Abstract
Background/Objectives
Hypothalamic obesity (HO) is a common complication of hypothalamic tumors and effective therapies are lacking. The objective of this pilot study was to investigate changes in body weight before and during treatment with exenatide.
Subjects/Methods
This was a prospective, open-label 52-week pilot study of exenatide (10 mcg b.i.d.) in adults with HO. Ten patients enrolled and 8 completed the study. Study measures included indirect calorimetry, body composition, buffet meals, diet recall, actigraphy and hormone assays.
Results
Participants were obese with a baseline weight of 137.2 ± 37.6 kg. Exenatide therapy was well tolerated. Change in weight with exenatide therapy was not significant (−1.4 ± 4.3 kg [95% CI −2.2 to 4.9], p=0.40) but 6 of 8 completers lost weight (−6.2 to −0.2 kg). Participants reported significantly lower intake on food recall during treatment compared with baseline (7837.8 ± 2796.6 vs. 6258.4 ± 1970.7 kJ [95% CI −2915.8 to −242.6], p= 0.027) but there was no change in intake during buffet meals.
Conclusions
We did not observe significant weight loss in patients with HO treated with exenatide but 75% of completers had stable or decreasing weight. Further studies are needed to evaluate weight loss efficacy in patients with HO.
Keywords: Hypothalamic obesity, GLP-1, Energy balance
Introduction
Hypothalamic obesity occurs in up to 60% of patients with tumors in the hypothalamic region, most commonly craniopharyngiomas. Despite excellent overall survival rates, craniopharyngioma patients have a 5-times greater overall mortality rate and a 3-times greater cardiovascular mortality rate than the general population.(1) Survivors who develop obesity have greater morbidity and mortality than normal weight survivors.(2)
Hypothalamic obesity is characterized by reduced energy expenditure, insulin resistance and leptin resistance though increased energy intake is not a consistent finding.(3–7) Treatments for hypothalamic obesity are limited and successful prevention and treatment is vital to decrease associated morbidity and mortality. Exenatide (Byetta®) is a GLP-1 receptor agonist (GLP1RA) approved for treatment of type 2 diabetes (T2D). GLP1RAs improve insulin sensitivity, slow gastric emptying and increase satiety, resulting in an average sustained weight loss of 4.4 kg.(8) GLP1RA may also increase energy expenditure.(9–11) There are case reports of successful weight loss after treatment with GLP1RAs in patients with hypothalamic obesity, but no prospective pilot studies.(12–14) The goal of this clinical trial was to investigate changes in body weight before and during treatment with exenatide.
Methods
Participants
Participants were 18–40 y.o. with a history of hypothalamic lesion >6 months post-treatment and BMI >30 kg/m2. Detailed enrollment information is included in the supplement.
Experimental procedure
A comprehensive assessment occurred during weeks 0 – 2 (baseline) and weeks 50 – 52 (during treatment). Following a 2 week baseline assessment, participants received exenatide subcutaneous twice daily for 50 weeks. Exenatide was initiated at 5 mcg/dose and increased to 10 mcg/dose after 8 weeks (study week 10), unless the patient had persistent nausea/vomiting. Monitoring visits occurred at study week 6, 10, 14, 22, 30 and 38. Detailed study procedures are included in the supplement.
Statistical Analysis
Continuous variables were expressed as mean ± SD. The primary endpoint was change in body weight over time as assessed by paired t-test. Statistical analysis was performed using SPSS version 22.
For this pilot study, it was estimated that 7 subjects would be adequate to achieve 80% power to detect a difference of change in weight of 5.7 kg, presuming a standard deviation of 4.5 kg. Based on previous studies with exenatide, we presumed a 30% dropout rate and recruited 10 subjects.
Results
Enrollment and Adverse Events
Ten subjects enrolled in the study. All patients were on treatment for panhypopituitarism (≥2 hormone deficiencies). Baseline characteristics are detailed in Table 1.
TABLE 1.
Baseline characteristics of participants. Results are presented as mean ± SD. All patients were on stable treatment for hormone deficiencies except one patient with untreated growth hormone deficiency. Homeostasis model assessment of insulin resistance (HOMA) was calculated using the formula insulin (mU/L) x glucose (mg/dL)/405.(18) Insulin sensitivity was estimated by calculating the fasting glucose insulin ratio by quantitative insulin-resistivity check index (QUICKI= 1/[log insulin (mU/L) + log glucose (mg/dL)]).
| Age (years) | 27.5 ± 7.8 |
| Race (% White) | 100 |
| Gender (% female) | 70 |
| BMI (kg/m2) | 47.5 ± 10.8 |
| Body fat (%) | 50.6 ± 5.3 |
| Hemoglobin A1C (%) | 5.4 ± 0.5 |
| HOMA | 5.94 ± 3.55 |
| QUICKI | 0.30 ± 0.24 |
| Hypothalamic Injury | Craniopharyngioma (n= 6) Astrocytoma (n= 1) Other pituitary/hypothalamic tumor (n= 3) |
| Years since diagnosis | 11 ± 9.5 |
| Childhood onset (%) | 60 |
| Hormone deficiencies | Central hypothyroidism (n= 10) Gonadotropin deficiency (n= 9) Growth hormone deficiency (n= 5) Diabetes insipidus (n= 7) Adrenal insufficiency (n= 7) |
The most common adverse events were nausea/vomiting (7/10 subjects), joint pain (3/10 subjects) and injection site reactions (3/10 subjects). One patient did not increase to the 10 mcg dose and subsequently withdrew. Examination of returned pens showed 60% with high adherence (>75% medication use) and 24% with moderate adherence (50–75% medication use). Medication compliance did not correlate with weight loss.
Two subjects withdrew at 14–22 weeks. One 18 y.o. male patient (baseline weight 149.8 kg) withdrew due to increased irritability and mood swings. Symptoms improved after cessation of treatment. One 18 y.o. female patient (baseline weight of 115.3 kg) withdrew due to kidney stones (self-resolving without intervention).
Baseline vs. Exenatide therapy
Weight
Patients were morbidly obese (138.3 ± 41.5 kg, range 79.4 to 225.5 kg). Patients treated with exenatide lost an average of −1.4 ± 4.3 kg ([95% CI −2.2 to 4.9], p=0.40, n=8). Peak weight loss was at week 22 (−3.0 kg ± 5.8, range −13.2 to 3.8 kg). Six patients lost an average of 3.3 kg (range −6.2 to −0.2 kg), equivalent to 2.4% of their body weight (range −4.6% to −0.1%). The two patients who withdrew gained 1.7 kg and 2.5 kg over the 1-year post enrollment. The patterns of weight change over time are depicted in figure 1. Body composition data is presented in supplemental table 1.
FIGURE 1.
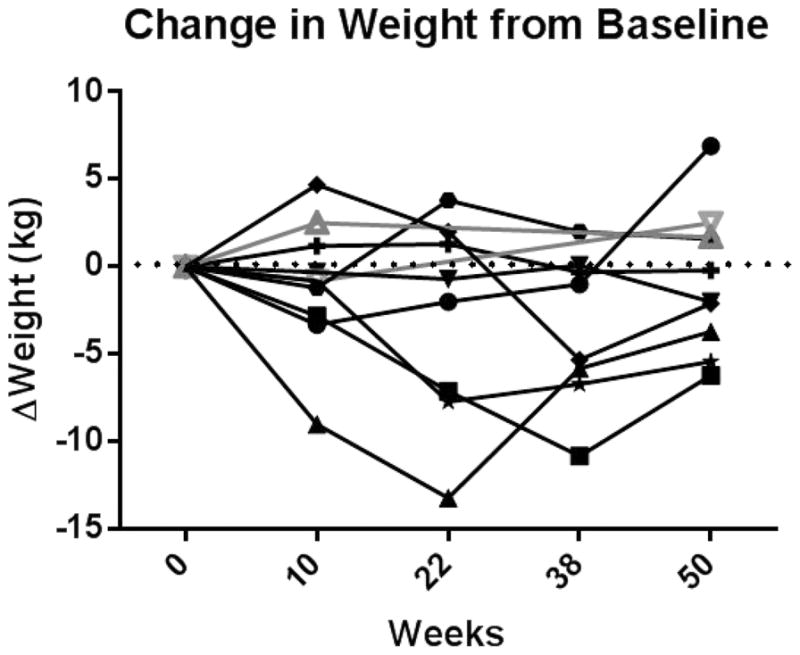
Change in weight from baseline in the 8 patients treated with exenatide (black, solid markers and lines) and the 2 patients who withdrew from the study after week 14 (grey, open markers and lines).
Food Intake
On 24-hour dietitian led food recall, participants reported significantly lower energy intake during treatment with exenatide compared with baseline (7837.8 ± 2796.6 vs. 6258.4 ± 1970.7 kJ [95% CI −2915.8 to −242.6], p= 0.027). The change in free-living energy intake was highly correlated with weight change (R2= 0.70, p= 0.009). All 6 patients who lost weight had reduced food intake (−2276.3 ± 1111.4 kJ) versus an increase of 509.4 ± 301.8 kJ in the 2 patients that gained weight. Buffet meal food consumption did not change with treatment (Table 2). Macronutrient proportions were consistent and accordance with dietary recommendations.(15) Gastric emptying time did not change significantly with treatment.
TABLE 2.
Energy and macronutrients consumed during admission to the Vanderbilt Clinical Research Center. Meals contained 5651 kJ and energy consumed was determined using the Nutrition Data System for Research (2011, University of Minnesota, Minneapolis, MN). Results are presented as mean ± SD and p-values were determined using paired t-test.
| Week 0 | Week 50 | p-value | |
|---|---|---|---|
| Dinner (n= 8) | |||
| Total kJ | 4183.6 ± 1054.4 | 4538.8 ± 1213.8 | 0.33 |
| Carbohydrates (%) | 54.8 ± 4.7 | 55.7 ± 3.7 | 0.55 |
| Protein (%) | 18.1 ± 3.9 | 17.7 ± 3.3 | 0.67 |
| Fat (%) | 27.0 ± 3.5 | 26.3 ± 1.8 | 0.58 |
| Breakfast (n= 6) | |||
| Total kJ | 3446.8 ± 1193.7 | 3515.4 ± 1207.5 | 0.87 |
| Carbohydrates (%) | 48.2 ± 5.6 | 50.9 ± 8.8 | 0.62 |
| Protein (%) | 16.0 ± 1.8 | 15.1 ± 3.4 | 0.60 |
| Fat (%) | 35.8 ± 4.2 | 34.0 ± 5.6 | 0.64 |
Energy Expenditure
Due to a technical issue, one patient did not have resting energy expenditure (REE) data available. REEmeasured (n= 7) did not change significantly during treatment with exenatide (−157.7 ± 657.3 kJ [95% CI −766.1 to 450.6], p= 0.40). There was also no difference in REE%expected (86.1 ± 12.2% vs. 85.4% ± 13.0%, p= 0.51).
Participants wore accelerometers an average of 11.3 valid days per 2-week period. Light activity accounted for 22.4 ± 4.4% (baseline) and 23.3 ± 5.2% (treatment) of accelerometer wear time. Participants spent 35.5 ± 40.8 min/day in moderate/vigorous activity (baseline) versus 25.9 ± 10.9 minutes/day during exenatide therapy (p= 0.48). At baseline, 4 participants met or exceeded the 2008 Physical Activity Guidelines for Americans recommendation of 150 minutes of moderate activity per week (range 74.6 to 942 min/week). During exenatide therapy, 5 participants met or exceeded that recommendation (range 65.0 to 261.0 minutes).
Hormone Assays
The baseline metabolic parameters are listed in table 1. There was no significant change in any metabolic parameter over time. Change in metabolic parameters did not correlate with weight loss. There was not a significant relationship between fat mass and leptin at either time point.
Discussion
This is the first pilot study to assess the efficacy of exenatide as a treatment for adults with HO. Participants tolerated the drug well. We did not observe significant weight loss in patients with HO over 50 weeks of treatment with exenatide with a 95% CI of −2.2 kg to 4.9 kg. Eight patients completed the study and six had stable or decreasing weight (−6.2 to −0.2 kg). While weight loss was small, untreated patients continued to gain weight and our measured weight change may be an underestimation. Previous case reports showed greater than expected weight loss of 12.6 ± 7.1 kg in 11 patients with HO treated with GLP1RAs for ≥ 6 months.(12–14) All but one patient had T2D, while our participants were treated for obesity. It is possible that patients with diabetes have a better response to exenatide, or there may be a publication bias with only successful cases reported in the literature.
Exenatide is thought to cause weight loss through reduction in food intake. Proposed mechanisms include slowing of gastric emptying and stimulation of GLP-1 receptors on vagal afferents, brainstem and hypothalamus.(16) While the hypothalamus does express GLP-1 receptors, it may not play a critical role in GLP-1 signaling, therefore GLP1RA may still be efficacious in the setting of hypothalamic obesity. For example, a decerebrate rat model showed that caudal brainstem processing may be sufficient to preserve GLP-1 mediated food intake reduction and delayed gastric emptying.(17) In our study, while treatment with exenatide did not reduce energy intake during a buffet meal, there was a significant reduction in free-living kJ consumption which correlated with weight loss. Self-report of decreased food intake may be a marker for response to exenatide in this population.
Limitations of this pilot study include the small sample size and lack of separate control group. Our subjects were white and the majority female which may limit generalizability. In order to more clearly evaluate the drug effect, we did not implement any additional diet or lifestyle modifications during this pilot study. The addition of such interventions may improve the weight loss with exenatide and should be considered in future studies. Medication adherence was moderate and it is possible that long-acting GLP1RA could have better efficacy. HO is a heterogeneous disorder and better understanding of each patient’s hypothalamic damage may identify patients with improved responsiveness to GLP1RAs. The majority of our patients developed HO in childhood and longstanding obesity may be more refractory to treatment.
In summary, we observed a reduction in energy intake, but not a significant reduction in weight in patients with HO treated with exenatide for 50 weeks. This is in contrast to previous case reports. Further studies are needed to evaluate if varying GLP1RA doses, early initiation of treatment or stratification based on location of hypothalamic damage can improve weight loss efficacy in patients with HO.
Supplementary Material
STUDY IMPORTANCE.
What is already known about this subject?
Hypothalamic obesity, due to tumors such as craniopharyngiomas, is a form of severe, treatment-resistant obesity
Patients with hypothalamic obesity are at increased risk of morbidity and mortality compared with other tumor survivors
There are case reports that report weight loss with GLP-1 receptor agonists in patients with hypothalamic obesity but there are no prospective, longitudinal studies.
What does this study add?
This pilot study is the first longitudinal, prospective study to evaluate the effects of the GLP-1 receptor agonist exenatide on patients with hypothalamic obesity
We did not observe significant weight loss in adults with hypothalamic obesity treated with exenatide for one year but 75% of completers had stable or decreasing weight.
Acknowledgments
Funding: This study was supported by the CTSA award number KL2TR000446 and UL1TR00445 from the National Center for Advancing Translational Sciences and the NIH grant K23DK101689 from the National Institute of Diabetes, Digestive and Kidney Diseases (AHS). Additional support was provided by the Vanderbilt Physician Scientist Development Program (AHS). Study drug was provided by an investigator initiated grant from AstraZeneca (AHS). Assays were performed by the VUMC Hormone Assay and Analytical Services Core which is supported by NIH grants DK059637 and DK020593. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: AHS serves on a hypothalamic injury associated obesity advisory board for Zafgen, Inc.
References
- 1.Bulow B, Attewell R, Hagmar L, Malmstrom P, Nordstrom CH, Erfurth EM. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab. 1998;83(11):3897–904. doi: 10.1210/jcem.83.11.5240. Epub 1998/11/14. [DOI] [PubMed] [Google Scholar]
- 2.Muller HL, Bueb K, Bartels U, Roth C, Harz K, Graf N, et al. Obesity after childhood craniopharyngioma--German multicenter study on pre-operative risk factors and quality of life. Klin Padiatr. 2001;213(4):244–9. doi: 10.1055/s-2001-16855. Epub 2001/08/31. [DOI] [PubMed] [Google Scholar]
- 3.Roth CL, Gebhardt U, Muller HL. Appetite-regulating hormone changes in patients with craniopharyngioma. Obesity (Silver Spring) 2011;19(1):36–42. doi: 10.1038/oby.2010.80. Epub 2010/04/10. [DOI] [PubMed] [Google Scholar]
- 4.Kim RJ, Shah R, Tershakovec AM, Zemel BS, Sutton LN, Grimberg A, et al. Energy expenditure in obesity associated with craniopharyngioma. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2010;26(7):913–7. doi: 10.1007/s00381-009-1078-1. Epub 2010/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guran T, Turan S, Bereket A, Akcay T, Unluguzel G, Bas F, et al. The role of leptin, soluble leptin receptor, resistin, and insulin secretory dynamics in the pathogenesis of hypothalamic obesity in children. Eur J Pediatr. 2009;168(9):1043–8. doi: 10.1007/s00431-008-0876-x. Epub 2008/12/02. [DOI] [PubMed] [Google Scholar]
- 6.Harz KJ, Muller HL, Waldeck E, Pudel V, Roth C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003;88(11):5227–31. doi: 10.1210/jc.2002-021797. Epub 2003/11/07. [DOI] [PubMed] [Google Scholar]
- 7.Holmer H, Pozarek G, Wirfalt E, Popovic V, Ekman B, Bjork J, et al. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010;95(12):5395–402. doi: 10.1210/jc.2010-0993. Epub 2010/09/10 .2010-0993 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–47. doi: 10.1111/j.1463-1326.2006.00602.x. Epub 2006/06/17 DOM602 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Shalev A, Holst JJ, Keller U. Effects of glucagon-like peptide 1 (7–36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Invest. 1997;27(1):10–6. doi: 10.1046/j.1365-2362.1997.540613.x. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 10.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–58. doi: 10.2337/db14-0302. Epub 2014/06/12. [DOI] [PubMed] [Google Scholar]
- 11.Tan TM, Field BC, McCullough KA, Troke RC, Chambers ES, Salem V, et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131–8. doi: 10.2337/db12-0797. Epub 2012/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons JH, Shoemaker AH, Roth CL. Treatment with glucagon-like Peptide-1 agonist exendin-4 in a patient with hypothalamic obesity secondary to intracranial tumor. Hormone research in paediatrics. 2012;78(1):54–8. doi: 10.1159/000339469. Epub 2012/07/27. [DOI] [PubMed] [Google Scholar]
- 13.Zoicas F, Droste M, Mayr B, Buchfelder M, Schofl C. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol. 2013;168(5):699–706. doi: 10.1530/EJE-12-0997. Epub 2013/02/09. [DOI] [PubMed] [Google Scholar]
- 14.Ando T, Haraguchi A, Matsunaga T, Natsuda S, Yamasaki H, Usa T, et al. Liraglutide as a potentially useful agent for regulating appetite in diabetic patients with hypothalamic hyperphagia and obesity. Intern Med. 2014;53(16):1791–5. doi: 10.2169/internalmedicine.53.1646. Epub 2014/08/19. [DOI] [PubMed] [Google Scholar]
- 15.Medicine Io. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington (DC): The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 16.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. Epub 2007/10/12. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–68. doi: 10.1210/en.2007-1743. Epub 2008/04/19 en.2007-1743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. Epub 1985/07/01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


