Abstract
Objective
This study examined vascular actions of angiotensin 1–7 (ANG 1–7) in human atrial and adipose arterioles.
Approach and Results
The endothelial-derived hyperpolarizing factor of flow mediated dilation (FMD) switches from anti-proliferative nitric oxide (NO) to pro-atherosclerotic hydrogen peroxide (H2O2) in arterioles from humans with coronary artery disease (CAD). Given the known vasoprotective properties of ANG 1–7, we tested the hypothesis that overnight ANG 1–7 treatment restores the NO-component of FMD in arterioles from CAD patients. Endothelial telomerase activity is essential for preserving the NO-component of vasodilation in the human microcirculation, thus we also tested whether telomerase activity was necessary for ANG 1–7 mediated vasoprotection by treating separate arterioles with ANG 1–7 ± the telomerase inhibitor BIBR-1532. ANG 1–7 dilated arterioles from patients without CAD, whereas dilation was significantly reduced in arterioles from CAD patients. In atrial arterioles from CAD patients incubated with ANG 1–7 overnight, the NO synthase inhibitor L-NAME abolished FMD while the H2O2 scavenger PEG catalase had no effect. Conversely, in vessels incubated with ANG 1–7 + BIBR-1532, L-NAME had no effect on FMD but PEG catalase abolished dilation. In cultured human coronary artery endothelial cells, ANG 1–7 significantly increased telomerase activity. These results indicate that ANG 1–7 dilates human microvessels, and dilation is abrogated in the presence of CAD. Further, ANG 1–7 treatment is sufficient to restore the NO component of FMD in arterioles from CAD patients in a telomerase-dependent fashion.
Conclusion
ANG 1–7 exerts vasoprotection in the human microvasculature via modulation of telomerase activity.
Keywords: Angiotensin 1–7, Vascular, Telomerase, Vasodilation, Coronary Artery Disease
Introduction
The vasoactive peptide angiotensin 1–7 (ANG 1–7) has been widely studied over the past two decades. Largely the protective actions of ANG 1–7 oppose the pro-oxidative, proliferative, and pressor actions of angiotensin II (ANG II), making it an attractive target for cardioprotective therapies. ANG 1–7 has been demonstrated to be a potent vasodilator in multiple vascular beds; however the majority of these studies have been performed in rodents. The vasodilator properties of ANG 1–7 have scarcely been described in humans, and those reports that do exist are conflicting. For example, Sasaki et al. have shown that intra-arterial infusion of ANG 1–7 significantly increases forearm blood flow in healthy subjects and that the response is blunted in hypertensive subjects;1 while Wilsdorf et al conversely showed that intra-brachial ANG 1–7 infusion had no effect on forearm blood flow and also had no potentiating effect on bradykinin-induced vasodilation.2 In this study, we first sought to determine if ANG 1–7 causes vasodilation in the human coronary and peripheral microcirculations, and if this response is altered in patients with coronary artery disease (CAD).
We have previously demonstrated that human atrial and adipose arterioles from subjects with CAD invoke a compensatory vasodilator pathway in response to increased intraluminal flow (flow mediated dilation; FMD), and that this pathway releases endothelial-derived hydrogen peroxide (H2O2) rather than nitric oxide (NO) as the vasoactive mediator.3–6 The long-term consequences of invoking a vasodilator pathway which repeatedly generates H2O2, a molecule which favors a proliferative and oxidative state in the endothelium, vs. NO, a molecule which is anti-proliferative and anti-inflammatory, remains unknown. However, it could be surmised that long term exposure to H2O2 in the vascular milieu exacerbates an already damaged vascular endothelium, thus it may be beneficial to restore NO-mediated vasodilation to promote vascular homeostasis. Previous studies have shown plasma ANG 1–7 levels positively correlate with brachial artery FMD in human subjects7, and recent findings by Zhang et al. demonstrated that treating renal arterioles from diabetic patients with ANG 1–7 overnight restores acetylcholine mediated dilation.8 In light of these findings, we also tested the hypothesis that exposing human atrial vessels from subjects with CAD to ANG 1–7 would restore the NO-component of the FMD response.
Telomerase is a ribonucleoprotein that has been classically described as the protein responsible for elongating telomeres at the ends of chromosomes and is a regulator of cellular senescence. However, emerging evidence indicates that telomerase also has non-nuclear roles, which include protecting against mitochondrial dysfunction.9, 10 Recently, telomerase activity has been shown to be critically involved in maintaining the NO-component of vasodilation in atrial and adipose microvessels from humans with CAD.11 While maintaining telomerase activity may be vital to preserving the NO component of FMD in the human microcirculation, the regulation of telomerase in the human vasculature is not well understood. Pro-atherosclerotic factors such as ANG II have been shown to down-regulate telomerase activity in a number of cell types, including the endothelium, by mechanisms directly related to ROS accumulation.12–14 Conversely, the effect of ANG 1–7 on telomerase activity has not been described. Since ANG 1–7 largely opposes the effects of ANG II, and in light of the recent finding that telomerase activity is essential in maintaining NO-mediated dilation,11 we hypothesized that ANG 1–7 exerts vasoprotective actions in the human microcirculation by increasing vascular telomerase activity.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Adipose and atrial resistance arterioles were obtained from 34 subjects with a clinical diagnosis of CAD and 16 subjects without known CAD. The age, sex, body mass index (BMI), location of adipose tissue, and underlying conditions of the subjects are shown in Table 1. There was no age difference between the subjects in the CAD vs. the non-CAD group (66±1.7 years vs. 64±3.5 years, respectively; P>0.05; t-test). A total of 45 vessels were used for analysis of vasodilation in this study. The vessels had an average internal diameter of 166±8.8 µm and were constricted to 40±1.3 % of their maximal diameter prior to assessing vasodilation. The size of vessels used between CAD and non-CAD tissues was not different, nor was the percent constriction (P>0.05; t-test).
Table 1.
Patient demographics.
| Subject Group | |||
|---|---|---|---|
| Non CAD (n=16) | CAD (n=33) | Total (n=49) | |
| Age | 64±3.5 | 66±1.7 | 65±2.5 |
| Male/Female | 7/9 | 23/10 | 30/19 |
| Body Mass Index | 30±1.7 | 31±1.1 | 31±0.9 |
| Underlying Condition | |||
| Coronary Artery Disease | 0 | 33 | 33 |
| Hypertension | 5 | 27 | 32 |
| Valve Disease | 4 | 11 | 15 |
| Atrial Fibrillation | 3 | 2 | 5 |
| Peripheral Vascular Disease | 0 | 4 | 4 |
| Diabetes | 4 | 9 | 13 |
| Hyperlipidemia | 4 | 9 | 13 |
| Tobacco Use | 2 | 9 | 11 |
| Cancer | 6 | 1 | 7 |
| Bowel Disease | 9 | 2 | 11 |
| Adipose Depot | |||
| Mesenteric | 7 | 1 | 8 |
| Omental | 3 | 0 | 3 |
| Pericardial | 2 | 4 | 6 |
| Perirectal | 0 | 1 | 1 |
n=number of subjects. Age and body mass index are presented as mean ± SEM.
ANG 1–7 vasodilation is mediated via the Mas receptor in the human microcirculation
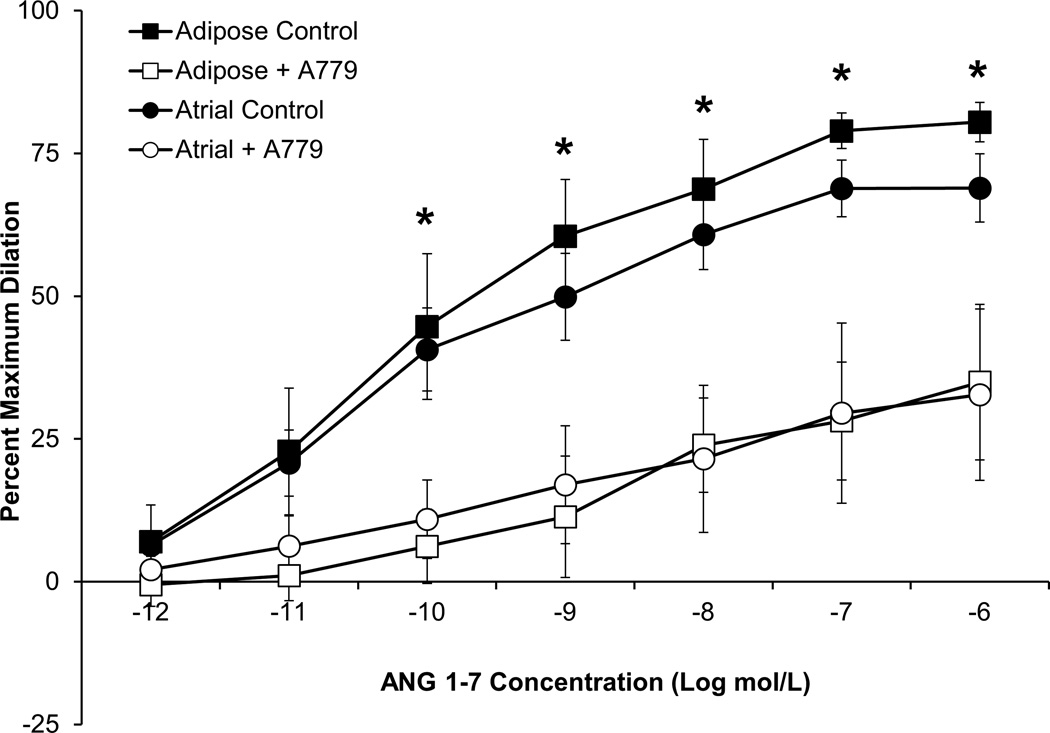
ANG 1–7 dilated both human adipose and atrial microvessels from subjects without CAD in a dose-dependent manner (Figure 1). Importantly, previous studies have shown human plasma concentrations of ANG 1–7 to be in the range of 1–50 pmol/L, thus the concentrations of ANG 1–7 which caused measurable vasodilation were physiological.15–18 Dilation to ANG 1–7 was also mediated by Mas receptor activation as pre-incubation with the Mas receptor antagonist A779 significantly reduced dilation in both vascular beds. Vasodilation to the direct smooth muscle activator papaverine (10−4 mol/L) was unaffected by the presence of A779 (≥95% dilation in all groups, P>0.05 between groups; t-test; data not shown).
Figure 1.
ANG 1–7 dilated atrial and adipose arterioles from human subjects without CAD. The Mas receptor antagonist A779 (10−5 mol/L) significantly reduced the vasodilator response to ANG 1–7 in both vessel types. n=5 atrial, n=4 adipose. *P<0.05 vs. A779 treated. A779 vessels were paired with the control responses. n=number of vessels, each from a different individual.
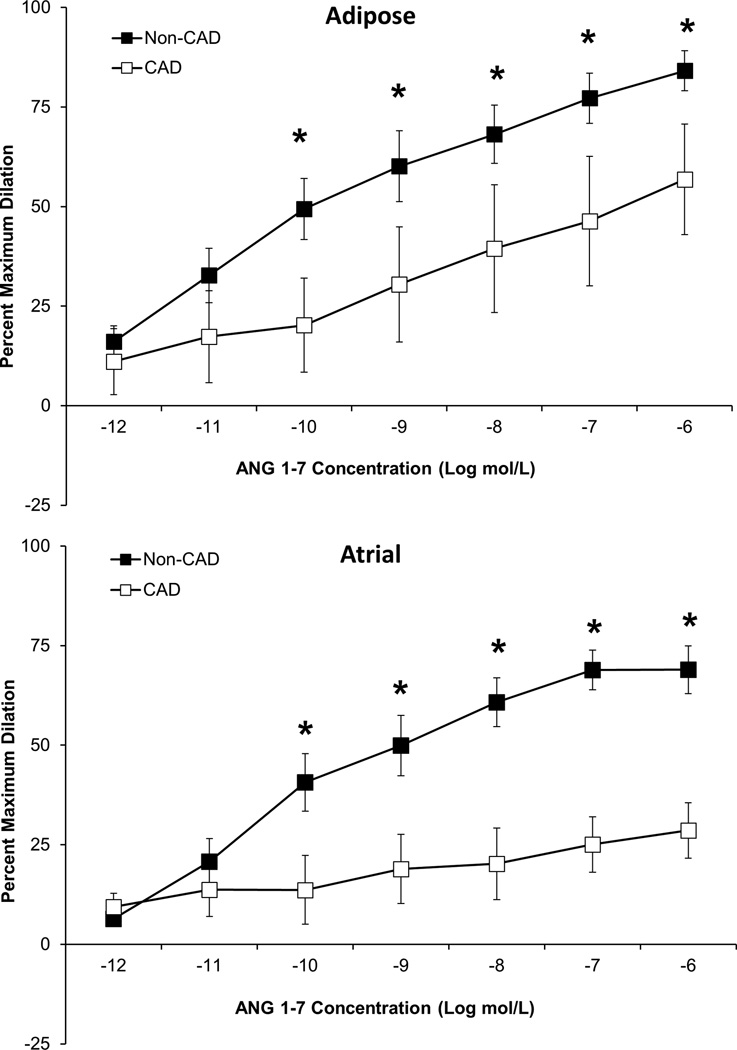
ANG 1–7 mediated vasodilation is reduced in microvessels from subjects with CAD
As shown in Figure 2, ANG 1–7 mediated vasodilation was significantly lower in atrial and adipose microvessels from subjects with CAD compared to subjects without CAD. These findings are consistent with studies performed in rodent models of cardiovascular disease which show reduced ANG 1–7 mediated vasodilation in the aorta of spontaneously hypertensive rats19 and in the coronary arteries from a rat model of cardiac hypertrophy20. The difference in ANG 1–7 mediated dilation in microvessels from CAD and non-CAD subjects is likely not due to reduced Mas receptor density, as the receptor is robustly expressed in both the endothelial and vascular smooth muscle cell layer of vessels from both CAD and non-CAD subjects (Supplemental Figure I). Dilation to papaverine was unaffected by CAD (≥95% dilation in both CAD and non CAD vessels, P>0.05; t-test; data not shown).
Figure 2.
ANG 1–7 dilated atrial (n=5) and adipose (n=8) arterioles from human subjects without CAD while the response was significantly abrogated in arterioles from subjects with CAD. CAD atrial n=4 and CAD adipose n=5. n=number of vessels, each from a different individual. *P<0.05 vs. CAD.
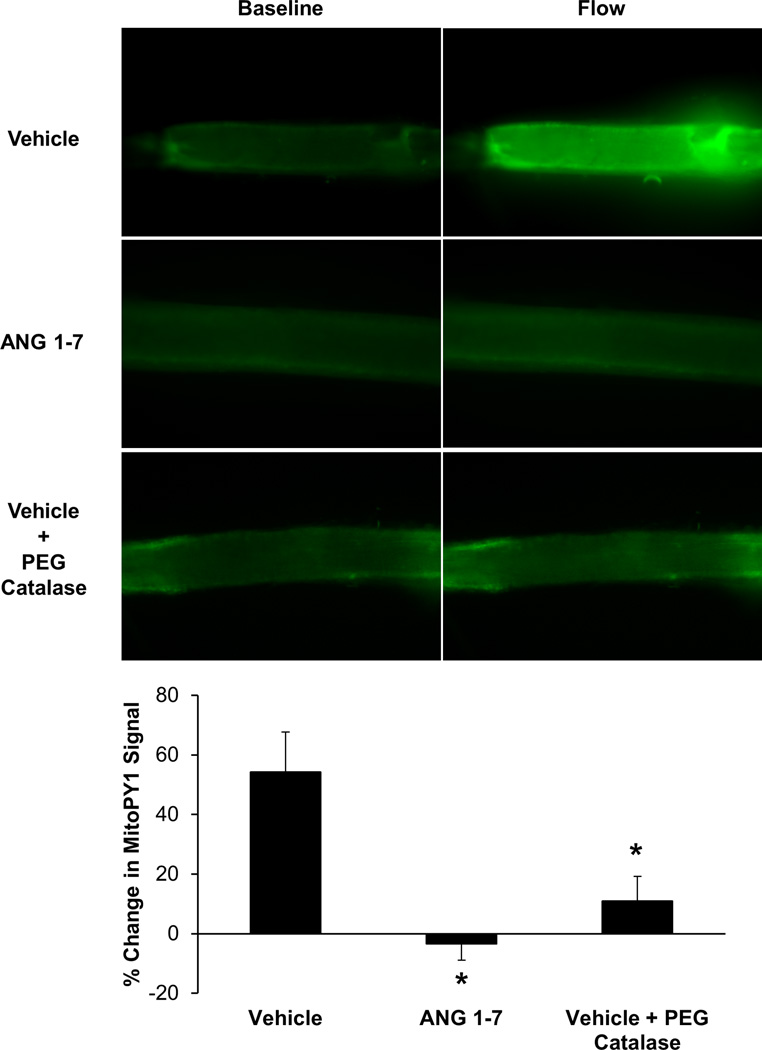
ANG 1–7 prevents flow-induced mitochondrial H2O2 generation in atrial vessels from CAD subjects
To measure mitochondrial H2O2 generation, the fluorescent dye MitoPY1 was used. It has previously been shown that mitochondrial H2O2 is the primary mediator of FMD in adipose and coronary arterioles from subjects with CAD.3, 5, 6, 11, 21 This was confirmed in Figure 3 because when flow was induced through the lumen of vehicle-treated, cannulated coronary arterioles from subjects with CAD, a significant increase in MitoPY1 fluorescence was observed. Conversely, the increase in MitoPY1 fluorescence was attenuated in vessels treated with ANG 1–7 overnight or vehicle-treated vessels that had the H2O2 scavenger PEG catalase added to the organ bath. To confirm the specificity of MitoPY1 for mitochondrial ROS, the mitochondrial electron transport chain uncoupler antimycin A caused a time-dependent increase in MitoPY1 fluorescence in separate vessels (Supplemental Figure III).
Figure 3.
(A) Representative images of cannulated human atrial arterioles from subjects with CAD with MitoPY1, a fluorescent probe specific for mitochondrial H2O2, in the lumen before and after intraluminal flow was induced. (B) Vehicle-treated atrial vessels from CAD subjects (n=7) showed a significant increase in MitoPY1 fluorescence after flow compared to arterioles that were treated with ANG 1–7 overnight (n=5) or vehicle treated vessels which had PEG-catalase added to the organ bath (n=5). n=number of vessels, each from a different individual. *P<0.05 vs. Vehicle.
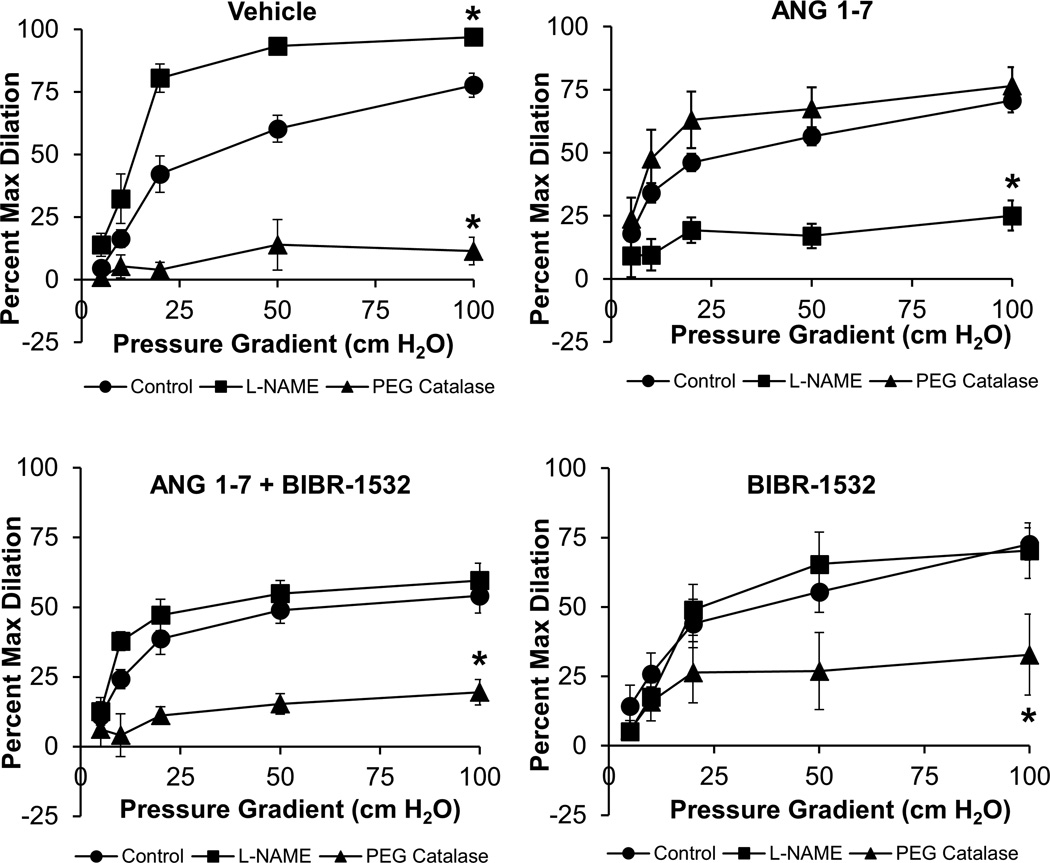
ANG 1–7 restores physiological NO-mediated vasodilation in a telomerase-dependent manner
Our group has previously shown that the magnitude of FMD in adipose and atrial arterioles from subjects with CAD is similar compared to subjects without CAD, however the mediator of vasodilation switches from NO to H2O2 during CAD.3–6 This finding was confirmed in Figure 4A, as PEG Catalase inhibited FMD in atrial vessels from patients with CAD, while L-NAME had a potentiating effect on the magnitude of FMD. The effect of L-NAME to potentiate dilation is not altogether unexpected and may be explained as eNOS-derived NO is a physiological regulator of mitochondrial oxygen consumption and ROS formation in endothelial cells.22–24 The FMD response in atrial arterioles from CAD subjects requires mitochondrial H2O2 as the vasoactive mediator of dilation, thus in the absence of NO, shear-induced mitochondrial ROS formation may increase more than when physiological levels of NO are present, resulting in potentiated dilation as observed in Figure 4A.
Figure 4.
(A) In human atrial vessels from CAD subjects incubated overnight in cell culture media + PBS vehicle, PEG-catalase significantly reduced FMD while L-NAME potentiated dilation, indicating that the primary mediator of vasodilation was H2O2. (B) Overnight treatment with ANG 1–7 (10−9 mol/L) restored the NO-component of FMD in atrial arterioles from subjects with CAD as L-NAME significantly reduced dilation while PEG catalase had no effect. (C) ANG 1–7 no longer restored the NO-component of dilation when vessels were co-treated with the telomerase inhibitor BIBR-1532 (10−5 mol/L) as L-NAME had no effect on dilation while PEG catalase significantly reduced dilation. (D) BIBR-1532 treatment alone had no effect on the mediator of FMD as PEG-catalase reduced FMD similarly to untreated vessels. n=4–6, all groups. n=number of vessels, each from a different individual. *P<0.05 vs. Control.
As shown in Figure 4B, overnight incubation with ANG 1–7 (10−9 mol/L, the approximate ED50 of ANG 1–7 in human microvessels; Figure 1) restored the NO component of FMD as evidenced by the observation that L-NAME abrogated vasodilation while the H2O2 scavenger PEG-catalase had no effect. The effect of ANG 1–7 to restore the NO-component of dilation was abolished when the arterioles were co-incubated with the specific telomerase inhibitor BIBR-1532 (Figure 4C), indicating that the protective effects of ANG 1–7 require telomerase activity. Incubation with BIBR-1532 alone had no effect on the mediator of FMD as PEG-catalase significantly reduced FMD (Figure 4D), similar to untreated atrial vessels from CAD patients. BIBR-1532 is the most specific pharmacological inhibitor of human TERT currently available (reviewed by Phatak and Burger),25 and cytotoxicity in TERT-treated cells has not been observed using the dose reported here.26 Further, BIBR-1532 does not directly affect eNOS mRNA, protein expression, or nitric oxide levels in human endothelial cells,11 or have an acute effect on mitochondrial ROS in human microvessels (Supplemental Figure III), thus the effects of BIBR-1532 on the mechanism of FMD are most likely due to its telomerase-inhibiting effects and not off-target effects which neither reduce eNOS expression/activity and acutely increase mitochondrial ROS.
Effects of ANG 1–7 on endothelial TERT expression and telomerase activity
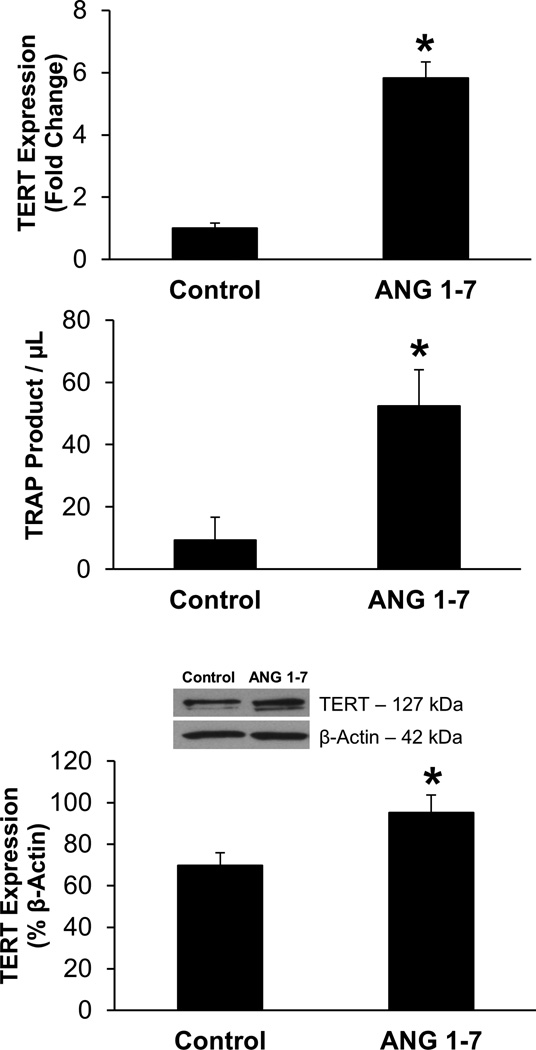
As shown in Figure 5A, TERT mRNA is significantly increased in cultured human coronary artery endothelial cells (HCAECs) treated overnight with ANG 1–7 (16–20 hours, 10−9 mol/L). ANG 1–7 treatment also caused a significant increase in telomerase activity in cultured HCAECs as assessed and quantified by modified TRAP assay using ddPCR (Figure 5B). ANG 1–7 treatment also caused a significant increase in TERT protein expression as assessed by Western Blot analysis (Figure 5C). Together these data indicate that ANG 1–7 increases telomerase activity by increasing transcription and translation of TERT, the catalytic subunit of telomerase.
Figure 5.
(A) Overnight ANG 1–7 treatment (10−9 mol/L) increased TERT mRNA expression in HCAECs compared to untreated cells. n=3 technical replicates performed in triplicate, both groups. (B) ANG 1–7 (n=3) increased telomerase activity in HCAECs compared to untreated cells (n=6). Telomerase activity was quantified by measuring telomerase extension product (TRAP) using ddPCR. All experiments were performed in duplicate. (C) ANG 1–7 treatment increased TERT expression in HCAECs as evaluated by Western Blot analysis. n=6 both groups. *P<0.05 vs. Control. n=number of technical replicates.
Discussion
There are four novel findings in this study. First, we show that ANG 1–7 is a potent vasodilator in both the human coronary and peripheral microcirculations, and that this response is dependent on the Mas receptor. Second, ANG 1–7 exerts cardioprotective effects by restoring the NO-component of FMD in coronary microvessels from humans with CAD. These vessels typically invoke compensatory H2O2-mediated vasodilation in response to increased flow.3–6 The observed changes induced by ANG 1–7 are endothelial in nature as smooth muscle function (tested by papaverine) was not altered. Third, the protective actions of ANG 1–7 on the human endothelium require telomerase activity, as the protective effects of ANG 1–7 are completely inhibited in the presence of BIBR-1532, a specific inhibitor of telomerase activity. Lastly, the mechanism of action of ANG 1–7 appears to be via increased telomerase activity, which is induced (at least in part) via transcriptional activation of TERT, the catalytic subunit of telomerase.
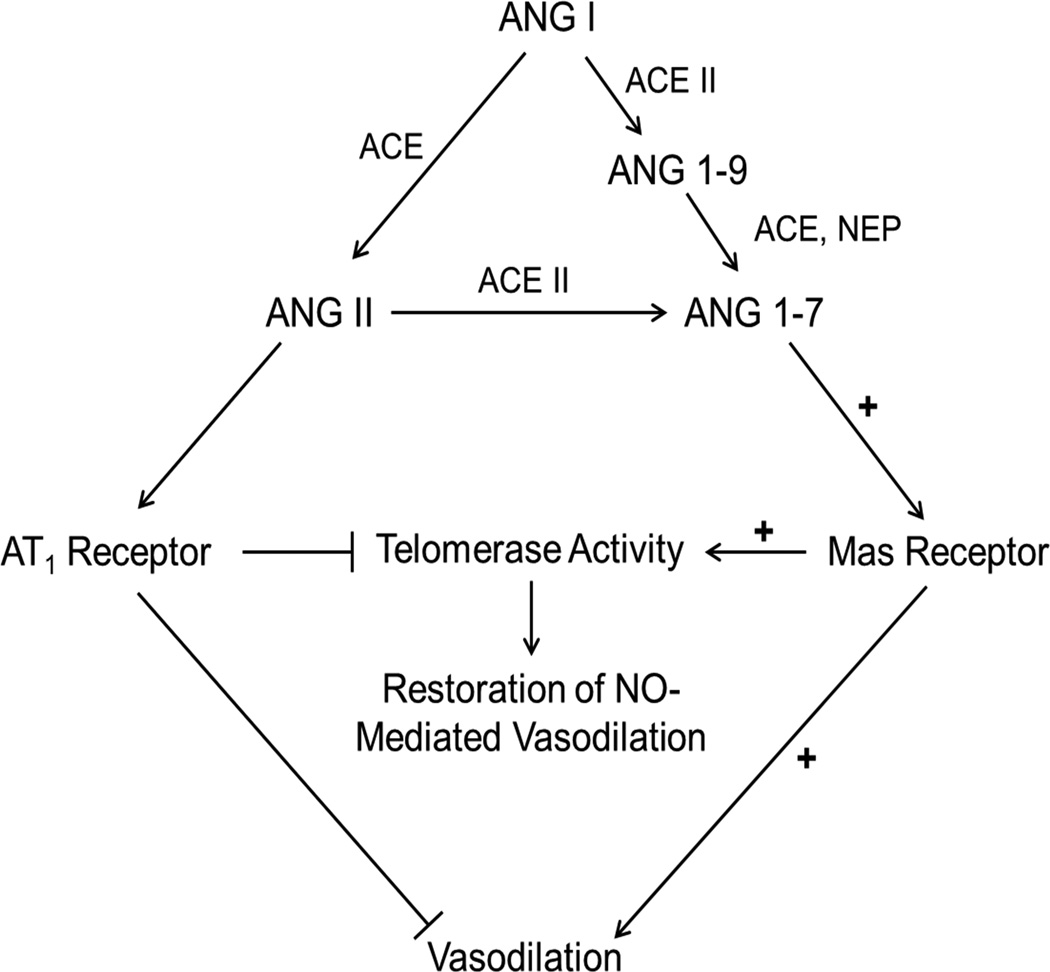
Our understanding of the role of the renin-angiotensin system (RAS) in cardiovascular homeostasis is continually evolving, but the general consensus is the vasoprotective ACE2 – ANG 1–7 – Mas receptor axis opposes many of the actions of the classically defined ACE – ANG II – angiotensin type 1 receptor axis (Figure 6). Much of the data which examines the beneficial cardiovascular actions of the ANG 1–7 – Mas receptor axis comes from animal models, with a limited number of studies evaluating the direct actions of ANG 1–7 in humans. An important observation made by Zisman et al. demonstrated a putative role for ANG 1–7 in the human heart as intracoronary infusion of ANG I (a precursor for both ANG II and ANG 1–7) resulted in a marked increase in ANG 1–7.27 Another important observation from that study was that ANG 1–7 generation from ANG I was dependent on ANG II as a substrate, as ANG 1–7 formation was suppressed by co-administration of enalaprilat, an ACE inhibitor. While that study didn’t examine a functional role for ANG 1–7, it importantly showed that formation of ANG 1–7 also results in a net reduction of ANG II in the human heart.
Figure 6.
Proposed vascular actions of ANG 1–7 in the human microvasculature. ACE, angiotensin converting enzyme; ANG, angiotensin; AT1, angiotensin type 1 receptor; NEP, neutral endopeptidase; NO, nitric oxide.
ACE inhibitors are a front line therapeutic to combat high blood pressure and congestive heart failure, and much of the blood pressure lowering and cardioprotective effects of ACE inhibitors are attributed to their ability to lower plasma ANG II levels. Interestingly, a number of studies have shown that ACE inhibitors also dramatically increase plasma ANG 1–7 levels (between 5- and 25-fold) in both animals28–31 and humans32. To what degree the beneficial effects of ACE inhibitors can be attributed to their ANG II lowering effects vs. their ability to increase ANG 1–7 levels remains to be determined. Interestingly, in hypertensive humans treated with captopril, subjects who had the largest increases in plasma ANG 1–7 concentrations also had the greatest reductions in diastolic blood pressure.32 Our results show clear evidence that ANG 1–7 both potently dilates the human coronary microcirculation and exerts a chronic vasoprotective role by promoting NO-mediated vasodilation during disease. Taken together with the recent study by Zhang et al. which showed that exogenous ANG 1–7 treatment restores vasodilation in renal arterioles from diabetic patients,8 it is conceivable that increased ANG 1–7 levels in the plasma during ACE inhibitor therapy may significantly contribute to their cardiovascular benefit.
ANG 1–7 signaling has been shown to be primarily mediated via the Mas receptor; however numerous studies have shown that ANG 1–7 can also signal through the AT2 receptor,33–35 most likely through transactivation of the receptor as the binding affinity of ANG 1–7 to the AT2 receptor is low.36, 37 Furthermore, it has been also shown that while ANG 1–7 has an even lower binding affinity for the AT1 receptor,36 some effects of ANG 1–7 can be mediated through a binding site which is losartan-sensitive, suggesting ANG 1–7 may also signal via the ACE – ANG II – AT1 receptor axis under certain conditions.38, 39 The contributions of the AT1 and AT2 receptors to ANG 1–7-mediated vasodilation were not assessed in this study, thus we cannot say with certainty that these receptor subtypes do not partially contribute to ANG 1–7 mediated signaling in the human microvasculature. A779, a specific Mas-receptor antagonist, inhibited vasodilation >50% in both atrial and adipose arterioles however, indicating that the Mas receptor is primarily responsible for effecting the actions of ANG 1–7 in these vascular beds.
Our findings that ANG 1–7 both exerts vasoprotection in a telomerase-dependent manner and directly increases telomerase activity in the human endothelium, coupled with the recent observation that telomerase activity is necessary to maintain NO-mediated dilation in the human microcirculation,11 suggests that the ACE2 – ANG 1–7 – Mas receptor axis of the RAS may be critically involved in promoting NO-mediated dilation in the human heart via modulation of telomerase activity. The direct mechanism by which ANG 1–7 modulates telomerase activity remains to be elucidated, but it is important to note that NO activates endothelial cell telomerase activity,40, 41 and increased telomerase activity elevates NO production and eNOS expression,11 suggesting a potential feed-forward mechanism. Thus, studies aimed at elucidating the direct role of ANG 1–7-mediated NO generation on modulating telomerase activity would be valuable.
If telomerase sits at a critical junction to regulate vascular health,11, 42 it is likely that ANG 1–7 is one of many positive regulators of endothelial cell telomerase activity. For example, peroxisome proliferator-activated receptor-γ (PPAR-γ) activators (thiazolidinediones, TZDs) are well studied agents which increase endothelial nitric oxide,43, 44 improve vascular function in humans,45, 46 and prevent atherosclerotic disease progression.47 While TZDs suppress telomerase expression and activity in proliferating cell types,48, 49 the opposite is observed in endothelial cells,50 which are non-proliferating cells. In support of this, ex vivo rosiglitazone treatment is also sufficient to restore NO-mediated vasodilation in CAD adipose arterioles in a BIBR-1532-inhibitable manner (Supplemental Figure IV). There is also evidence that chronic ANG 1–7 treatment increases PPAR-γ expression in spontaneously hypertensive rats,51 thus it is possible that PPAR-γ may serve as a downstream signaling effector through which ANG 1–7 modulates endothelial cell telomerase activity.
As our data indicates, the increase in endothelial cell telomerase activity is due to a transcriptional increase in TERT, the catalytic subunit of telomerase. There are several regulatory layers which modulate TERT protein expression and activity, including transcriptional,52, 53 post-transcriptional,54 and epigenetic55 modifiers. Complete understanding of these pathways is still not defined (reviewed by Akincilar, Unal and Tergaonkar)56. Future studies to decipher these complex interactions and their contribution to the development of cardiovascular disease are needed to completely understand how ANG 1–7, via TERT, contributes to the regulation of vascular tone in health and disease.
Study Limitations
It cannot be said with certainty if the difference in the magnitude of ANG 1–7 mediated dilation in vessels from CAD and non-CAD subjects is due to the presence of CAD itself, or a combination of cardiovascular risk factors which contributed to the onset of the disease. For example, of the CAD subjects, 27 had hypertension, which is known to reduce ANG 1–7 mediated dilation in animals.57 To determine which cardiovascular diseases reduce ANG 1–7 mediated dilation in the human microvasculature, larger, prospective studies would need to be undertaken. Similarity, a larger study would need to be performed to determine if age, sex, or adipose location affects microvascular dilation to ANG 1–7.
We also did not test the time-course needed for ANG 1–7 to restore NO-mediated vasodilation in this study. Faria-Silva and colleagues showed in conscious Wistar rats that infusion of either ANG 1–7 or the Mas-receptor agonist AVE0991 for as little as 30 minutes is sufficient to improve endothelial function in a NO-dependent manner.58 Overnight treatment with ANG 1–7 was chosen for our ex-vivo studies to allow for sufficient time for transcription and translation of proteins which could be modulated by ANG 1–7 (such as TERT). While a full examination of these mechanisms is beyond the scope of this study, it is important to note that treatment with ANG 1–7 for as little as 16–20 hours was sufficient to reverse a phenotype which took years to decades to develop – i.e. CAD and the associated switch in vasodilator mechanism which favors H2O2.
Due to the limited size of the human microvessels used in this study (each vessel has an approximate wet weight of 10 µg) we cannot accurately measure telomerase activity in the vessels. As a surrogate, we demonstrated that ANG 1–7 increases both TERT mRNA and protein expression, as well as telomerase activity in cultured HCAECs. Because of the inability to accurately measure telomerase activity in human arterioles, we are also unable to say with certainty that telomerase activity was inhibited by BIBR-1532; however, the dose which was used (10 µM) is more than 100-fold greater than the reported EC50 by Damm and colleagues (93 nM).59 It is also important to note that BIBR-1532 was used at doses up to 50 µM in that study and no cytotoxic effects were observed.
Supplementary Material
Highlights.
This study is the first to show that ANG 1–7 causes vasodilation in the human coronary and peripheral microcirculations and promotes NO-mediated dilation in coronary arterioles from subjects with CAD.
These effects are mediated by increasing telomerase activity.
Based on its high vasodilator potency and attenuation with disease, these findings indicate that ANG 1–7 may have an important physiological role in human cardiovascular pathophysiology. Thus, pharmacological targeting of the ACE2 – ANG 1–7 – Mas receptor axis of the RAS may promote NO-mediated vasodilation during cardiovascular disease.
Acknowledgments
The authors thank the Froedtert/MCW Department of Pathology tissue bank, the surgeons and nurses at Froedtert Memorial Lutheran Hospital, the Division of Cardiothoracic Surgery at the Medical College of Wisconsin, the Cardiothoracic Surgery Division at the Zablocki Veterans Affairs Medical Center in Milwaukee, the Aurora Medical Group Cardiovascular and Thoracic Surgery, the Wheaton Franciscan Health Group, and the Cardiothoracic Surgery Group of Milwaukee for providing tissue.
Sources of Funding
This work was supported by National Institutes of Health Grants R01-HL-113612 (D.D.G), R21-OD-018306 (A.M.B.), T32-HL-007792 and American Heart Association Grant 14POST18780022 (M.J.D.).
List of Abbreviations
- ACE
Angiotensin Converting Enzyme
- ANG
Angiotensin
- AT1
Angiotensin Type 1 Receptor
- BIBR-1532
2-[[(2E)-3-(2-Naphthalenyl)-1-oxo-2-butenyl1-yl]amino]benzoic acid
- BMI
Body Mass Index
- BSA
Bovine Serum Albumin
- CAD
Coronary Artery Disease
- cDNA
Complimentary Deoxyribonucleic Acid
- ddPCR
Droplet Digital Polymerase Chain Reaction
- DNA
Deoxyribonucleic Acid
- eNOS
Endothelial Nitric Oxide Synthase
- FMD
Flow Mediated Dilation
- H2O2
Hydrogen Peroxide
- HCAEC
Human Coronary Artery Endothelial Cells
- HPLC
High Performance Liquid Chromatography
- L-NAME
NG-Nitro-L-arginine methyl ester
- MitoPY1
Mitochondria peroxy yellow 1
- NEP
Neutral Endopeptidase
- NO
Nitric Oxide
- PCR
Polymerase Chain Reaction
- PEG
Polyethylene Glycol
- PPAR-γ
Peroxisome Proliferator-Activated Receptor-γ
- RCF
Relative Centrifugal Force
- RNA
Ribonucleic Acid
- ROS
Reactive Oxygen Species
- RT-qPCR
Reverse Transcription Polymerase Chain Reaction
- TERT
Catalytic Subunit of Telomerase
- TRAP
Telomeric Repeat Amplification Protocol
- TZD
Thiazolidinediones
Footnotes
Author Disclosure Statement: No competing financial interests exist.
References
- 1.Sasaki S, Higashi Y, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effects of angiotensin-(1–7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension. 2001;38:90–94. doi: 10.1161/01.hyp.38.1.90. [DOI] [PubMed] [Google Scholar]
- 2.Wilsdorf T, Gainer JV, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin-(1–7) does not affect vasodilator or tpa responses to bradykinin in human forearm. Hypertension. 2001;37:1136–1140. doi: 10.1161/01.hyp.37.4.1136. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of h2o2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ. Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 4.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 5.Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am. J. Physiol Heart Circ. Physiol. 2003;285:H2345–H2354. doi: 10.1152/ajpheart.00458.2003. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during cad. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan JC, Rodriguez-Miguelez P, Zimmerman MA, Harris RA. Differences in angiotensin (1–7) between men and women. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1171–H1176. doi: 10.1152/ajpheart.00897.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Liu J, Luo JY, Tian XY, Cheang WS, Xu J, Lau CW, Wang L, Wong WT, Wong CM, Lan HY, Yao X, Raizada MK, Huang Y. Upregulation of angiotensin (1–7)-mediated signaling preserves endothelial function through reducing oxidative stress in diabetes. Antioxid Redox Signal. 2015;23:880–892. doi: 10.1089/ars.2014.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, Gordon DM, Holt IJ, Santos JH. Human telomerase acts as a htr-independent reverse transcriptase in mitochondria. Nucleic acids research. 2012;40:712–725. doi: 10.1093/nar/gkr758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelsohn AR, Larrick JW. Telomerase reverse transcriptase and peroxisome proliferator-activated receptor gamma co-activator-1alpha cooperate to protect cells from DNA damage and mitochondrial dysfunction in vascular senescence. Rejuvenation Res. 2015;18:479–483. doi: 10.1089/rej.2015.1780. [DOI] [PubMed] [Google Scholar]
- 11.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparii M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Critical role for telomerase in the mechanism of flow mediated dilation in the human microcirculation. Circ. Res. 2015 doi: 10.1161/CIRCRESAHA.115.307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imanishi T, Hano T, Nishio I. Angiotensin ii accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23:97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Imanishi T, Kobayashi K, Kuroi A, Ikejima H, Akasaka T. Pioglitazone inhibits angiotensin ii–induced senescence of endothelial progenitor cell. Hypertens Res. 2008;31:757–765. doi: 10.1291/hypres.31.757. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Imanishi T, Akasaka T. Endothelial progenitor cell differentiation and senescence in an angiotensin ii-infusion rat model. Hypertens Res. 2006;29:449–455. doi: 10.1291/hypres.29.449. [DOI] [PubMed] [Google Scholar]
- 15.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine. 2002;18:239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 16.Velloso EP, Vieira R, Cabral AC, Kalapothakis E, Santos RA. Reduced plasma levels of angiotensin-(1–7) and renin activity in preeclamptic patients are associated with the angiotensin i-converting enzyme deletion/deletion genotype. Braz J Med Biol Res. 2007;40:583–590. doi: 10.1590/s0100-879x2007000400018. [DOI] [PubMed] [Google Scholar]
- 17.Nussberger J, Brunner DB, Nyfeler JA, Linder L, Brunner HR. Measurement of immunoreactive angiotensin-(1–7) heptapeptide in human blood. Clin. Chem. 2001;47:726–729. [PubMed] [Google Scholar]
- 18.Sullivan JC, Rodriguez-Miguelez P, Zimmerman MA, Harris RA. Differences in angiotensin (1–7) between men and women. Am. J. Physiol Heart Circ. Physiol. 2015;308:6. doi: 10.1152/ajpheart.00897.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva DM, Gomes-Filho A, Olivon VC, Santos TM, Becker LK, Santos RA, Lemos VS. Swimming training improves the vasodilator effect of angiotensin-(1–7) in the aorta of spontaneously hypertensive rat. J Appl Physiol. 2011;111:1272–1277. doi: 10.1152/japplphysiol.00034.2011. [DOI] [PubMed] [Google Scholar]
- 20.Souza AP, Sobrinho DB, Almeida JF, Alves GM, Macedo LM, Porto JE, Vencio EF, Colugnati DB, Santos RA, Ferreira AJ, Mendes EP, Castro CH. Angiotensin ii type 1 receptor blockade restores angiotensin-(1–7)-induced coronary vasodilation in hypertrophic rat hearts. Clin. Sci. (Lond.) 2013;125:449–459. doi: 10.1042/CS20120519. [DOI] [PubMed] [Google Scholar]
- 21.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ. Res. 2014;115:525–532. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clementi E, Brown GC, Foxwell N, Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinou E, Weisse M, Chen Q, Souza JM, Hertkorn C, Selak M, Daikhin E, Yudkoff M, Sowa G, Sessa WC, Ischiropoulos H. Dynamic regulation of metabolism and respiration by endogenously produced nitric oxide protects against oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11575–11580. doi: 10.1073/pnas.201293198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phatak P, Burger AM. Telomerase and its potential for therapeutic intervention. Br. J. Pharmacol. 2007;152:1003–1011. doi: 10.1038/sj.bjp.0707374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, Niestroj C, Daiber C, Enenkel B, Guilliard B, Lauritsch I, Muller E, Pascolo E, Sauter G, Pantic M, Martens UM, Wenz C, Lingner J, Kraut N, Rettig WJ, Schnapp A. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001;20:6958–6968. doi: 10.1093/emboj/20.24.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zisman LS, Meixell GE, Bristow MR, Canver CC. Angiotensin-(1–7) formation in the intact human heart: In vivo dependence on angiotensin ii as substrate. Circulation. 2003;108:1679–1681. doi: 10.1161/01.CIR.0000094733.61689.D4. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence AC, Clark IJ, Campbell DJ. Increased angiotensin-(1–7) in hypophysial-portal plasma of conscious sheep. Neuroendocrinology. 1992;55:105–114. doi: 10.1159/000126103. [DOI] [PubMed] [Google Scholar]
- 29.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 30.Kohara K, Brosnihan KB, Chappell MC, Khosla MC, Ferrario CM. Angiotensin-(1–7). A member of circulating angiotensin peptides. Hypertension. 1991;17:131–138. doi: 10.1161/01.hyp.17.2.131. [DOI] [PubMed] [Google Scholar]
- 31.Kohara K, Brosnihan KB, Ferrario CM. Angiotensin(1–7) in the spontaneously hypertensive rat. Peptides. 1993;14:883–891. doi: 10.1016/0196-9781(93)90063-m. [DOI] [PubMed] [Google Scholar]
- 32.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J. Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1–7) and low-dose angiotensin ii infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1024–H1033. doi: 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1–7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1341–H1352. doi: 10.1152/ajpheart.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin ii type 2 receptors in conscious rats. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 36.Rowe BP, Saylor DL, Speth RC, Absher DR. Angiotensin-(1–7) binding at angiotensin ii receptors in the rat brain. Regul. Pept. 1995;56:139–146. doi: 10.1016/0167-0115(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 37.Bouley R, Perodin J, Plante H, Rihakova L, Bernier SG, Maletinska L, Guillemette G, Escher E. N- and c-terminal structure-activity study of angiotensin ii on the angiotensin at2 receptor. Eur. J. Pharmacol. 1998;343:323–331. doi: 10.1016/s0014-2999(97)01549-5. [DOI] [PubMed] [Google Scholar]
- 38.Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB. Characterization of angiotensin-(1–7) receptor subtype in mesenteric arteries. Peptides. 2003;24:455–462. doi: 10.1016/s0196-9781(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 39.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with at1 and at2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 40.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ. Res. 2000;87:540–542. doi: 10.1161/01.res.87.7.540. [DOI] [PubMed] [Google Scholar]
- 41.Farsetti A, Grasselli A, Bacchetti S, Gaetano C, Capogrossi MC. The telomerase tale in vascular aging: Regulation by estrogens and nitric oxide signaling. J Appl Physiol. 2009;106:333–337. doi: 10.1152/japplphysiol.91360.2008. [DOI] [PubMed] [Google Scholar]
- 42.Spyridopoulos I. Is telomerase a potential target for vascular rejuvenation? Atherosclerosis. 2011;216:19–20. doi: 10.1016/j.atherosclerosis.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Goya K, Sumitani S, Otsuki M, Xu X, Yamamoto H, Kurebayashi S, Saito H, Kouhara H, Kasayama S. The thiazolidinedione drug troglitazone up-regulates nitric oxide synthase expression in vascular endothelial cells. J. Diabetes Complications. 2006;20:336–342. doi: 10.1016/j.jdiacomp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: Evidence for involvement of peroxisome proliferator-activated receptor (ppar) gamma-dependent and ppargamma-independent signaling pathways. J. Biol. Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 45.Rizza S, Cardellini M, Porzio O, Pecchioli C, Savo A, Cardolini I, Senese N, Lauro D, Sbraccia P, Lauro R, Federici M. Pioglitazone improves endothelial and adipose tissue dysfunction in pre-diabetic cad subjects. Atherosclerosis. 2011;215:180–183. doi: 10.1016/j.atherosclerosis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Martens FM, Visseren FL, de Koning EJ, Rabelink TJ. Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J. Cardiovasc. Pharmacol. 2005;46:773–778. doi: 10.1097/01.fjc.0000187176.13403.05. [DOI] [PubMed] [Google Scholar]
- 47.Pfutzner A, Marx N, Lubben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: Results from the pioneer study. J. Am. Coll. Cardiol. 2005;45:1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Rashid-Kolvear F, Taboski MA, Nguyen J, Wang DY, Harrington LA, Done SJ. Troglitazone suppresses telomerase activity independently of ppargamma in estrogen-receptor negative breast cancer cells. BMC Cancer. 2010;10:390. doi: 10.1186/1471-2407-10-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toaldo C, Pizzimenti S, Cerbone A, Pettazzoni P, Menegatti E, Daniela B, Minelli R, Giglioni B, Dianzani MU, Ferretti C, Barrera G. Ppargamma ligands inhibit telomerase activity and htert expression through modulation of the myc/mad/max network in colon cancer cells. J. Cell. Mol. Med. 2010;14:1347–1357. doi: 10.1111/j.1582-4934.2009.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE, Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ. Res. 2006;98:e50–e59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 51.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1–7) prevents diabetes-induced attenuation in ppar-gamma and catalase activities. Eur. J. Pharmacol. 2010;638:108–114. doi: 10.1016/j.ejphar.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of tert transcription by c-myc. Nat. Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 54.Zhou J, Dai W, Song J. Mir-1182 inhibits growth and mediates the chemosensitivity of bladder cancer by targeting htert. Biochem. Biophys. Res. Commun. 2016;470:445–452. doi: 10.1016/j.bbrc.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Cheng D, Zhao Y, Wang S, Jia W, Kang J, Zhu J. Human telomerase reverse transcriptase (htert) transcription requires sp1/sp3 binding to the promoter and a permissive chromatin environment. J. Biol. Chem. 2015;290:30193–30203. doi: 10.1074/jbc.M115.662221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cell. Mol. Life Sci. 2016 doi: 10.1007/s00018-016-2146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva DM, Gomes-Filho A, Olivon VC, Santos TM, Becker LK, Santos RA, Lemos VS. Swimming training improves the vasodilator effect of angiotensin-(1–7) in the aorta of spontaneously hypertensive rat. J Appl Physiol. 2011;111:1272–1277. doi: 10.1152/japplphysiol.00034.2011. [DOI] [PubMed] [Google Scholar]
- 58.Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1–7) receptor Mas stimulation improves endothelial function in normotensive rats. Hypertension. 2005;46:948–952. doi: 10.1161/01.HYP.0000174594.17052.33. [DOI] [PubMed] [Google Scholar]
- 59.Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K, Schnapp A. Mechanism of human telomerase inhibition by bibr1532, a synthetic, non-nucleosidic drug candidate. J. Biol. Chem. 2002;277:15566–15572. doi: 10.1074/jbc.M201266200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.