Abstract
Background
Colorectal cancer (CRC) screening has been shown to decrease the incidence of late-stage colorectal cancer, yet a substantial proportion of Americans do not receive screening. Those in rural areas may face barriers to colonoscopy services based on travel time, and previous studies have demonstrated lower screening among rural residents. Our purpose was to assess factors associated with late-stage CRC, and specifically to determine if longer travel time to colonoscopy was associated with late-stage CRC among an insured population in Iowa.
Methods
SEER-Medicare data were used to identify individuals ages 65 to 84 years old diagnosed with CRC in Iowa from 2002-2009. The distance between the centroid of the ZIP code of residence and the ZIP code of colonoscopy was computed for each individual who had continuous Medicare fee-for-service coverage for a 3- to 4-month period prior to diagnosis, and a professional claim for colonoscopy within that time frame. Demographic characteristics and travel times were compared between those diagnosed with early versus late-stage CRC. Also, demographic differences between those who had colonoscopy claims identified within 3-4 months prior to diagnosis (81%) were compared to patients with no colonoscopy claims identified (19%).
Results
5,792 subjects met inclusion criteria; 31% were diagnosed with early stage versus 69% with late-stage CRC. Those divorced or widowed (vs married) were more likely to be diagnosed with late-stage CRC (OR: 1.20, 95% CI: 1.06-1.37). Travel time was not associated with diagnosis of late-stage CRC.
Discussion
Among a Medicare-insured population, there was no relationship between travel time to colonoscopy and disease stage at diagnosis. It is likely that factors other than distance to colonoscopy present more pertinent barriers to screening in this insured population. Additional research should be done to determine reasons for non-adherence to screening among those with access to CRC screening services, given that over two-thirds of these insured individuals were diagnosed with late-stage CRC.
Keywords: access to care, colorectal cancer, geography, health services research, Medicare
Colorectal cancer (CRC) is the third most common cancer in men and women in the United States, and it is the third leading cause of cancer death.1 With proper screening, CRC is largely detectable at an early stage or preventable with polypectomy, and it is largely curable if detected early. There are several screening modalities that have been shown to be effective at reducing morbidity and mortality from CRC including colonoscopy every 10 years or annual fecal occult blood testing.2 Colonoscopy became the most common screening method in the US following the implementation of a Centers for Medicare and Medicaid Services (CMS) policy that provided coverage for this method beginning in 2001,3 and the push by gastroenterologists that this was the “best” test because it detected pre-cancerous polyps that could be removed, thus preventing CRC.4 Subsequent studies have shown that colonoscopy with polypectomy have reduced CRC incidence and mortality.5-7 Despite this evidence, only 65% of age eligible adults in the US had been screened in 2012 by any method, including colonoscopy, sigmoidoscopy, double contrast barium enema, fecal occult blood tests (FOBT) or fecal immunochemical tests (FIT).8 This is below the Healthy People 2020 target of 70.5% and well below the CDC's Colorectal Cancer Program goal of 80% by 2014.8 Iowa trends of CRC screening mirror these national trends. In 2012, 67% of Iowans 50 years and older reported having had a FOBT or FIT in the past year, sigmoidoscopy in the past 5 years, or a colonoscopy in the past 10 years; 69% of Iowans 50 years and older reported ever having had a colonoscopy or sigmoidoscopy (of these, greater than 97% had colonoscopy).9
There are a number of barriers to receiving CRC screening, including socioeconomic status, race, education, lack of health insurance and cost, lack of usual source of care, ineffective communication between health care providers and patients, lack of understanding and knowledge about CRC screening, pain/discomfort associated with colonoscopy, access to colonoscopy services, and rural residence.10-14
Two of these factors—access to colonoscopy services and rural residence—are interrelated. Lack of health insurance and distance to health care services are barriers to care that have been associated with rural populations.15-19 In a national study, rural residents were less likely to be up-to-date on screening for CRC.18 However, studies have not found an association between rurality and stage at diagnosis, though none have focused exclusively on an insured population to determine the impact of distance when insurance status is not a factor.20,21
The bowel preparation for the colonoscopy procedure and the typical requirements that accompany procedural sedation may cause anxiety and enhance logistical barriers for those who have to drive long distances to colonoscopy services. It is possible that rural residents may delay this procedure until they are experiencing troubling symptoms such as rectal bleeding, anemia or signs of bowel obstruction associated with later stage CRC, rather than having the procedure done for screening purposes to detect polyps or asymptomatic early stage cancer. Given the well-established relationship between receipt of recommended screening colonoscopy and early detection or prevention of CRC, an objective of this study was to characterize the relationship between travel time to colonoscopy and stage at cancer diagnosis in a group of insured individuals residing in a rural state with a well-established Surveillance, Epidemiology and End Results (SEER) cancer registry, and a relatively large network of critical access hospitals to serve less populated areas. We also examined the proportion of people who traveled past the nearest colonoscopy service to a more distant provider, and the association between doing so and stage at diagnosis.
Methods
Data Sources
A retrospective analysis of Iowa SEER Cancer Registry data linked to Medicare claims was conducted. The Iowa SEER Registry is a population-based cancer registry that attempts to capture all cancer diagnoses occurring among Iowa residents, and it has been in existence since 1973. The SEER data file contains extensive information on every cancer case diagnosed within a SEER region, such as demographic information (age at diagnosis, sex, race, marital status), detailed information on stage at diagnosis, and site and histology of the tumor.
Medicare is a federally funded program administered by CMS that provides health insurance for 97% of people age 65 and older in the United States. Of all SEER cases diagnosed with cancer at age 65 years or older, 94% are matched with their Medicare enrollment records. Most Medicare beneficiaries have fee-for-service coverage, particularly in Iowa where Medicare Advantage uptake has been relatively low (Iowa: 9% of total Medicare population enrolled in Medicare Advantage in 2009; overall US: 23%),22 resulting in health claims for each unique service provided. The Medicare Enrollment file contains information including months of coverage and demographic information. The Medicare National Claims History (NCH) file contains date of service, diagnosis codes, and a procedural code on each professional claim that represents the specific service received, as well as the ZIP code of the beneficiary and the ZIP code of the physician providing the service. This file was used to define the date and location of the colonoscopy and the residential ZIP code of the beneficiary.
Study Population
Subjects included in the analysis were Iowa residents ages 65 to 84 years at the time of a histologically confirmed diagnosis of in situ or invasive CRC cancer between 2002 and 2009, with no previous history of CRC, a known stage of CRC, and enrolled in both Parts A and B Medicare Fee-For Service plans for at least 3 months prior to the month of diagnosis.
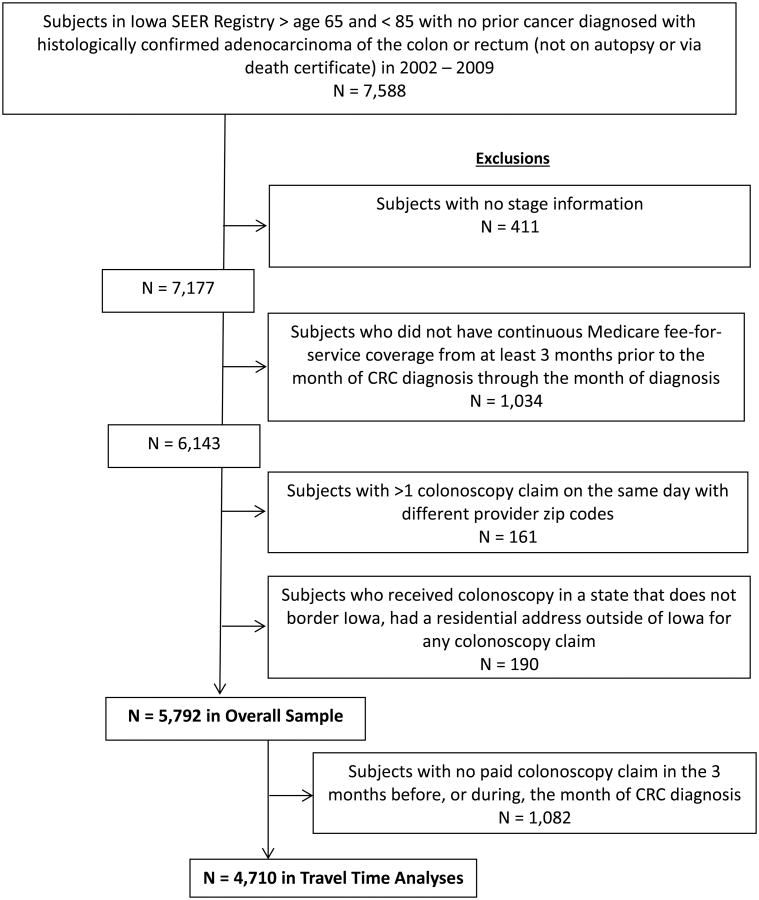
Subjects with multiple colonoscopy claims on the same day with different provider ZIP codes (N=161) were excluded because it could not be determined which ZIP code was the most accurate. Subjects who had a residential ZIP code outside of Iowa on their colonoscopy claim were excluded from this study because our focus was on Iowa residents only. Similarly, those who received a colonoscopy in another state that does not border Iowa were excluded because it was unlikely that full-time Iowa residents would travel to a non-contiguous state for the sole purpose of receiving colonoscopy services and they may have been living elsewhere for at least part of the study period (N = 190). These criteria yielded an overall cohort of 7,588 patients. See Figure 1 for a detailed flowchart of inclusion/exclusion criteria. This study received approval by the University of Iowa Institutional Review Board.
Figure 1.
Flowchart of eligibility criteria and study population.
Study Variables
Patient Characteristics
Age, sex, race, American Joint Commission on Cancer (AJCC)23 cancer stage, and month/year of diagnosis were taken from the Patient Entitlement and Diagnosis Summary File (PEDSF). In addition, state buy-in, an indicator that the subject is also enrolled in Medicaid or receives some other type of state-based assistance with health care coverage, was taken from the PEDSF file. ZIP-code-level characteristics including the proportion of residents in a ZIP code that were: living below 200% of the federal poverty level; age 25+ with no education beyond a high school education; and in a minority race were also taken from the PEDSF.
Access to primary care physicians (PCPs) was measured in each ZIP code by 1) counting the number of PCPs (i.e., physicians with an active clinical practice in the specialties of Family Medicine, General Internal Medicine, or General Practice) and adults residing within 30 minutes of each given ZIP code, 2) calculating the PCP to population ratio of the ZIP code, and 3) normalizing the right-skewed ratio by taking its log. The population information was derived from the 2000 United States Census Data, and adults were considered to be those 15 years of age and older because the Census ages are reported in 5-year age-categories. The ZIP code of each PCP in Iowa was determined from the Iowa Physician Information Inventory file, which contains demographic, educational, and professional information for over 11,000 actively practicing Iowa physicians. The tracking system is monitored and updated on a continuous basis, incorporating changes in the workforce due to deaths, retirements, relocations, and new practitioners entering practice.24
To determine if the colonoscopies were performed with diagnostic, surveillance, or screening intent, ICD-9 diagnosis codes on non-colonoscopy claims one month prior to colonoscopy (N=33 were not included in this analysis because one month prior to their colonoscopy extended beyond the 3-4 month look back from diagnosis) were evaluated (see Appendix A (available online only) for listing of ICD-9 codes) for beneficiaries who received any health care services associated with symptoms or conditions warranting increased surveillance for CRC. Each beneficiary was assigned a category based on the following hierarchy: 1) Diagnostic (based on evidence of potential CRC symptoms), 2) Surveillance (no evidence of symptoms but had a condition warranting more frequent surveillance for CRC), or 3) Screening (no evidence of symptoms or conditions warranting surveillance). Due to the small number of surveillance conditions detected, subjects who received diagnostic or surveillance colonoscopies were grouped into one category.
Travel Time Variables
The index colonoscopy was defined as the first claim for a colonoscopy in the Medicare NCH file occurring within the 3-4 months prior to the month of diagnosis. The first day of the month of diagnosis was considered to be the date of diagnosis because the specific date of diagnosis is not included in SEER-Medicare data. The index colonoscopy claim was used to define both the provider and subject ZIP codes. The ZIP code of each colonoscopy service was defined as the 5-digit ZIP code associated with the physician who provided the service, and the ZIP code for each subject was defined as the 5-digit ZIP code where the patient resided at the time of the colonoscopy. For those who had multiple colonoscopy claims identified on different dates and with different provider ZIP codes (n=67), the claim with the earliest date was chosen.
Address-level data were not available. Travel time (in minutes) was computed between the geographic center of subject ZIP code of residence and the colonoscopy provider ZIP code, both listed on the colonoscopy claim in the NCH file. Travel time from a subject's residence ZIP code to the nearest colonoscopy provider ZIP code was also computed using this methodology. These travel times were calculated using an online routing database containing known typical speed limits for each segment of road (Bing Maps Route Application Programming Interface). This process is described elsewhere.25 The matrix of travel times was calculated from the road segment vertex nearest to the centroids of the ZIP code polygons. The comprehensive listing of ZIP codes used to determine nearest colonoscopy services was derived from the NCH file; the provider ZIP codes associated with at least one claim for colonoscopy were included. If a participant did not receive a colonoscopy in the nearest ZIP code, they were categorized as having bypassed the closest services. We then created 2 variables: 1) a binary variable to address the question, “Did the patient travel more than 30 minutes beyond the nearest colonoscopy provider,” and 2) a continuous variable that quantifies the additional bypass time of the patients who bypassed the nearest provider. Travel time was analyzed both continuously and categorically. In instances where the actual or nearest colonoscopy was associated with the same ZIP code of patient residence, an arbitrary travel time of 1 minute was assigned for the continuous analyses. Sensitivity analyses using values of 10 or 20 minutes instead of 1 minute confirmed there was no impact on results. These cases were assigned to the “Within ZIP Code” for categorical analyses, with the “Other” category including all other ZIP codes. Those with missing provider or subject ZIP codes were excluded from the travel time portion of the analyses (n = 16). Also, many subjects had no identifiable colonoscopy claim prior to diagnosis (n = 1,082 (18.7%)) and were excluded from the travel time analysis. These subjects with no colonoscopy claim identified were compared to the remainder of the study population to determine if there were differences in demographic or clinical factors.
To establish rural status, each subject was assigned a Rural – Urban Commuting Area (RUCA) code based on their residential ZIP code. Then, each RUCA code was classified as rural or urban using “Categorization C” as described by the developers of the RUCA classification system.26
Outcome Variables
The primary outcome was diagnosis of early versus late-stage CRC. Early stage CRC was defined as AJCC Stage 0 (in situ; intraepithelial or invasion of lamina propria) or Stage I (tumor has invaded submucosa and has not gone all the way through the muscularis propria), and late-stage CRC was defined as AJCC Stage II (tumor has invaded through the muscularis propria into peri-colorectal tissues, surface of visceral peritoneum or to adjacent organs), Stage III (metastases in regional lymph nodes) or IV (metastases to other distant organs).23
Data Analysis
Descriptive analyses and significance tests comparing continuous variables were done on the median using a Wilcoxon rank-sum test or Mood's median test if differences in distribution shape prevented the interpretation of the rank-sum test as a comparison of medians. Variables were also compared by early stage versus late-stage CRC (Table 2) through univariable analysis in binary logistic regression models stratified by presence/absence of a colonoscopy claim within 3-4 months prior to diagnosis. The significance of the predictors was assessed through Wald tests. Univariable analysis was also used to compare those diagnosed with early versus late-stage CRC by travel time (continuous and categorical) to the ZIP code centroid of the performed colonoscopy, travel time beyond the nearest colonoscopy service available if the subject bypassed the nearest colonoscopy provider, PCP to population ratio, and diagnostic/surveillance versus screening colonoscopy. Lastly, an interaction term was used to check if the effect of travel time (continuous) varied by rurality status. These variables were also compared between the population that had a colonoscopy claim identified within 3-4 months prior to CRC diagnosis and the population that did not by using Pearson chi-square tests and independent samples t-tests. To explore any non-linear relationships, continuous predictors were finely categorized, entered with polynomial terms, and visually examined with LOESS curves of the predictor against the dichotomous response; decreases in AIC were used to assess improvements in fit over the assumed linear relationship. All models were examined for the effects of influential points and a substantial number of observations per level of categorical predictors by the response. All tests were 2-tailed, and α was set at 0.05. Statistical analyses were performed using SAS® statistical software version 9.3 (SAS Institute Inc., Cary, North Carolina). In addition, Stata version 11.2 (StataCorp LP, College Station, Texas) was used to calculate Kaplan-Meier curves for those with early versus late-stage disease by calculating the proportion of individuals who traveled at least x minutes, where x is the time shown on the x-axis. The log-rank test was used to assess whether there were differences in early versus late-stage disease with respect to time required to drive to the location of the colonoscopy.
Table 2.
Colonoscopy characteristics by subjects' residence in urban vs. rural areas and by early vs. late stage colorectal cancer.
| Urban | Rural | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic | N (%) | Early Stage (N = 787) | Late Stage (N = 1,521) | p | Early Stage (N = 851) | Late Stage (N = 1,551) | p |
|
| |||||||
| Category of colonoscopy | Diagnostic/Surveillance | 381 (49.0) | 972 (64.2) | < 0.0001 | 352 (41.8) | 935 (60.6) | < 0.0001 |
| Screening | 396 (51.0) | 542 (35.8) | 491 (58.2) | 608 (39.4) | |||
|
| |||||||
| Travel time (min) (continuous) | Median | 15.4 | 16.4 | 0.07 | 39.5 | 41.5 | 0.09 |
| Mean (SD) | 23.2 (30.3) | 24.9 (33.1) | 43.8 (35.5) | 46.7 (37.4) | |||
|
| |||||||
| Bypassed nearest colonoscopy provider by > 30 min | Yes | 106 (13.5) | 193 (12.8) | 0.63 | 297 (35.5) | 545 (36.1) | 0.78 |
| No | 678 (86.5) | 1312 (87.2) | 539 (64.5) | 965 (63.9) | |||
|
| |||||||
| Additional time if bypass >30 min = yes (min) | Median | 69.4 | 79.8 | 50.5 | 49.4 | ||
| Mean (SD) | 76.6 (36.6) | 75.4 (31.3) | 0.81 | 58.6 (26.7) | 60.0 (28.8) | 0.80 | |
Results
There were 5,792 Iowa beneficiaries who met all inclusion criteria for the study. The number of total CRC cases steadily declined each year in both groups with 242 (29%) early stage and 593 (71%) late-stage cases in 2002 to 177 (33%) early stage cases and 363 (67%) late-stage cases in 2009. Overall, 31% were diagnosed with early stage CRC (302 Stage 0 and 1,487 Stage Icases), and 69% with late-stage CRC (1,762 Stage II, 1,360 Stage III, 881 Stage IV cases). Among those with a colonoscopy claim identified prior to diagnosis, 35% were diagnosed with early stage and 65% with late-stage. Among those with no colonoscopy claim identified, 14% were diagnosed with early stage and 86% with late-stage disease.
Characteristics of the study population by early stage versus late-stage diagnosis, and by identification of a colonoscopy claim prior to diagnosis, are displayed in Table 1. Among the subjects who had a colonoscopy claim identified, those diagnosed with late-stage CRC were more likely to be separated, widowed or divorced (OR 1.20, 95% CI: 1.06-1.37) compared to those who were married at diagnosis. There were no significant differences in the ZIP-code-level variables (percent below 200% of the federal poverty level, percent minority, and percent with no more than a high school education), so these variables are not shown in Table 1.
Table 1.
Subject characteristics by early vs. late stage colorectal cancer and by presence or absence of a colonoscopy claim within 3-4 months prior to diagnosis.
| Colonoscopy claim identified within 3-4 months prior to diagnosis (N=4,710) | No colonoscopy claim identified within 3-4 months prior to diagnosis (N=1,082) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Characteristic | N (%) | Early Stage (N = 1,638) | Late Stage (N = 3,072) | Odds Ratio (95% CI) | p | Early Stage (N = 151) | Late Stage (N = 931) | Odds Ratio (95% CI) | p |
|
| |||||||||
| Age at diagnosis | 65-69 | 328 (20.0) | 585 (19.0) | Reference | - | 30 (19.9) | 158 (17.0) | Reference | - |
| 70-74 | 436 (26.6) | 730 (23.8) | 0.94 (0.78, 1.12) | 0.49 | 36 (23.8) | 227 (24.4) | 1.20 (0.71, 2.03) | 0.50 | |
| 75-79 | 466 (28.5) | 890 (29.0) | 1.07 (0.90, 1.28) | 0.45 | 48 (31.8) | 285 (30.6) | 1.13 (0.69, 1.85) | 0.64 | |
| 80-84 | 408 (24.9) | 867 (28.2) | 1.19 (1.00, 1.43) | 0.06 | 37 (24.5) | 261 (28.0) | 1.34 (0.80, 2.25) | 0.27 | |
|
| |||||||||
| Sex | Male | 781 (47.7) | 1442 (46.9) | Reference | - | 92 (60.9) | 454 (48.8) | Reference | - |
| Female | 857 (52.3) | 1630 (53.1) | 1.03 (0.91, 1.16) | 0.63 | 59 (39.1) | 477 (51.2) | 1.64 (1.15, 2.33) | < 0.01 | |
|
| |||||||||
| Race | White | 1,608 (98.3) | 3,036 (98.9) | Reference | - | -- | -- | Reference | - |
| Other | 28 (1.7) | 35 (1.1) | 0.66 (0.40, 1.09) | 0.11 | -- | -- | 0.81 (0.18, 3.73) | 0.79 | |
|
| |||||||||
| Marital status* | Married | 1,054 (65.4) | 1,859 (61.2) | Reference | - | 83 (55.7) | 502 (54.6) | Reference | - |
| Separated/Divorced/Widowed | 480 (29.8) | 1,019 (33.5) | 1.20 (1.06, 1.37) | <0.01 | 54 (36.2) | 348 (37.9) | 1.07 (0.74, 1.54) | 0.74 | |
| Single (never married) | 77 (4.8) | 162 (5.3) | 1.19 (0.90, 1.58) | 0.22 | 12 (8.1) | 69 (7.5) | 0.95 (0.49, 1.83) | 0.88 | |
|
| |||||||||
| State Buy-In (SBI)* | Yes | 108 (6.6) | 235 (7.7) | 1.17 (0.93, 1.49) | 0.18 | 20 (13.3) | 100 (10.7) | 0.79 (0.47, 1.32) | 0.36 |
| No | 1,530 (93.4) | 2,837 (92.4) | Reference | - | 131 (86.8) | 831 (89.3) | Reference | - | |
|
| |||||||||
| Rurality | Urban | 787 (48.1) | 1,521 (49.5) | Reference | - | 68 (45.0) | 454 (48.8) | Reference | - |
| Rural | 851 (52.0) | 1,551 (50.5) | 0.94 (0.84, 1.06) | 0.34 | 83 (55.0) | 477 (51.2) | 0.86 (0.61, 1.22) | 0.39 | |
|
| |||||||||
| Access to Primary Care Physician** | Mean (SD) | 0.93 (1.34) | 0.95 (1.35) | 1.01 (0.97, 1.06) | 0.53 | 0.86 (1.27) | 0.89 (1.31) | 1.01 (0.89, 1.16) | 0.84 |
-- Cell sizes <=10 were suppressed to protect patient confidentiality.
Significantly different between those with colonoscopy claims identified vs. those without; marital status: p< 0.001; SBI: p< 0.001.
Access to PCPs was measured in each ZIP code by 1) counting the number of primary care physicians and persons aged 15 and older within 30 minutes of each given ZIP code, 2) calculating the ZIP code's physician to population ratio, and 3) attempting to normalize the right-skewed ratio by taking the log.
Among those with no colonoscopy claim identified, 14% were diagnosed with early stage and 86% with late-stage disease, and a significantly greater proportion were not married or received state assistance compared to the group with colonoscopy claims identified. Female gender (OR 1.64, 95% CI: 1.15-2.33) was the only characteristic associated with greater odds of late-stage diagnosis.
Table 2 contains descriptive statistics of reason for colonoscopy, travel time, and bypass status by urban versus rural status, and early versus late diagnosis. As expected, a higher proportion of late-stage CRC subjects had a diagnostic/surveillance colonoscopy as opposed to a screening colonoscopy (urban subjects: 64% vs 36%, respectively, P < .0001; rural subjects: 60% vs 40%, respectively, P < .0001). Also as expected, the median travel time for rural patients was substantially greater than that of urban patients, regardless of stage at diagnosis (40 vs. 16 minutes; P < .0001). The proportion of rural patients who bypassed the nearest colonoscopy provider by >30 minutes was greater than the proportion of urban patients who bypassed the nearest provider, regardless of stage at diagnosis (36% vs 13%, respectively; P < .0001). However, the additional travel time for those who bypassed the closest provider by >30 minutes was smaller in rural patients compared to urban patients, regardless of stage at diagnosis (50 minutes vs 75 minutes; P < .0001).
In addition, separate models were constructed for rural versus urban residents to assess relationships between bypass and stage at diagnosis, and reason for colonoscopy and stage at diagnosis, and no effect of rurality was detected. There were no significant differences in the reason for colonoscopy with respect to age, sex, race, year of diagnosis (data not shown), or rurality, though the odds of late-stage diagnosis were nearly statistically significantly greater in those 80-84 years of age relative to 65-69 (P = .06). The interaction term for travel time by residential rurality status was not significant (P = .83). Consequently, no multivariate models were presented.
Figure 2 illustrates the proportion of individuals traveling at least x minutes, where x is the time shown on the x-axis. There was no difference in the travel times between those diagnosed with early versus late-stage CRC (Log rank test, P =.11). In addition, there were no significant differences in travel times found after stratifying by urban versus rural residence.
Figure 2.
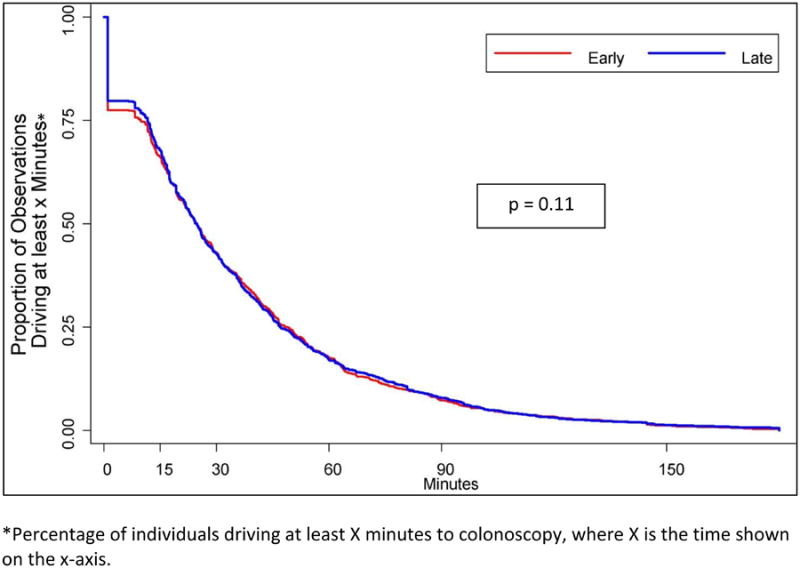
Travel time to colonoscopy by early vs. late-stage colorectal cancer diagnosis.
Overall, 24% (n=1,141) of subjects bypassed the nearest colonoscopy service by more than 30 minutes. Of those who bypassed the nearest colonoscopy service, the average time of bypass was 20 minutes beyond the nearest service; there were no differences in early versus late-stage diagnosis among bypassers versus non-bypassers.
Discussion
Overall, 69% of Iowa residents aged 65-84 with CRC were diagnosed with late-stage disease, which begs the question of why the proportion of largely preventable late-stage disease is so high in this insured group of people. Individuals were less likely to be diagnosed with late-stage CRC if they were married compared to those who were divorced or single. There was no relationship between travel time to colonoscopy and diagnosis of late-stage CRC. In addition, we found no difference in stage at diagnosis by rural versus urban residence. These results are consistent with studies conducted in Utah which found that distance to colonoscopy did not account for adherence to CRC screening guidelines, but those authors did not examine CRC stage of diagnosis by distance to colonoscopy.16 Results were also consistent with studies in Texas and Georgia that found no association between rurality and stage at diagnosis.20,21 In contrast, a study of national CRC screening showed that rural residents were less likely to be adherent with screening as compared with urban residents, but that study did not examine CRC stage by rural versus urban residence.18
There are a number of potential reasons why a significant relationship between stage at diagnosis, rural residence, and distance to colonoscopy was not detected. First, there may be adequate access to colonoscopy services in Iowa among those enrolled in Medicare. Specifically, Iowa has at least one hospital in nearly all 99 counties, meaning that no one is located great distances from a colonoscopy provider. Also, approximately one-quarter of subjects bypassed the nearest colonoscopy provider, so other factors are likely being considered in the decision regarding where to go for colonoscopy. Most colonoscopies in Iowa require a referral from a primary care physician, so that physician may be selecting a colonoscopist that they are familiar with and not considering distance the patient must travel as an overriding influence.
Second, the process of undergoing and recovering from a colonoscopy essentially takes a full day whether you drive 10 minutes or 1 hour to reach the colonoscopist, so the decision about whether you can devote a full day to having the procedure or not may not be heavily influenced by how far you choose to travel to receive services. It is possible that the association between marital status and early versus late-stage is driven by the spouse's encouragement to get screening and/or by having a spouse who can serve as the “responsible adult” to accompany the patient to the colonoscopy appointment, particularly since Medicare does not cover non-emergency transportation in most cases, unless there is a written order from the patient's physician stating that a non-emergent ambulance ride to health care services is necessary due to the patient's medical condition.27
While ZIP-code-level socioeconomic status indicators and the state buy-in variable were not significant in the analyses of patients with colonoscopy claims, it is still possible that individual-level education, ethnicity, and income, along with social support (beyond marital status), could play more major roles in the decision to get screened than travel time. It could also be that general health status is more strongly related to CRC screening than distance one needs to travel. Furthermore, the level of effort and counseling techniques put forth by the primary care physician to get patients screened might be a major explanatory factor that could not be accounted for in this analysis. These potentially mediating or modifying factors may all be more important than the actual distance that must be traveled by the patient to receive colonoscopy in this Medicare population.
Third, it is important to consider that screening colonoscopies can prevent the development of CRC, so many of the beneficiaries receiving regular colonoscopies and polypectomies for potentially problematic polyps would never develop cancer and thus never enter the SEER database. This potentially leaves a greater population of beneficiaries who were non-compliant with CRC screening in the SEER-Medicare colorectal cancer dataset. It is possible that travel time or rurality has less of an impact on stage at diagnosis in this population, but more of an impact on receipt of CRC screening services in the overall population, which could not be assessed in this study.
Subjects with no colonoscopy claims identified appeared to have a higher rate of late-stage cancer, be more impoverished (as evidenced by state buy-in), and not married compared to those with colonoscopy claims. Due to issues with identifying services provided during inpatient stays related to DRG-based reimbursement, only professional claims in the NCH file were examined, and it is possible that these professional claims were reimbursed by another payor or were bundled with other procedures. It is also possible that some of these subjects presented with bowel obstructions secondary to CRC, underwent a computerized tomography scan, and were taken to the operating room where colorectal cancer was diagnosed without a colonoscopy. In addition, some subjects may have had competing medical and/or psychosocial issues and decided to forgo a colonoscopy for a variety of reasons, but it is important to note that all cases included in this analysis were categorized to be histologically confirmed in the SEER database. Finally, it is possible that the colonoscopy occurred more than 3-4 months prior to the month of diagnosis. However, longer periods of up to 1 year were tested prior to the decision to select a window of 3-4 months, and it was determined that very few colonoscopies could be identified by lengthening the period of time prior to month of diagnosis.
Several limitations should be considered when interpreting results. Nearly 20% of individuals with CRC were excluded from the travel time analysis because a professional Medicare claim for a colonoscopy could not be identified, and thus we could not assess whether distance to colonoscopy service impacted their stage at diagnosis. Also, Medicare beneficiaries in a single state were included in the analysis, so results may not generalize to beneficiaries in other states, or to uninsured or younger populations. In addition, the ZIP code of the physician who performed the colonoscopy service, which was almost always available on the professional claim, was used in travel time analyses instead of the ZIP code of the facility where the service was performed, which was often not available on the facility claim. It is possible that some gastroenterologists travel to multiple distant sites to provide services but list the ZIP code of their usual location on the claim. Furthermore, travel times were computed based on ZIP code centroids because specific address information was not available in the data, and this introduces uncertainty. However, there is no reason to believe that the uncertainty associated with the travel behavior of the late-stage patients would be any different from the uncertainty of the early stage patients, or for any of the other factors we examined, so uncertainty is likely to be evenly distributed among all cases.
There were also limitations related to classification of colonoscopies into diagnostic/surveillance versus screening. This classification was based on claims 3-4 months prior to diagnosis, so misclassification could have occurred if symptoms occurred prior to that time period or were not specifically listed on the claim. However, the results were consistent with the idea that screening colonoscopies were significantly more likely to identify early stage disease when compared to diagnostic/surveillance colonoscopies (OR 2.01, P < .0001). Also, as stated previously, factors such as general health status and physician practices related to screening were not measured. Finally, we did not examine other screening modalities because our research question focused on the impact of travel time to colonoscopy services, which are almost always done in order to diagnose CRC. While it is possible that some of beneficiaries in this analysis received a positive FOBT or other screening test prior to, or instead of, receiving a colonoscopy, previous studies have shown that use of FOBT and flexible sigmoidoscopy are negligible in Iowa (6%-8%).12,28
Conclusions
In this study of Iowans with Medicare coverage, there was no association with late-stage diagnosis of CRC and travel time to colonoscopy services. Iowa has a robust distribution of colonoscopy services across the state that may account for these findings. These null results may provide some caution to policy makers and public health professionals who may assume that providing health coverage to a population will automatically lead to a lower proportion of late-stage CRC in and of itself, or that adding more colonoscopy services in locations that do not currently have them will substantially increase the proportion of early stage CRC. Given that over two-thirds of this insured sample was diagnosed with late-stage CRC, suggesting that beneficiaries were not receiving CRC screening as recommended, further investigation into factors that enable or modify CRC screening is warranted. Also, given that distance to colonoscopy provider did not have a significant impact on whether a colorectal cancer patient received a diagnosis of late or early stage cancer, and that socioeconomic status and marital status were associated with higher rates of late-stage diagnosis, it is important to educate PCPs on these disparities and develop programs to increase CRC screening in these populations specifically, as well as overall.
In addition, development of strategies for increasing CRC screening via other less invasive modalities such as fecal immunochemical tests may lead to lower proportions of late-stage diagnoses among people who find these at-home tests to be more acceptable and convenient. Canada, Australia and many European countries have adopted a FIT/FOBT approach as part of their national screening programs.29-38
Supplementary Material
Acknowledgments
Source of Funding: This work was funded by a pilot award from the University of Iowa Holden Comprehensive Cancer Center, which is supported in part by NIH/NCI P30 CA086862. Technical assistance was provided by the HCCC Population Research Core.
Footnotes
Author Contributions: All authors have made: a. substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; b. drafting the article or revising it critically for important intellectual content; c. final approval of the version to be published.
The authors have no conflicts of interest to declare.
Disclaimers: This manuscript is original and neither published, accepted, or submitted for publication elsewhere.
References
- 1.American Cancer Society. Cancer Facts and Figures, 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2008;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 3.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2004;2(1):72–77. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Annals of internal medicine. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England Journal of Medicine. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296(23):2815–2822. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 8.Klabunde CN, Joseph DA, King JB, White A, Plescia M. Vital Signs: Colorectal Cancer Screening Test Use — United States, 2012. Morbidity and Mortality Weekly Report (MMWR) 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 9.Iowa Department of Public Health. Health in Iowa Annual Report from the Behavioral Risk Factor Surveillance System. Iowa: 2012. [Accessed on May 15, 2015]. Available at: http://www.idph.state.ia.us/brfss/common/pdf/2012BRFSSannual.pdf. [Google Scholar]
- 10.Garcia-Dominic O, Lengerich EJ, Wray LA, et al. Barriers to CRC screening among Latino adults in Pennsylvania: ACCN results. American Journal of Health Behavior. 2012;36(2):153–167. doi: 10.5993/AJHB.36.2.2. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. American journal of preventive medicine. 2006;30(4):313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Levy BT, Dawson J, Hartz AJ, James PA. Colorectal cancer testing among patients cared for by Iowa family physicians. American Journal of Preventive Medicine. 2006;31(3):193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.McLachlan SA, Clements A, Austoker J. Patients' experiences and reported barriers to colonoscopy in the screening context--a systematic review of the literature. Patient Education and Counseling. 2012;86(2):137–146. doi: 10.1016/j.pec.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Preventive medicine. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Aboagye JK, Kaiser HE, Hayanga AJ. Rural-Urban Differences in Access to Specialist Providers of Colorectal Cancer Care in the United States: A Physician Workforce Issue. JAMA Surgery. 2014;149(6):537–543. doi: 10.1001/jamasurg.2013.5062. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(5):526–533. doi: 10.1016/j.cgh.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett KJ, Probst JC, Bellinger JD. Receipt of cancer screening services: surprising results for some rural minorities. J Rural Health. 2012;28(1):63–72. doi: 10.1111/j.1748-0361.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 18.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998-2005 data from the Centers for Disease Control's Behavioral Risk Factor Surveillance Study. Cancer Medicine. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James TM, Greiner KA, Ellerbeck EF, Feng C, Ahluwalia JS. Disparities in colorectal cancer screening: a guideline-based analysis of adherence. Ethnicity & Disease. 2006;16(1):228–233. [PubMed] [Google Scholar]
- 20.Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. American Journal of Public Health. 2014;104(3):e63–e71. doi: 10.2105/AJPH.2013.301572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojinnaka CO, Choi Y, Kum HC, Bolin JN. Predictors of Colorectal Cancer Screening: Does Rurality Play a Role? J Rural Health. 2015;31(3):254–268. doi: 10.1111/jrh.12104. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser Family Foundation. [Accessed December 19, 2014];Medicare Advantage Enrollees as a Percent of Total Medicare Population. 2014 Available at: http://kff.org/medicare/state-indicator/enrollees-as-a-of-total-medicare-population/
- 23.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 24. [Accessed July 22, 2014];University of Iowa Carver College of Medicine Office of Statewide Clinical Education Programs. Available at: http://www.medicine.uiowa.edu/oscep/products/
- 25.Fairchild G, Polgreen PM, Foster E, Rushton G, Segre AM. How many suffice? A computational framework for sizing sentinel surveillance networks. Int J Health Geogr. 2013;12:56. doi: 10.1186/1476-072X-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Washington Rural Health Resource Center. [Accessed December 1, 2014];RUCA Data. Available at: http://depts.washington.edu/uwruca/ruca-uses.php.
- 27.Centers for Medicare & Medicaid Services. [Accessed July 20, 2015];Medicare Coverage of Ambulance Services. 2015 Available at: https://www.medicare.gov/Pubs/pdf/11021.pdf.
- 28.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 29.Malila N, Anttila A, Hakama M. Colorectal cancer screening in Finland: details of the national screening programme implemented in Autumn 2004. Journal of Medical Screening. 2005;12(1):28–32. doi: 10.1258/0969141053279095. [DOI] [PubMed] [Google Scholar]
- 30.Malila N, Palva T, Malminiemi O, et al. Coverage and performance of colorectal cancer screening with the faecal occult blood test in Finland. Journal of Medical Screening. 2011;18(1):18–23. doi: 10.1258/jms.2010.010036. [DOI] [PubMed] [Google Scholar]
- 31.Hoff G, Dominitz JA. Contrasting US and European approaches to colorectal cancer screening: which is best? Gut. 2010;59(3):407–414. doi: 10.1136/gut.2009.192948. [DOI] [PubMed] [Google Scholar]
- 32.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39(2):168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 33.McClements PL, Madurasinghe V, Thomson CS, et al. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiology. 2012;36(4):e232–e242. doi: 10.1016/j.canep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Goulard H, Boussac-Zarebska M, Ancelle-Park R, Bloch J. French colorectal cancer screening pilot programme: results of the first round. Journal of Medical Screening. 2008;15(3):143–148. doi: 10.1258/jms.2008.008004. [DOI] [PubMed] [Google Scholar]
- 35.Costantini AS, Martini A, Puliti D, et al. Colorectal cancer mortality in two areas of Tuscany with different screening exposures. Journal of the National Cancer Institute. 2008;100(24):1818–1821. doi: 10.1093/jnci/djn404. [DOI] [PubMed] [Google Scholar]
- 36.Grazzini G, Castiglione G, Ciabattoni C, et al. Colorectal cancer screening programme by faecal occult blood test in Tuscany: first round results. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2004;13(1):19–26. doi: 10.1097/00008469-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Major D, Bryant H, Delaney M, et al. Colorectal cancer screening in Canada: results from the first round of screening for five provincial programs. Current Oncology. 2013;20(5):252–257. doi: 10.3747/co.20.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pignone MP, Flitcroft KL, Howard K, Trevena LJ, Salkeld GP, St John DJ. Costs and cost-effectiveness of full implementation of a biennial faecal occult blood test screening program for bowel cancer in Australia. The Medical journal of Australia. 2011;194(4):180–185. doi: 10.5694/j.1326-5377.2011.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.