Abstract
Objectives
Dietary deficiency in polyunsaturated fatty acids (PUFA), including omega-3 fatty acids eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), and excesses in omega-6 fatty acids including linoleic acid (LA, 18:2n-6) and arachidonic acid (AA, 20:4n-6), may be associated with the pathophysiology of bipolar disorder. In an effort to provide clarification regarding the relationship between PUFA biostatus and bipolar disorder, this meta-analysis investigated studies comparing erythrocyte (red blood cell) membrane PUFA composition in patients with bipolar disorder and healthy controls.
Methods
A meta-analysis was performed on case–control studies comparing erythrocyte PUFA (EPA, DHA, LA, AA) levels in patients with bipolar I disorder. Standardized effect sizes were calculated and combined using a random effects model.
Results
Six eligible case–control studies comprising patients with bipolar I disorder (n = 118) and healthy controls (n = 147) were included in the analysis. Compared with healthy controls, patients with bipolar I disorder exhibit robust erythrocyte DHA deficits (p = 0.0008) and there was a trend for lower EPA (p = 0.086). There were no significant differences in LA (p = 0.42) or AA (p = 0.64).
Conclusions
Bipolar I disorder is associated with robust erythrocyte DHA deficits. These findings add to a growing body of evidence implicating omega-3 PUFA deficiency in the pathophysiology of bipolar disorder.
Keywords: arachidonic acid, bipolar disorder, omega-3 fatty acids, omega-6 fatty acids
A growing body of evidence suggests that lower habitual dietary intake of omega-3 polyunsaturated fatty acids (PUFA), including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), is associated with the pathophysiology of bipolar disorder. Cross-national epidemiological surveys suggest that greater habitual dietary intake of fish/seafood, primary dietary sources of preformed EPA and DHA, are associated with reduced lifetime prevalence rates of bipolar disorder (1). A meta-analysis of controlled trials suggest that short-term fish oil supplementation is more effective than placebo for reducing depressive, but not manic, symptom severity in patients with bipolar disorder (2). Fish oil supplementation has also been shown to reduce manic and depression symptom severity in bipolar youth (3, 4). Emerging translational evidence additionally suggests that DHA is required for normal brain development (5). Additional evidence also suggest that patients with bipolar disorder exhibit abnormalities in the metabolism of the omega-6 PUFA linoleic acid (LA, 18:2n-6) (6). Moreover, arachidonic acid (AA, 20:4n-6), a derivative of LA, has pro-inflammatory effects which are blocked by mood-stabilizer medications (7).
Erythrocyte (red blood cell) membrane EPA and DHA composition is highly correlated with habitual intake of fish or fish oil (8, 9) and represents a valid and reliable biomarker of omega-3 PUFA biostatus (10). In contrast, plasma fatty acid levels are potentially confounded by prior meals and exhibit larger within-subject variability (11, 12). Moreover, erythrocyte DHA is correlated with cortical gray matter DHA composition (13, 14), and neuroimaging studies suggest that DHA intake and erythrocyte biostatus are correlated with corticolimbic structure and function (15–18). Low erythrocyte EPA+DHA levels are also associated with increased risk of cardiovascular mortality (19), and cardiovascular-related diseases are a primary cause of excess premature mortality in bipolar disorder (20, 21). These and other findings suggest that erythrocyte EPA and/or DHA levels may be relevant to the pathophysiology of bipolar disorder.
To provide clarification regarding the relationship between PUFA biostatus and bipolar disorder, the present meta-analysis was conducted on studies that compared erythrocyte PUFA composition in patients with bipolar I disorder and healthy comparison subjects.
Methods
A computerized search was performed to identify eligible peer-reviewed studies published up to December 2015 using PubMed at the National Library of Medicine. The following search terms were used: (bipolar or bipolar disorder) and (erythrocyte or red blood cell) and (fatty acid or omega-3 fatty acid). We focused our analysis on the primary omega-3 PUFAs DHA and EPA and the primary omega-6 PUFAs LA and AA. Effect sizes were based on unadjusted comparisons of fatty acid levels between cases and controls. Standardized effect sizes (d) were calculated and random effects models estimated. In addition to point and 95% confidence intervals of each mean effect, we computed estimates of I2 (the percentage of total variation attributable to heterogeneity) and p-values under the null hypothesis of homogeneous effect sizes. As the power of these tests is generally low, we did not accept the validity of a fixed effect (homogeneous) model when the heterogeneity test was not significant but instead report results under both fixed and random effect models for comparison. We assessed potential internal sensitivity by repeated analyses with one-out cross-validation (i.e., each study omitted in turn). Models were estimated by restricted maximum likelihood using PROC MIXED in the SAS system, version 9.4.
Results
The literature search yielded six case–control studies that compared erythrocyte PUFA levels in patients with bipolar I disorder (n = 118) and healthy controls (n = 147) (22–27) and all of these studies were included in the present analysis. In one study (23), a subset of patients with bipolar II disorder (n = 3) and bipolar disorder not-otherwise-specified (n = 5) were included in the bipolar disorder group (23). The remaining studies included only patients meeting DSM-IV criteria for bipolar I disorder. The demographic characteristics of study participants are presented in Table 1. Based on reported depression and mania symptom severity scores, four studies employed patients experiencing a manic or mixed episode (22, 24, 25, 27). One study did not report mania or depression symptom severity (23), and one study reported patients were euthymic (26).
Table 1.
Demographic characteristics of participants
| Study | Number, n | Mean age, years | % female | % medicated |
Diagnostic instrument |
Country | |||
|---|---|---|---|---|---|---|---|---|---|
| Control | Case | Control | Case | Control | Case | ||||
| McNamara et al. 2015 (25) | 40 | 40 | 18.5 | 17.8 | 50 | 50 | 0 | DSM-IV | USA |
| Ross et al. 2011 (27) | 25 | 15 | 36.0 | 42.0 | 46 | 47 | 100 | DSM-IV | Scotland |
| McNamara et al. 2010 (24) | 20 | 18 | 36.2 | 30.7 | 50 | 33 | 0 | DSM-IV | USA |
| Clayton et al. 2008 (23) | 15 | 15 | 14.7 | 13.6 | 60 | 60 | 100 | DSM-IV | Australia |
| Ranjekar et al. 2003 (26) | 27 | 10 | 39.7 | 40.9 | 0 | 0 | 100 | DSM-IV | India |
| Chiu et al. 2003 (22) | 20 | 20 | 38.7 | 39.0 | 55 | 50 | 75 | DSM-IV | Taiwan |
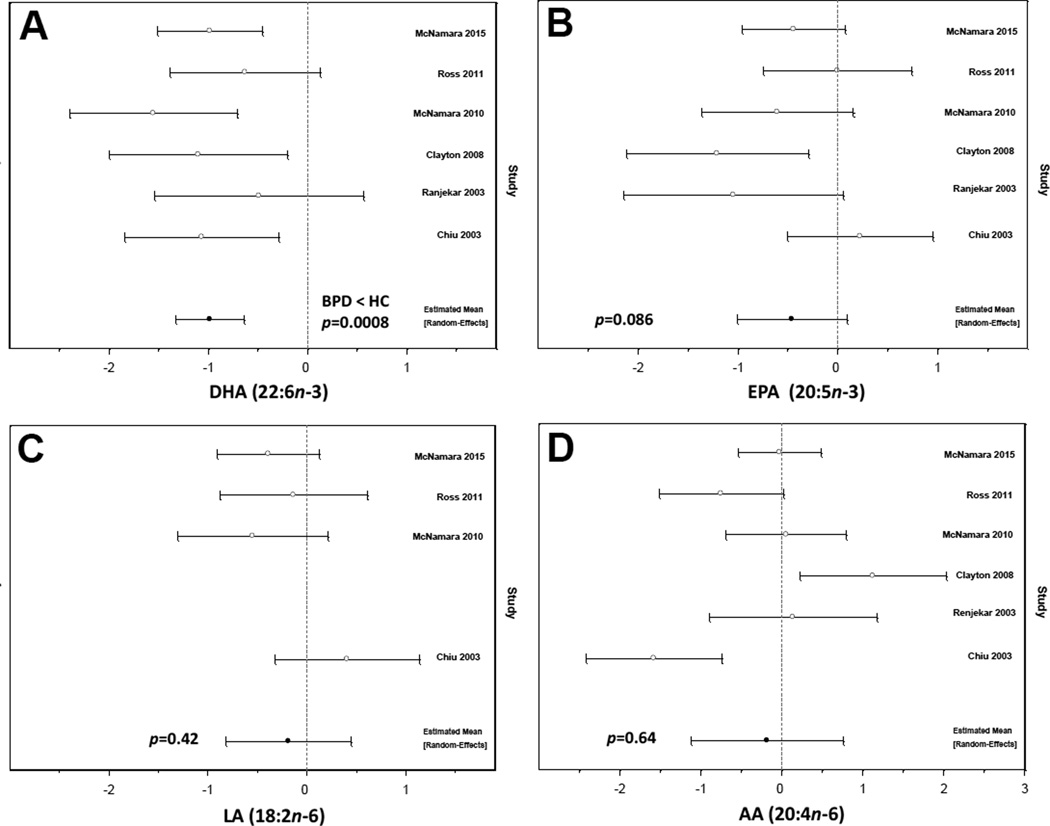
As the number of studies was small, methods based on the funnel plot were unlikely to be informative about potential publication bias and were not employed. For the same reason, we did not consider potential study-level covariates that might account for heterogeneity. Forest plots of standardized effect sizes are shown in Figure 1. Effect sizes for DHA were the least heterogeneous of the four fatty acids analyzed (I2 = 0.0%, p = 0.4358). The mean standardized effect size was significantly different from zero, with bipolar cases exhibiting lower levels than controls [d = −0.98 (−1.33, −0.63), p = 0.0008] (Fig. 1A). The point estimate of among-study variance was zero, and the fixed effect results were identical. Effect sizes for EPA were significantly heterogeneous (I2 = 57.4%, p = 0.0385). The mean standardized effect size was not significantly different from zero [d = −0.46 (−1.01, 0.09), p = 0.0857] (Fig. 1B). This was the only finding that was qualitatively altered by inclusion of a single study (22), which slightly lowered this trending p-value to below 0.05 [d = −0.58 (−1.13, −0.04 0, p = 0.0414]. However, since the average effect was only modestly increased, the results cannot be considered particularly sensitive. Two of the six identified studies did not report LA (23, 26). Effect sizes exhibited moderate, though not statistically significant, heterogeneity (I2 = 44.9%, p = 0.1418). The mean standardized effect size was not significantly different from zero [d = −0.18 (−0.82, 0.45), p = 0.4240) (Fig. 1C). When a fixed effect model was adopted for these data, the results remained qualitatively similar [d = −0.21 (−0.67, 0.25), p = 0.249]. Effect sizes for AA were highly heterogeneous (I2 = 83.5%, p < 0.0001). The mean standardized effect size was not significantly different from zero [d = −0.18 (−1.12, 0.76), p = 0.6447] (Fig. 1D).
Fig. 1.
Forest plot illustrating standardized effect sizes (d) and 95% confidence intervals for individual studies comparing erythrocyte docosahexaenoic acid (DHA, 22:6n-3) (A), eicosapentaenoic acid (EPA, 20:5n-3) (B), linolenic acid (LA, 18:2n-6) (C), and arachidonic acid (AA, 20:4n-6) (D) levels in patient with bipolar I disorder (BPD) and healthy controls (HC). Pooled results and associated p-values are presented.
Discussion
The primary finding of this meta-analysis is that patients with bipolar I disorder exhibit robust erythrocyte DHA deficits compared with demographically similar healthy controls. While there was a trend for lower erythrocyte EPA in patients versus controls, differences in erythrocyte EPA levels were more variable across studies and did not reach significance. Moreover, there were no significant differences in the erythrocyte omega-6 PUFAs LA or AA. Similar erythrocyte DHA deficits were observed across studies conducted in five different countries, including both Western and Eastern countries. Moreover, similar erythrocyte DHA deficits were observed in studies employing adolescent or adult patients, studies employing medicated or unmedicated patients. Although plasma fatty acid levels are more variable, it is also worth noting that a prior case-control study (28), but not others (6, 29, 30), similarly observed DHA deficits in plasma from patients with bipolar disorder. These present results suggest that bipolar I disorder is associated with robust and selective erythrocyte DHA deficits, and add to a growing body of evidence implicating omega-3 PUFA deficiency in the pathophysiology of bipolar I disorder.
A limitation of this meta-analysis is the relatively small number of studies included in the analysis, and the data obtained may not be representative of all individuals with bipolar disorder. However, the effect sizes for DHA were homogeneous and pooled analysis had a large sample size. Second, the cross-sectional design of the studies preclude evaluation of causality, and prospective longitudinal studies will be required to clarify the role of low erythrocyte DHA biostatus in bipolar risk progression. Third, several studies did not include data regarding potential confounders, including cigarette smoking and body mass index (BMI), to evaluate their contribution to the present findings. Nevertheless, studies that specifically investigated the contribution of cigarette smoking did not observe an association with erythrocyte PUFA status (22, 25), and two studies that reported BMI did not observe a difference between cases and controls (22, 25). While gender differences in blood DHA levels have been observed (31), the majority of studies employed both male and female subjects, and two studies that specifically investigated the contribution of gender did not observe an association with erythrocyte DHA status (24, 25).
The etiology of the erythrocyte DHA deficit observed in patients with bipolar disorder across studies may be multifactorial. Selective erythrocyte DHA deficits in the absence of EPA and AA deficits are consistent with impaired peroxisome function which is required for the final biosynthesis of DHA and associated with erythrocyte DHA deficits (32–34). However, peroxisomal-mediated DHA biosynthesis from fatty acid precursors is negligible even in healthy human subjects (35), and first-episode manic patients do not exhibit other lipid abnormalities characteristic of peroxisomal disorders (36). Alternatively, elevations in lipid peroxidation/oxidation may contribute to erythrocyte membrane DHA deficits (37). However, extant evidence suggests that erythrocyte DHA deficits in bipolar disorder are dissociable from indices of lipid peroxidation (27, 38). Medication effects are unlikely since erythrocyte DHA deficits are observed in chronically medicated, medication-withdrawn, and medication-naïve patients with bipolar disorder. Moreover, one study found that erythrocyte DHA deficits in medication-naïve patients with bipolar disorder were not significantly altered following 52-week treatment with either lithium or quetiapine (25). The low erythrocyte DHA levels observed in patients may reflect dietary DHA insufficiency. This is supported by the observations that (i) dietary intake of preformed DHA from fish or fish oil supplements increases erythrocyte DHA levels in a linear dose-dependent manner (8, 9), (ii) patients with bipolar disorder consume less DHA in their diet (6, 23), and (iii) fish oil supplementation significantly increases erythrocyte DHA levels in patients with bipolar disorder (3, 4). While to role of EPA and/or DHA supplementation in the treatment of manic or depressive symptoms is requires additional investigation, extant evidence suggests that DHA and EPA deficiency can be corrected with fish oil supplementation.
Because bipolar disorder is associated with recurrent episodes of depression, it is notable that a meta-analysis of case-control studies of patients with major depressive disorder (MDD) observed significant EPA and DHA deficits, and no difference in AA, compared with controls (39). It is also notable that a large percentage of patients with bipolar disorder have a history of psychotic symptoms (40) and a meta-analysis of case–control studies observed significant erythrocyte DHA as well as AA deficits in first-episode psychosis patients (41). Furthermore, there is a high rate of attention-deficit hyperactivity disorder (ADHD) comorbidity in youth with bipolar disorder (42), and a meta-analysis found that youth with ADHD also exhibit robust DHA deficits (43). Taken collectively, these and the present results suggest that erythrocyte DHA deficits are not unique to bipolar disorder, and are also associated with different psychiatric symptoms that are frequently comorbid with bipolar disorder.
The present findings may take on additional significance in view of evidence that DHA is the primary omega-3 fatty acid found in mammalian gray matter and erythrocyte DHA is correlated with cortical gray matter DHA composition (13, 14). Moreover, emerging neuroimaging evidence suggests that DHA intake and erythrocyte biostatus is correlated with corticolimbic structure and function (15–18). Additionally, erythrocyte EPA and DHA biostatus is positively correlated with immune cell (i.e., monocyte) EPA and DHA composition (44) and inversely correlated with pro-inflammatory cytokine levels (45). It is notable therefore that elevated pro-inflammatory cytokine levels have been observed in patients with bipolar disorder (46). Moreover, prospective studies have found that lower baseline DHA levels, or a higher AA/EPA + DHA ratio, are associated with increased risk for developing a major depressive episode (47, 48), as well as manic-like symptoms including anger and irritability increase (49), during treatment with the pro-inflammatory cytokine IFN-γ. Additional studies are therefore warranted to investigate the links between DHA deficiency, pro-inflammatory signaling, and alterations in cortical structural and functional maturation in bipolar disorder.
Conclusions
In summary, the results of this meta-analysis demonstrate that bipolar I disorder is associated with robust erythrocyte DHA deficits compared with demographically similar healthy subjects. This result was obtained from studies conducted in five different countries, and adds to a growing body of cross-national and cross-sectional findings implicating dietary omega-3 PUFA deficiency in the pathophysiology and potentially pathoetiology of bipolar disorder. These and other data further suggest that erythrocyte DHA deficits may represent a candidate prodromal risk biomarker warranting additional investigation in prospective studies. Additionally, prospective supplementation studies are warranted to determine whether correcting low erythrocyte DHA biostatus by increasing fish or fish oil intake can mitigate risk progression in youth at high-risk for developing bipolar disorder. This approach is supported by the observation that fish oil supplementation prevented or delayed the onset of psychosis in ultra-high risk youth (50).
Acknowledgments
This study was supported, in part, by National Institute of Health (NIH)/NIDDK grant DK097599 to RKM; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Disclosures
RKM has received research support from Martek Biosciences, Inc., Inflammation Research Foundation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly & Co., and NARSAD; and has served on the scientific advisory board of the Inflammation Research Foundation. JAW does not have any conflicts of interest to report.
References
- 1.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 2.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 3.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 5.McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J Psychiatry. 2015;5:15–34. doi: 10.5498/wjp.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. doi: 10.1016/j.jpsychires.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5:459–467. doi: 10.1021/cn500058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, von Schacky C, Park Y. Standardizing methods for assessing omega-3 fatty acid biostatus. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Nova Science Publishers, Inc.; 2013. pp. 385–398. [Google Scholar]
- 11.Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem. 2010;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Harris WS, Varvel SA, Pottala JV, Warnick GR, McConnell JP. Comparative effects of an acute dose of fish oil on omega-3 fatty acid levels in red blood cells versus plasma: implications for clinical utility. J Clin Lipidol. 2013;7:433–440. doi: 10.1016/j.jacl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 14.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 15.Conklin SM, Gianaros PJ, Brown SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 16.McNamara RK, Able JA, Jandacek R, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara RK, Jandacek RJ, Rider T, et al. Low docosahexaenoic acid status is associated with indices of cortical integrity in the anterior cingulate of healthy male children: A 1H MRS study. Nutr Neurosci. 2013;16:183–190. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology. 2014;82:435–442. doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 21.Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 22.Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 23.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara RK, Jandacek RJ, Tso P, et al. First-episode bipolar disorder is associated with erythrocyte membrane docosahexaenoic acid deficits: Dissociation from clinical response to lithium or quetiapine. Psychiatry Res. 2015;230:447–453. doi: 10.1016/j.psychres.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranjekar PK, Hinge A, Hegde MV, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 27.Ross BM, Maxwell R, Glen I. Increased breath ethane levels in medicated patients with schizophrenia and bipolar disorder are unrelated to erythrocyte omega-3 fatty acid abundance. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:446–453. doi: 10.1016/j.pnpbp.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Pomponi M, Janiri L, La Torre G, et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47:337–342. doi: 10.1016/j.jpsychires.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Saunders EF, Reider A, Singh G, Gelenberg AJ, Rapoport SI. Low unesterified:esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment. Bipolar Disord. 2015;17:729–742. doi: 10.1111/bdi.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 32.Martinez M, Mougan I, Roig M, Ballabriga A. Blood polyunsaturated fatty acids in patients with peroxisomal disorders. A multicenter study. Lipids. 1994;29:273–280. doi: 10.1007/BF02536332. [DOI] [PubMed] [Google Scholar]
- 33.Moser AB, Jones DS, Raymond GV, Moser HW. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem Res. 1999;24:187–197. doi: 10.1023/a:1022549618333. [DOI] [PubMed] [Google Scholar]
- 34.Wanders RJA. Peroxisomal biosynthesis of omega-3 fatty acids and human peroxisomal diseases. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. U.S.A.: Nova Science Publishers, Inc.; 2013. pp. 19–30. [Google Scholar]
- 35.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC International Society for the Study of Fatty Acids and Lipids, ISSFAL. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 36.McNamara RK, Strawn JR, Stahl L, et al. Society of Biological Psychiatry Meeting. Vol. 75. New York, NY: 2014. Long-chain omega-3 fatty acid deficits in youth with or at high or ultra-high risk for bipolar disorder are not due to impaired peroxisomal function; p. S319. [Google Scholar]
- 37.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 38.Scola G, McNamara RK, Croarkin PE, et al. Lipid peroxidation biomarkers in adolescents with or at high-risk for bipolar disorder. J Affect Disord. 2016;192:176–183. doi: 10.1016/j.jad.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Dunayevich E, Keck PE., Jr Prevalence and description of psychotic features in bipolar mania. Curr Psychiatry Rep. 2000;2:286–290. doi: 10.1007/s11920-000-0069-4. [DOI] [PubMed] [Google Scholar]
- 41.Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 42.Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8:710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 43.Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34:496–505. doi: 10.1016/j.cpr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browning LM, Walker CG, Mander AP, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontes JD, Rahman F, Lacey S, et al. Red blood cell fatty acids and biomarkers of inflammation: a cross-sectional study in a community-based cohort. Atherosclerosis. 2015;240:431–436. doi: 10.1016/j.atherosclerosis.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 47.Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2013;31:48–53. doi: 10.1016/j.bbi.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su KP, Huang SY, Peng CY, et al. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha treatment is moderated by the ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013;75:475–483. doi: 10.1016/j.jpsychores.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]