Abstract
Objective
To formally test whether insulin sensitivity mediates the relationship between fitness and brain integrity.
Methods
Eighty-four non-diabetic, middle-aged participants received a 6-minute walk test from which maximal oxygen uptake (VO2 max) was derived, a structural MR scan, and a medical evaluation including fasting glucose and insulin levels.
Results
We showed significant associations between fitness, abdominal obesity, and insulin sensitivity and anterior cingulate cortex (ACC) volume as well as between ACC thickness and quantitative insulin-sensitivity check index (QUICKI). We showed that the relationship between ACC volume and VO2 max was completely mediated through QUICKI, see Figure 3. Further, this strong association was confirmed by a single and very significant cluster on the ACC linking gray matter volume and QUICKI in a voxel-based morphometry analysis.
Conclusions
As expected, increased abdominal obesity was associated with reductions in fitness, ACC volumes, and insulin sensitivity. Importantly, we demonstrate a significant mediation of the relationship between VO2 max and ACC volume by QUICKI. This suggests that the links between impaired insulin sensitivity and brain abnormalities in adults carrying excess weight could be alleviated through increased physical activity and fitness.
Keywords: Obesity, fitness, VO2 max, insulin resistance, anterior cingulate gyrus, mediation
Introduction
Over one-third of U.S. adults have obesity, and 86.3% are projected to have either overweight or obesity by 2030 (1). Insulin resistance (IR) is among the most common metabolic complications related to obesity, and may be the link between obesity and associated co-morbid conditions such as type 2 diabetes (T2DM), cardiovascular disease, and hypertension (2). IR has been shown to independently predict all three conditions (3). Even in the absence of these associated co-morbidities, individuals carrying excess weight tend to be insulin resistant, but this can be improved through weight loss (4). While the mechanisms causing IR remain unclear, evidence suggests that excess adiposity, particularly around the midriff (5), and physical inactivity are important predictors of IR (2).
A growing body of literature links obesity and associated metabolic problems to brain abnormalities (6, 7). We have shown that even in adolescents, who may have relatively short duration of metabolic impairment and are without clinically manifest vascular disease, obesity and IR are associated with reductions in cognitive performance (8, 9) and with brain changes (8, 10). These observations underscore the importance of preventative health measures to promote brain integrity and overall health.
Diet and exercise represent the first line of intervention for facilitating weight loss and attenuation of obesity-related metabolic disorders. High levels of physical activity and fitness are associated with health benefits that include reduced abdominal adiposity, improved insulin sensitivity and lipid profile, and lowered blood pressure (11). Moreover, fitness has been shown to support brain health, especially among aging adults. Studies have consistently reported increased hippocampal and prefrontal cortex volume as a function of fitness (12, 13). Other brain regions positively influenced by fitness include the temporal, parietal (12), and cingulate (14) cortices. Exercise interventions seem to improve many cognitive domains, particularly executive function (15). Associations between exercise and executive function may be partly explained by the larger prefrontal cortex volumes seen among individuals with high levels of fitness, but the direct role of gray matter (GM) volume on cognitive function among normal individuals remains speculative (15).
Neuroimaging studies exploring the benefits of physical fitness on brain health have focused on maximal oxygen uptake, or VO2 max, which is considered an accurate, objective measure of cardiorespiratory fitness (CRF) (15). Although the gold-standard measurement of VO2 max is obtained through graded exercise testing, this method of assessment presents barriers, such as equipment costs and risk of injury. The 6-minute walk test (6MWT) is a simpler, more accessible assessment that is well established for use in diverse populations, including healthy adults and patients with chronic obstructive pulmonary disease (16). The distance covered during a 6MWT provides an excellent estimate of VO2 max that has been validated against direct measurements of VO2 max during graded exercise testing (17), and its predictive value increases when combined with other easily obtained characteristics (16).
The purpose of the current study was to investigate the relationships between central obesity, insulin sensitivity, and CRF relative to frontal lobe structure. We recently reported reduced orbitofrontal cortex (OFC) (9, 10) and anterior cingulate cortex (ACC) (9) thicknesses in adolescents with obesity. We hypothesized that adults with high CRF (high VO2 max) would have greater GM volume and cortical thickness in these two brain regions, which have been found to be impacted by both fitness (10, 12) and obesity (9), and are known to be involved in the reward circuit and eating behaviors (18). We further hypothesized that insulin sensitivity would explain some of the relationships between VO2 max and brain structure, thereby suggesting a possible mechanism by which fitness affects brain integrity.
Methods
Participants
Adults between the ages of 45 and 63 years were evaluated as part of an NIH-sponsored study at the Brain, Obesity, and Diabetes Laboratory (BODyLab), NYU School of Medicine. The study was approved by the local Institutional Review Board, and informed consent was obtained from all study participants. Participants were recruited via internet advertisement, or referred by other participants. Active medical (other than T2DM, hypertension, or dyslipidemia), neurological, or psychiatric conditions were exclusionary. Participants underwent medical, endocrine, neurological, psychiatric, neuropsychological, and magnetic resonance imaging (MRI) assessments completed over four visits within two months. The parent study included individuals with T2DM, however for the current analysis we were interested in ascertaining the relationships between fitness, obesity, insulin sensitivity, and brain structure, and therefore excluded individuals with T2DM. Of the 136 adults evaluated, 52 were excluded from analysis (38 had T2DM, 6 were missing MRI, and 8 were missing VO2 max data). Individuals missing MRI data dropped out of the study before completing the MRI, and VO2 max data was missing for participants unable to perform the 6MWT due to mobility limitations. This left a final set of 84 non-diabetic, middle-aged adults.
Physical assessment
Height and weight were measured on the first day of study with the participant wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Using adult cutoffs, participant BMI was classified as lean (BMI<25 kg/m2), overweight (BMI≥25 and <30 kg/m2), or obese (BMI≥30 kg/m2). Abdominal obesity was assessed by waist-to-height ratio (WHtR), a reportedly stronger predictor of diabetes and cardiovascular risk than BMI and/or waist circumference alone (19). While computed tomography and MRI are the gold standards for measuring visceral adipose tissue (VAT), WHtR has been shown to strongly predict direct measurements of VAT (20). For WHtR, waist circumference was measured at the level of the umbilicus over a single layer of light clothes. Body fat percentage was estimated using a body composition 2.1 RJL Portable System and Quantum IV Bioelectrical Impedance Body Composition Analyzer (RJL Systems, Clinton Township, MI, USA). Sitting blood pressure was measured using standardized conditions during the first and third visits using a random-zero sphygmomanometer, and the two readings were averaged.
Blood samples
Following a 9-12 hour overnight fast, blood was collected from participants at the NYU Medical Center Outpatient Laboratory. Standard blood tests, including comprehensive metabolic panel with glucose and insulin levels, complete blood count, lipid profile, and high sensitivity C-reactive protein (hs-CRP) were performed. Insulin sensitivity was estimated by the quantitative insulin-sensitivity check index calculation (QUICKI; 1/(log fasting glucose [mg/dL]+log fasting insulin [μU/mL])), which has been validated against the hyperinsulinemic-euglycemic glucose clamp (21). Higher QUICKI values reflect higher insulin sensitivity, and QUICKI ≤ 0.350 is suggestive of IR.
6MWT and VO2 max calculation
The 6MWT was administered following the American Thoracic Society guidelines (22) as closely as possible. A corridor, where every 1 meter was marked, served as the walking course. Participants, in comfortable clothing and shoes, were instructed to walk as fast as possible for 6 minutes between two cones spaced 20 m apart. Standardized verbal encouragements were provided. Blood pressure, heart rate, blood oxygenation, and ratings of perceived exertion were recorded before and immediately after the test. The total distance covered in meters was also recorded. To estimate VO2 max, we utilized the following formula: VO2 max (mL•kg-1 •min-1)=70.161 +(0.023 × 6MWT distance [m]) – (0.276 × weight [kg] – (6.79 × sex, where m=0, f=1) – (0.193 × resting HR [beats per minute]) – (0.191 × age [years]) (17).
MRI Acquisition
All participants were studied on the same 3.0T Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) using a standard protocol described previously (10). T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (TR 2200 ms; TE 2.3 ms; TI 1100 ms; FOV 240 × 240; 192 slices; slice thickness 0.90 mm; NEX 1; flip angle 12°) was utilized for brain structural evaluation. Together with the MPRAGE image, the fluid-attenuated inversion recovery image (TR 9000 ms; TE 81 ms; TI 2500 ms; FOV 220 × 220; 1 average and 2 concatenations; flip angle 120°) was used to rule out primary neurological abnormalities. Three participants were unable to complete the MRI due to claustrophobia, and one was excluded from analyses involving neuroimaging data due to a brain abnormality.
Region of interest (ROI)-based cortical thickness and volume assessment
Cortical thickness and GM volume estimates were derived from the MPRAGE images using FreeSurfer image analysis suite version 5.1.0 (http://surfer.nmr.mgh.harvard.edu/). Details of the fully automated and well-validated procedures have been described previously (23, 24). Briefly, steps included skull-stripping, automated Talairach registration, gray/white matter segmentation, and construction of a model gray-white matter boundary. The cerebral cortex was then parcellated into ROIs based on gyral and sulcal structures (25).
The OFC (lateral and medial combined) and ACC (rostral and caudal combined) ROIs were selected a priori for our analyses. The rostral middle frontal gyrus (rMFG), which has been linked to fitness (26), was used as a control region. This will allow us to demonstrate the anatomical specificity of our findings. Total GM volume was computed across the left and right hemispheres for each ROI. The average cortical thickness of each ROI was determined by averaging the cortical thickness of the subregions weighted by the corresponding surface areas for both hemispheres. Total intracranial vault (ICV) volume was used to adjust the data for individual variability in head size.
Voxel-based morphometry (VBM)
All MPRAGE images were preprocessed and analyzed using Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.18 (MathWorks, Natick, MA, USA). We created a custom template from a population of 167 middle-aged adults studied at the BODyLab (including participants from the current study); MPRAGE images were normalized to the standard Montreal Neurological Institute (MNI) template, segmented into GM, white matter, and cerebrospinal fluid partitions, and averaged. Individual scans were then processed using the custom template and high-dimensional DARTEL registration. To preserve relative differences in GM regional volume, Jacobian modulation was applied. Finally, the modulated GM images were smoothed with a Gaussian kernel of 8-mm full-width at half maximum.
Statistical analysis
Statistical analyses of demographic and segmented brain data were performed with SPSS 20.0 software for Windows (SPSS, Chicago, IL, USA). For segmented brain data, values greater than 3 standard deviations (SDs) from the mean were excluded.
Although group differences were not evaluated, mean values (± SD) for individuals with lean BMIs and overweight or obesity (O/O) BMIs are presented separately for descriptive purposes. However, analyses were conducted on the total set of participants. Given that normal aging is associated with increased BMI and fat mass (27), decreased physical activity levels (28), and brain tissue loss (29) and that we have observed differential effects of metabolic impairment on brain by sex (30), we controlled for age and sex in all analyses. Brain volumes and thicknesses were residualized to ICV using linear regression.
Spearman rank correlations adjusting for age and sex were used to first establish relationships among variables. A mediation analysis was then conducted using the PROCESS macro in SPSS (31) to evaluate whether QUICKI mediated the relationship between fitness and ACC volume. The indirect (mediation) effect was tested using a bias-corrected, nonparametric bootstrapping procedure. 5,000 bootstrap resamples were utilized to generate a 95% confidence interval. If the confidence interval did not contain zero, we concluded that the indirect effect was statistically significant.
Voxel-based multiple regression analysis was performed with SPM8 to explore the association between whole-brain GM volume and QUICKI. Age (log transformed to follow normal distribution), sex, and segmentation-derived ICV volume were included in the model as nuisance covariates. An absolute threshold mask of 0.7 was applied to the GM images. Statistical significance was set at p < .001 (uncorrected) at the voxel-level and p < .05 (uncorrected) at the cluster-level, with a minimum cluster size of 100 contiguous voxels. MNI coordinates of significant peak and sub-peak voxels (greater than 8mm apart) were labeled with the AAL template (32) available within MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/).
Results
Subject Characteristics
BMI levels varied widely (18.08 - 50.66 kg/m2; 32 lean, 18 overweight, and 34 obese). Thirty-two participants were classified as insulin resistant by QUICKI with 30 either having overweight or obesity. Participants with overweight or obesity were pooled into an O/O group. Background characteristics of the lean and O/O groups are presented in Table 1 for descriptive purposes. Although group differences were not evaluated for statistical significance, the two groups were demographically comparable with unequal metabolic characteristics; lean adults had a healthier metabolic profile (lower fasting glucose and insulin levels) and anthropometric measures (lower BMI, WHtR, and estimated fat %), lower blood pressure, and better fitness (higher VO2 max).
Table 1.
Selected Subject Characteristics
| Lean (n=32) | O/O (n=52) | |
|---|---|---|
| Measures | Mean ± SD | Mean ± SD |
| Age (years) | 52.21 ± 4.46 (45.10-60.80) | 50.48 ± 3.63 (45.04-60.89) |
| Sex (Male/Female) | 15/17 | 26/26 |
| Race (Black/White/Asian/Hispanic/Other) | 5/19/1/7/0 | 19/23/1/7/2 |
| Education (years completed) | 15.77 ± 2.21 (12.00-20.00) | 15.64 ± 2.27 (11.00-21.00) |
| BMI (kg/m2) | 22.23 ± 1.88 | 33.17 ± 5.97 |
| WHtR | 0.49 ± 0.04 | 0.63 ± 0.09 |
| Estimated fat (%) | 24.23 ± 6.43 | 35.39 ± 9.34 |
| Systolic blood pressure (mmHg) | 112.98 ± 9.49 | 120.59 ± 12.37 |
| Diastolic blood pressure (mmHg) | 70.41 ± 7.04 | 74.23 ± 8.39 |
| HDL cholesterol (mg/dL) | 61.22 ± 16.42 | 52.37 ± 13.05 |
| Triglycerides (mg/dL) | 81.63 ± 33.34 | 118.98 ± 65.03 |
| Hemoglobin A1C (%) | 5.53 ± 0.29 | 5.66 ± 0.42 |
| Fasting glucose (mg/dL) | 86.88 ± 9.05 | 90.31 ± 6.35 |
| Fasting insulin (μU/mL) | 4.32 ± 1.78 | 11.26 ± 9.91 |
| QUICKI | 0.396 ± 0.030 | 0.347 ± 0.033 |
| hs-CRP (mg/L) | 1.75 ± 3.16 | 3.02 ± 3.12 |
| VO2 max (mL/kg/min) | 38.83 ± 4.18 | 28.08 ± 7.48 |
BMI, body mass index; WHtR, waist-to-height ratio; HDL, high-density lipoprotein; QUICKI, quantitative insulin-sensitivity check index; hs-CRP, high sensitivity C-reactive protein; VO2 max, maximal oxygen uptake.
Associations between central obesity, insulin sensitivity, and CRF
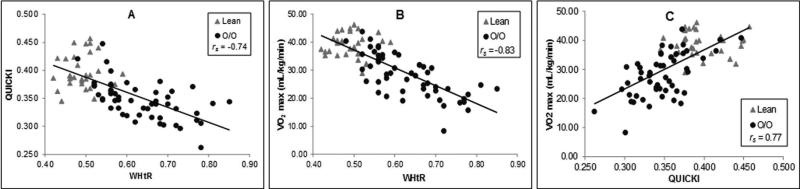
We examined the relationships between central obesity measured by WHtR, insulin sensitivity measured by QUICKI, and CRF measured by VO2 max independent of age and sex. As expected, WHtR had significant negative associations with QUICKI (rs(80) = −0.74, p < .001; see Fig.1A) as well as VO2 max (rs(80)= −0.83, p < .001; see Fig.1B). A significant positive correlation was found between QUICKI and VO2 max (rs(80) = 0.77, p < .001; see Fig.1C).
Figure 1.
Panel A is a plot of the very robust inverse relationship between insulin sensitivity (quantitative insulin-sensitivity check index, QUICKI) and our measure of fitness (maximal oxygen uptake, VO2 max). Please note that the value for the strength of the association is a partial r (after adjusting for age and sex). The partial correlation coefficients (rps; accounting for age and sex) for the relationships between VO2 max and waist-to-height ratio (WHtR) (B) and QUICKI C), respectively are shown. Please note that for all scatter plots the gray triangles represent participants with lean body mass indices (BMIs), and the black circles represent participants with overweight and obese (O/O) BMIs.
Neuroimaging assessment
In Table 2, we present the raw segmented brain data for the lean and O/O groups.
Table 2.
Segmented Data by group
| Lean (n=31) | O/O (n=49) | |
|---|---|---|
| Measures | Mean ± SD | Mean ± SD |
| ICV volume (cc) | 1496.18 ± 145.34 | 1515.85 ± 158.80 |
| OFC gray matter volume (cc) | 25.71 ± 2.54 | 25.70 ± 2.79 |
| ACC gray matter volume (cc) | 8.76 ± 1.29 | 8.31 ± 1.25 |
| rMFG gray matter volume (cc) | 31.07 ± 3.68 | 31.60 ± 3.98 |
| OFC cortical thickness (mm) | 2.64 ± 0.09 | 2.64 ± 0.13 |
| ACC cortical thickness (mm) | 2.79 ± 0.14 | 2.74 ± 0.16 |
| rMFG cortical thickness (mm) | 2.43 ± 0.10 | 2.44 ± 0.11 |
ICV, intracranial vault; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; rMFG, rostral middle frontal gyrus.
However, for the following analyses we used ICV-adjusted brain volumes and thicknesses. Correlation analysis of WHtR, QUICKI, and VO2 max with a priori selected ROIs is presented in Table 3.
Table 3.
Spearman's partial correlation analysis (rs values) between segmented brain measures & cardiometabolic factors independent of age and sex
| Measures | WHtR | QUICKI | VO2 max |
|---|---|---|---|
| OFC gray matter volume | −0.16 | 0.21 | 0.13 |
| ACC gray matter volume | −0.26* | 0.36*** | 0.29* |
| rMFG gray matter volume | −0.02 | 0.10 | 0.06 |
| OFC cortical thickness | −0.16 | 0.19 | 0.16 |
| ACC cortical thickness | −0.14 | 0.30** | 0.20 |
| rMFG cortical thickness | 0.02 | 0.13 | 0.16 |
p < .05
p < .01
p = .001.
ICV, intracranial vault; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; rMFG, rostral middle frontal gyrus.
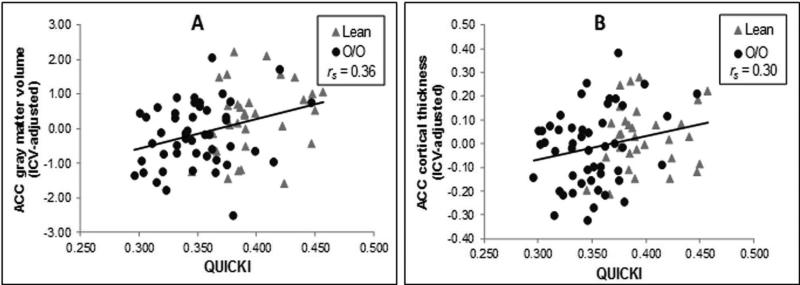
After accounting for age and sex, ACC volume correlated positively with QUICKI (rs(74)= 0.36, p = .001; see Fig. 2A) and VO2 max (rs(74)= 0.29, p = .01) and inversely with WHtR (rs (74)= −0.26, p = .02). ACC thickness showed a significant correlation with QUICKI (rs(76)= 0.30, p = .008; see Fig. 2B). In contrast, no significant associations were detected with OFC volume or thickness. The control region variables, rMFG volume and thickness, also failed to show significant associations with the cardiometabolic factors (WHtR/QUICKI/VO2 max).
Figure 2.
Figure 2 shows the relationships between the intracranial vault volume (ICV)-adjusted (residual) anterior cingulate (ACC) volume (panel A) and thickness (panel B) and maximal oxygen uptake (VO2 max).
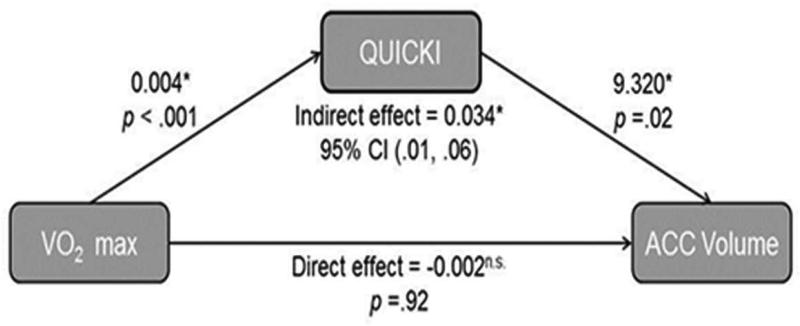
Given that VO2 max significantly correlated with both QUICKI (rs (80) = 0.77, p < .001) and ACC volume (rs (73) = 0.29, p = .01), we tested our hypothesis that insulin sensitivity would mediate the association between fitness and brain volume, after controlling for age and sex. Results indicated that the overall model was significant (total effect = 0.032, p = .03) and that QUICKI was a significant mediator of the relationship between VO2 max and ICV-adjusted ACC volume (indirect effect= 0.034, 95% CI [0.01, 0.06], however after the mediation effect, the direct effect was no longer significant; see Fig. 3).
Figure 3.
A schematic of the complete mediation effect, demonstrating that the indirect effect (mediation) of insulin sensitivity (quantitative insulin-sensitivity check index, QUICKI) on intracranial vault volume (ICV)-adjusted (residual) anterior cingulate (ACC) volume is significant, but that the direct effect of maximal oxygen uptake (VO2 max) is not significant after the indirect effect is accounted for.
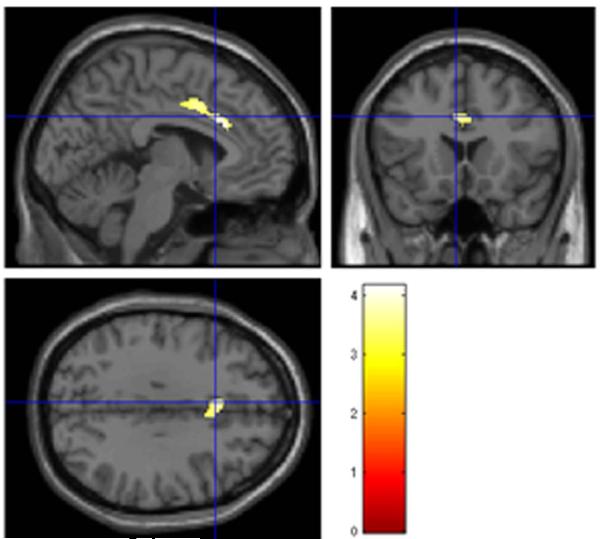
To further explore and validate our findings, we ran a whole-brain voxel-wise analysis correlating QUICKI and GM volume. Age (log-transformed), sex, and ICV volume were entered as confounding variables. Consistent with the ROI-based segmentation analyses, the voxel-wise analysis revealed a significant positive association between QUICKI and GM volume in the left ACC and bilateral median cingulate (Table 4, Fig. 4). This finding did not survive family-wise error correction.
Table 4.
| Cluster-level |
Peak-level |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates |
|||||||||
| k | p FWE | p uncorr | Region | x | y | z | T | p FWE | p uncorr |
| 505 | 0.08 | 0.04 | L. anterior cingulate | −3 | 24 | 27 | 4.18 | 0.12 | < .001 |
| — | L. median cingulate | −6 | 8 | 40 | 3.68 | 0.44 | < .001 | ||
| — | R. median cingulate | 6 | 14 | 34 | 3.55 | 0.55 | < .001 | ||
Regions that exhibit a significant (p < .05 uncorrected at cluster level, k > 100 voxels; p < .001 uncorrected at voxel-level) correlation with QUICKI are listed. —indicates sub-peak within a given cluster; k, cluster size (voxels); R, right; L, left.
Figure 4.
Statistical parametric maps showing increased gray matter volume of the anterior cingulate region associated with higher insulin sensitivity (quantitative insulin-sensitivity check index, QUICKI) after correcting for age, sex, and intracranial vault volume. Results are shown p < .001 (uncorrected).
Discussion
To our knowledge, this is the first report documenting a clear relationship between structural integrity of the frontal lobe with insulin sensitivity and CRF in middle-aged adults. The goal of our study was to examine whether the degree of CRF, as ascertained by an estimate of VO2 max, helped explain the integrity of three specific frontal lobe cortical regions (ACC, OFC, rMFG) and whether insulin sensitivity mediated those effects. Our results support our hypothesis that adults with high CRF (VO2 max) have larger GM volume in the ACC. Further analyses confirmed that insulin sensitivity significantly mediated the effect of VO2 max on ACC volume (Figure 3). We confirmed these relationships by utilizing voxel-wise analyses, a completely different analytical approach, finding a significant positive association between our measure of insulin sensitivity (QUICKI) and ACC volume with the correlation cluster extending into both hemispheres (Figure 4). QUICKI significantly correlated with ACC cortical volume and thickness and these associations were quite specific, since none were present to either thickness or volume of the OFC and rMFG regions.
As expected, we found a strong negative relationship between WHtR with QUICKI and VO2 max, indicating that those individuals with central obesity have more IR and are less fit. Similarly, we found a robust positive correlation between QUICKI and VO2 max, indicating that individuals who are fitter also have higher insulin sensitivity (See Figure 1). It has been well documented that lack of physical activity is the strongest predictor of low CRF and that this is associated with abdominal obesity (33), and that visceral adiposity is the leading cause of IR (5, 34). Further, studies of fitness have shown that physical inactivity can cause IR (2) and increasing exercise can improve insulin sensitivity (35). Consequently, exercise remains an effective intervention to attenuate IR and its associated co-morbidities.
As predicted, QUICKI, VO2 max and WHtR were associated with ACC GM volume. This is consistent with studies that have demonstrated an increase in ACC GM volume in physically active adults (14). QUICKI also showed a significant positive correlation with ACC cortical thickness. This is consistent with previous findings from our lab where adolescents with obesity, many of whom have significant levels of IR, show reductions in cortical thickness of the ACC (8, 9). The results of the current study confirm, now in adults, our previous findings in adolescents that QUICKI is the strongest predictor of brain structural integrity (8, 10). In contrast to our previous findings of increased cortical thickness of the OFC being associated with VO2 max and QUICKI among adolescents (10), we found no associations among middle-aged individuals. As expected, the control region, the rMFG, did not correlate with our measures of abdominal obesity, insulin sensitivity or CRF.
Consistent with our predictions, the mediation analysis confirms our hypothesis that the effect of VO2 max on ACC volume is mediated by insulin sensitivity (QUICKI). Further, we found a complete mediation, suggesting that most of the influence of CRF on ACC volume is mediated by how insulin sensitive individuals are. Although we did not directly test it here, it has been suggested that the enhanced neural and synaptic plasticity induced by aerobic exercise (36) is a result of improved growth factor signaling (37). This improved signaling is a result of increased growth factor levels and reduction of pro-inflammatory cytokines. Insulin-like growth factor 1 (IGF-1) is one such growth factor that is reportedly inhibited by pro-inflammatory cytokines that may lead to IR (38). Exercise increases IGF-1 levels leading to better insulin sensitivity and healthier brain integrity. While the exact mechanisms are unknown and likely multifactorial, our results provides support for one possible pathway through which CRF and insulin sensitivity affect brain volume.
The results of our whole-brain VBM analysis showed a significantly larger GM volume in the ACC region in participants with higher levels of QUICKI. This supports a previous voxel-wise analysis that showed greater levels of IR predicting less GM in the cingulate cortex (39). Furthermore, using VBM, Moran et al (2013) demonstrated GM cingulate region volume loss attributable to T2DM (40). The results of our voxel-wise analysis validate our volumetric analysis results showing a significant positive correlation between ACC volume and QUICKI.
This study has significant strengths and some limitations. Although our sample size was only modest, we had adequate statistical power to test our hypotheses. Our lean and O/O groups were well matched on age, sex, race and years of education. Furthermore, our participants were recruited from the community and were not a clinical population, thus improving our ability to generalize these results. It must be noted that our measure of VO2 max is an indirect estimation of aerobic fitness based on the 6MWT (17). Although this estimate has been validated against direct measures of VO2 max (16), future studies should use more rigorous measurements of VO2 max. Our confidence regarding the validity of our brain findings is strengthened by the fact that they were confirmed by two different types of analyses; both segmentation and voxel-wise analyses detected subtle changes in ACC GM volume associated with varying levels of insulin sensitivity (QUICKI). Furthermore, by means of mediation analysis we demonstrated that the relationship between VO2 max and ACC volume was mediated through insulin sensitivity (QUICKI, Figure 3). With this said, although our results suggest that insulin sensitivity is important in the relationship between fitness and ACC volume, by itself a single mediation analysis is only hypothesis generating and cannot be used to draw any conclusions regarding a causation.
In summary, these data suggests that higher levels of CRF are associated with greater ACC volumes in adults and that insulin sensitivity mediates this effect. Our findings suggest that by improving CRF, which will very likely result in improvements in insulin sensitivity, healthier brain structure may be maintained. Future studies should employ a longitudinal design to track whether improving CRF and insulin sensitivity can improve brain structural integrity or reverse existing atrophy.
What is already known about this subject?
There are known associations between abdominal obesity, lower fitness (VO2 max), and insulin resistance.
Fitness is known to be associated with overall frontal lobe integrity.
What does your study add?
In middle-aged adults, VO2 max explains the volume of the anterior cingulate gyrus (ACC).
Insulin sensitivity significantly mediates the association between VO2 max and ACC volume.
The association between VO2 max and ACC volume are specific as orbitofrontal and rostral middle frontal gyrus volumes are not related to fitness.
Acknowledgments
Funding: This study was supported by grants from the National Institutes of Health DK064087 and supported in part by grant 1UL1RR029893 from the National Center for Research Resources
Footnotes
Disclosures: None of the authors have any conflicts to disclose
Author Contributions: MGC collected some of the data, conducted data analyses and drafted manuscript; CV collected some of the data, conducted data analyses and edited manuscript; PLY conducted the image analyses and oversaw statistical analyses and data interpretation, edited the manuscript; AC conceived and supervised the study, obtained funding, interpreted the data, and edited manuscript.
References
- 1.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 4.Niskanen L, Uusitupa M, Sarlund H, Siitonen O, Paljarvi L, Laakso M. The effects of weight loss on insulin sensitivity, skeletal muscle composition and capillary density in obese non-diabetic subjects. Int J Obes Relat Metab Disord. 1996;20:154–160. [PubMed] [Google Scholar]
- 5.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 6.Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. Obesity is associated with memory deficits in young and middle-aged adults. EatWeightDisord. 2006;11:e15–e19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- 7.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22:1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite. 2015 doi: 10.1016/j.appet.2015.03.033. E- pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 13.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burr JF, Bredin SS, Faktor MD, Warburton DE. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. The Physician and sportsmedicine. 2011;39:133–139. doi: 10.3810/psm.2011.05.1904. [DOI] [PubMed] [Google Scholar]
- 17.Cataneo DC, Kobayasi S, Carvalho LR, Paccanaro RC, Cataneo AJ. Accuracy of six minute walk test, stair test and spirometry using maximal oxygen uptake as gold standard. Acta cirurgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2010;25:194–200. doi: 10.1590/s0102-86502010000200013. [DOI] [PubMed] [Google Scholar]
- 18.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro Roriz AK, Santana Passos LC, Cunha de Oliveira C, Eickemberg M, de Almeida Moreira P, Ramos Sampaio L. Discriminatory power of indicators predictors of visceral adiposity evaluated by computed tomography in adults and elderly individuals. Nutr Hosp. 2014;29:1401–1407. doi: 10.3305/nh.2014.29.6.7185. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 22.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 25.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, et al. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer's disease. Brain Imaging Behav. 2015;9:639–649. doi: 10.1007/s11682-014-9325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 28.Riebe D, Blissmer BJ, Greaney ML, Garber CE, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159–1178. doi: 10.1177/0898264309350076. [DOI] [PubMed] [Google Scholar]
- 29.Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 30.Ha J, Cohen JI, Tirsi A, Convit A. Association of obesity-mediated insulin resistance and hypothalamic volumes: possible sex differences. Dis Markers. 2013;35:249–259. doi: 10.1155/2013/531736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. Guilford Press; New York: 2013. [Google Scholar]
- 32.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 33.Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med. 2014;127:717–727. e712. doi: 10.1016/j.amjmed.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 35.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 36.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, et al. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- 39.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, et al. Brain Atrophy in Type 2 Diabetes: Regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]