Abstract
Testosterone (T) exposure during midgestation differentiates neural circuits controlling sex-specific behaviors and patterns of gonadotropin secretion in male sheep. T acts through androgen receptors (AR) and/or after aromatization to estradiol and binding to estrogen receptors. The current study assessed the role of AR activation in male sexual differentiation. We compared rams that were exposed to the AR antagonist flutamide (Flu) throughout the critical period (i.e. day 30 – 90 of gestation) to control rams and ewes that received no prenatal treatments. The external genitalia of all Flu rams were phenotypically female. Testes were positioned subcutaneously in the inguinal region of the abdomen, exhibited seasonally impaired androgen secretion and were azospermic. Flu rams displayed male-typical precopulatory and mounting behaviors, but could not intromit or ejaculate because they lacked a penis. Flu rams exhibited greater mounting behavior than control rams, and like controls, showed sexual partner preferences for estrous ewes. Neither control nor Flu rams responded to estradiol treatments with displays of female-typical receptive behavior or LH surge responses; whereas all control ewes responded as expected. The ovine sexually dimorphic nucleus in Flu rams was intermediate in volume between control rams and ewes and significantly different from both. . These results indicate that prenatal antiandrogen exposure is not able to block male sexual differentiation in sheep and suggest that compensatory mechanisms intervene to maintain sufficient androgen stimulation during development.
Keywords: sexual differentiation, sexual partner preference, sexually dimorphic nucleus, gonadotropin, gonadal steroids, sexual behavior
Introduction
Sheep, like other mammals, exhibit profound sex differences in the control of gonadal function and behavior by the central nervous system that result from brain sexual differentiation. Females display estrous cycles characterized by an estrogen-evoked surge in gonadotropin that leads to ovulation and facilitates female-typical sexual attraction and receptive reproductive behaviors. Males, on the other hand, exhibit steady-state gonadotropin secretion that sustains continual sperm production and testosterone secretion and supports male-typical sexual attraction and copulatory behaviors necessary for successful fertilization.
Physiological and behavioral sex differences are programmed during early fetal life and are the consequence of interactions between sex chromosome genes and gonadal hormones acting during a limited critical period in development. Sexual differentiation in sheep occurs from approximately gestational days (GD) 30 to 90 of the 147 day pregnancy (1,2). Circulating concentrations of testosterone (T) are significantly higher in males than in females during this critical period (3–7). As in other long-gestation mammals, T secreted by the fetal lamb testes masculinizes and defeminizes the genitalia and brain systems that control gonadotropin release and sex behavior (8–11). Masculinization refers to the expression of masculine physical characteristics and the potential to exhibit the adult male-typical neuroendocrine and behavioral patterns. Defeminization relates to the suppression of adult feminine characteristics such as the estrogen-stimulated LH surge and female-typical sexual behaviors and is normally regulated together with masculinization.
Many of the organizational effects of T on sexual differentiation in mammals result from the neural actions of estradiol formed via local aromatization of T in the developing male brain (12,13). Like other species, the fetal lamb hypothalamus and preoptic area express appreciable levels of aromatase and estrogen receptor (ER)(6,7). Aromatization is presumed to be obligatory for masculinization and defeminization of the sheep brain and behavior. Prenatal treatment with aromatizable T, but not with the non-aromatizable androgen receptor (AR) agonist dihydrotestosterone, programs the development of copulatory and aggressive behaviors, blocks the development of the LH surge mechanism and decreases the capacity of females to show receptive behaviors (14,15). The role of estrogens (or estrogenic metabolites of testosterone) in brain sexual differentiation of sheep has not been studied directly because maternal estrogen treatments needed to alter fetal brain development disrupt uterine function and imperil the pregnancy (16). Thus, inferences about estrogen’s role for masculinization and defeminization in sheep have been made using subtractive approaches. One strategy is to block estrogen synthesis with the aromatase inhibitor 1,4,6-Androstatriene-3,17-dione (ATD) during the gestational critical period and compare the consequences between treated and control offspring (7). Rams exposed to ATD prenatally exhibit a modest but significant decrease in mounting behavior at 18 mo of age signifying that prenatal estrogen plays a role in masculinizing copulatory behavior in sheep similar to other species (17). However, ATD-exposed rams, like control rams, do not respond to estrogen treatments with either displays of receptive behavior or LH surges indicating that ATD does not disrupt the defeminization process in males. These results fail to support the hypothesis that prenatal estrogens generated by aromatization defeminize behavioral and neuroendocrine responses in male sheep (18). A complimentary experimental approach used the AR antagonist flutamide (Flu) to eliminate the androgenic actions of T while sparing T availability for aromatization to estradiol (16,19,20). Prenatal exposure to T blocks the LH surge mechanism in adult ewes. Simultaneous prenatal exposure to T plus the antiandrogen Flu restores LH surges indicating that estrogens are not sufficient to defeminize the surge mechanism and androgens are necessary (19,20). Neither pharmacological approach excludes the possibility that the combination of androgens and estrogens may be necessary.
Sexual partner preference is another aspect of sexual behavior that is highly sexually dimorphic and organized by perinatal exposure to gonadal hormones in many mammalian species (21). Typically, females prefer males over females and males prefer estrous females over males. Testosterone exposure during both prenatal and postnatal life masculinizes and defeminizes partner preference behavior in rats and ferrets (13). Testosterone acts perinatally to organize sexual partner preferences after conversion to estrogen in rats and mice (17,22). In male pigs, estradiol can defeminize sexual partner preferences by acting as late as 3 months postnatally (23). The expression of sexual preferences requires integration of social chemosensory signals and steroid hormone cues that involves neurons in the medial amygdala and medial preoptic area/anterior hypothalamus (mPOA/AH) (24,25) In male ferrets the mPOA/AH contains a cluster of large neurons in nissl-stained brain sections that is not seen in females (26). Either excitotoxic (27) or electrolytic (28) lesions centered in the this so-called male nucleus of male ferrets caused them to prefer to approach and interact sexually with another male, as opposed to a female, in T-maze tests of partner preference. A cluster of neurons exists in the mPOA/AH of rats that is larger in males than in females and has been named the sexually dimorphic nucleus (SDN) of the preoptic area(29). Perinatal experimental manipulations that alter sexual preferences produced significant positive correlations between an animal’s preference for a receptive female and the volume of the SDN (30). In humans, the volume of the third interstitial nucleus of the anterior hypothalamus is larger in heterosexual men than in women homosexual men (31).
Male and female sheep are typically motivated to approach opposite sex conspecifics in response to visual and olfactory cues (32). However, roughly 8% of rams show a spontaneous preference for other males i.e., male-oriented rams (33). Sexual partner preferences in sheep are also assumed to be organized prenatally by T because the ovine sexually dimorphic nucleus (oSDN), a hallmark of gestational masculinization (34), is significantly smaller in male-oriented rams than in rams that are attracted to receptive females i.e., female-oriented rams (35).
A clear picture of the steroid requirements for programming the masculinization and defeminization of the sheep brain and behavior remains elusive. Prenatal T clearly masculinizes copulatory behavior and defeminizes receptive behavior and neuroendocrine responses such as the LH surge mechanism. Prenatal mechanisms program sensitivity to negative feedback actions of estradiol and the timing of neuroendocrine puberty (14,16). The case for a role of estrogens in defeminization is mixed (14,19,36). Estrogens may play a role in masculinization of copulatory behavior but it is unlikely sufficient to act alone (18). There are no behavioral studies to prove that gestational T masculinizes/defemininzes sexual partner preferences in sheep, although it does masculinize the oSDN volume, which correlates with sexual partner preference (34). Prenatal exposure to the aromatase inhibitor ATD does not change adult male sexual preferences or disrupt masculinization of the oSDN (11), which fails to support a role for estrogens. However, we recently reported that mean oSDN volume of Flu ram fetuses is intermediate between that of control male and females indicating that ARs mediate the effect of T on oSDN masculinization (37). In this earlier experiment, maternal Flu treatment was used to study oSDN development during fetal gestation and did not assess the effect of the treatment on adult behavior. Thus, the aim of the current investigation was to test the hypothesis that T acts through an AR-mediated mechanism to masculinize sexual partner preferences in male sheep. To accomplish this goal, pregnant sheep were treated with Flu and their male offspring tested for signs of anatomical, behavioral and neuroendocrine masculinization and defeminization.
Materials and Methods
Animals and treatments
Suffolk lambs born in April were obtained from the Reproductive Sciences Program Sheep Research Facility at the University of Michigan (Ann Arbor, MI). Seven ram lambs were exposed in utero to anti-androgen by treating their mothers with a daily 15 mg/kg s.c. injection of Flu from d 30 to 90 of gestation (term pregnancy = 147 days) as described previously (16). Control ram lambs (n=7) and ewe lambs (n=6) were obtained from untreated mothers. Lambs were weaned at 60 days of age and shipped to Corvallis, OR (OSU) at 3 mo. of age in June. During the winter, they were fed alfalfa hay supplemented with barley-based concentrate. They were maintained on pasture grass during the spring and summer. The sheep had ad libitum access to water and a mineral supplement and were regularly treated with antihelminthics to control internal parasite infection. Flu-treated rams, control rams and control ewes were raised separately and kept isolated from each other throughout the study. Lambs were weighed at 3 and 10 mo. of age and jugular blood samples (5 ml) were collected weekly during this period to obtain a profile of androgen secretion during the first breeding season. All subsequent testing was done after the sheep were 1 yr. or older. When the rams were 20 months old they were bilaterally castrated. For castrations, anesthesia was induced with ketamine and diazepam i.v. and maintained with inhaled isoflurane and oxygen. The rams were placed in dorsal recumbence and wool was widely removed from the perineal and inguinal areas followed by a three stage prep of the skin over the surgical site. A roughly 10 cm incision was made through the skin over each testis. The parietal and visceral layers of the tunica vaginalis were carefully excised to expose the spermatic cord. The remaining loose connective tissue surrounding each spermatic cord was stripped off with dry gauze and the cord was double ligated with absorbable transfixation sutures. The cords were severed between the ligatures and the testes removed for histological processing. Animal husbandry and experimental protocols were conducted according to the specifications of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of the University of Michigan and the Oregon State University.
LH surge response to estradiol
The mechanism that governs the LH surge response to an acute increase in estradiol in ewes is rendered permanently inoperative in rams by testicular hormones secreted during the critical period of sexual differentiation (38). To determine whether testicular T acts through AR- mediated mechanisms in male fetuses to suppress the surge mechanism, the LH surge response to an estradiol challenge was tested in males that were treated prenatally with the androgen antagonist Flu and compared to control males and females at 12 months of age (first season anestrous period) and again at 23 months of age 3 mo. after the rams were gonadectomized. All sheep were given subcutaneous implants containing 0.3 gm. of progesterone (Eazi-Breed CIDR Sheep Inserts, Pfizer, New York, NY). The CIDRs were removed after 6 days and 24 h later the sheep were given an intramuscular injection of 50 μg 17β-estradiol (Sigma-Aldrich Corp. St. Louis, MO.) in 1 ml of corn oil. Jugular blood samples were collected every 2 h from 4 h before until 30 h after the estradiol injection and serum LH concentrations determined.
GnRH challenge
To determine whether prenatal antagonism of the AR altered anterior pituitary sensitivity, sheep were administered exogenous GnRH (i.e. GnRH challenge test) to evaluate the LH response. The test was conducted in testes-intact rams one month after the initial estrogen-induced surge response was tested. Sheep were given 100 μg GnRH intravenously (Cystorelin, Merial Limited, Duluth, GA). Blood samples were collected every 15 min. starting 1 h before injection and then at 30 min intervals for the following 2 h Serum was used to measure LH concentrations.
Male-typical sexual behaviors and sexual partner preferences
Sexually naïve testes-intact rams were 16 to 18 months old when they underwent male sexual behavior tests and sexual partner preference tests between September and October of their second breeding season. Estrus was induced in ovariectomized ewes by sequential treatments with controlled internal drug release (CIDR) devices containing 0.3 g progesterone followed by estradiol injections as described above. Sexual behavior tests began 24 h after the estradiol injection when rams were paired individually with an estrous ewe in a 10 m x 10 m pen. Rams were observed for 20 min during which time the latency and frequency of pre-copulatory courtship behaviors (genital sniffs, foreleg kicks and nudges, flehmen and vocalizations) and consummatory behaviors (mounts and ejaculations) were recorded. Each ram was tested once on any day and three times during a week for three weeks or a total of nine separate tests. Two weeks after the final sexual behavior test, rams were observed in a sexual partner preference test as described previously (18,33,39). Briefly, rams were isolated for 5 d to prevent mounting and courtship behavior between male pen mates prior to the preference test. Then each ram was observed for 10-min in a testing pen that contained two restrained rams and two restrained estrous ewes (18). The sex of the preferred stimulus animal and the frequency of courtship and consummatory behaviors were recorded and the test was repeated three times at five day intervals.
Female-typical sexual behaviors
Like the LH surge response, estrogen-stimulated expression of female sexual behaviors is suppressed (i.e., defeminized) in normal male and in female sheep exposed to T prenatally. To determine whether prenatal AR activation also mediates this effect, control and Flu-exposed sheep were given CIDR implants followed by estradiol injections as described above. The test was conducted twice, first in testes-intact rams at approximately 20 months of age and then after the rams were castrated 3 mo. later during the second estradiol challenge test. Female sexual behaviors were recorded 24 h after estradiol stimulation during both evaluations of the LH surge system as described previously (3;4). Briefly, each animal was paired with a sexually vigorous ram for 5 min. during which time proceptive (sniffs, head turns, tail fans) and receptive (standing) behaviors were recorded.
Hormone Assays
Serum concentrations of T, dihydrotestosterone (DHT), androstenedione and progesterone were measured by RIA after ether extraction and chromatography on Sephadex LH-20 (Sigma- Aldrich) using previously validated methodologies (40,41). Briefly, serum samples (100–500 μl) were extracted with 5 ml diethyl ether, dried under a stream of air and redissolved in column solvent (hexane:methanol:ether = 62:20:10) and fractionated. Each column fraction contained a specific isolated steroid and was subjected to RIA with steroid-specific antibodies. The percentages of recovery, water blanks and intra-assay coefficients of variation for each steroid were: T, 78%, 2 pg and 11%; DHT, 57%, 12pg and 6%; androstenedione, 84%, 11 pg and 2% and progesterone, 63%, 8 pg and 5%. Concentrations of LH were measured in duplicate 40 μl aliquots of serum using an RIA developed by Niswender et al. (42). Assay sensitivities averaged 0.2 ng/ml National Institutes of Health oLH-S19 and the average mid-point intra-assay variation was 9.76%, and interassay CV was 8.6% using the same plasma pool.
Testicular Histology
Testicular biopsies were obtained at the time of castration, immersion fixed overnight in buffered formalin and then stored in ethanol until processed for histology. Tissues were imbedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin by the Histopathology Shared Resource at OHSU.
In situ hybridization and oSDN volume measurement
To achieve equivalent physiologic concentrations of serum T for anatomical comparisons among groups, castrated rams and control anestrous ewes were given 2 injections of testosterone-cypionate (1.5 mg/kg i.m.) spaced 2 weeks apart. Ten days after the last injection, jugular blood samples (10 ml) were taken after which the sheep were euthanized with an overdose (15 mg/kg) of sodium pentobarbital (Euthasol; Delmarva Laboratories, Inc. Midlothian, VA). The head was perfused with saline and 4% paraformaldehyde as described previously (43). The brain was then removed, blocked, postfixed overnight, cryoprotected, frozen and stored at −80°C. Fixed tissues were sectioned coronally (40 μm thick) into parallel series and mounted onto Superfrost microscope slides (Fisher Scientific Co., Pittsburgh, PA). Slides were desiccated under vacuum and stored frozen at −80°C. Adjacent series of brain sections were stained with thionin and processed for in situ hybridization using a sheep-specific [33P] aromatase cRNA as described previously (35). After hybridization the slides were exposed to Kodak Biomax MR film (Kodak, Rochester, NY) for 9 days. The volume of the oSDN was measured bilaterally from both the thionin stained sections and film autoradiograms using NIH Image J software (version 1.48). Volumes were estimated by multiplying cross-sectional area x number of sections containing oSDN x distance between section (120 μm). Thionin sections were used to confirm the location and boundaries of the oSDN. No statistical differences between brain sides were found (data not shown). Thus, bilateral oSDN measurements for each animal were averaged and used for statistical comparisons among treatment groups.
Statistical Analyses
Data on hormone concentrations and behaviors collected over time or with repeated testing were analyzed by 2-way ANOVA for repeated measures followed by Bonferroni posttests to compare treatments when appropriate. Mean oSDN volumes were compared by 1-way ANOVA followed by Newman-Keuls post hoc test. In all tests P< 0.05 was considered significant.
Results
Reproductive anatomy and testicular histology
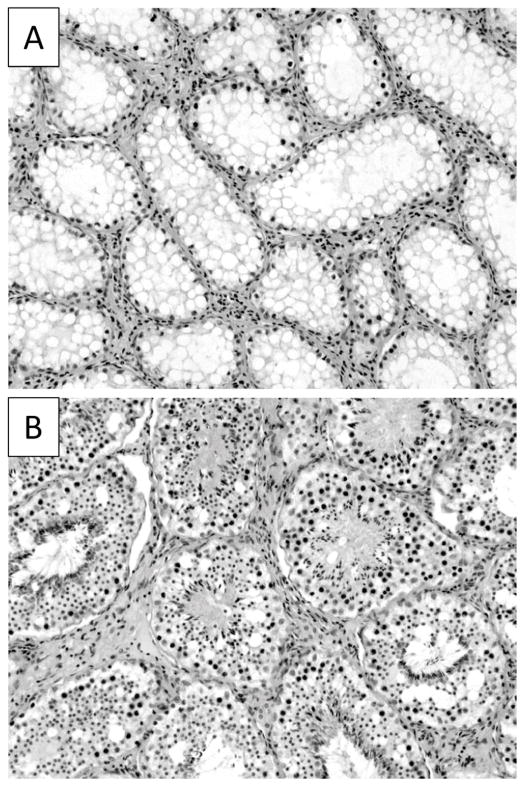
The external genitalia of all male lambs exposed to Flu prenatally were phenotypically female with no evidence of penile or scrotal development. The testes of Flu rams had descended through the inguinal canal and were positioned beneath the skin in the inguinal area. Testicular weights were significantly smaller (P < .001) in Flu rams (192 ± 23 gm.) compared to control rams (438 ± 26 gm.). Prenatal Flu treatment did not affect animal size or rate of growth in males as measured by body weight (data not shown). Histological examination of Flu-exposed testes showed cardinal signs associated with cryptorchidism (44) highlighted by a complete absence of mature sperm, reduced numbers of germ cells and vacuolated Sertoli cells (Fig. 1).
Figure 1.
Cross sections of testis from Flu rams (A) and control rams (B). Digital image shows that seminiferous tubules of Flu rams are devoid of developing germ cells and spermatozoa in comparison to control tubules. 20X magnification.
Steroid hormone concentrations
Control and Flu ram lambs had similar serum levels of T prior to the seasonal increase that began in the Fall at 20 weeks of age (i.e. September 1). Between September 1 and November 24, serum T concentrations increased faster and reached significantly greater concentrations (F11,167 = 2.8; P<.005) in control than in Flu rams (Fig. 2). After November, serum concentrations dropped dramatically and were not different between groups. Nearly identical group differences were observed for seasonal concentrations of serum DHT and androstenedione (data not shown).
Figure 2.
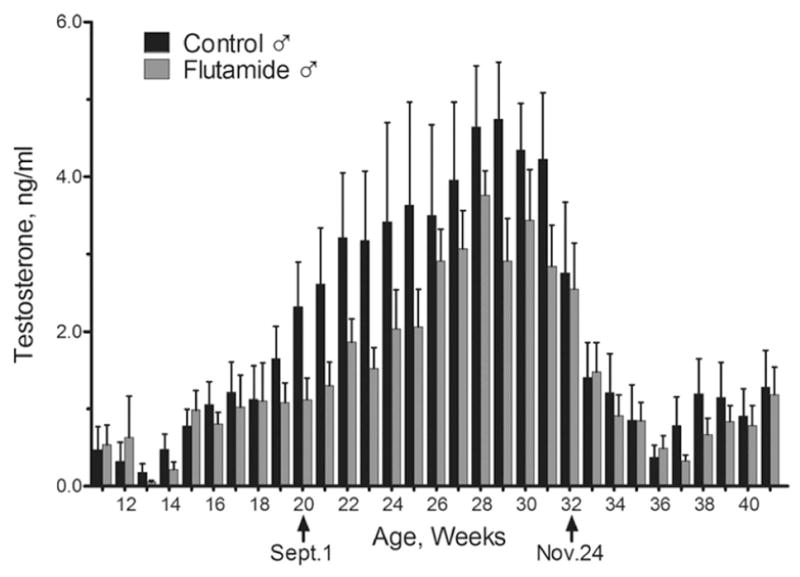
Mean ± SEM serum concentrations of T in control (n=7) and Flu ram (n=7) lambs from 3 months of age through the end of January during the first breeding season. 2-way ANOVA for repeated measures revealed that T concentrations were significantly greater (P < 0.05) in control vs. Flu ram lambs between September 1 and November 24.
LH responsiveness to estradiol and GnRH
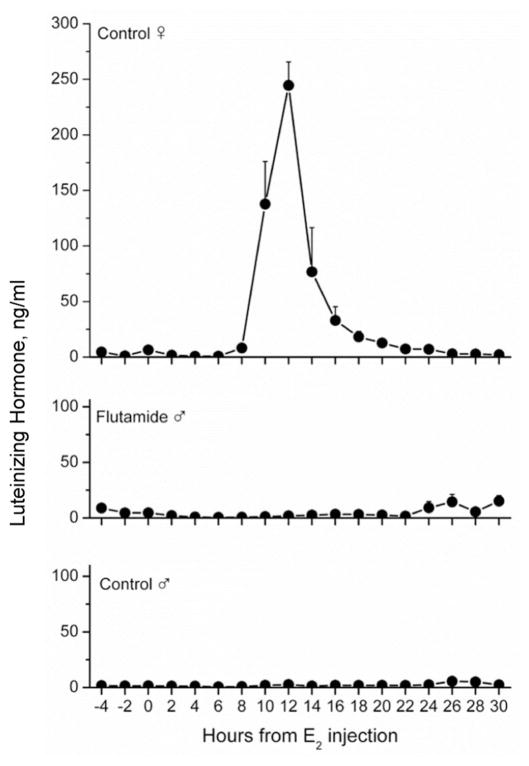
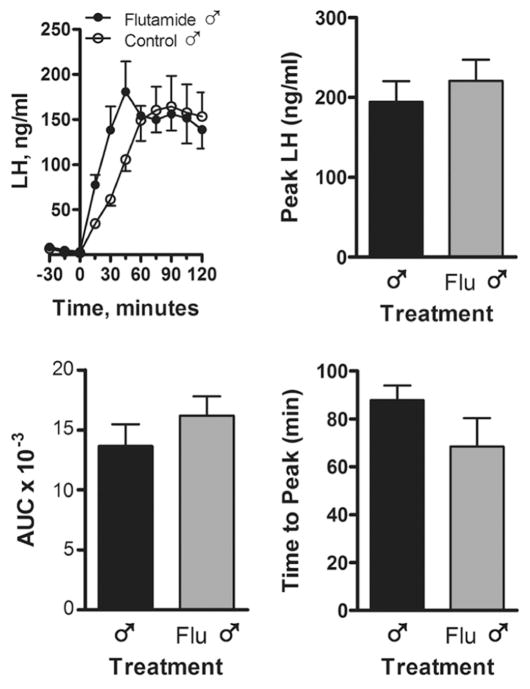
A positive LH surge response to estradiol was elicited in all 12 month-old control intact ewes (Fig. 3) during Spring seasonal anestrus. In contrast, none of the intact Flu rams or control rams exhibited an LH surge. The LH surge mechanism was tested again when all animals were 23 months old, gonadectomized and treated sequentially with progesterone and estradiol. Similar to the first test, only females exhibited an LH surge (data not shown). The LH response to a GnRH challenge was evaluated one month after the first estrogen challenge (Fig. 4). Injection of GnRH caused a significant increase in LH secretion in both intact Flu-exposed and control groups. No differences were apparent in the area under the curve, peak LH response or time to peak LH.
Figure 3.
LH secretion in response to a surge-inducing dose of estradiol (50 μg/animal) in control female sheep (n=6), Flu rams (n=7) and control rams (n=7). Blood samples were collected every 2 h from 4 h before estradiol was injected until 30 h after injection. All 6 females met the criterion for an LH surge defined as LH values exceeding twice the average pre-estradiol baseline for a minimum of 6h. No males met this criterion. Values are mean ± SEM.
Figure 4.
LH secretion in response to an i.v. injection of GnRH (100 μg/animal) in control and Flu rams (n=7 rams per group). Blood samples were collected every 15 min before and after GnRH administration. Time = minutes before or after GnRH injection. AUC, area under the curve. Values are mean ± SEM.
Male-typical sexual behavior and partner preference
After nine separate tests of sexual behavior all but one ram in each group mounted estrous females. Testes-intact control and Flu rams displayed comparable levels of pre-copulatory courtship behaviors such as genital sniffs, nudges, foreleg kicks. Flu males exhibited significantly greater mounting frequencies than control males, but were unable to ejaculate because their external genitalia were feminized (Fig. 5). Prenatal Flu exposure did not alter sexual partner preferences (Fig. 6). Control and Flu rams interacted with both male and female stimulus animals. Flu males exhibited greater female-directed pre-copulatory behaviors and mounts than controls. Out of the seven animals in each treatment group, two were exclusively female-oriented, four mounted both males and females and one showed no interest in either stimulus gender. No rams in either group exhibited exclusive male-oriented sexual preference.
Figure 5.
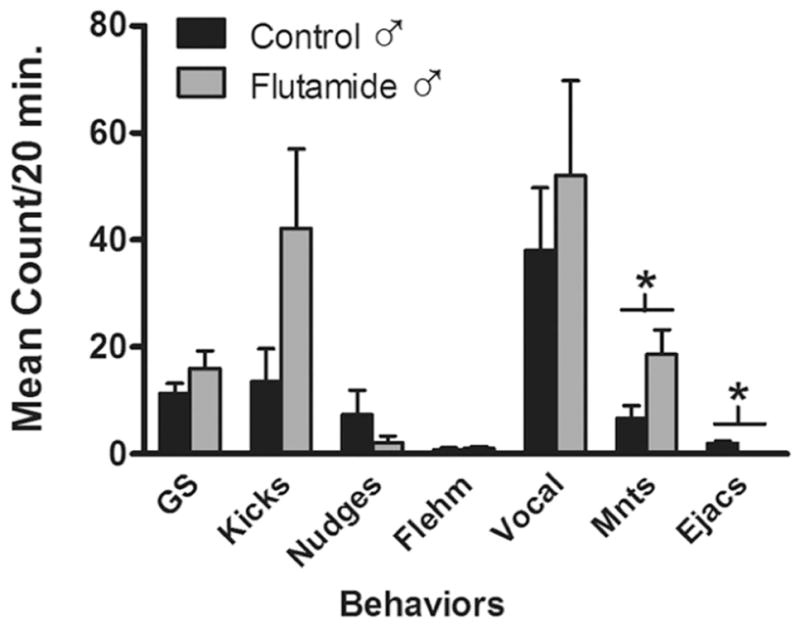
The frequency of male reproductive behaviors exhibited in serving capacity tests. Rams were placed with two sexually receptive ewes and behaviors recorded for 20 min on 9 separate occasions. Data represent the mean ± SEM frequencies on the final 3 tests (n=6 rams per group). *, P < 0.05 control versus Flu ram. Abbreviations: GS, anogenital sniffs; kicks, foreleg kicks; Flehm, flehmen; Vocal, vocalizations; Mnts, mounts; Ejacs, ejaculations.
Figure 6.
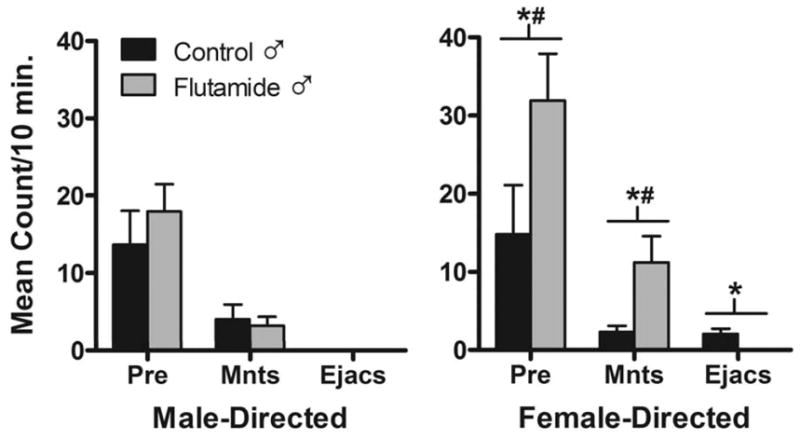
Sexual preference tests. Each ram was given 3 preference tests at ~19 months of age (yr. 2). The frequencies of precopulatory (Pre) behaviors (anogenital sniffs, foreleg kicks, nudges, flehmen, and vocalizations), mounts (Mnts) and ejaculations (Ejacs) directed at either estrous female stimulus animals (Female-directed) or male stimulus animals (Male-directed) were recorded. Data represent the mean ± SEM frequencies on 3 tests (n=6 rams per group). *, P < 0.05 control versus Flu ram; #, P < 0.05 female-directed versus male-directed.
Female-typical sexual behavior
All intact control ewes displayed receptive behavior (i.e. stands) and received mounts from the test ram; 80% received ejaculations. When tested before and after castration, neither control nor Flu rams exhibited female-typical receptive behaviors and neither group received mounts or ejaculations (data not shown).
oSDN volume
One-way ANOVA revealed significant differences between groups in the volume of the oSDN F(2,19) = 9.80, P < 0.01, while no group differences in serum T concentrations were observed (Fig. 7). Newman-Keuls multiple comparison test showed that the volume of the oSDN was significantly greater (P <0.05) in control rams than in Flu rams and significantly larger (P < 0.05) in Flu rams than in ewes. Thus, the oSDN volume of Flu rams was intermediate between control males and females and significantly different from both.
Figure 7.
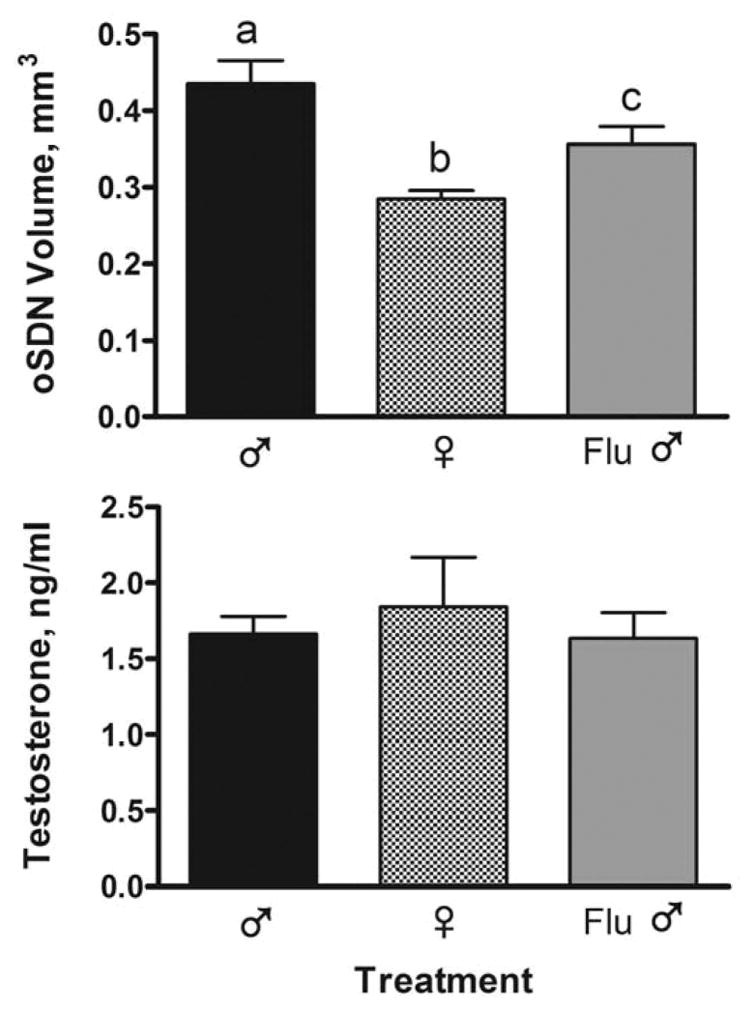
Effect of prenatal exposure to Flu from GD 30 to 90 on oSDN development in adult castrated rams and intact ewes treated with T (n=6–7 sheep per group). Data are mean volumes (± SEM) calculated from autoradiograms of aromatase mRNA expression. Serum testosterone concentrations were measured in jugular blood sampled on the day of brain perfusion, 1-wk after the final testosterone cypionate (1.5 mg/kg) injection. Bars with different superscripts are significantly different, P < 0.05.
Discussion
It has long been recognized that when the female lamb fetus is exposed to T during a sensitive period of in utero development (~GD 30–90) the neural mechanisms regulating gonadotropin secretion and sexual behavior become masculinized and defeminized (45). T acts on ARs directly or after reduction to DHT and on ERs after aromatization to estradiol (12). In general, short-gestation mammals such as rats, mice and ferrets rely on aromatization more extensively for male sexual differentiation than do long-gestation mammals such as guinea pigs and monkeys (13,46). In sheep, the T-treated female has proved valuable for determining the timing, type and dose of steroid needed to masculinize neural mechanisms, structures and behaviors (15). However, an equally useful approach is to block the actions of endogenous androgen in males by prenatally administering an antiandrogen to study the effects on sexually dimorphic adult responses. We chose to use the antiandrogen Flu that is rapidly metabolized to its primary active form hydroxyflutamide, which competes with T for AR binding. We showed previously that the dose of Flu used in the current study crosses the placenta and produces therapeutic levels of hydroxyflutamide in the fetal circulation (37). The antiandrogen effects of Flu were clearly apparent and, as expected, the external genitalia of prenatally exposed males were feminized. However, Flu did not significantly disrupt masculinization or defeminization of hormonal and behavioral responses.
One possible reason why the external genitalia were susceptible to Flu is that they develop before the hypothalamic-pituitary-gonadal (HPG) axis becomes active (37). In males, but not females, the HPG axis is tonically suppressed by T secreted by the fetal testes after the first trimester of gestation (37,47,48). Thus, it is likely that after the genitalia developed, Flu blocked negative feedback resulting in elevated concentrations of LH and T in the fetal blood, which then competed with Flu for binding and activation of ARs in the brain and pituitary. Two observations support this reasoning. First, we demonstrated previously that Flu-exposure from GD 60 to 84 elicited exactly this type of compensation in eugonadal males (37). Secondly, masculinization of the oSDN was partially blocked in the previous and current studies as would be expected if the compensatory rise in T interferes with the antiandrogen at the receptor level. Although this is the most parsimonious explanation of the results, we cannot exclude the possibility that endogenous T in males acted outside of the Flu treatment period. Several reports suggest that T remains elevated in male compared to female lamb fetuses throughout most of gestation and into early postnatal life (5,18,34) during which androgens could have additional effects. Published reports suggest that a surge of T occurring in the first few hours after birth contributes to male sexual differentiation in several mammalian species (49–52). Evidence also exists for a postnatal period of estrogen sensitivity in sheep that completes defeminization in T-exposed females and consolidates masculinization in males (1,53,54). In spite of the clearly defined critical period that occurs between GD 60 to 90 for exogenous administration of T (55), no systematic studies have yet been undertaken to determine whether sensitivity to T extends into late gestation or early postnatal life in sheep.
Prenatal Flu exposure did not block behavioral masculinization, but rather increased mount frequency. These results are consistent with previous reports in rats that used prenatal treatments with Flu or cyproterone acetate (56,57). It is possible that the elevation in mounting behavior resulted from sexual excitement due to inability of Flu rams to intromit and ejaculate. Published data demonstrate that mounting behavior in rats increases when intromission is blocked by applying lidocaine to the penis and reducing sensory feedback (58). In general, studies that blocked endogenous androgen receptors prenatally in short gestation mammals report that copulatory behaviors are either unaffected or reduced, but not eliminated (56,59–61). Fetal Flu exposure also failed to block masculinization of mating behaviors in male ferrets (62). Male hyenas exposed to Flu during prenatal development exhibit abnormal penile development and marked decrements in intromission and ejaculations (63). In rhesus monkeys, prenatal Flu radically alters penile development (64), but does not diminish sexual behavior in most males nor does it preclude ejaculation (65).
Another experimental strategy employed to assess the role of AR activity in masculinizing copulatory behavior is to examine spontaneous mutant rats and mice with reduced AR function (testicular feminized or Tfm) or engineered AR knockout mice. Early studies in Tfm animals and global AR knockout mice found severe deficits in male copulatory behaviors but like the pharmacological approach were unable to distinguish between peripheral versus neural-specific function of AR (66). A recent study using conditional neuron-specific AR deletion (ARNes/Cre) suggests that AR is not essential for the perinatal programming of male sexual behavior, but rather is involved in behavioral activation in adulthood (67). Taken together these data indicate that across species functional ARs are not essential for the masculinization of copulatory behaviors. Our previous study (18) suggests there may be a role for ER during developmental masculinization, but this cannot be considered definitive because normal ewes have an inherent capacity to show male copulatory behavior in response to continuous T stimulation although its expression can be enhanced by prenatal T exposure (45,68).
Gestational Flu exposure did not alter sexual partner preferences in rams, with the exception that Flu rams showed significantly greater female-directed precopulatory behaviors and mounts. The latter observation probably reflects the fact the Flu males were unable to ejaculate as discussed. However, in general, rams in the present study did not exhibit a robust female preference and only two were exclusively female-oriented in each treatment group. Previous studies using Western sheep breeds found ~55% of yearling ram lambs were exclusively female-oriented (69). The reason for this difference is not known but may relate to differences in sheep breed or rearing conditions. Similar to sheep, early prenatal treatment with Flu had no effect on partner preferences in rhesus monkeys (65). Most of the data collected in rats, however, indicate that developmental estrogens are required for male-typical partner preference behavior (70). In mice, it is unclear whether T or estradiol plays the greater role in development of adult partner preferences. Several studies favor a role for androgens. Male Tfm mice, which lack a functional AR, show a preference for male-soiled bedding over female-soiled bedding like WT females, while WT males show a strong preference for female bedding (71). DHT administered to females at birth masculinizes partner preferences whereas estradiol does not (72). Female mice lacking alpha fetoprotein (AFP-KO) that are exposed to maternal estrogens sufficient to masculinize and defeminize sexual behaviors show unaltered female-typical odor preferences (73). However, ARNes/Cre male mice show normal male-typical preferences and Fos responses in the medial amygdala and medial preoptic area suggesting androgens are not essential (67,74). The reasons for these discrepancies are not yet understood but may relate to aspects of experimental design, completeness of neural AR ablation or the ability of the organism to compensate for disruptions in the developmental program.
A sexually dimorphic nucleus (SDN) that is larger in males than in females has been identified in the preoptic area/ anterior hypothalamus of several species including humans (75). Neural lesion studies in rats and ferrets have linked males’ preference for sexual interaction with females as opposed to other males to the function of the male-typical preoptic nucleus (76). In sheep, the volume of the sexually dimorphic oSDN is significantly larger in males than in females (35). The volume of the oSDN is also larger in rams that express a male-typical sexual preference for ewes than in rams that prefer other rams (35). The association between the volume of oSDN and sexual preferences suggests that this region somehow contributes to male-typical preferences for female sexual partners. Previous findings established that prenatal T masculinizes the oSDN and that aromatization is not required for T to act (2,18,34). The current results showing that the volume of oSDN in Flu rams was significantly different from both control rams and ewes is consistent with the interpretation that Flu exerted only a partial block on ARs (37) and may explain why sexual preference was not altered in the present study. Although inconclusive, these results are consistent with androgenic programming at the organizational level.
Like control rams, Flu rams did not exhibit LH surges or female-typical receptive behavior in response to estrogen treatment. These results indicate that neuroendocrine and behavioral defeminization is not interrupted by Flu in eugonadal males. As explained above, the fetal hypothalamus-pituitary-testicular axis is sufficiently developed during the period of sexual differentiation that it can react to AR inhibition at this age with a compensatory increase in T secretion that mitigates disruptions in brain differentiation. Such compensation is not possible in female lamb fetuses because the ovary does not normally secrete T or exert negative feedback. This is probably the reason that co-treatment with Flu blocks the defeminizing actions of prenatal exposure to exogenous T in female lamb fetuses but not to endogenous androgens in ram fetuses (19,20,77). Despite the ability of prenatal Flu to restore LH surges to androgenized ewes, the surges were reduced in magnitude. It has been inferred that estrogens program LH surge magnitude because prenatal treatment with nonaromatizable DHT failed to reduce the LH surge magnitude (77). Thus, it is possible that both androgens and estrogens synergize to organize the LH surge system in sheep.
The steroid requirements for defeminization of sexual receptivity have not been extensively studied in sheep. The potential for estrogenic programming is suggested by the absence of an effect of prenatal DHT on female receptivity (14). However, males exposed prenatally to the aromatase inhibitor ATD did not show receptive behavior in response to adult treatment with estradiol, which contradicts this assumption (18). Thus, the question of whether androgen and/or ER activation programs behavioral defeminization in sheep needs to be further addressed.
The LH surge mechanism is sexually differentiated and nonfunctional in males of several rodent species, including rats, mice and hamsters. Defeminization of the LH surge in rodents depends predominantly upon aromatization of T to estradiol and ER activation (12) although there is evidence for an androgenic contribution as well in rats (78). By contrast, ARs appear to primarily mediate defeminization of the LH surge mechanism in male guinea pigs (46), whereas in male monkeys, goats and men the LH surge mechanism is functional (79–81). Most experiments in rats, mice and guinea pigs support the conclusion that defeminization of sexual receptivity depends on aromatization (66,82,83). By contrast, prenatal treatment with either T or DHT defeminizes female sexual behavior in monkeys (84). Thus, the roles of T and its estrogenic metabolites assume different roles in organizing LH secretion and sexual behaviors across species.
The anatomical effects of prenatal Flu in sheep agree with those described previously using a paradigm that combined treatments with exogenous T and Flu (16) and confirm the role that fetal androgens and the AR plays in masculinization of the external genitalia. The current study shows that the testes of Flu rams retain the capacity for androgen synthesis although the testes are positioned subcutaneously in the caudal abdominal region and are azospermic. Differences in the concentrations of serum androgen between control and Flu rams only became evident during the fall breeding season (i.e., between September and November) when androgens rose precipitously in control rams, but were not different among groups in the summer and winter. Flu exposure did not alter pituitary responsiveness to a bolus injection of GnRH. Thus the reduced capacity for T secretion suggests that prenatal Flu exposure compromised the development and function of Leydig cells either directly or by altering the endocrine milieu during gestation (85).
In summary, we found that prenatal exposure to the antiandrogen Flu only partially interfered with masculinization of the oSDN. Although Flu rams displayed enhanced mounting behavior as adults, there was no effect on the display of other aspects of male-typical sexual behavior, including sexual partner preference. Moreover, Flu rams, like control rams, did not exhibit female-typical receptive behavior or LH surges in response to estrogen treatment. Prenatal Flu completely blocked masculinization of the external genitalia and decreased the seasonal increase in androgen secretion. These results indicate that prenatal exposure to antiandrogens that are sufficient to block masculinization of the external genitalia were insufficient to totally block male sexual differentiation in sheep and suggest that compensatory mechanisms intervene in fetal ram lambs to maintain sufficient androgen stimulation during development.
Acknowledgments
This work was supported by NIH R01OD011047 (CER).
The authors wish to acknowledge Dr. Vasantha Padmanhaban and her associates for providing the sheep used in this study. We also wish to thank the numerous students at OSU who helped care for and test these animals.
Footnotes
There are no conflicts of interest for any of the authors.
References
- 1.Ford JJ, D’Occhio MJ. Differentiation of sexual behavior in cattle, sheep, and swine. J Anim Sci. 1989;67:1816–1823. doi: 10.2527/jas1989.6771816x. [DOI] [PubMed] [Google Scholar]
- 2.Foster DL, Padmanabhan V, Wood RI, Robinson JE. Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: concepts derived from sheep models. Reprod Suppl. 2002;59:83–99. [PubMed] [Google Scholar]
- 3.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intra-uterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee VWK, Cumming IA, De Kretser DM, Findlay JK, Hudson B, Keogh EJ. Regulation of gonadotrophin secretion in rams from birth to sexual maturity. J Reprod Fertil. 1976;46:1–6. doi: 10.1530/jrf.0.0460001. [DOI] [PubMed] [Google Scholar]
- 5.Pomerantz DK, Nalbandov AV. Androgen levels in the sheep fetus during gestation. Proc Soc Exp Biol Med. 1975;149:413–420. doi: 10.3181/00379727-149-38818. [DOI] [PubMed] [Google Scholar]
- 6.Reddy RC, Estill CT, Meaker M, Stormshak F, Roselli CE. Sex differences in expression of oestrogen receptor alpha but not androgen receptor mRNAs in the foetal lamb brain. J Neuroendocrinol. 2014;26:321–328. doi: 10.1111/jne.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68:370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 8.Clarke IJ, Scaramuzzi RJ, Short RV. Sexual differentiation of the brain: endocrine and behavioral responses of androgenized ewes to oestrogen. J Endocrinol. 1976;71:175–176. doi: 10.1677/joe.0.0710175. [DOI] [PubMed] [Google Scholar]
- 9.Fabre-Nys C, Venier G. Sexual differentiation of sexual behaviour and preovulatory LH surge in ewes. Psychoneuroendocrinology. 1991;16:383–396. doi: 10.1016/0306-4530(91)90003-c. [DOI] [PubMed] [Google Scholar]
- 10.Foster DL, Jackson LM, Padmanabhan V. Reproduction in Domestic Ruminants. Nottingham: Nottingham University Press; 2007. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model; pp. 83–107. [DOI] [PubMed] [Google Scholar]
- 11.Roselli CE, Stormshak F. Prenatal programming of sexual partner preference: The ram model. J Neuroendocrinol. 2009;21:359–364. doi: 10.1111/j.1365-2826.2009.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 13.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Elsevier Science (USA); 2002. pp. 385–423. [Google Scholar]
- 14.Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- 15.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- 16.Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum MJ. Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand J Psychol. 2003;44:213–220. doi: 10.1111/1467-9450.00338. [DOI] [PubMed] [Google Scholar]
- 18.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 19.Padmanabhan V, Veiga-Lopez A, Herkimer C, Abi Salloum B, Moeller J, Beckett E, Sreedharan R. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve LH durge dynamics in prenatal testosterone-treated sheep. Endocrinology. 2015;156:2678–2692. doi: 10.1210/en.2015-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmer KM, Jackson LM, Foster DL. Sexual differentiation of the LH surge response to estradiol in sheep treated prenatally with testosterone plus and antiandrogen. Biol Reprod. 2008;177:518. [Google Scholar]
- 21.Henley CL, Nunez AA, Clemens LG. Hormones of choice: The neuroendocrinology of partner preference in animals. Front Neuroendocrinol. 2011;32:146–154. doi: 10.1016/j.yfrne.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp 19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 23.Adkins-Regan E. Sex hormones and sexual orientation in animals. Psychobiol. 1988;16:335–347. [Google Scholar]
- 24.Petrulis A. Chemosignals, hormones and mammalian reproduction. Horm Behav. 2013;63:723–741. doi: 10.1016/j.yhbeh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 2009;55:579–588. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobet SA, Zahniser DJ, Baum MJ. Sexual dimorphism in the preoptic/anterior hypothalamic area of ferrets: effects of adult exposure to sex steroids. Brain Res. 1986;364:249–257. doi: 10.1016/0006-8993(86)90837-1. [DOI] [PubMed] [Google Scholar]
- 27.Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kindon HA, Baum MJ, Paredes RG. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm Behav. 1996;30:514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- 29.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 30.Houtsmuller EJ, Brand T, De Jonge FH, Joosten RNJMA, Van De Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 31.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay DR. The importance of olfactory stimuli in the mating behaviour of the ram. Anim Behav. 1965;13:75–78. [Google Scholar]
- 33.Price EO, Katz LS, Wallach SJR, Zenchak JJ. The relationship of male-male mounting to the sexual preferences of young rams. Appl Anim Behav Sci. 1988;21:347–355. [Google Scholar]
- 34.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 35.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 36.Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roselli CE, Reddy R, Estill C, Scheldrup M, Meaker M, Stormshak F, Montilla HJ. Prenatal influence of an androgen agonist and antagonist on the differentiation of the ovine sexually dimorphic nucleus in male and female lamb fetuses. Endocrinology. 2014;155:5000–5010. doi: 10.1210/en.2013-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology. 1975;97:373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- 39.Perkins A, Fitzgerald JA, Price EO. Luteinizing hormone and testosterone response of sexually active and inactive rams. J Anim Sci. 1992;70:2086–2093. doi: 10.2527/1992.7072086x. [DOI] [PubMed] [Google Scholar]
- 40.Resko JA, Malley A, Begley D, Hess DL. Radioimmunoassay of testosterone during fetal development of the rhesus monkey. Endocrinology. 1973;93:156–161. doi: 10.1210/endo-93-1-156. [DOI] [PubMed] [Google Scholar]
- 41.Roselli CE, Resko JA. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology. 1984;114:2183–2189. doi: 10.1210/endo-114-6-2183. [DOI] [PubMed] [Google Scholar]
- 42.Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- 43.Roselli CE, Stormshak F, Resko JA. Distribution of aromatase mRNA in the ram hypothalamus: an in situ hybridization study. J Neuroendocrinol. 2000;12:656–664. doi: 10.1046/j.1365-2826.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- 44.Hochereau-de Reviers MT, Blanc MR, Cahoreau C, Courot M, Dacheux JL, Pisselet C. Histological testicular parameters in bilateral cryptochid adult rams. Ann Biol Anim Bioch Biophys. 1979;79:1141–1146. [Google Scholar]
- 45.Clarke IJ, Scaramuzzi RJ. Sexual behaviour and LH secretion in spayed androgenized ewes after a single injection of testosterone or oestradiol-17β. J Reprod Fertil. 1978;52:313–320. doi: 10.1530/jrf.0.0520313. [DOI] [PubMed] [Google Scholar]
- 46.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesiano S, Hart CS, Heyer BW, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XXVI A sex difference in the effect of castration on the hypothalamic-pituitary gonadotropin unit in the ovine fetus. Endocrinology. 1991;129:3073–3079. doi: 10.1210/endo-129-6-3073. [DOI] [PubMed] [Google Scholar]
- 48.Matwijiw I, Faiman C. Control of gonadotropin secretion in the ovine fetus. II A sex difference in pulsatile luteinizing hormone secretion after castration. Endocrinology. 1989;124:1352–1358. doi: 10.1210/endo-124-3-1352. [DOI] [PubMed] [Google Scholar]
- 49.Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- 50.Baum MJ, Carroll RS, Tobet SA. Steroidal control of behavioural, neuroendocrine and brain sexual differentiation: studies in a carnivore, the ferret. J Neuroendocrinol. 1990;2:401–418. doi: 10.1111/j.1365-2826.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 51.Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod. 1985;32:855–864. doi: 10.1095/biolreprod32.4.855. [DOI] [PubMed] [Google Scholar]
- 52.Roffi J, Chami F, Corbier P, Edwards DA. Testicular hormones during the first few hours after birth augment the tendency of adult male rats to mount receptive females. Physiol Behav. 1987;39:625–628. doi: 10.1016/0031-9384(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 53.Jackson LM, Mytinger A, Roberts EK, Lee TM, Foster DL, Padmanabhan V, Jansen HT. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154:1612–1623. doi: 10.1210/en.2012-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattner PE, George JM, Braden AWH. Testosterone treatment of ram lambs: effect on adult libido. Theriogenology. 1976;6:613. [Google Scholar]
- 55.Roselli CE, Estill C, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152:2409–2415. doi: 10.1210/en.2010-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10:40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- 57.Matuszczyk JV, Larsson K. Sexual preference and feminine and masculine sexual behavior of male rats prenatally exposed to antiandrogen or antiestrogen. Horm Behav. 1995;29:191–206. doi: 10.1006/hbeh.1995.1014. [DOI] [PubMed] [Google Scholar]
- 58.Sachs BD, Barfield RJ. Temporal patterning of sexual behavior in the male rat. J Comp Physiol Psychol. 1970;73:359–364. doi: 10.1037/h0030243. [DOI] [PubMed] [Google Scholar]
- 59.Thornton JE, Irving S, Goy RW. Effects of prenatal antiandrogen treatment on masculinization and defeminization of guinea pigs. Physiol Behav. 1991;50:471–475. doi: 10.1016/0031-9384(91)90532-s. [DOI] [PubMed] [Google Scholar]
- 60.Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75:337–346. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- 61.Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiol Behav. 2003;79:633–641. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 62.Baum MJ, Tobet SA. Effect of prenatal exposure to aromatase inhibitor, testosterone, or antiandrogen on the development of feminine sexual behavior in ferrets of both sexes. Physiol Behav. 1986;37:111–118. doi: 10.1016/0031-9384(86)90392-6. [DOI] [PubMed] [Google Scholar]
- 63.Drea CM, Place NJ, Weldele ML, Coscia EM, Licht P, Glickman SE. Exposure to naturally circulating androgens during foetal life incurs direct reproductive costs in female spotted hyenas, but is prerequisite for male mating. Proc Biol Sci. 2002;269:1981–1987. doi: 10.1098/rspb.2002.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- 65.Herman RA, Zehr JL, Wallen K. Prenatal androgen blockade accelerates pubertal development in male rhesus monkeys. Psychoneuroendocrinology. 2006;31:118–130. doi: 10.1016/j.psyneuen.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda SI, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Signoret JP. Effects of oestrogen and androgen on the sexual behaviour responses of the ovariectomized ewe. Psychoneuroendocrinology. 1975;1:179–184. doi: 10.1016/0306-4530(75)90009-8. [DOI] [PubMed] [Google Scholar]
- 69.Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–620. doi: 10.1016/j.yhbeh.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olvera-Hernandez S, Fernandez-Guasti A. Perinatal administration of aromatase inhibitors in rodents as animal models of human male homosexuality: similarities and differences. Adv Neurobiol. 2015;10:381–406. doi: 10.1007/978-1-4939-1372-5_18. [DOI] [PubMed] [Google Scholar]
- 71.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 72.Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakker J, De Mees C, Szpirer J, Szpirer C, Balthazart J. Exposure to oestrogen prenatally does not interfere with the normal female-typical development of odour preferences. J Neuroendocrinol. 2007;19:329–334. doi: 10.1111/j.1365-2826.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 74.Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooke BM, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 76.Baum MJ. Mammalian animal models of psychosexual differentiation: when is ‘translation’ to the human situation possible? Horm Behav. 2006;50:579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Abi Salloum B, Herkimer C, Lee JS, Veiga-Lopez A, Padmanabhan V. Developmental programming: prenatal and postnatal contribution of androgens and insulin in the reprogramming of estradiol positive feedback disruptions in prenatal testosterone-treated sheep. Endocrinology. 2012;153:2813–2822. doi: 10.1210/en.2011-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475–1483. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- 79.Westfahl PK, Stadelman HL, Horton LE, Resko JA. Experimental induction of estradiol positive feedback in intact male monkeys: absence of inhibition by physiologic concentrations of testosterone. Biol Reprod. 1984;31:856–862. doi: 10.1095/biolreprod31.5.856. [DOI] [PubMed] [Google Scholar]
- 80.Dorner G. Neuroendocrine response to estrogen and brain differentiation in heterosexuals, homosexuals, and transsexuals. Arch Sex Behav. 1988;17:57–75. doi: 10.1007/BF01542052. [DOI] [PubMed] [Google Scholar]
- 81.Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, Wakabayashi Y, Okamura H, Maeda KI, Uenoyama Y, Tsukamura H, Ohkura S. The luteinising hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol. 2015;27:57–65. doi: 10.1111/jne.12235. [DOI] [PubMed] [Google Scholar]
- 82.Choate JVA, Resko JA. Prenatal inhibition of aromatase activity affects luteinizing hormone feedback mechanisms and reproductive behaviors of adult guinea pigs. Biol Reprod. 1994;51:1273–1278. doi: 10.1095/biolreprod51.6.1273. [DOI] [PubMed] [Google Scholar]
- 83.Hines M, Goy RW. Estrogens before birth and development of sex-related reproductive traits in the female guinea pig. Horm Behav. 1985;19:331–347. doi: 10.1016/0018-506x(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 84.Pomerantz SM, Roy MM, Thornton JE, Goy RW. Expression of adult female patterns of sexual behavior by male, female, and pseudohermaphroditic female rhesus monkeys. Biol Reprod. 1985;33:878–889. doi: 10.1095/biolreprod33.4.878. [DOI] [PubMed] [Google Scholar]
- 85.Kotula-Balak M, Hejmej A, Kopera I, Lydka M, Bilinska B. Prenatal and neonatal exposure to flutamide affects function of Leydig cells in adult boar. Domestic Anim Endocrinol. 2012;42:142–154. doi: 10.1016/j.domaniend.2011.11.002. [DOI] [PubMed] [Google Scholar]