Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers).
Abbreviations
- JNK
c-Jun NH2-terminal kinases
- LDLR
LDL-receptor
- FLC
fetal liver cells
- PTEN
phosphatase and tensin homolog
Footnotes
Disclosure: None of the authors have a financial interest related to these studies
References
- 1.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Davis RJ. Signal transduction by the jnk group of map kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Vallerie SN, Hotamisligil GS. The role of jnk proteins in metabolism. Sci Transl Med. 2010;2:60–65. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran DN, Reddy EP. Jnk signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch U-K. C-jun n-terminal kinases (jnks) mediate pro-inflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 8.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between jnk1 and jnk2 isoforms in obesity and insulin resistance. Proc Nat Acad Sci USA. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karin M, Gallagher E. From jnk to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 10.Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, Flavell RA, Le Gros G, Davis RJ, Rincón M. C-jun nh2-terminal kinase (jnk)1 and jnk2 have distinct roles in cd8+ t cell activation. J Exp Med. 2002;195:811–823. doi: 10.1084/jem.20011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for jnk in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 12.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. Jnk expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel Varman T, Shulman Gerald I. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernia S, Cavanagh-Kyros J, Garcia-Haro L, Sabio G, Barrett T, Jung Dae Y, Kim Jason K, Xu J, Shulha Hennady P, Garber M, Gao G, Davis Roger J. The pparα-fgf21 hormone axis contributes to metabolic regulation by the hepatic jnk signaling pathway. Cell Metabolism. 2014;20:512–525. doi: 10.1016/j.cmet.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricci R, Sumara G, Sumara I, et al. Requirement of jnk2 for scavenger receptor a-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Lin A. Role of jnk activation in apoptosis: A double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Minemoto Y, Lin A. C-jun n-terminal protein kinase 1 (jnk1), but not jnk2, is essential for tumor necrosis factor alpha-induced c-jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of jnk for stress- induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 20.Duronio V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. Akt/pkb signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/akt and c-jun nh2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145:4522–4531. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 23.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. Jnk antagonizes akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of jnk. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 25.Du H, Sun X, Guma M, Luo J, Ouyang H, Zhang X, Zeng J, Quach J, Nguyen DH, Shaw PX, Karin M, Zhang K. Jnk inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc Nat Acad Sci. 2013;110:2377–2382. doi: 10.1073/pnas.1221729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the jnk signaling pathway as a functional target of the tumor suppressor pten. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage ep4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metabolism. 2008;8:492. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated akt-1 is vital for the survival of human monocyte-differentiated macrophages: Role of mcl-1, independent of nuclear factor (nf)-{kappa}b, bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danial NN. Bad: Undertaker by night, candyman by day. Oncogene. 2009;27:S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 30.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma Y-C, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Survival factor-mediated bad phosphorylation raises the mitochondrial threshold for apoptosis. Developmental Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- 32.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent pten inhibitors. FEBS letters. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 33.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 34.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo J-L, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. Jnk1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metabolism. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-jun amino terminal kinase (jnk) in the regulation of systemic insulin sensitivity. PLoS ONE. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metzler B, Hu Y, Dietrich H, Xu Q. Increased expression and activation of stress-activated protein kinases/c-jun nh2-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol. 2000;156:1875–1886. doi: 10.1016/S0002-9440(10)65061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolley CD, Dietschy JM, Turley SD. Genetic differences in cholesterol absorption in 129/sv and c57bl/6 mice: Effect on cholesterol responsiveness. Am J Physiol - Gastrointest Liver Phys. 1999;276:G1117–G1124. doi: 10.1152/ajpgi.1999.276.5.G1117. [DOI] [PubMed] [Google Scholar]
- 38.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD. Genetic background determines the extent of atherosclerosis in apoe-deficient mice. Arterioscl Thromb Vasc Biol. 1999;19:1960–1968. doi: 10.1161/01.atv.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Ding Y, Tanimoto A, Wang K-Y, Guo X, Li Z, Tasaki T, Nabesima A, Murata Y, Shimajiri S, Kohno K, Ichijo H, Sasaguri Y. Apoptosis signal–regulating kinase 1 deficiency accelerates hyperlipidemia-induced atheromatous plaques via suppression of macrophage apoptosis. Arterioscler Thromb Vasc Biol. 2011;31:1555–1564. doi: 10.1161/ATVBAHA.111.227140. [DOI] [PubMed] [Google Scholar]
- 40.Shen J, Chandrasekharan UM, Ashraf MZ, Long E, Morton RE, Liu Y, Smith JD, DiCorleto PE. Lack of mitogen-activated protein kinase phosphatase-1 protects apoe-null mice against atherosclerosis. Circ Res. 2010;106:902–910. doi: 10.1161/CIRCRESAHA.109.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amini N, Boyle JJ, Moers B, Warboys CM, Malik TH, Zakkar M, Francis SE, Mason JC, Haskard DO, Evans PC. Requirement of jnk1 for endothelial cell injury in atherogenesis. Atherosclerosis. 2014;235:613–618. doi: 10.1016/j.atherosclerosis.2014.05.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croons V, Martinet W, Herman AG, Timmermans J-P, De Meyer GRY. The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase. J Pharm Exp Ther. 2009;329:856–864. doi: 10.1124/jpet.108.149948. [DOI] [PubMed] [Google Scholar]
- 43.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 44.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoe / and ldlr / mice lacking chop. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through irs-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Lee YH, Giraud J, Davis RJ, White MF. C-jun n-terminal kinase (jnk) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 47.Solinas G, Naugler W, Galimi F, Lee M-S, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by jnk-mediated phosphorylation of insulin-receptor substrates. Proc Nat Acad Sci. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. Jnk is required for effector t-cell function but not for t-cell activation. Nature. 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 49.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J-P, Jochum W, Wagner EF, Karin M. Jnk2 is required for efficient t-cell activation and apoptosis but not for normal lymphocyte development. Current Biology. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 50.Arbour N, Naniche D, Homann D, Davis RJ, Flavell RA, Oldstone MBA. C-jun nh2-terminal kinase (jnk)1 and jnk2 signaling pathways have divergent roles in cd8+ t cell–mediated antiviral immunity. J Exp Med. 2002;195:801–810. doi: 10.1084/jem.20011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam F, Kauskot A, Nurden P, Sulpice E, Hoylaerts MF, Davis RJ, Rosa J-P, Bryckaert M. Platelet jnk1 is involved in secretion and thrombus formation. Blood. 2010;115:4083–4092. doi: 10.1182/blood-2009-07-233932. [DOI] [PubMed] [Google Scholar]
- 52.Weston CR, Davis RJ. The jnk signal transduction pathway. Current Opinion in Cell Biology. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Hübner A, Mulholland DJ, Standen CL, Karasarides M, Cavanagh-Kyros J, Barrett T, Chi H, Greiner DL, Tournier C, Sawyers CL, Flavell RA, Wu H, Davis RJ. Jnk and pten cooperatively control the development of invasive adenocarcinoma of the prostate. Proc Nat Acad Sci. 2012;109:12046–12051. doi: 10.1073/pnas.1209660109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50:S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.