Abstract
Objective
Angiotensin-converting enzyme (ACE) is present in many cell types of atherosclerotic lesions. This study determined whether ACE activity in endothelium and smooth muscle cells (SMCs), two major resident cell types of the aorta, contributes to hypercholesterolemia-induced atherosclerosis.
Approach and Results
All study mice were in LDL receptor -/- background. To determine the contribution of ACE on endothelial cells to atherosclerosis, female ACE floxed mice were bred to male Tie2-Cre transgenic mice. Endothelial cell-specific deletion of ACE significantly decreased serum ACE activity, but had no effect on systolic blood pressure and atherosclerosis. Since ACE protein is present on SMCs, the most abundant cell type of the aorta, we then determined whether ACE on SMCs contributes to atherosclerosis. ACE was depleted from SMCs by breeding female ACE floxed mice with male SM22-Cre transgenic mice. SMC-specific deficiency of ACE did not affect ACE activity in serum, but ablated its presence and activity in the aortic media. Although SMC-specific deficiency of ACE had no effect on systolic blood pressure, it significantly attenuated hypercholesterolemia-induced atherosclerosis in both male and female mice.
Conclusions
These studies provide direct evidence that ACE derived from endothelial cells does not play a critical role in atherosclerosis. Rather, smooth muscle cell-derived ACE contributes to atherosclerosis, independent of circulating ACE activity and blood pressure.
Keywords: angiotensin-converting enzyme, angiotensin, smooth muscle cells, endothelial cells, atherosclerosis
Introduction
Angiotensin (Ang)II is a major regulator of blood pressure and atherosclerosis.1 Endogenous AngII is derived exclusively from angiotensinogen.2 While there are evolving complexities of the renin angiotensin system, the major initial step is renin cleavage to release AngI. Angiotensin-converting enzyme (ACE) is the major enzyme that converts AngI into AngII.3 The role of ACE in blood pressure and atherosclerosis has been demonstrated in both experimental studies4-10 and human trials11,12 using ACE inhibitors.
ACE is abundant in atherosclerotic lesions where it is expressed in many cell types.13-17 A potential role of local ACE in atherosclerosis has been studied previously using genetically manipulated mice.18 One approach used ACE2/2 mice that lack membrane-bound ACE.19 Inability of ACE to tether to cellular membranes in apolipoprotein E -/- mice led to low serum ACE activity, lack of ACE activity in lung and vessels, low blood pressure, and less atherosclerosis.18 Another approach is the ACE3/3 mouse, in which ACE expression is found predominantly in liver and minimally in kidney, but absent in lung and arteries.20 This manipulation of ACE does not affect serum ACE activity, and has no effects on blood pressure and atherosclerosis. While findings from ACE2/2 mice implicate the importance of local ACE on atherosclerosis, findings from ACE3/3 mice do not support this assumption. It is worth noting that ACE3/3 mice have 50-100-fold higher expression of ACE in hepatocytes than wild type mice,20 which may not represent a pathophysiological relevant condition. Additionally, neither mouse model provides direct evidence whether ACE on resident cells of the arterial wall contributes to atherosclerosis.
Macrophages are the predominant cell type in atherosclerotic lesions. We have shown that ACE inhibition by enalapril ablates atherosclerosis,10 but leukocyte-derived ACE only has a modest contribution to atherosclerosis in hypercholesterolemic mice,21 suggesting that other cell types may also play roles on atherogenesis. In human atherosclerotic lesions, ACE is present in both endothelium and smooth muscle cells.22-24 To determine cell-specific effects of ACE in atherosclerosis, we developed ACE floxed mice and bred them to Cre trangenic mice in a low-density lipoprotein (LDL) receptor -/- background. Although endothelial cell-specific deficiency of ACE profoundly reduced systemic ACE activity, it did not reduce atherosclerosis. In contrast, ACE activity in smooth muscle cells has a major impact on atherosclerotic lesion size.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Endothelial cell-specific ACE deficiency had no effects on systolic blood pressure and atherosclerosis
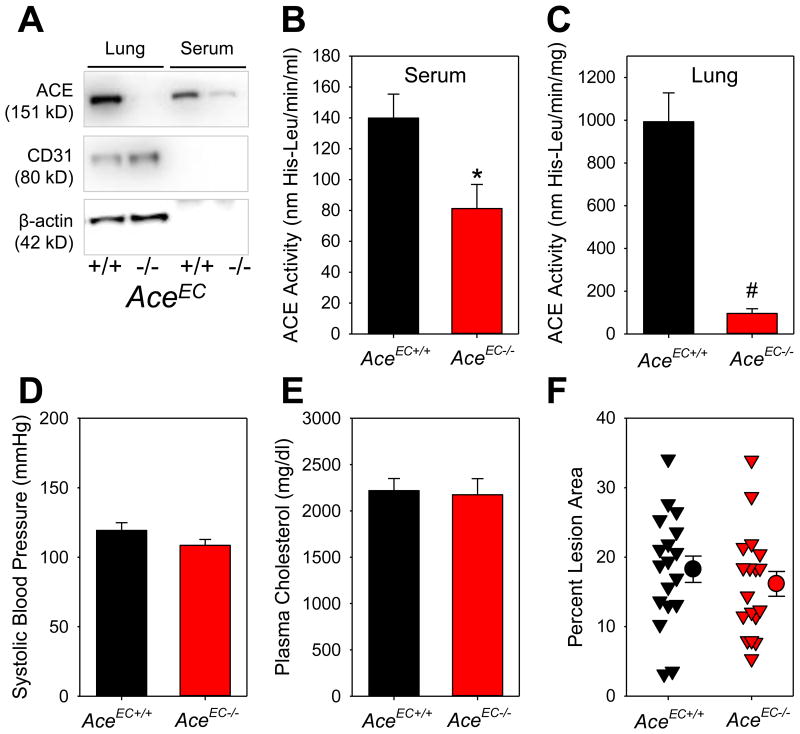
All study mice were in an LDL receptor -/- background. Endothelial cell-specific deficiency of ACE was achieved by cross-mating female ACE floxed mice to male Tie2-Cre hemizygous mice. ACE deficiency in endothelial cells (AceEC-/-) markedly reduced ACE protein and activity in lung and serum, and ACE activity in whole aorta but not in aortic media, compared to their wild type littermates (Figure 1A-C and Figure II in the online-only Data Supplement).
Figure 1. Endothelial cell-specific ACE deficiency diminished circulating ACE activity, but had no effects on systolic blood pressure and atherosclerosis in LDL receptor -/- mice.
(A) Western blotting of ACE, CD31, and β-actin in serum and lung; Serum (B) and (C) lung ACE activity was measured using a fluorimetric method. * P = 0.001 by Student's t-test, and # P = 0.002 by Mann-Whitney Rank Sum Test; (D) Systolic blood pressure was measured using a tail-cuff system. P = 0.2 by Student's t-test; (E) Plasma total cholesterol concentrations were measured enzymatically using a commercial kit. P = 0.8 by Student's t-test; (F) Atherosclerotic lesion area from the ascending aorta to 3 mm below the subclavian artery was measured by an en face method. Percent lesion area = (total lesion area/total intimal area) × 100%. Triangles are individual data, circles represent means of each genotype, and error bars are SEM. P = 0.4 by Student's t-test.
Consistent with the findings in mice fed normal diet, serum ACE activity was significantly lower in AceEC-/- mice than that in AceEC+/+ mice fed a Western diet for 12 weeks (AceEC+/+ versus AceEC-/-: 165 ± 16 versus 71 ± 11 nmol His-Leu/min/ml, P < 0.001 by Mann-Whitney Rank Sum Test). ACE deficiency in endothelial cells had no effect on plasma AngI and AngII concentrations (Figure III in the online-only Data Supplement), systolic blood pressure (Figure 1D) and plasma total cholesterol concentrations (Figure 1E). The low serum ACE activity in AceEC-/- mice did not mitigate atherosclerosis, as demonstrated by both en face measurements of the ascending, arch and part of descending thoracic regions (Figure 1F) and sections throughout aortic roots (Figure IV in the online-only Data Supplement).
Smooth muscle cell-specific ACE deficiency had no effect on systolic blood pressure, but attenuated atherosclerosis
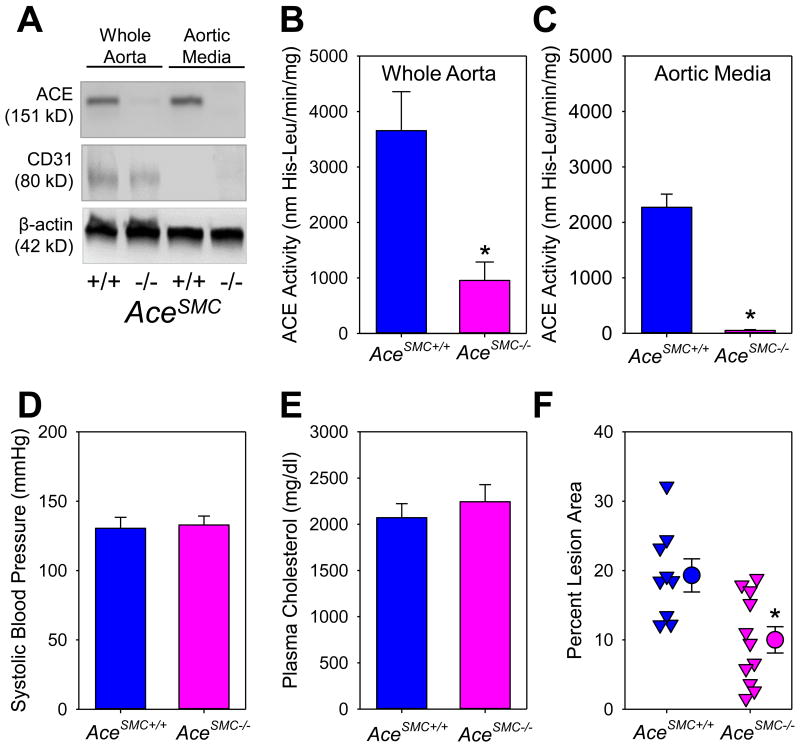
ACE is abundant in aortic SMCs.21 To determine whether ACE in SMCs plays a critical role on atherosclerosis, we developed SMC-specific ACE deficient (AceSMC-/-) mice by cross-mating female ACE floxed mice to male SM22-Cre hemizygous mice. Western blotting demonstrated ablation of ACE protein in aortic media of AceSMC-/- mice (Figure 2A). Consistently, ACE activity in whole aorta (containing both endothelium and media) of AceSMC-/- mice was profoundly reduced (Figure 2B), while it was ablated in the aortic media (Figure 2C), compared to their wild type littermates. ACE depletion in SMCs did not affect either serum or lung ACE activity (Figure V in the online-only Data Supplement).
Figure 2. Smooth muscle cell-specific ACE deficiency reduced aortic ACE activity and atherosclerosis in LDL receptor -/- mice.
(A) Western blotting of ACE, CD31, and β-actin in whole aorta (consisting of endothelium and aortic media) and aortic media alone; ACE activity was measured using a fluorimetric method in whole aorta (B) and aortic media alone (C). * P = 0.01 (B) and < 0.001(C) by Student's t-test, respectively; (D) Systolic blood pressure was measured using a tail-cuff system. P = 0.8 by Mann-Whitney Rank Sum Test; (E) Plasma total cholesterol concentrations were measured enzymatically using a commercial kit. P = 0.5 by Student's t-test; (F) Atherosclerotic lesion area from the ascending aorta to 3 mm below the subclavian artery was measured by an en face method. Percent lesion area = (total lesion area/total intimal area) × 100%. Triangles are individual data, circles represent means of each genotype, and error bars are SEM. * P = 0.005 by Student's t-test.
In mice fed Western diet for 12 weeks, no significant difference in serum ACE activity was found between AceSMC+/+ and AceSMC-/- mice (AceSMC+/+ versus AceSMC-/-: 133 ± 18 versus 114 ± 7 nmol His-Leu/min/ml, P = 0.3 by Mann-Whitney Rank Sum Test). ACE deficiency in SMCs had no effect on plasma AngI and AngII concentrations, systolic blood pressure (Figure 2D) and plasma total cholesterol concentrations (Figure 2E). ACE deficiency in SMCs significantly ameliorated atherosclerosis, compared to wild type littermates, as assessed by en face method (Figure 2F). Similarly in female mice, ACE deficiency in SMCs had no effects on serum ACE activity, systolic blood pressure and plasma total cholesterol concentrations, but reduced atherosclerosis (Figure VI in the online-only Data Supplement). Consistent with previous findings,10,25-27 macrophages, as demonstrated by positive immunostaining of CD68 (Figure VII in the online-only Data Supplement), were the major cell type of atherosclerotic lesions in mice with either genotype.
Discussion
It has been speculated that ACE in serum is originated from pulmonary vascular endothelium.28 To our knowledge, this is the first study to provide direct evidence that ACE derived from endothelial cells contributes to its systemic presence. However, this contribution accounts for only 50% of systemic ACE activity. We have demonstrated that Tie2 promoter in Cre transgenic mice leads to depletion of target genes on endothelial cells of lungs and major arteries including the aorta. SMC-derived ACE does not account for its systemic presence because its deficiency does not affect serum ACE activity. In our previous study using bone marrow transplantation approach, we have demonstrated that serum ACE activity is not influenced by leukocyte-derived ACE.21 Several mouse models have been developed with specific cell-restricted overexpression of ACE including ACE overexpression in hepatocytes (ACE3/3), cardiac myocytes (ACE8/8), and macrophages (ACE10/10). All these 3 mouse models, with absence of ACE in lung, aorta, and other vessels, have comparable serum ACE activity as wild type littermates,20,29,30 implicating that ACE expression on arterial wall is not necessary for maintaining normal systemic ACE activity. However, all these mouse models have 50-100-fold higher ACE expression in restricted cell types than wild type littermates. It is unclear whether these cell types, under a physiological condition or a pathophysiological relevant state, contribute to systemic ACE activity. Therefore, it is currently unknown, in addition to endothelial cells, which cell types contribute to the other 50% of circulating ACE.
Pharmacological inhibition or global genetic depletion of ACE in mice reduces blood pressure, which is consistent in both human studies and animal models.10,11,31,32 These previous studies do not define the source of ACE that contributes to blood pressure regulation. In the present study, profound reductions of ACE activity in serum of AceEC-/- mice do not affect systolic blood pressure. Blood pressure is also not regulated by ACE in leukocytes21 or SMCs (the present study). AngII regulates blood pressure through its interaction with AT1a receptors. While AngII-induced increases in blood pressure have been assumed to be via stimulation of AT1a receptors on SMCs, deletion of AT1a receptors on this cell type had no effect on blood pressure during infusion of AngII.33,34 Rather, AT1a receptor in proximal convoluted tubules of kidney contributes to AngII-mediated blood pressure changes.33 It would be interesting to determine whether the lack of effects on blood pressure in SMC-specific ACE deficient mice is due to its regulation by other cell types, such as epithelial cells of the proximal convoluted tubules in kidney.33
In addition to blood pressure, another unexpected finding in the present study is that endothelial cell-derived ACE has no effects on atherosclerosis, although it attributes to 50% of circulating ACE. SMCs are the predominant cell type of the arterial wall. ACE is readily detectable in vascular SMCs as demonstrated in our previous21,25 and the present studies. ACE is also abundant in SMCs of atherosclerotic lesions, and is elevated during progression of atherosclerosis.13,14,35 Although ACE deletion in SMCs does not change serum ACE activity and blood pressure, it leads to 50% reduction of atherosclerotic lesions. This is not a gender-specific effect since there are also significant reductions of atherosclerosis in female AceSMC-/- mice. These findings, combined with our previous findings that ACE deficiency on leukocytes has no effects on serum ACE activity and blood pressure, but reduces atherosclerosis (approximately 50% reductions),21 have provided insights into several aspects. First, locally derived ACE, rather than systemic ACE, is a contributor to hypercholesterolemia-induced atherosclerosis. Second, blood pressure is not a contributor to ACE-mediated atherosclerosis. Third, macrophages and SMCs are the two major cell types for ACE to promote atherosclerosis in hypercholesterolemic mice.
There have been consistent demonstrations that inhibition of AngII and AT1a receptor pathway profoundly reduces atherosclerosis in hypercholesterolemic mice.25,36 However, the location of AngII synthesis and cell type(s) in which AT1a receptors are activated have yet to be completely resolved.26,37-40 SMC-specific deletion of AT1a receptor does not influence atherosclerosis.40 Therefore, it is unlikely that reduced atherosclerosis in mice with SMC-specific deficiency of ACE is attributed to ablated interaction between AngII and AT1a receptor in SMCs. AngII has both autocrine and paracrine effects and hence may affect neighboring cells. To understand by which mechanisms ACE derived from SMCs contributes to atherosclerosis will require the determination of which cell type is stimulated via AT1a receptors to promote atherogenesis.
In summary, using two cell-specific ACE deficient mouse models, we show that ACE derived from SMCs contributes to the development of atherosclerosis in a blood pressure- and gender-independent mechanism.
Supplementary Material
Highlights.
We developed angiotensin-converting enzyme (ACE) floxed mouse to study cell-specific effects of ACE.
Endothelial cell-derived ACE contributes to 50% of circulating ACE activity.
Systolic blood pressure is not regulated by circulating ACE and ACE derived from either endothelial cells or smooth muscle cells.
ACE derived from smooth muscle cells, rather than endothelial cells, contributes to hypercholesterolemia-induced atherosclerosis, which is independent of blood pressure, and is not gender-specific.
Acknowledgments
None.
Sources of Funding: This study was supported by the National Institutes of Health under award number R01 HL062846, grants from Zhejiang Provincial Natural Science Foundation of China under Grant numbers LY14H020001 and LY12H02002, and National Natural Science Foundation of China under Grant number 81400325. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- AngII
angiotensin II
- AT1a
angiotensin type 1a receptor
- EC
endothelial cells
- LDL
low-density lipoprotein
- SMC
smooth muscle cells
Footnotes
Disclosures: None.
References
- 1.Lu H, Cassis LA, Daugherty A. Atherosclerosis and arterial blood pressure in mice. Curr Drug Targets. 2007;8:1181–1189. doi: 10.2174/138945007782403829. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston) 2011;4:183–190. doi: 10.7156/v4i4p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216:762–766. doi: 10.1038/216762a0. [DOI] [PubMed] [Google Scholar]
- 4.Aberg G, Ferrer P. Effects of captopril on atherosclerosis in cynomolgus monkeys. J Cardiovasc Pharmacol. 1990;15:S65–S72. [PubMed] [Google Scholar]
- 5.Campbell JH, Fennessy P, Campbell GR. Effect of perindopril on the development of atherosclerosis in the cholesterol-fed rabbit. Clin Exp Pharmacol Physiol. 1992;19:13–17. doi: 10.1111/j.1440-1681.1992.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 6.Charpiot P, Rolland PH, Friggi A, Piquet P, Scalbert E, Bodard H, Barlatier A, Latrille V, Tranier P, Mercier C, Luccioni R, Calaf R, Garcon D. ACE inhibition with prindopril and atherogenesis-induced structural and functional changes in minipig arteries. Arterioscler Thromb. 1993;13:1125–1138. doi: 10.1161/01.atv.13.8.1125. [DOI] [PubMed] [Google Scholar]
- 7.Kowala MC, Grove RL, Aberg G. Inhibitors of angiotensin converting enzyme decrease early atherosclerosis in hyperlipidemic hamsters. Fosinopril reduces plasma cholesterol and captopril inhibits macrophage-foam cell accumulation independently of blood pressure and plasma lipids. Atherosclerosis. 1994:61–72. doi: 10.1016/0021-9150(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 8.Hayek T, Attias J, Smith J, Breslow JL, Keidar S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 1998;31:540–544. doi: 10.1097/00005344-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Hayek T, Attias J, Coleman R, Brodsky S, Smith J, Breslow JL, Keidar S. The angiotensin-converting enzyme inhibitor, fosinopril, and the angiotensin II receptor antagonist, losartan, inhibit LDL oxidation and attenuate atherosclerosis independent of lowering blood pressure in apolipoprotein E deficient mice. Cardiovasc Res. 1999;44:579–587. doi: 10.1016/s0008-6363(99)00239-4. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Balakrishnan A, Howatt DA, Wu C, Charnigo R, Liau G, Cassis LA, Daugherty A. Comparative effects of different modes of renin angiotensin system inhibition on hypercholesterolaemia-induced atherosclerosis. Br J Pharmacol. 2012;165:2000–2008. doi: 10.1111/j.1476-5381.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 12.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 13.Ohishi M, Ueda M, Rakugi H, Naruko T, Kojima A, Okamura A, Higaki J, Ogihara T. Enhanced expression of angiotensin-converting enzyme is associated with progression of coronary atherosclerosis in humans. J Hypertens. 1997;15:1295–302. doi: 10.1097/00004872-199715110-00014. [DOI] [PubMed] [Google Scholar]
- 14.Ribichini F, Pugno F, Ferrero V, Bussolati G, Feola M, Russo P, Di Mario C, Colombo A, Vassanelli C. Cellular immunostaining of angiotensin-converting enzyme in human coronary atherosclerotic plaques. J Am Coll Cardiol. 2006;47:1143–1149. doi: 10.1016/j.jacc.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Diet F, Pratt RE, Berry GJ, Momose N, Gibbons GH, Dzau VJ. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–2767. doi: 10.1161/01.cir.94.11.2756. [DOI] [PubMed] [Google Scholar]
- 16.Mitani H, Bandoh T, Kimura M, Totsuka T, Hayashi S. Increased activity of vascular ACE related to atherosclerotic lesions in hyperlipidemic rabbits. Am J Physiol. 1996;271:H1065–71. doi: 10.1152/ajpheart.1996.271.3.H1065. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara M, Geary RL, Diz DI, Gallagher PE, Wilson JA, Glazier SS, Dean RH, Ferrario CM. Angiotensin-converting enzyme expression in human carotid artery atherosclerosis. Hypertension. 2000;35:353–359. doi: 10.1161/01.hyp.35.1.353. [DOI] [PubMed] [Google Scholar]
- 18.Weiss D, Bernstein KE, Fuchs S, Adams J, Synetos A, Taylor WR. Vascular wall ACE is not required for atherogenesis in ApoE(-/-) mice. Atherosclerosis. 2010;209:352–358. doi: 10.1016/j.atherosclerosis.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole J, Quach du L, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial Angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90:87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 21.Chen XC, Lu H, Zhao M, Tashiro K, Cassis LA, Daugherty A. Angiotensin-converting enzyme promotes atherosclerosis through an angiotensin I to angiotensin II pathway involving leukocytes. Arterioscler Thromb Vasc Biol. 2013;33:2075–2080. doi: 10.1161/ATVBAHA.113.301777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakugi H, Wang DS, Dzau VJ, Pratt RE. Potential importance of tissue angiotensin-converting enzyme inhibition in preventing neointima formation. Circulation. 1994;90:449–455. doi: 10.1161/01.cir.90.1.449. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell PR, Seegal BC, Hsu KC, Das M, Soffer RL. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976;191:1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- 24.Orfanos SE, Armaganidis A, Glynos C, Psevdi E, Kaltsas P, Sarafidou P, Catravas JD, Dafni UG, Langleben D, Roussos C. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102:2011–2018. doi: 10.1161/01.cir.102.16.2011. [DOI] [PubMed] [Google Scholar]
- 25.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Rateri DL, Feldman DL, Charnigo RJ, Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Wu C, Howatt DA, Balakrishnan A, Charnigo RJ, Jr, Cassis LA, Daugherty A. Differential effects of dietary sodium intake on blood pressure and atherosclerosis in hypercholesterolemic mice. J Nutr Biochem. 2013;24:49–53. doi: 10.1016/j.jnutbio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng KK, Vane JR. The conversion of angiotensin I to angiotensin II in vivo. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259:188–189. doi: 10.1007/BF00537778. [DOI] [PubMed] [Google Scholar]
- 29.Cole JM, Xiao H, Adams JW, Disher KM, Zhao H, Bernstein KE. New approaches to genetic manipulation of mice: tissue-specific expression of ACE. Am J Physiol Renal Physiol. 2003;284:F599–607. doi: 10.1152/ajprenal.00308.2002. [DOI] [PubMed] [Google Scholar]
- 30.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 32.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 33.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnal JF, Battle T, Rasetti C, Challah M, Costerousse O, Vicaut E, Michel JB, Alhenc-Gelas F. ACE in three tunicae of rat aorta: expression in smooth muscle and effect of renovascular hypertension. Am J Physiol. 1994;267:H1777–1784. doi: 10.1152/ajpheart.1994.267.5.H1777. [DOI] [PubMed] [Google Scholar]
- 36.Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- 37.Wu C, Xu Y, Lu H, Howatt DA, Balakrishnan A, Moorleghen JJ, Kooi CW, Cassis LA, Wang JA, Daugherty A. Cys18-Cys137 disulfide bond in mouse angiotensinogen does not affect AngII-dependent functions in vivo. Hypertension. 2015;65:800–805. doi: 10.1161/HYPERTENSIONAHA.115.05166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, Feldman DL, Cassis LA, Vander Kooi CW, Daugherty A. Angiotensinogen Exerts Effects Independent of Angiotensin II. Arterioscler Thromb Vasc Biol. 2016;36:256–265. doi: 10.1161/ATVBAHA.115.306740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 40.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One. 2012;7:e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.