Abstract
Objectives
To explore the association between the rate of physical health deterioration, operationalized as rising multi-morbidity overtime, and the longitudinal decline in cognitive function in non-demented older adults.
Design
Longitudinal Study (Baltimore Longitudinal Study of Aging, BLSA)
Setting
Community
Participants
756 BLSA participants, aged 65 or older, followed for an average of 3 years and free of dementia or mild cognitive impairment both at baseline and follow-ups.
Measurements
Standardized neurocognitive tests evaluating mental status, memory, executive function, processing speed and verbal fluency were administered. Multi-morbidity was assessed at each visit as number of diagnosed chronic diseases from a pre-defined list. Faster accumulation of chronic diseases was defined as upper quartile of rate of change in number of diseases over time (≥0.25 diseases/year).
Results
Faster accumulation of chronic diseases was significantly associated with greater rate of decline in Category and Letter Fluency Tests (P=.015 and P=.013 respectively). Similar trends were also found for Trail Making Tests A and B (P<0.1), while no association was found with rate of change in visual and verbal memory.
Conclusion
Although further investigations are required to validate our results and fully understand the underlying mechanisms, these findings suggest that accelerated deterioration of physical health is associated with accelerated decline with aging in specific cognitive domains in non-demented older adults.
Keywords: multimorbidity, cognition, cognitive decline, aging, older adults
INTRODUCTION
Multi-morbidity, the co-occurrence of multiple chronic diseases, affects roughly 3 in 4 individuals aged 65 years and older, and has been recognized as the most common “chronic medical condition” in older persons1.
From a gerontological perspective, the rising burden of multi-morbidity with aging can be interpreted as the clinical manifestation of progressive loss of resilience and homeostatic dysregulation in multiple organs and systems, which characterize aging itself. In fact, when a certain threshold of dysfunction is reached, the multisystem effect of aging becomes evident as multi-morbidity2. Noteworthy, especially in the context of longitudinal studies, the complexity of multisystem effect of aging has been partially resolved and clustered in discrete domains or aging phenotypes3. In particular, neurodegeneration, whose main expression is cognitive decline, is considered one of the typical aging phenotypes. Therefore, if we assumed that multi-morbidity is a proxy measure of age-related multisystem failures, it is reasonable to hypothesize an association between burden of multi-morbidity and cognitive decline.
In addition, at a population level, multi-morbidity presents new challenges to clinical decision making in clinical practice and health care organizations, which tend to be organized around specific disease diagnoses4-6. The co-occurrence of multiple chronic conditions, in fact, is a well-known risk factor for hospital admission, longer hospital stays, polypharmacy, physical function decline and premature death7. Besides, it is a common clinical observation that older patients with accelerated accumulation of multi-morbidity tend to have an accelerated worsening of their cognition, including slight chronic cognitive decline accompanying multiple chronic diseases and episodes of acute delirium. This cognitive deterioration may not evolve to frank dementia and may even improve if the underlying diseases are successfully controlled. However, whether deterioration of physical health, operationalized as rising multi-morbidity, can be one of the multiple pathways leading to cognitive decline in older adults has not been fully investigated. Previous studies examining the influence of co-morbidities on clinical progression of dementia provided contradictory results and only one attempt to explore the effect of incident medical conditions on cognitive function in a normal adult population was reported8-13. However, in this study, cognitive assessment was limited solely to verbal memory and psychomotor speed13. Therefore, while previous literature largely explored the effect of single chronic diseases, such as, for example, diabetes14, congestive heart failure15, COPD16 and chronic kidney disease17 on cognition, knowledge about neuropsychological deficits associated with the co-presence of multiple medical conditions is still limited. Indeed, understanding whether the accumulation of multiple chronic diseases, independent of their type, accelerates the decline of cognitive performance, even in non-demented older adults, may help to better understand pathological mechanisms leading to cognitive deterioration in older adults and to develop preventive strategies.
Using data from the Baltimore Longitudinal Study of Aging (BLSA), including participants aged 65 years or older and free of diagnosis of mild cognitive impaired (MCI) or dementia, we explored the relationship between the rate of change in number of chronic diseases and rate of change in cognitive performance. In particular, the aims of the present study were (1) to test whether participants with accelerated deterioration of physical health, operationalized as faster accumulation of chronic diseases over time, experience concurrent accelerated longitudinal decline in cognitive performance compared to the rest of the population and independent of potential confounders, and (2) to determine whether specific cognitive domains are differentially affected by faster accumulation of chronic diseases over time.
METHODS
Study design and setting
The BLSA is a study of human aging established in 1958 and conducted by the National Institute on Aging (NIA) Intramural Research Program. A general description of the study population and enrollment procedures and criteria has been previously reported18. Briefly, the BLSA continuously enrolls healthy volunteers aged 20 and older who are followed for life regardless of changes in health and functional status. Presently, participants are examined over three days of testing at the NIA Clinical Research Unit in Baltimore at intervals of 1 to 4 years, with more frequent follow up visits for older ages. Certified nurse practitioners and certified technicians administer all assessments according to standardized protocols.
Participants
The sample for the current study consisted of 756 participants, aged 65 or older, who attended their first visit between May 2005 and September 2013 and were followed for an average of 3 years (range 0-8 years). Of these, 515 (68.12%) underwent at least one follow up visit. The number of participants by length of follow up and the number of observations by follow up time (years) are also provided in TableS1a and TableS1b, respectively. All data used in these analyses were from participants who were considered cognitively non-impaired and non-demented both at baseline and at follow up visits. Visits after the onset of symptoms of cognitive impairment indicative of MCI or prevalent or incident dementia defined by standardized consensus criteria procedures for the BLSA, were excluded from analysis19-20.
Measurements
Cognitive assessment
At each study visit, standard neuropsychological tests were administered by highly trained psychometricians. The Mini Mental State Examination (MMSE; n = 702) assessed global cognitive function. Letter Fluency (n = 652) and Category Fluency (n = 652) examined phonemic and semantic fluency, respectively. The Benton Visual Retention Test (BVRT; n =749) evaluated short term visual memory and visuo-constructional skills. The California Verbal Learning Test (CVLT), including Learning and Immediate Free Recall (n =684), Short Delay Free Recall (n=683) and Long Delay Free Recall (n=679), measured verbal learning and memory. The Digit Span Forward (n = 750), Backward (n = 750) and Digit Symbol Substitution Test (N=697) tests assessed attention and working memory. The Trail Making Test Part A (n = 653) and Part B (n = 647) evaluated psychomotor speed, attention, and executive functions. The Card Rotations Test (n = 715) measured spatial ability.
For BVRT and the Trail Making Test, worse performance was indicated by higher scores. For all other neuropsychological measures, worse performance was indicated by lower scores.
Multi-morbidity
In recent years, the study of multi-morbidity has gained considerable interest in the literature although no standard and widely acknowledged operational definition of multi-morbidity has emerged. One of the most common approaches is to define multi-morbidity as a count of number of diseases21. For this purpose, Fortin et al suggested considering (at least) the 12 most prevalent chronic diseases with high impact or burden in a given population22. Following these recommendations, we selected “a priori” a list of 13 candidate chronic conditions that could be reliably adjudicated based on the data available that are known to have high prevalence and associated with high disability and mortality risk in older adults. Most of these conditions (hypertension, diabetes, coronary artery disease, congestive heart failure, stroke, chronic obstructive pulmonary disease, cancer, Parkinson’s disease, history of hip fracture and lower extremities joint disease) were defined using standard criteria and algorithms similar to those used in the Women’s Health and Aging Study23. In addition, anemia was defined as hemoglobin < 12g/dl in women and < 13 g/dl in men24; chronic kidney disease was defined as glomerular filtration rate estimated using the MRDR equation <60 ml/l25; peripheral arterial disease was defined as ankle-brachial index measured by Doppler stethoscope < 0.926. The presence of each chronic condition was ascertained at baseline and follow-ups. Details about the diagnostic criteria for each condition and their prevalence in our study population are also presented in TableS2. As in previous studies, we operationalized multi-morbidity as number of diagnosed diseases according to standard clinical criteria27.
Covariates
Baseline age and education were assessed in years. Binary covariates included sex (male = 1; female = 0) and race (white = 1; nonwhite = 0). Baseline number of diseases, among those included in the definition of multi-morbidity presented above, was treated as an ordinal variable (range 0-8). Depression was defined as a score of 16 or greater on the Center for Epidemiologic Studies-Depression (CES-D) Scale28. Current and former smokers were ascertained by self-reported questionnaire. Alcohol use was ascertained by self-reported questionnaire as number of drink per week.
Statistical analyses
Summary statistics of the population at the baseline are presented as mean ± standard deviation (SD) or percentage. Baseline prevalence of each disease was also calculated and presented in Supplemental Materials.
Linear mixed models were used to explore the longitudinal association between rising multi-morbidity and decline in cognitive performance overtime. First of all, using linear mixed models, we estimated individual longitudinal rate of change (or slopes) in multi-morbidity, operationalized as number of diagnosed chronic diseases. Multi-morbidity was used as dependent variable while intercept and time (of follow up) were used both as fixed effects and random effects. Unstructured covariance structure was assumed on the random effects. Secondly, linear mixed models were used again with each longitudinal cognitive measure as outcome to explore association between changes in multi-morbidity and changes in cognition. In particular, person-specific rates of change overtime in number of diseases, which were estimated from the first step, were used as predictors of rate of change overtime in cognitive tests scores. The individual slopes of rising multi-morbidity overtime were ranked and dichotomized as “group with faster accumulation of multi-morbidity” versus the rest of the population. Group with faster accumulation of multi-morbidity was defined as rate of accumulation greater or equal to the upper quartile cut-off of the sample population (≥0.25 diseases/year). Main predictors included group with faster accumulation of multi-morbidity, time and group with faster accumulation of multi-morbidity*time. We also included the following covariates baseline age, sex, race, education and baseline number of chronic diseases and their interactions with time. We also included the following covariates baseline age, sex, race, education and baseline number of chronic diseases and their interactions with time. Results are presented as β coefficients and P values corresponding to “group with faster accumulation of multi-morbidity *time” interactions for all the neuropsychological tests. Baseline age and education were used as continuous covariates and centered on the average value. The other covariates were used as binary/ordinal variables.
Moreover, actual annual changes in cognitive tests scores were also calculated, according to group with faster accumulation of multi-morbidity versus the rest of the population, in order to verify whether the statistical models appropriately fitted the crude data. In particular, we firstly calculated crude changes per year as individual difference between last visit and first visit scores, divided by number of years of follow up. Then, the crude changes were adjusted by baseline age and sex, weighted on length of follow up and presented as means (± standard deviations) according to the two groups.
In addition, sensitivity analyses were performed to investigate whether incidental diagnoses of specific diseases included in metric of multi-morbidity may be associated with longitudinal changes in cognitive performance, suggesting that our original results may be driven by those conditions instead of global multi-morbidity. Therefore, we tested for each of the 13 diseases included in our list of candidate conditions whether their presence/absence at each time of the study (time-dependent predictive variable) may be associated with decline in cognitive function overtime (outcome variable), adjusting for the same covariates. Additionally, we also fitted 13 different variants of the original model, obtained by removing each time one of 13 conditions, to test whether the results were still consistent with the original ones.
To exclude the possibility that participants who were followed longer were followed longer were younger or healthier and the rate of change in number of diseases could be a biased estimated, we compute additional Spearman correlation analyses.
To verify whether the association observed was explained by the future development of MCI or dementia, additional sensitivity analyses were performed excluding from our sample population all individuals with prevalent or incident diagnosis of MCI and dementia.
Finally, a fully-adjusted model including depression, smoke status and alcohol use as covariates was run to exclude the influence of these additional potential confounders.
All analyses were performed using the SAS statistical package, version 9.3 (SAS institute Inc., Cary, NC) and R 3.1.2.
RESULTS
Baseline results
Baseline population included 756 BLSA participants (391 men and 365 women) aged 65-95 years (mean age ± SD = 73.8 ± 7.4 years). Of these, 531 (70.2%) were white. Average years of education were 16.6 ± 2.5. The mean number of diseases was 2.4 ±1.6, ranging from 0 to 8. Additionally, characteristics of the baseline population (including age, sex and number of diseases) are also presented according to different cognitive tests in Supplemental Materials (TableS3). The most prevalent diseases were hypertension (67.2%), lower extremities joint diseases (51.8%) and chronic kidney disease (34.1%) (TableS2).
Baseline age-adjusted means of all neuropsychological test scores are presented in Table1, according to baseline number of diseases (0-1 vs ≥2 diseases). After adjustment for baseline age, participants with at least 2 diseases had significantly lower performance on the Digit Symbol Test and Trail Making Test B compared to those with none or one disease. However, the association was no longer significant when the analysis was also adjusted for sex, race and education.
Table1.
Age adjusted means (±Standard Deviations) for all neuropsychological measures according to number of diseases (0-1 vs ≥2 diseases) in the baseline population.
|
Baseline population
|
||||||
|---|---|---|---|---|---|---|
| Domain | Neuropsychological Measure |
N | Possible range of values (best –worse) |
0-1 disease (mean, SD) |
≥2 diseases (mean, SD) |
Age adjusted P value |
|
Global
cognition |
MMSE | 702 | 30-0 | 28.7 (0.1) | 28.6 (0.1) | .192 |
|
| ||||||
| Verbal fluency | Category Fluency | 652 | 35*-0 | 15.6 (0.2) | 15.5 (0.1) | .784 |
| Letter Fluency | 652 | 31.7*-0 | 14.1 (0.3) | 14.3 (0.2) | .534 | |
|
| ||||||
|
Figural
Memory |
Benton Visual Retention Test (BVRT) |
749 | 0-30# | 7.2 (0.3) | 6.9 (0.2) | .397 |
|
| ||||||
|
Verbal
Memory |
California Verbal Language (CVLT) Learning & Immediate Free Recall |
684 | 80-0 | 50.8 (0.9) | 51.5 (0.6) | .498 |
| CVLT Short Delay Free Recall |
683 | 16-0 | 9.9 (0.2) | 10.2 (0.2) | .284 | |
| CVLT Long Delay Free Recall |
679 | 16-0 | 10.3 (0.3) | 10.7 (0.2) | .179 | |
|
| ||||||
|
Attention and
Working Memory |
Digitspan Forward | 750 | 14-0 | 7.7 (0.2) | 7.71 (0.1) | .929 |
| Digitspan Backward | 750 | 14-0 | 6.8 (2.6) | 6.6 (2.7) | .507 | |
| Digit Symbol | 697 | 93-0 | 45.3 (0.7) | 42.9 (0.4) | .005 | |
|
| ||||||
|
Executive
functions |
Trail Making Test A | 653 | 5-300 | 32.8 (0.9) | 34.8 (0.6) | .069 |
| Trail Making Test B | 647 | 5-300 | 80.4 (3.2) | 90.8 (2.0) | .006 | |
|
| ||||||
| Spatial Ability | Card Rotations Test | 715 | 224-(−224) | 82.9 (2.5) | 82.8 (1.7) | .959 |
best value in our study population;
worse value in our study population (for tests that do not have a standardized range of possible values)
SD=standard deviation; MMSE=Mini Mental State Examination; BVRT=Benton Visual Retention Test; CVLT= California Verbal Language Test.
Longitudinal results
In our sample population, the number of chronic diseases significantly increased over the follow up time (β=0.19, P<.001). Informative statistics on annual rates of change in number of chronic diseases are reported in the Supplemental Material (TableS4). Estimated beta coefficients (standard errors) and corresponding P values for the average annual rate of change of cognitive measures (“time”) are presented in Table 2. Estimated beta coefficients (standard errors) and corresponding P values for the comparison in rates of change in cognitive measures between the group with faster accumulation of multi-morbidity and the rest of the population (“group with faster accumulation of multi-morbidity *time”) are also presented in the same table. After adjusting for baseline age, sex, race, education and baseline number of diseases, participants who experienced faster accumulation of chronic diseases over the follow up had significantly greater longitudinal rate of decline in Category and Letter fluency performance compared to those with a lower rate of change in number of diseases over time (P=.015 and P=.013, respectively, figure1A and 1B). Trail Making Test Part A and Part B showed similar associations that did not reach statistical significance (P=.084 and P=.075, respectively). Faster accumulation of diseases was not significantly associated with rates of change in other neuropsychological measures. In addition, the baseline number of chronic diseases, used as a covariate in the present analysis, was not significantly predictive of accelerated longitudinal decline in performance for any of the neuropsychological tests assessed.
Table2.
Results from multiple linear mixed models, testing the relationship between longitudinal decline in cognitive performance and faster accumulation of chronic diseases, conceptualized as group with faster accumulation of multi-morbidity (upper quartile of the sample population, 25%) versus the rest of the sample (75%).
| Longitudinal analyses | |||||
|---|---|---|---|---|---|
| Effect of faster accumulation of multi-morbidities on longitudinal decline in cognitive performance (“Group with faster accumulation of multi-morbidity*time” vs rest of the population), after adjustment for covariates† | |||||
| Time | Group with faster accumulation of multi- morbidity *time (Vs rest of the population) |
||||
| Domain | Neuropsychological Measure |
β (SE) | P value | β (SE) | P value |
|
Global
cognition |
MMSE | −0.07 (0.02) | .015 | −0.04 (0.04) | .249 |
| Verbal fluency | Category Fluency | −0.18 (0.03) | <.001 | −0.13 (0.05) | .015 |
| Letter Fluency | −0.02 (0.04) | .658 | −0.16 (0.06) | .013 | |
|
Figural
Memory |
Benton Visual Retention Test (BVRT) |
0.37 (0.05) | <.001 | 0.03 (0.08) | .752 |
| Verbal Memory | California Verbal Language (CVLT) Learning & Immediate Free Recall |
−0.24 (0.13) | .079 | −0.25 (0.22) | .269 |
| CVLT Short Delay Free Recall |
−0.07 (0.04) | .079 | −0.06 (0.07) | .393 | |
| CVLT Long Delay Free Recall |
−0.02 (0.04) | .524 | −0.05 (0.07) | .448 | |
|
Attention and
Working Memory |
Digitspan Forward | −0.11 (0.03) | <.001 | 0.06 (0.05) | .232 |
| Digitspan Backward | −0.09 (0.03) | .002 | 0.07 (0.05) | .179 | |
| Digit Symbol | −1.26 (0.08) | <.001 | 0.05 (0.13) | .672 | |
|
Executive
functions |
Trail Making Test A | 0.28 (0.24) | .236 | 0.71 (0.41) | .084 |
| Trail Making Test B | 1.48 (0.49) | .003 | 1.46 (0.82) | .075 | |
| Spatial Ability | Card Rotations Test | −1.05 (0.25) | <.001 | 0.53 (0.42) | .208 |
covariates included baseline age, sex, race, education, baseline number of diseases, time, baseline age*time, sex*time, race*time, education*time, baseline number of diseases*time.
β=beta coefficient; SE=standard error
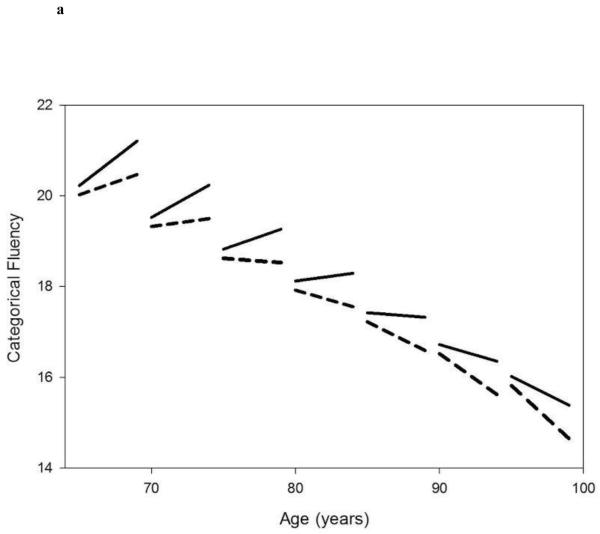
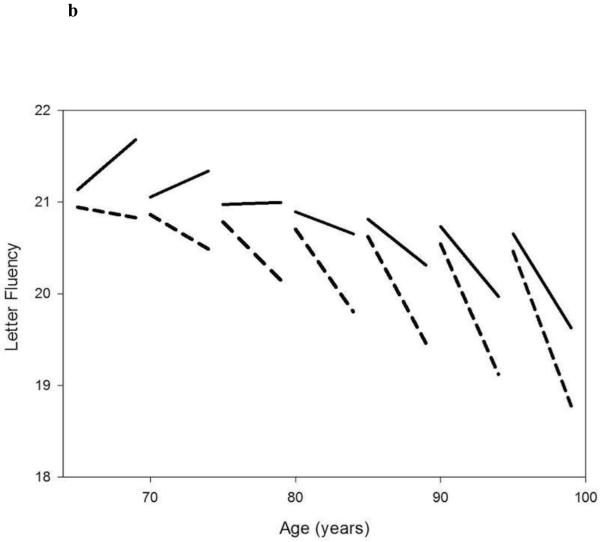
Figures 1A and 1B.
Linear mixed model estimated trajectories of decline in cognitive performance in Category Fluency Test (fig 1A) and Letter Fluency Test (fig 1B) in participants with faster accumulation of diseases overtime (25%, red lines) versus the rest of the population (75%, dark lines), according to different baseline age groups.
Note: Possible range of values (best-worse) for Categorical and Letter Fluency are 35-0 and 31.7-0, respectively.
Sensitivity analyses
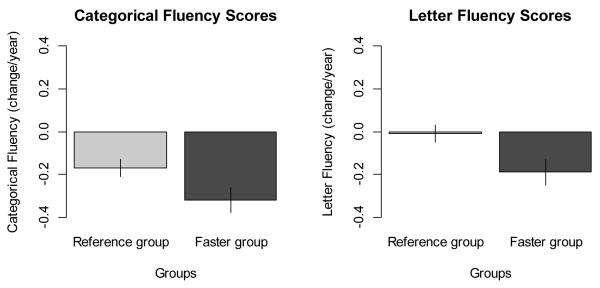
Firstly, in order to get a sense of the magnitude and clinical significance of the findings from the models, the actual annual changes in Fluency tests scores were also calculated and shown in Figure 2A and 2B. We found that the actual changes according to group with faster accumulation of multi-morbidity versus the rest of the population were consistent to the ones estimated by statistical analyses (i.e., −0.31 vs −0.18 [statistical analyses] and −0.32 vs −0.17 [actual changes] for Categorical Fluency; −0.18 vs −0.02 [statistical analyses] and −0.19 vs −0.01 [actual changes] for Letter Fluency], confirming that the statistical models appropriately fitted the crude data.
Figure 2A and 2B.
Actual annual changes in Categorical and Letter Fluency tests score according to group with faster accumulation of multi-morbidity (“faster group”, i.e. ≥0.25 diseases/year or ≥1 diseases in 4 years) versus the rest of the population (“reference group”), adjusted by baseline age and sex and weighted on length of follow up.
Note: Possible range of values (best-worse) for Categorical and Letter Fluency are 35-0 and 31.7-0, respectively.
Sensitivity analyses were also performed to exclude the possibility that the results were driven by specific diseases. In particular, we tested for each of the 13 diseases included in our list of candidate conditions whether their incidental diagnosis may be specifically associated with accelerated decline in Category and Letter fluency performance overtime, independent of covariates. None of the individual diseases was significantly associated with worse decline over time in fluency performance. In addition, we also fitted 13 different models, obtained by removing each time one of 13 conditions included in the original list of candidate diseases, whose results, summarized and presented in Table 3 as median (± interquartile range, IQR) of β coefficients and P values for “group with faster accumulation of multi-morbidity*time” interaction across the 13 models, were consistent with the original ones.
Table3.
Results from Sensitivity Analyses for Letter and Category Fluency Tests, presented as mean and median (± interquartile range, IQR) of β coefficient and P value for “group with faster accumulation of multi-morbidity *time” interaction across 13 different models obtained by removing each time one of 13 conditions included in the original list of candidate diseases.
|
Sensitivity Analyses
|
||
|---|---|---|
| Neurophysiological Measure |
“Group with faster accumulation of multi-morbidity *time” interaction | |
|
| ||
| β median (IQR) |
P value median (IQR) |
|
|
|
||
| Category Fluency | −0.14 (0.13-0.14) | .012 (.009-.017) |
| Letter Fluency | −0.19 (0.16-0.19) | .004 (.003-.021) |
β = beta coefficient
IQR= inter-quartile range
Moreover, to exclude that participants with higher multi-morbidity tend to have a shorter follow up time because of death or losses during the follow up, additional correlation analyses were also run and we found that the duration of the follow up (years) was not correlated to either baseline age of participants or level of multi-morbidity (TableS5) , making it unlikely that differential censoring in participants with different severity of multi-morbidity may have biased the results of our analysis.
Furthermore, additional sensitivity analyses were performed excluding from our sample population all individuals with prevalent or incident diagnosis of MCI and dementia, confirming that individuals with faster accumulation of diseases over the follow up showed a longitudinal steeper rate of decline overtime in Categorical and Letter Fluency tests scores compared to the rest of the population.
Finally, a fully-adjusted model including depression, smoke status and alcohol use as additional covariates was run. The significant association between faster accumulation of multi-morbidities and greater rate of decline in Fluency tests performance remained even after adjusting for these potential confounders (β=−0.14, P=.011 for Categorical Fluency and β=−0.17, P=.009 for Letter Fluency, respectively).
DISCUSSION
The present analysis investigated the association between accelerated deterioration of physical health, operationalized as faster accumulation of multi-morbidity over time, and longitudinal changes in cognitive function over the same time frame in older BLSA participants, free of MCI or dementia over the study period. We found that faster accumulation of multi-morbidity was significantly associated with a faster rate of decline in Category and Letter Fluency Tests, with similar trends for Trail Making Test A and B. In contrast, no effect on visual and verbal memory decline was found.
In particular, we found that, independent of the baseline level of morbidity, participants who accumulated at least one more disease over 5 years of follow up presented a significantly accelerated decline in Fluency tests performance compared to the average rate of decline of the rest of the population (i.e. almost double rate of decline for Categorical Fluency and 9-times faster rate of decline for Letter Fluency).
Our study contributes to previous literature providing new insight to understand the contribution of declining physical health to age-associated cognitive decline in non-demented older persons. Except for a previous study exploring the effect of incident medical conditions on cognitive function in a normal adult population, with the important limitation of restriction of the investigation solely to verbal memory and processing speed, the interrelated connections between physical health deterioration and cognitive deterioration in non-demented older adults were still largely un-explored13. In particular, to the best of our knowledge, the longitudinal relationship between rate of increase in number of chronic diseases and rate of decline in cognitive function in older adults without dementia or cognitive impairment had never been tested.
Exploring decline in cognitive function throughout a comprehensive range of neuropsychological test, our study pointed out that accelerated physical health deterioration, operationalized as faster accumulation of multi-morbidity over time, accelerates the normal effect of aging on decline in verbal fluency in non-demented older adults. In contrast, accelerated physical health deterioration seems to have no effect on memory decline. The decline in processing speed, suggested by Trail Making Test A and B, but surprisingly not confirmed by Digit Symbol, may also be associated with accelerated physical health deterioration.
Both executive function and semantic processing ability contribute to performance in verbal fluency, which represents the ability to initiate, generate and articulate a word in response to a specific cue and is considered to reflect problem solving abilities29-31. Previous studies showed that verbal fluency declines in normal aging, with some evidence showing a greater effect of age on category than letter fluency32-33. Variations of performance in executive function and processing speed have been associated with pathologic brain changes, in particular increase of periventricular white matter hyper intensities34-38. Moreover, speed-of-processing has been proposed as a potential mediator of the relation between aging and decline in fluid intelligence, defined as abstract reasoning or the capacity to face challenges and solve problems in new situations, independent of acquired knowledge39-41. Age-effects on fluid intelligence are robust even in the absence of cognitive impairment and, according to our findings, may be accelerated by physical health deterioration.
However, new research is required to fully understand the underlying mechanisms and validate these exploratory findings. If future investigations in larger and diverse population confirm the contribution of accelerated deterioration of physical health to longitudinal decline in cognitive performance, it may be possible to develop preventive strategies. In addition, from a clinical perspective, it may become important to monitor changes in specific cognitive domains in older adults with multi-morbidity, even in absence of diagnoses of mild cognitive impairment or dementia. Finally, the relevance of our findings also concerns ethical implications of decision-making in older patients with multiple comorbidities.
The strengths of our study include the large community dwelling study sample, the longitudinal design and the availability of multiple cognitive tests. However, several limitations need to be addressed. First, because of the demands of attending a clinic visit and enduring performance testing, the BLSA population may have lower prevalence of some illnesses and disability compared to other populations of the same age. Therefore, findings should be confirmed and validated by further studies, in more diverse populations. Second, we acknowledge that no standard methodological approach to measure multi-morbidity exists. Counts of diseases are the most common approach, but they may not be the most valid and reliable, especially when they rely exclusively on self-report. As a consequence, in the present analysis diagnoses of diseases were based on algorithms which integrated different sources of data (i.e. not only self-reported diseases and medication but also, when possible, laboratory and instrumental tests). Of note, we did not include information on disease severity in considering multi-morbidity. However, we used strict thresholds for defining diseases to minimize the chance of over-diagnosis and to increase specificity. Also, severity classification systems that homogeneously quantify the impact of diseases on health are not currently available. Third, the average length of the follow up time was quite limited, mainly due to the relatively recent introduction of some assessments included in the large pool of information used for the diagnosis of multi-morbidity.
Moreover, another important limitation was the variable duration of the follow up for the participants included in our analysis. If participants with higher multi-morbidity had a shorter follow up time because of death or losses during the follow up, there would be the potential for an important bias in our analysis which might influence the interpretation of our results. In particular, if participants who were followed longer were younger or healthier, you could expect that they had both lower multi-morbidity, and less likely to have or develop cognitive problems. To address this important issue we conducted additional correlation analyses and we found that the duration of the follow up (years) was not correlated to either baseline age of participants or level of multi-morbidity, making it unlikely that differential censoring in participants with different severity of multi-morbidity may have biased the results of our analysis. Of relevance, mortality could have been a competing factor in the analyses of cognitive decline in association with rising multi-morbidity, only if participants with higher multi-morbidity were more likely to die and therefore to be lost during the follow-up. Because no significant correlation could be found between lengths of follow up and level of morbidity, we can conclude that mortality rate did not affect our results”.
In addition, because multi-morbidity increase and decline in cognitive performance occur in the same time frame, a temporal relationship cannot be established. As a consequence, further studies in larger populations and over a longer follow-up are required to validate our results and to better understand the temporal direction of the described association. Furthermore, due to the characteristics of our study population (community-dwelling older adults), we purposely did not focused on the association between multi-morbidity and delirium, although we acknowledge that it should be specifically examined in further investigations, using data from hospitalized older adults or nursing home residents.
Finally, because this study was largely exploratory, adjustments were not carried out for multiple comparisons.
In conclusion, accelerated deterioration of physical health is associated with accelerated decline over time in verbal fluency in older adults, without mild cognitive impairment or dementia. Although further investigations are required to fully explain the underlying mechanisms and validate our results, our findings suggest that accelerated deterioration of physical health may be one of the multiple pathways that can lead to age-related cognitive deterioration and point to future development of possible strategies to prevent and decrease the decline in cognitive performance in older adults with multi-morbidity.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging (grant number: 03-AG-0325).
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors contributed to this paper.
REFERENCES
- 1.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri E, Zoli M, Gonzalez-Freire M, et al. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J Am Med Dir Assoc. 2015;16:640–647. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrucci L, Studenski S. Chapter 72. Clinical Problems of Aging. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison's Principles of Internal Medicine, 18e. McGraw-Hill; New York, NY: 2012. Available at: http://accessmedicine.mhmedical.com/content.aspx. [Google Scholar]
- 4.Smith SM, O'Dowd T. Chronic diseases: What happens when they come in multiples? Br J Gen Pract. 2007;57:268–270. [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin M, Soubhi H, Hudon C, et al. Multimorbidity's many challenges. BMJ. 2007;334:1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menotti A, Mulder I, Nissinen A, et al. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly) J Clin Epidemiol. 2001;54:680–686. doi: 10.1016/s0895-4356(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 8.Bäckman L, Jones S, Small BJ, et al. Rate of cognitive decline in preclinical Alzheimer's disease: The role of comorbidity. J Gerontol B Psychol Sci Soc Sci. 2003;58(B):P228–236. doi: 10.1093/geronb/58.4.p228. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald SW, Karlsson S, Fratiglioni L, et al. Trajectories of cognitive decline following dementia onset: What accounts for variation in progression? Dement Geriatr Cogn Disord. 2011;31:202–209. doi: 10.1159/000325666. [DOI] [PubMed] [Google Scholar]
- 10.Solomon A, Dobranici L, Kåreholt I, et al. Comorbidity and the rate of cognitive decline in patients with Alzheimer dementia. Int J Geriatr Psychiatry. 2011;26:1244–1251. doi: 10.1002/gps.2670. [DOI] [PubMed] [Google Scholar]
- 11.Leoutsakos JM, Han D, Mielke MM, et al. Effects of general medical health on Alzheimer's progression: The Cache County Dementia Progression Study. Int Psychogeriatr. 2012;24:1561–1570. doi: 10.1017/S104161021200049X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melis RJ, Marengoni A, Rizzuto D, et al. The influence of multimorbidity on clinical progression of dementia in a population-based cohort. PLoS One. 2013;8:e84014. doi: 10.1371/journal.pone.0084014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarts S, van den Akker M, Tan FE, et al. Influence of multimorbidity on cognition in a normal aging population: A 12-year follow-up in the Maastricht Aging Study. Int J Geriatr Psychiatry. 2011;26:1046–1053. doi: 10.1002/gps.2642. [DOI] [PubMed] [Google Scholar]
- 14.Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38. doi: 10.1016/j.nbd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol. 2007;16:171–174. doi: 10.1111/j.1076-7460.2007.06563.x. [DOI] [PubMed] [Google Scholar]
- 16.Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7:32. doi: 10.1186/s13195-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugnicourt JM, Godefroy O, Chillon JM, et al. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–363. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 18.Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. doi: 10.1093/geronj/21.4.575. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll I, Resnick SM, Troncoso JC, et al. Impact of Alzheimer's pathology on cognitive trajectories in non-demented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. Review. [DOI] [PubMed] [Google Scholar]
- 21.Goodman RA, Posner SF, Huang ES, et al. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013 Apr 25;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann Fam Med. 2012;10:142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. pp. 95–4009. [Google Scholar]
- 24.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Fried L, Simonsick E, et al. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The women's health and aging study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri E, An Y, Zoli M, Simonsick EM, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70A:63–70. doi: 10.1093/gerona/glu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977:385–401. [Google Scholar]
- 29.Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 30.Bryan J, Luszcz MA, Crawford JR. Verbal knowledge and speed of information processing as mediators of age differences in verbal fluency performance among older adults. Psychol Aging. 1997;12:473–478. doi: 10.1037//0882-7974.12.3.473. [DOI] [PubMed] [Google Scholar]
- 31.Elgamal SA, Roy EA, Sharratt MT. Age and verbal fluency: The mediating effect of speed of processing. Can Geriatr J. 2011;14:66–72. doi: 10.5770/cgj.v14i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomer R, Levin BE. Differential effects of aging on two verbal fluency tasks. Percept Mot Skills. 1993;76:465–466. doi: 10.2466/pms.1993.76.2.465. [DOI] [PubMed] [Google Scholar]
- 33.Clark LJ, Gatz M, Zheng L, et al. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2010 Dec;Jan;24:461–468. doi: 10.1177/1533317509345154. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and subjective cognitive dysfunction: The Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JH, Reed BR, Mungas D, et al. Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2002;72:217–220. doi: 10.1136/jnnp.72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 37.Vannorsdall TD, Waldstein SR, Kraut M, et al. White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol. 2009;24:209–217. doi: 10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 40.Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10:131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Bugg JM, Zook NA, DeLosh EL, et al. Age differences in fluid intelligence: Contributions of general slowing and frontal decline. Brain Cogn. 2006;62:9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.