Abstract
Objective
During inflammation, P-selectin expressed on activated endothelial cells and platelets mediates rolling adhesion of leukocytes. Atherosclerosis-prone mice crossed with P-selectin-deficient (Selp−/−) mice develop smaller lesions. Cytokines such as tumor necrosis factor-α increase Selp transcripts and augment atherosclerosis in mice. However, they decrease SELP transcripts in humans, challenging assumptions that human P-selectin is atherogenic. We used mice expressing a human SELP transgene to examine the atherogenic role of P-selectin.
Approach and results
We crossed apolipoprotein E-deficient (Apoe−/−) mice with Selp−/− mice and/or transgenic mice expressing the entire human SELP gene (TgSELP+/−). Aortas developed larger, macrophage-rich atheromas in Apoe−/−Selp−/−TgSELP+/− mice than in Apoe−/−Selp−/− mice after 8 or 16 weeks on a Western diet. Confocal microscopy of Apoe−/−Selp−/−TgSELP+/− aortas revealed staining for human P-selectin in endothelial cells overlying atheromas, but not in lesional macrophages. We also observed staining for human P-selectin in aortic endothelial cells of 3–4-week-old Apoe−/−Selp−/−TgSELP+/− weanlings before atheromas developed. Furthermore, human SELP transcripts were ~3-fold higher in aortas of Apoe−/−Selp+/−TgSELP+/− weanlings than in Selp+/−TgSELP+/− weanlings, whereas murine Selp and Sele transcripts were equivalent in weanlings of both genotypes. Human SELP transcripts in aortas of Apoe−/−Selp+/−TgSELP+/− mice remained nearly constant during 16 weeks on a Western diet, whereas murine Selp and Sele transcripts progressively increased. Bone marrow transplantation in Apoe−/−Selp−/− and Apoe−/−Selp−/−TgSELP+/− mice demonstrated that both platelets and endothelial cells must express human P-selectin to promote atherogenesis.
Conclusions
P-selectin expressed by human SELP is atherogenic in Apoe−/− mice, suggesting that P-selectin contributes to atherogenesis in humans.
Keywords: cell adhesion, inflammation, leukocyte, platelet, endothelial cell
Subject codes: animal models of human disease, genetically altered and transgenic models, inflammation, atherosclerosis
Introduction
Atherosclerosis is a chronic inflammatory disorder of the arterial wall1. An early event is the recruitment of circulating monocytes. These cells differentiate into macrophages and become foam cells upon engulfing excessive lipids. During physiological inflammation, adhesion receptors and chemokines enable leukocytes to adhere to and migrate through postcapillary venules2. Selectin-ligand interactions mediate leukocyte rolling3. Integrin-ligand interactions slow rolling velocities and promote leukocyte arrest, intraluminal crawling, and transendothelial migration in response to chemokine gradients2. In murine models of atherosclerosis, adhesion receptors and chemokines are thought to direct monocyte recruitment into arteries4. As in humans, this occurs primarily at bifurcation sites where lower and oscillatory shear stresses permit leukocyte adhesion5. Mice lacking selectins, selectin ligands, leukocyte integrins, integrin ligands, chemokine receptors, or chemokines that interact with monocytes are protected from atherosclerosis to varying degrees4. However, extrapolation of these data to human atherosclerosis remains uncertain6.
In both mice and humans, P-selectin is constitutively expressed by platelets and venular endothelial cells3. It is stored in the membranes of α-granules of platelets and Weibel-Palade bodies of endothelial cells. Agonists such as thrombin, histamine, or oxygen-derived radicals rapidly induce translocation of P-selectin to the plasma membrane, where it interacts with P-selectin glycoprotein ligand-1 on neutrophils, monocytes, and effector lymphocytes to initiate rolling7. Thus, P-selectin is one of the earliest mediators of acute inflammation. In mice and all other non-primate mammals that have been studied, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), or bacterial lipopolysaccharide markedly increases mRNA and protein for P-selectin8–13, supporting a prominent role for P-selectin in chronic as well as acute inflammation. TNF-α is a major contributor to murine atherosclerosis14, perhaps in part by augmenting expression of P-selectin. P-selectin-deficient (Selp−/−) mice bred with apolipoprotein E-deficient (Apoe−/−) mice or with low-density lipoprotein (LDL) receptor-deficient (Ldlr−/−) mice develop smaller atherosclerotic lesions than control mice15–18. Bone marrow transplantation experiments suggest that P-selectin on non-hematopoietic, presumably endothelial cells contributes more to atherosclerosis than P-selectin on hematopoietic cells19. However, infusion of activated platelets initiates atherosclerosis, in part through P-selectin20, 21, and it was reported that macrophages in murine atheromas express P-selectin22. These combined data support an important role for P-selectin in murine atherogenesis.
Nevertheless, significant differences in the inducible expression of P-selectin in mice and humans reduce confidence in whether P-selectin contributes to human atherosclerosis. Unlike in mice, TNF-α, IL-1β, or lipopolysaccharide decreases rather than increases mRNA for P-selectin in humans and in non-human primates23–25. The altered response to these mediators is primarily due to sequence differences in the proximal promoter of the human SELP gene, which lacks the binding sites for nuclear factor κB (NF-κB) and activating transcription factor-2 that are in the murine Selp promoter26–30. In vitro, oxidized LDL increases expression of SELP mRNA and P-selectin protein in human aortic endothelial cells31, and oncostatin M, IL-4, IL-13, or substance P increases SELP mRNA and/or P-selectin protein in human umbilical vein endothelial cells or human dermal microvascular endothelial cells24, 32, 33. Whether these mediators augment P-selectin expression in vivo has not been determined. Expression of P-selectin was observed in endothelial cells overlying human atheromas, but not in macrophages within the lesions31, 34. Whether P-selectin has a causal role in human atherogenesis has not been examined.
Transgenic mice bearing the entire human SELP gene constitutively express human P-selectin in platelets and venular endothelial cells, which after mobilization to the cell surface, mediates rolling of murine leukocytes like murine P-selectin35. TNF-α or lipopolysaccharide infused into transgenic mice that retain the endogenous Selp gene markedly increases mRNA for murine P-selectin but decreases mRNA for human P-selectin in many organs35. Thus, the basal and inducible expression of the SELP transgene recapitulates that of the native gene in humans. Here, we crossed the transgenic mice with Apoe−/− mice to directly examine the role of human P-selectin in atherosclerosis.
Materials and methods
Materials and methods are available in the on-line only Data Supplement.
Results
Apoe−/−Selp−/−TgSELP+/− mice develop larger atherosclerotic plaques than Apoe−/−Selp−/− mice
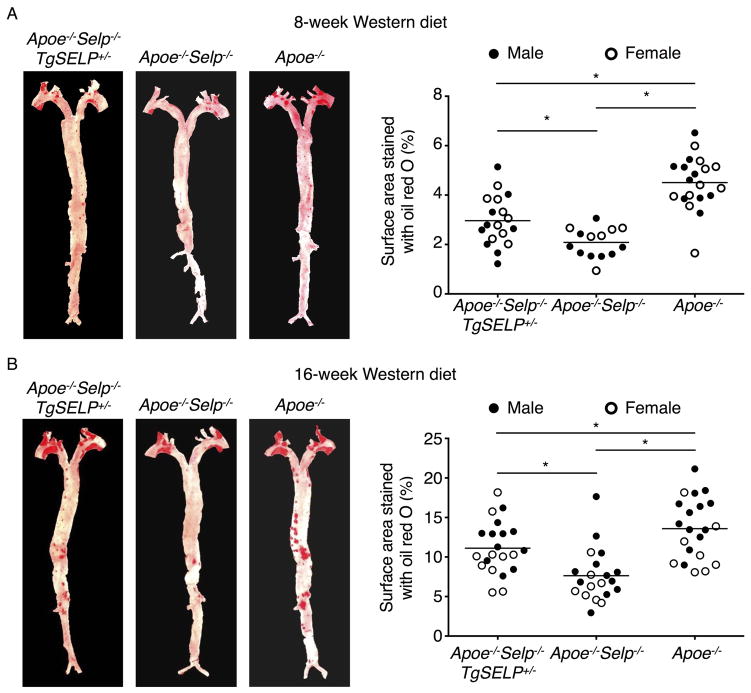
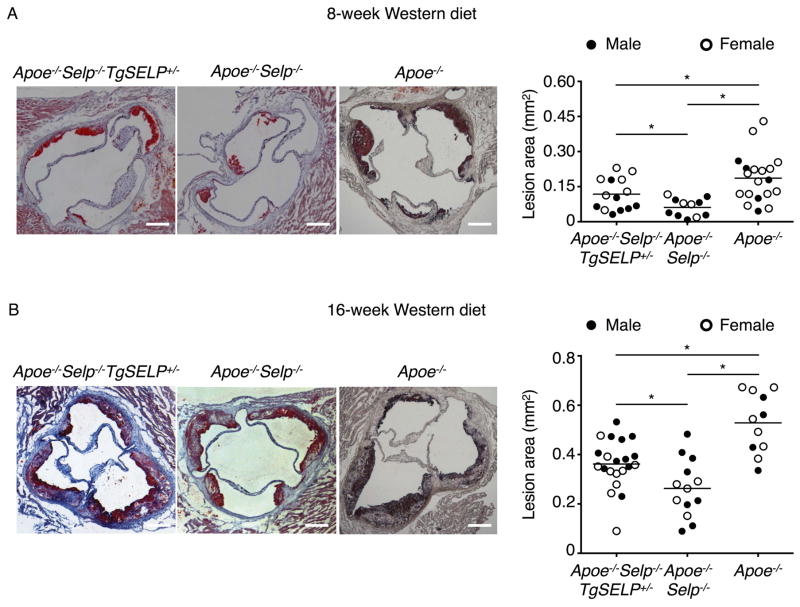
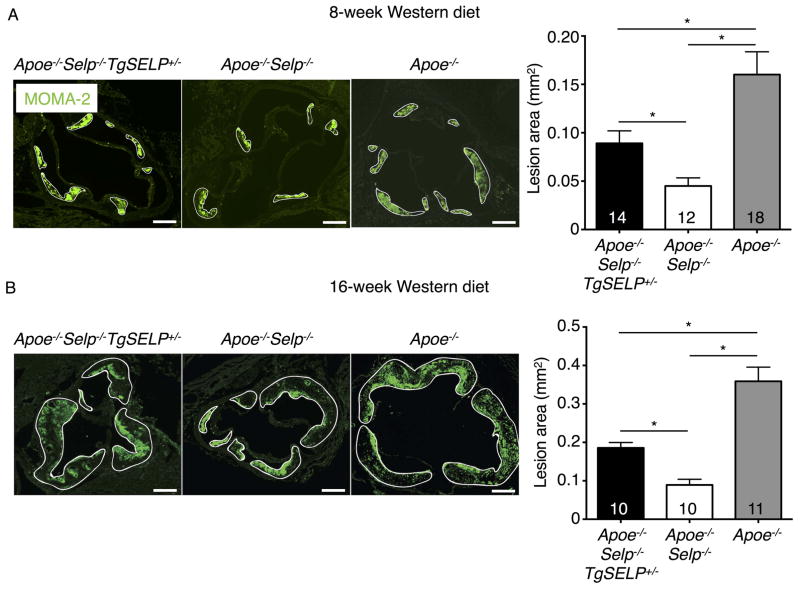
We previously generated transgenic mice that express the entire human SELP gene comprising all 17 exons and 16 introns plus 70 kb of 5′ flanking sequence and 29 kb of 3′ flanking sequence. Quantitative polymerase chain reaction (qPCR) documented a single copy of the transgene35. We crossed homozygous mice expressing two human SELP alleles (TgSELP+/+) or heterozygous mice expressing one human SELP allele (TgSELP+/−) with Selp−/− mice to generate mice expressing human but not murine P-selectin (Selp−/−TgSELP+/− or Selp−/−TgSELP+/+)35. No phenotypic differences have been detected in mice expressing one or two human SELP alleles after prolonged breeding. Nevertheless, to avoid confounding effects from inadvertent disruption of both alleles of an endogenous gene after integration of the transgene, we used Selp−/−TgSELP+/− mice. Selp−/−TgSELP+/− or Selp−/− mice were crossed with Apoe−/− mice to generate Apoe−/−Selp−/−TgSELP+/− or Apoe−/−Selp−/− mice. Immediately after weaning at age 3 weeks, these two genotypes and control Apoe−/− mice were fed a Western diet. Apoe−/−Selp−/−TgSELP+/− and Apoe−/−Selp−/− mice had comparable body weights, leukocyte counts, and plasma cholesterol and triglycerides (Supplemental Fig. I). Platelet counts are slightly higher in Selp−/−TgSELP+/− than in wild-type mice35. Similarly, we observed slightly higher platelet counts in Apoe−/−Selp−/−TgSELP+/− than in Apoe−/−Selp−/− mice (Supplemental Fig. I). After 8 or 16 weeks on the Western diet, the mice were sacrificed and the aortas were isolated. Plaques were highlighted by Oil Red O staining. We observed plaques throughout the aortas, particularly at the aortic arch, with larger plaques after 16 weeks than 8 weeks (Fig. 1). Plaques throughout the aorta were significantly larger in Apoe−/− mice than in Apoe−/−Selp−/− mice, confirming a role of murine P-selectin in atherogenesis. Plaques throughout the aorta were also significantly larger in Apoe−/−Selp−/−TgSELP+/− mice than in Apoe−/−Selp−/− mice. Plaque sizes were similar for males and females of each genotype (Fig. 1). Similarly, plaques in the aortic root were larger in Apoe−/−Selp−/−TgSELP+/− mice than in Apoe−/−Selp−/− mice (Fig. 2). Furthermore, immunofluorescence revealed more macrophages expressing the marker MOMA-2 in the aortic root lesions of Apoe−/−Selp−/−TgSELP+/− mice than in Apoe−/−Selp−/− mice (Fig. 3). Plaques were smaller in Apoe−/−Selp−/−TgSELP+/− mice than in Apoe−/− mice (Figs. 1–3). Nevertheless, these data demonstrate that human P-selectin contributes to atherosclerosis after 8 or 16 weeks on a Western diet.
Figure 1. Apoe−/−Selp−/−TgSELP+/− mice develop larger atherosclerotic plaques throughout aortas than Apoe−/−Selp−/− mice after 8- and 16-week Western diet.
Left, aortas with plaques highlighted by Oil Red O from Apoe−/−Selp−/−TgSELP+/−, Apoe−/−Selp−/−, and Apoe−/− mice after 8-week (A) and 16-week (B) Western diet. Right, quantification of lesion areas. Solid circles, male mice. Open circles, female mice. Horizontal line represents mean of all mice. *P < 0.05.
Figure 2. Apoe−/−Selp−/−TgSELP+/− mice develop larger atherosclerotic plaques at aortic root than Apoe−/−Selp−/− mice after 8- and 16-week Western diet.
Left, cryosections of aortic roots with plaques highlighted by Oil Red O from Apoe−/−Selp−/−TgSELP+/−, Apoe−/−Selp−/−, and Apoe−/− mice after 8-week (A) and 16-week (B) Western diet. Right, quantification of lesion areas. Solid circles, male mice. Open circles, female mice. Horizontal line represents mean of all mice. Scale bar, 200 μm. *P < 0.05.
Figure 3. Apoe−/−Selp−/−TgSELP+/− mice have more plaque macrophages than Apoe−/−Selp−/− mice after 8- and 16-week Western diet.
Left, cryosections of aortic roots with macrophages stained by MOMA-2 mAb (green) from Apoe−/−Selp−/−TgSELP+/−, Apoe−/−Selp−/−, and Apoe−/− mice after 8-week (A) and 16-week (B) Western diet. Solid white lines highlight plaques. Right, quantification of macrophage-positive areas. Numbers of mice from each genotype are indicated. Scale bar, 200 μm. *P < 0.05.
Aortic endothelial cells in Apoe−/−Selp−/−TgSELP+/− mice express human P-selectin before weaning and after 16-week Western diet
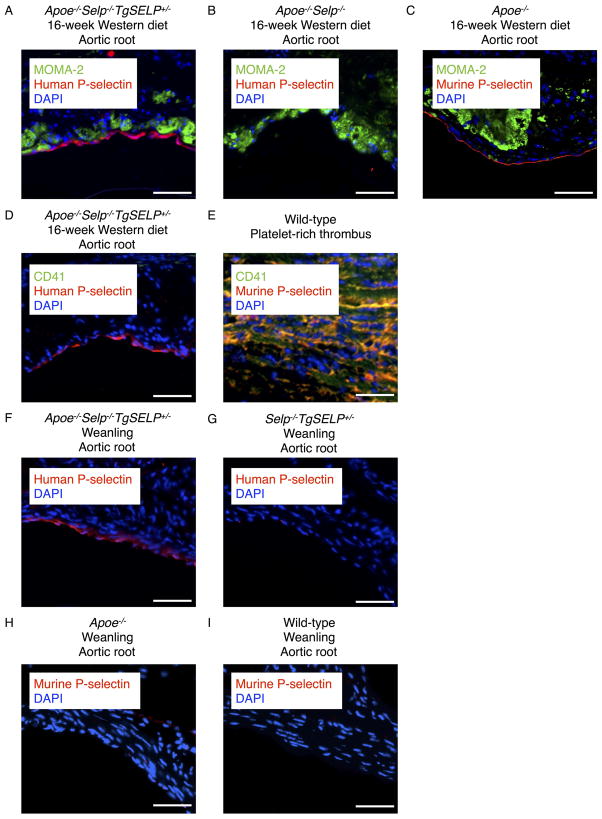
We used confocal immunofluorescence microscopy to probe expression of human P-selectin in aortas. Goat polyclonal IgG that recognizes both human and murine P-selectin30, 36 identified human P-selectin in endothelial cells overlying aortic root plaques of Apoe−/−Selp−/−TgSELP+/− mice after 16 weeks on a Western diet (Fig. 4A). The staining was specific for human P-selectin, because no staining was observed in endothelial cells overlying plaques of Apoe−/−Selp−/− mice (Fig. 4B). We also noted staining for murine P-selectin in endothelial cells overlying aortic root plaques of Apoe−/− mice (Fig. 4C). Expression of P-selectin on platelets was excluded by negative staining with antibody to the platelet marker CD41 (integrin αIIb subunit) (Fig. 4D), although the antibody readily colocalized CD41 with P-selectin in platelet-rich venous thrombi from wild-type mice (Fig. 4E). We observed no staining for human or murine P-selectin in lesional macrophages (Fig. 4, A and C). Resident macrophages (F4/80hiCD11bhiICAM-2hi)37, 38 from the peritoneum of Apoe−/−Selp−/−TgSELP+/− or Apoe−/− mice expressed human or murine P-selectin, respectively (Supplemental Fig. II). In both genotypes, however, inflammatory macrophages (F4/80intCD11bintICAM-2lo)37, 38 recovered from the peritoneum after challenge with thioglycollate did not express P-selectin (Supplemental Fig. II).
Figure 4. Aortic endothelial cells in Apoe−/−Selp−/−TgSELP+/− mice express human P-selectin before weaning and after 16-week Western diet.
(A–C) Cryosections of aortic roots from Apoe−/−Selp−/−TgSELP+/−, Apoe−/−Selp−/−, and Apoe−/− mice after 16-week Western diet. MOMA-2 mAb stains macrophages green. Anti-human/murine P-selectin IgG stains red. DAPI stains nuclei blue. (D) Cryosection of aortic root from an Apoe−/−Selp−/−TgSELP+/− mouse after 16-week Western diet. Anti-P-selectin IgG stains red. DAPI stains nuclei blue. No platelet staining with anti-CD41 mAb (green) is observed. (E) Control cryosection of a platelet-rich thrombus from the inferior vena cava of a wild-type mouse. Antibodies to P-selectin (red) and CD41 (green) colocalize on platelets (yellow). (F–I) Cryosections of aortic roots from Apoe−/−Selp−/−TgSELP+/−, Selp−/−TgSELP+/−, Apoe−/−, and wild-type weanlings. Anti-P-selectin IgG stains red. DAPI stains nuclei blue. Images are representative of 3–8 experiments. Scale bar, 50 μm.
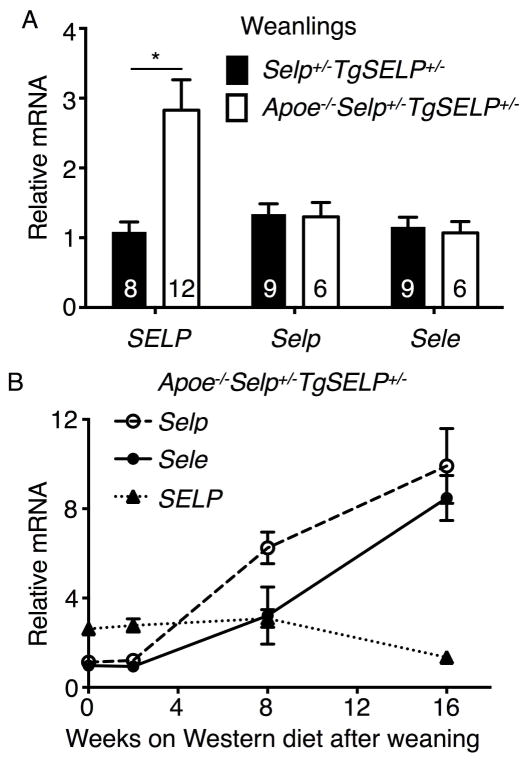
We next asked whether aortic endothelial cells in Apoe−/−Selp−/−TgSELP+/− mice upregulate expression of human P-selectin at an even earlier age. We consistently observed staining for human P-selectin in aortic root endothelial cells of 3–4-week-old Apoe−/−Selp−/−TgSELP+/− pups before weaning (Fig. 4F). In contrast, we observed no staining for human P-selectin in aortic root endothelial cells of 3–4-week-old Selp−/−TgSELP+/− pups (Fig. 4G). Unlike the staining for human P-selectin, we observed no staining for murine P-selectin in aortic root endothelial cells of Apoe−/− pups (Fig. 4H) or of wild-type pups (Fig. 4I). These data suggest that hyperlipidemia from ingestion of Apoe−/− maternal milk is sufficient to trigger inappropriate expression of human P-selectin, but not murine P-selectin, in aortic endothelial cells of weanlings before overt atherosclerosis develops. To further test this hypothesis, we quantified mRNA encoding human P-selectin, murine P-selectin, and murine E-selectin in aortas of 3–4-week-old Apoe−/−Selp+/−TgSELP+/− and Selp+/−TgSELP+/− weanlings. Human SELP transcripts were ~3-fold higher in Apoe−/−Selp+/−TgSELP+/− weanlings than in Selp+/−TgSELP+/− weanlings (Fig. 5A). In contrast, the relative levels of murine Selp and Sele transcripts were equivalent in weanlings of both genotypes. Human SELP transcripts in aortas of Apoe−/−Selp+/−TgSELP+/− mice remained nearly constant during 16 weeks on a Western diet, whereas murine Selp and Sele transcripts progressively increased (Fig. 5B). These data demonstrate that the Apoe−/− background upregulates human SELP expression in aortic endothelial cells before weaning, consistent with a causal role for human P-selectin early in atherogenesis.
Figure 5. Human SELP transcripts are elevated in aortas of Apoe−/−Selp−/−TgSELP+/− weanlings but do not increase further on Western diet.
(A) Quantification of human SELP, murine Selp, and murine Sele mRNA in aortas from Selp+/−TgSELP+/− and Apoe−/−Selp+/−TgSELP+/− weanlings. Numbers of aortas from each genotype are indicated. (B) Quantification of SELP, Selp, and Sele mRNA in aortas from Apoe−/−Selp+/−TgSELP+/− mice before and during Western diet. The data represent the mean ± SEM from 3–10 mice per group. *P < 0.05.
Human P-selectin on both platelets and endothelial cells is required to promote atherosclerosis
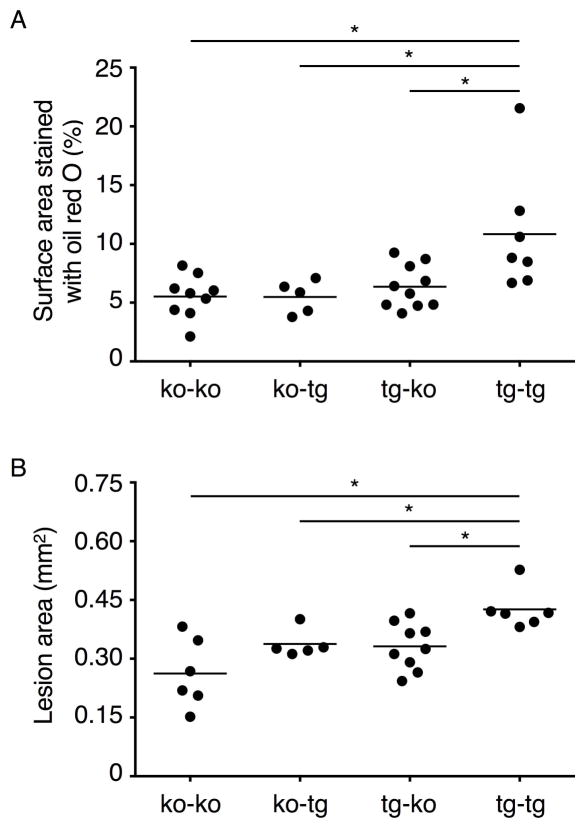
We used bone marrow transplantation to examine the relative contributions of human P-selectin on hematopoietic and non-hematopoietic cells to atherosclerosis. At age 6 weeks, Apoe−/−Selp−/− and Apoe−/−Selp−/−TgSELP+/− mice were lethally irradiated and reconstituted with bone marrow of either genotype to obtain 4 groups of animals: Apoe−/−Selp−/− bone marrow transplanted into Apoe−/−Selp−/− mice (ko-ko), Apoe−/−Selp−/− bone marrow transplanted into Apoe−/−Selp−/−TgSELP+/− mice (ko-tg), Apoe−/−Selp−/−TgSELP+/− bone marrow transplanted into Apoe−/−Selp−/− mice (tg-ko), and Apoe−/−Selp−/−TgSELP+/− bone marrow transplanted into Apoe−/−Selp−/−TgSELP+/− mice (tg-tg). At age 12 weeks (6 weeks after transplantation), flow cytometry confirmed that more than 90% of circulating platelets were of donor origin (Supplemental Fig. III). All groups were then fed a Western diet for 15 weeks before sacrifice. Body weights, blood platelet and leukocyte counts, and plasma cholesterol and triglycerides were equivalent in all groups (Supplemental Fig. IV). Plaque sizes along the aorta (Fig. 6A) and in the aortic root (Fig. 6B) were equivalent in the ko-ko, ko-tg, and tg-ko groups but were significantly larger in the tg-tg group. These results indicate that human P-selectin from either hematopoietic cells (presumably platelets) or non-hematopoietic cells (presumably endothelial cells) is insufficient to augment lesion area. Instead, human P-selectin from both cellular origins is required to promote atherogenesis.
Figure 6. Human P-selectin on both platelets and endothelial cells is required to promote atherosclerosis.
(A) Quantification of lesion areas in aortas. (B) Quantification of lesion areas at aortic root. Each solid circle represents one male mouse. Horizontal line represents the mean. Transplant groups are Apoe−/−Selp−/− bone marrow transplanted into Apoe−/−Selp−/− mice (ko-ko), Apoe−/−Selp−/− bone marrow transplanted into Apoe−/−Selp−/−TgSELP+/− mice (ko-tg), Apoe−/−Selp−/−TgSELP+/− bone marrow transplanted into Apoe−/−Selp−/− mice (tg-ko), and Apoe−/−Selp−/−TgSELP+/− bone marrow transplanted into Apoe−/−Selp−/−TgSELP+/− mice (tg-tg). ko, knockout; tg, transgenic. *P < 0.05.
Discussion
Our results demonstrate that expression of human SELP augments atherosclerosis in Apoe−/− mice (Figs. 1–3). This effect requires human P-selectin on both platelets and endothelial cells (Fig. 6).
Apoe−/− weanlings have elevated plasma cholesterol levels, which progressively increase after transfer to a high-fat/cholesterol diet39. The hyperlipidemic environment is likely responsible for the early pathological expression of human P-selectin in aortic endothelial cells of Apoe−/−Selp−/−TgSELP+/− weanlings and its persistent expression in aortic endothelial cells of Apoe−/−Selp−/−TgSELP+/− mice on the Western diet (Fig. 4). Our data are consistent with expression of P-selectin in arterial endothelial cells overlying human atheromas, strengthening the clinical relevance of our model31, 34. Expression of human P-selectin on the endothelium may facilitate recruitment of monocytes early during atherogenesis. In vitro, oxidized LDL increases SELP mRNA and P-selectin protein in human aortic endothelial cells31. Whether and if so, how modified forms of LDL increase human P-selectin in aortic endothelial cells in vivo remains to be determined. TNF-α and IL-1β, which initiate NF-κB signaling pathways40, upregulate expression of murine Selp and Sele but downregulate expression of human SELP25, 35. These cytokines are found in human atheromas41, 42. Their presence in developing murine atheromas14 may explain the progressive increase in transcripts for Selp and Sele, but not SELP, in aortas of Apoe−/−Selp+/−TgSELP+/− mice on the Western diet (Fig. 5). Indeed, Apoe−/− mice fed standard chow upregulate Selp transcripts in aortas during atherogenesis43. Apoe−/− mice formed larger, macrophage-rich lesions than Apoe−/−Selp−/−TgSELP+/− mice (Figs. 1–3), perhaps in part because Apoe−/− mice expressed murine P-selectin from Selp on both alleles, whereas Apoe−/−Selp−/−TgSELP+/− mice expressed human P-selectin from SELP on only one allele. The progressive increase in murine Selp transcripts on the Western diet may further increase atherogenesis.
Human P-selectin on both platelets and endothelial cells is required to augment atherogenesis (Fig. 6). We did not detect platelet markers in atheromas of Apoe−/−Selp−/−TgSELP+/− mice (Fig. 4). However, interactions of activated platelets with pre-lesional arterial endothelial cells may be transient. Activated platelets might bind to monocytes or monocyte fragments that first adhere to endothelial cell P-selectin. In venules, interactions of P-selectin on activated platelets with P-selectin glycoprotein-1 on adherent leukocytes mobilize endothelial cell P-selectin from Weibel-Palade bodies to the cell surface44. A similar mechanism could operate in arteries. Moreover, products released from adherent platelets may induce expression of other adhesion receptors or chemokines21. Deleting human P-selectin on either platelets or endothelial cells significantly reduced atherogenesis, suggesting that both cell types contribute equally (Fig. 6). In contrast, deleting murine P-selectin on non-hematopoietic cells reduces atherosclerosis much more than deleting murine P-selectin on hematopoietic cells19. This suggests that murine P-selectin on endothelial cells has the dominant role in atherogenesis. Progressive NF-κB-dependent expression of Selp in endothelial cells might explain this dominance.
Lesional macrophages in Apoe−/− mice were reported to express murine P-selectin22, which with murine P-selectin on platelets19, may comprise the hematopoietic cell component to atherogenesis. Unexpectedly, we did not detect murine P-selectin in lesional macrophages of Apoe−/− mice (Fig. 4). We cannot explain this discrepancy. We did not observe human P-selectin in lesional macrophages of Apoe−/−Selp−/−TgSELP+/− mice (Fig. 4), and P-selectin has not been detected in macrophages of human atheromas31, 34. In the absence of inflammation, resident macrophages from the peritoneum of wild-type or Selp−/−TgSELP+/− mice constitutively express murine or human P-selectin35, 45, which persisted in mice lacking apolipoprotein E (Supplemental Fig. II). However, inflammatory macrophages from the thioglycollate-challenged peritoneum of Apoe−/− or Apoe−/−Selp−/−TgSELP+/− mice did not express murine or human P-selectin (Supplemental Fig. II). Macrophages are heterogeneous46 and arise from different tissues47–49. Further study is required to determine whether hyperlipidemia or inflammation induces some lesional macrophages to express murine or human P-selectin during atherogenesis.
Genes encoding many adhesion receptors and chemokines have conserved NF-κB-responsive elements in humans, mice, and other mammals. Why these elements have diverged in the SELP promoters of humans and other primates remains unclear. Nevertheless, our results offer strong support for the role of P-selectin in human atherogenesis. Therapies that target pathological expression or function of human P-selectin might prevent or limit progression of this disease, which remains a major health challenge across the world.
Supplementary Material
Highlights.
A human SELP transgene (TgSELP) confers human-like expression of P-selectin in mice
Aortas develop larger, macrophage-rich atheromas in Apoe−/−Selp−/−TgSELP+/− mice expressing human but not murine P-selectin than in Apoe−/−Selp−/− mice
Apoe−/−Selp−/−TgSELP+/− mice express human P-selectin in aortic endothelial cells before atheromas develop
Apoe−/−Selp−/−TgSELP+/− mice must express human P-selectin on both platelets and endothelial cells to augment atherogenesis
Acknowledgments
We thank Sumith Panicker for technical assistance and helpful discussion. We also thank the Imaging Facility of the Oklahoma Medical Research Foundation for assistance with confocal microscopy and cryosections.
Sources of funding
This work was supported by National Institutes of Health grants HL034363 and HL085607 to R.P.M. and by American Heart Association SouthWest Affiliate predoctoral fellowship 14PRE1806001 to N.Z.
Abbreviations
- HBSS
Hanks’ balanced salt solution
- IL
interleukin
- LDL
low-density lipoprotein
- NF-κB
nuclear factor κB
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- TNF-α
tumor necrosis factor-α
Footnotes
Disclosures
R.P.M. has equity interest in Selexys and Tetherex Pharmaceutical Corporations. The other authors declare no conflicts of interest.
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders WE, Wilson RW, Ballantyne CM, Beaudet AL. Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood. 1992;80:795–800. [PubMed] [Google Scholar]
- 9.Weller A, Isenmann S, Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor. J Biol Chem. 1992;267:15176–15183. [PubMed] [Google Scholar]
- 10.Auchampach JA, Oliver MG, Anderson DC, Manning AM. Cloning, sequence comparison and in vivo expression of the gene encoding rat P-selectin. Gene. 1994;145:251–255. doi: 10.1016/0378-1119(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff J, Brasel C. Regulation of P-selectin by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1995;210:174–180. doi: 10.1006/bbrc.1995.1643. [DOI] [PubMed] [Google Scholar]
- 12.Dore M, Sirois J. Regulation of P-selectin expression by inflammatory mediators in canine jugular endothelial cells. Vet Pathol. 1996;33:662–671. doi: 10.1177/030098589603300605. [DOI] [PubMed] [Google Scholar]
- 13.Stocker CJ, Sugars KL, Harari OA, Landis RC, Morley BJ, Haskard DO. TNF-alpha, IL-4, and IFN-gamma regulate differential expression of P- and E-selectin expression by porcine aortic endothelial cells. J Immunol. 2000;164:3309–3315. doi: 10.4049/jimmunol.164.6.3309. [DOI] [PubMed] [Google Scholar]
- 14.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. Combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in apoe-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 18.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 20.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo YQ, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Sanders JM, Phan ET, Ley K, Sarembock IJ. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am J Pathol. 2005;167:1511–1518. doi: 10.1016/S0002-9440(10)61237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns SA, DeGuzman BJ, Newburger JW, Mayer JE, Jr, Neufeld EJ, Briscoe DM. P-selectin expression in myocardium of children undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1995;110:924–933. doi: 10.1016/s0022-5223(05)80159-x. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Pan J, Setiadi H, Patel KD, McEver RP. Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J Exp Med. 1996;184:81–92. doi: 10.1084/jem.184.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood. 1999;94:3820–3828. [PubMed] [Google Scholar]
- 26.Pan J, McEver RP. Characterization of the promoter for the human P-selectin gene. J Biol Chem. 1993;268:22600–22608. [PubMed] [Google Scholar]
- 27.Pan J, McEver RP. Regulation of the human P-selectin promoter by Bcl-3 and specific homodimeric members of the NF-κB/Rel family. J Biol Chem. 1995;270:23077–23083. doi: 10.1074/jbc.270.39.23077. [DOI] [PubMed] [Google Scholar]
- 28.Pan J, Xia L, McEver RP. Comparison of promoters for the murine and human P-selectin genes suggests species-specific and conserved mechanisms for transcriptional regulation in endothelial cells. J Biol Chem. 1998;273:10058–10067. doi: 10.1074/jbc.273.16.10058. [DOI] [PubMed] [Google Scholar]
- 29.Pan J, Xia L, Yao L, McEver RP. Tumor necrosis factor-α- or lipopolysaccharide-induced expression of the murine P-selectin gene in endothelial cells involves novel κB sites and a variant activating transcription factor/cAMP response element. J Biol Chem. 1998;273:10068–10077. doi: 10.1074/jbc.273.16.10068. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Zhang N, Shao B, Panicker SR, Fu J, McEver RP. Replacing the promoter of the murine gene encoding P-selectin with the human promoter confers human-like basal and inducible expression in mice. J Biol Chem. 2016;291:1441–1447. doi: 10.1074/jbc.M115.702126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vora DK, Fang ZT, Liva SM, Tyner TR, Parhami F, Watson AD, Drake TA, Territo MC, Berliner JA. Induction of P-selectin by oxidized lipoproteins - separate effects on synthesis and surface expression. Circ Res. 1997;80:810–818. doi: 10.1161/01.res.80.6.810. [DOI] [PubMed] [Google Scholar]
- 32.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- 33.Miyazaki Y, Satoh T, Nishioka K, Yokozeki H. Stat-6-mediated control of P-selectin by substance P and interleukin-4 in human dermal endothelial cells. Am J Pathol. 2006;169:697–707. doi: 10.2353/ajpath.2006.051211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Miner JJ, Yago T, Yao L, Lupu F, Xia L, McEver RP. Differential regulation of human and murine P-selectin expression and function in vivo. J Exp Med. 2010;207:2975–2987. doi: 10.1084/jem.20101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: A juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Ree JH, van den Broek WJ, Dahlmans VE, Groot PH, Vidgeon-Hart M, Frants RR, Wieringa B, Havekes LM, Hofker MH. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis. 1994;111:25–37. doi: 10.1016/0021-9150(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 40.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 41.Kishikawa H, Shimokama T, Watanabe T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima. Role of cell-mediated immunity in human atherogenesis. Virchows Archiv. A, Pathological anatomy and histopathology. 1993;423:433–442. doi: 10.1007/BF01606532. [DOI] [PubMed] [Google Scholar]
- 42.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 43.Molenaar TJ, Twisk J, de Haas SA, Peterse N, Vogelaar BJ, van Leeuwen SH, Michon IN, van Berkel TJ, Kuiper J, Biessen EA. P-selectin as a candidate target in atherosclerosis. Biochem Pharmacol. 2003;66:859–866. doi: 10.1016/s0006-2952(03)00387-3. [DOI] [PubMed] [Google Scholar]
- 44.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: Role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tchernychev B, Furie B, Furie BC. Peritoneal macrophages express both P-selectin and PSGL-1. J Cell Biol. 2003;163:1145–1155. doi: 10.1083/jcb.200310079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17:159–168. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 48.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.