Abstract
The golli proteins, products of the myelin basic protein gene, are widely expressed in oligodendrocyte progenitor cells and neurons during the postnatal development of the brain. While golli appears to be important for oligodendrocyte migration and differentiation, its function in neuronal development is completely unknown. We have found that golli proteins function as new and novel modulators of voltage-operated Ca++ channels (VOCCs) in neurons. In vitro, golli knock-out (KO) neurons exhibit decreased Ca++ influx after plasma membrane depolarization and a substantial maturational delay. Increased expression of golli proteins enhances L-type Ca++ entry and processes outgrowth in cortical neurons and pharmacological activation of L-type Ca++ channels stimulates maturation and prevents cell dead in golli-KO neurons. In situ, Ca++ influx mediated by L-type VOCCs was significantly decreased in cortical and hippocampal neurons of the golli-KO brain. These Ca++ alterations affect cortical and hippocampal development and the proliferation and survival of neural progenitor cells during the postnatal development of the golli-KO brain. The CA1/3 sections, and the dentate gyrus of the hippocampus were reduced in the golli-KO mice as well as the density of dendrites in the somatosensory cortex. Furthermore, the golli-KO mice display abnormal behavior including deficits in episodic memory and reduced anxiety. Because of the expression of the golli proteins within neurons in learning and memory centers of the brain, this work has profound implication in neurodegenerative diseases and neurological disorders.
Keywords: neuron development, golli-MBP, calcium influx, voltage-operated Ca++ channels, brain development
INTRODUCTION
Regulation of the golli-MBP gene involves two promoters and differential splicing. The downstream promoter transcript gives at least four myelin basic proteins (MBPs): the “classic” MBPs. Their function is mostly structural and all of them are found in compact myelin. The upstream promoter, which can be activated in very different cell types, drives the expression of the second family of proteins encoded by the MBP gene: the golli proteins. In the mouse, three golli isoforms have been identified: BG21, J37, and TP8 [1, 2]. Both BG21 and J37 have segments in common with the classic 18.5kDa isoform of MBP, but TP8 differ from this structure due to a frame shift mutation.
Golli proteins were mainly detected in neurons and oligodendrocytes of the central nervous system (CNS) as well as in T cells and macrophages in the thymus [3–5]. The expression of the golli sequences precedes the expression of the classic MBP and is detected during early embryogenesis [6]. During the first postnatal weeks, golli proteins were found in immature neurons of several cortical layers, hippocampus, thalamus, neostriatum and corpus callosum. Furthermore, golli proteins were expressed by young neurons in the mouse olfactory system and cerebellum [7, 8]. In humans, golli proteins were found in early neurons of the developing telencephalon, in the subventricular zone and in the future cerebral cortex [9].
Golli regulates oligodendrocyte progenitor cell migration and proliferation in vitro [10, 11], and studies of golli knock-out (KO) and golli-overexpressor brains indicate that its influence on oligodendrocyte development has important consequences for myelination in vivo [12–14]. Golli proteins modulate the entry of external Ca++ into oligodendrocytes through two classes of Ca++ channel, the voltage-operated Ca++ channels (VOCCs) and store-operated Ca++ channels (SOCCs) (For review see Fulton and coworkers [15]). We have found that golli regulates different aspects of oligodendrocyte function through distinct routes of Ca++ entry. For example, golli regulation of SOCCs influences oligodendrocyte proliferation [16], and its modulation of VOCCs controls process extension and migration [10, 11, 17].
In this work we showed that Ca++ homeostasis is altered in postnatal neurons from the golli-KO mice and that increased expression of golli proteins enhances Ca++ influx into neuronal cells. Golli proteins can modulate Ca++ influx through L-type voltage-operated Ca++ channels in the plasma membranes of cortical and hippocampal neurons and this intracellular Ca++ modulation affects Ca++ dependent functions such as neurites extension, proliferation and cell viability. Furthermore, we have found morphological changes in the cortex and hippocampus of golli-KO animals and more importantly these mice display abnormal behavior including deficits in episodic memory and reduced anxiety. Since the golli-MBP gene has been linked to both schizophrenia and bipolar disorder, this work has significant implication in understanding the molecular events governing psychiatric and cognitive disorders.
MATERIALS AND METHODS
Animals
All animals used in the present study were housed in the UB Division of Laboratory Animal Medicine vivarium, and procedures were approved by UB’s Animal Care and Use Committee, and conducted in accordance with the guidelines in “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health. The generation of the golli-KO mice in which the golli products of the MBP gene were selectively ablated while permitting normal expression of the classic MBPs was described in Jacobs and co-workers [12].
Primary culture of cortical neurons
Cortical neurons were prepared from the brains of 1 to 2-day-old mouse pups using established procedures [18] with minor modifications. Briefly, brains were removed aseptically and placed into DMEM/F12 (Life Technologies). After the brains were dissected, the blood vessels and meninges were carefully removed under a dissecting microscope. Brain cortices were isolated and dissociated by digestion with a solution of 0.05% trypsin (Sigma) containing DNase I (0.06%) (Sigma) in Neurobasal medium (Life Technologies) for 10min at 37°C. The digestion reaction was stopped with Neurobasal medium containing 10% fetal bovine serum (Omega Scientific) and triturated by repeated passages (20 times) through a 10ml pipette. The cell suspension was filtered through a sterile cell strainer (70μm; BD Biosciences) into a 50ml centrifuge tube. The cells were pelleted by centrifugation at 200g for 5min, and resuspended in Neurobasal medium plus 2% (v/v) B27 (Life Technologies) supplemented with 0.25mM GlutaMax I (Life Technologies), 0.25mM glutamine (Life Technologies), and 100μg/ml gentamicin (Omega Scientific). 2000 cells/mm2 were plated onto 12mm glass coverslips coated with poly-D-lysine (Sigma). The neurons were kept at 37°C in 95% air 5% CO2 for 7, 14 and 21 days.
Cortical neurons transfection
After 3 days in vitro, selected neuronal cultures were transfected with the full length and mutated J37 and BG21 golli isoforms using Lipofectamine 2000 (Life Technologies). The construction of the full length J37 and BG21 clones in pEGFP-N3 was described in Reyes and Campagnoni [19] and the construction of the J37 deletion 1 and 2, the myristolyation mutations and the deletion of the calmodulin binding region was described in Paez and co-workers [17]. Briefly, 1μg of plasmid DNA and 1.5μl of Lipofectamine were diluted in individual 25μl aliquots of Neurobasal medium (Life Technologies) and incubated for 5min at room temperature. The solutions were then mixed and incubated for 20min at room temperature. One μg of plasmid DNA was used to transfect 4×104 cells/coverslip. While the DNA was complexing, the cells were washed for 5min with Neurobasal medium. The complexed DNA mixture was then applied to the coverslips and incubated at 37°C for 6h. The cells were washed with Neurobasal medium and subsequently incubated at 37°C for 4 days prior to fixation in Neurobasal medium plus 2% (v/v) B27 (Life Technologies) supplemented with 0.25mM GlutaMax I (Life Technologies), 0.25mM glutamine (Life Technologies) and 100μg/ml gentamicin (Omega Scientific). Transfection efficiency was monitored by counting EGFP-positive neurons and DAPI-positive cells (total number of cells) in 20 randomly selected fields. In an average experiment about 35% of the cells were positive for EGFP.
Immunocytochemistry
Cells were stained with antibodies against several neuronal markers and examined by confocal microscopy. Briefly, the cells were rinsed in PBS and fixed in 4% buffered paraformaldehyde (Sigma) for 20min at room temperature. After rinsing in PBS, the cells were permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 2min at room temperature and then processed for immunocytochemistry following the protocol as outlined by Reyes and Campagnoni [19]. Essentially, fixed cells were incubated in a blocking solution (5% goat serum in PBS) followed by an overnight incubation at 4°C with the primary antibody. Cells were then incubated with the appropriate secondary antibodies (1:200; Jackson), nuclei were stained with the fluorescent dye DAPI (Life Technologies), mounted onto slides with Aquamount (Thermo Scientific), and fluorescent images were obtained using a Olympus spinning disc confocal microscope (Olympus, IX83-DSU). Quantitative analysis of the results was done counting the antigen-positive and DAPI-positive cells (total number of cells) in 20 randomly selected fields, which resulted in counts of >2000 cells for each experimental condition. Counts of antigen-positive cells were normalized to the counts of total DAPI-positive cells for each condition. For some antigens the total area covered by processes in a 1mm2 was assessed in 20 randomly selected fields by MetaMorph software (Molecular Devices) using a built-in auto inclusive threshold. This function was design to measure the morphology of fluorescent specimens and uses the histogram of gray values within the image to set the low and high limits. For all experiments involving quantification of positive cells and area covered by processes in culture, data represent pooled results from at least 5 independent cultures. Quantification was performed blind to the genotype of the sample. The primary antibodies used for immunocytochemistry were against: caspase-3 (1:2000; Cell Signaling), doublecortin (1:500; Santa Cruz), GAP43 (1:200; Millipore), golli (1:500; from Dr. Anthony T. Campagnoni lab), Ki67 (1:250; Abcam), MAP2 (1:200; Millipore), NeuN (1:200; Millipore), neurofilament H (1:100; Millipore), neurofilament M (1:100; Millipore), synapsin 1 (1:2000; Synaptic Systems) and Tuj1 (1:500; Covance).
Caspase-3 assay
NucView 488 Caspase-3 substrate, a cell membrane-permeable fluorogenic caspase substrate designed for detecting caspase-3 activity within live cells in real time, was used in accordance with the manufacturer’s recommendations (Biotium Inc). Briefly, neuronal primary cultures were incubated in medium containing NucView 488 Caspase-3 substrate (final concentration 5μM) in a stage top chamber with 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal inverted microscope (Olympus, IX83-DSU). Fluorescent field images were obtained with a specific GFP filter at 6min intervals for a total of 24h. MetaMorph software (Molecular Devices) was used to assess apoptotic cell death by calculating the percentage of Caspase-3 positive cells in a total of 5 experiments on 10 random fields.
Immunohistochemistry
Free-floating vibratome sections were incubated in a blocking solution (2% normal goat serum and 0.2% Triton X-100 in PBS) for 2h at room temperature and then incubated with the primary antibody overnight at 4°C. Sections were then rinsed in PBS and incubated with Alexa conjugated secondary antibodies (Life Technologies) for 2h at room temperature followed by a counterstain with the nuclear dye DAPI (Life Technologies). After washing, the sections were mounted on to Superfrost Plus slides (Fisher) using coverslips and mounting medium (Aquamount; Thermo Scientific). The total number of positive cells was stereologically quantified in the somatosensory cortex (0.6mm2, including all the cortical layers) and in the dentate gyrus of the hippocampus (0.4mm2, including upper and lower blades and the subgranular zone). For all experiments involving quantification of positive cells and fluorescent intensity in tissue sections, data represent pooled results from at least 5 brains per experimental group. Five slices per brain (50μm each) were used and quantification was performed blind to the genotype of the sample using an unbiased stereological sampling method. The primary antibodies used in the present study were against: caspase-3 (1:1000; Cell Signaling), doublecortin (1:250; Santa Cruz), Ki67 (1:250; Abcam), MAP2 (1:200; Millipore), NeuN (1:100; Millipore), neurofilament L (1:100; Millipore), Sox2 (1:250; Millipore) and Sox9 (1:500; Millipore).
Cortical and hippocampal thickness measurements
Cortical layer measurements were performed in coronal slices at approximately −2.1mm from bregma and in the region of the primary somatosensory cortex [20]. The thickness of hippocampal layers was measured in the same slice. At least 5 brains per experimental group and 5 slices per brain (50μm each) were used. Quantification was performed blind to the genotype of the sample and using an unbiased stereological sampling method.
Western blot
Total protein was collected from primary culture of cortical neurons, mouse cortex and hippocampus. The final protein pellet was homogenized in lysis buffer containing 50mM Tris-HCl, 0.25% (w/v) sodium deoxycholate, 150mM NaCl, 1mM EDTA, 1% (w/v) Triton X-100, 0.1% (w/v) SDS, 1mM sodium vanadate, 1mM AEBSF, 10μg/ml aprotinin, 10μg/ml leupeptin, 10μg/ml pepstatin and 4μM sodium fluoride. Western blots were performed as previously described [21]. Twenty-five micrograms of total protein was loaded onto a 4–20% Tris-glycine gel (Life Technologies). Protein bands were detected by chemiluminescence using the Amersham ECL kit (GE Healthcare) with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling). Relative intensities of the protein bands were quantified by scanning densitometry using the NIH Image Software Image J. Equal protein loading was verified by Ponceau S solution (Sigma) reversible staining of the blots and each extract was also analyzed for relative protein levels of β-actin and P84. Data represent pooled results from at least 5 brains or 5 independent cultures per experimental group. The primary antibodies used for Western blots were against: doublecortin (1:6000; Santa Cruz), GAP43 (1:1000; Millipore), NeuN (1:1000; Millipore), neurofilament L (1:1000; Millipore), P84 (1:10000; Genetex), synapsin 1 (1:1000; Synaptic Systems), Tuj1 (1:500; Covance) and β-actin (1:10000; Sigma).
Calcium imaging in vitro
Methods were similar to those described previously [17]. Briefly, primary culture of cortical neurons were washed in serum and phenol red-free DMEM (Life Technologies) containing a final concentration of 4μM fura-2 (AM) (Life Technologies) plus 0.08% Pluronic F127 (Life Technologies) to load dye into the cells, incubated for 25min at 37°C, 5% CO2, then washed four times in DMEM (Life Technologies) and stored in DMEM for 10min before been imaged. Calcium influx and resting Ca++ levels were measured in serum and phenol red-free HBSS containing 1.3mM Ca++ and 1mM Mg++ (Life Technologies). The fluorescence of fura-2 was excited alternatively at wavelengths of 340 and 380nm every 2s by means of a high-speed wavelength-switching device (Lambda DG4; Sutter Instruments). A spinning disc confocal inverted microscope (Olympus, IX83-DSU) equipped with a CCD camera (Hamamatsu ORCA-R2) measured the fluorescence. Calcium influx and resting Ca++ levels were measured on individual neuronal cell bodies using the image analysis software MetaFluor (Molecular Devices). More than 600 cells for each experimental condition were analyzed and the results from 5 separate experiments were pooled. To minimize bleaching, the intensity of excitation light and sampling frequency was kept as low as possible.
Calcium imaging in situ
Calcium imaging acquisitions of cortical and hippocampal neurons were performed on living slices at postnatal day 10, 20 and 30, as described elsewhere [22]. Briefly, mice were anesthetized with isoflurane, after which brains were rapidly removed and stored in ice cold slice solution containing: 110mM choline chloride, 25mM NaHCO3, 11.6mM sodium ascorbate, 7mM MgCl2, 3.1mM sodium pyruvate, 2.5mM KCl, 1.25 NaH2PO4, 0.5mM CaCl2 and 10mM glucose gassed with 95% O2 and 5% CO2. Coronal slices were cut at 150μm thickness on a vibratome (Leica VT1000S). Brain slices were kept for 2h at room temperature in artificial cerebrospinal fluid (ACSF) containing: 125mM NaCl, 3mM KCl, 2.5mM MgCl2, 1.25mM NaH2PO4, 26mM NaHCO3, 1.6mM CaCl2, and 10mM glucose gassed with 95% O2 and 5% CO2. Intracellular Ca++ measurements were made after loading the tissue with 20μM of fura-2 (AM) (Life Technologies) in ACSF for 30min at 36°C [23]. Then the slices were washed with ACSF for 30min before being imaged. Calcium influx and resting Ca++ levels were measured in ACSF containing 1.5mM MgCl2. For cell detection and identification, a z-stack using a stepsize of 1μm and image +/− 20μm around the imaged plane of focus was taken. Only on focus neuronal cell bodies were selected for the analysis. Five brains per experimental group and 6 slices per brain were used. About 100 individual neurons were evaluated in each slice. Measurements were made once every 2s.
SHIRPA
Before the actual behavioral tests started all animals underwent a qualitative analysis of their health status and reflexes. The protocol used was adopted and modified from the original SHIRPA protocol [24]. Home cage behavior (barbering, fighting, freezing, and grooming), general appearance (body weight, fur condition, missing whiskers, eye abnormality, and lesions), and neurological reflexes were analyzed. Muscular functions were analyzed in two tests. In the forepaw reaching test animals were held on tails 25cm above the surface and slowly neared a cage wall horizontally in the visual field of the mice. The ability to overstretch the back and reach out with both forepaws was rated. In the wire hang test animals were put on a wire mesh (standard cage lid) that was turned upside down approximately 25cm above a cage-floor. There was no possibility to escape the horizontal platform or bring the head into a vertical position. The animals had to hang on this mesh for maximal 2min. The time until the animals fell down to the wood-chip covered floor was measured. For this group of behavioral tests at least 25 male per experimental group were examined. In all the behavioral experiments described is this work, the researcher conducting the behavioral testing and scoring was blind to the experimental conditions.
Rotarod and beam-walking
Coordinated motor activity was measured by a rotarod apparatus following the standard procedure of EMPReSS (European Mouse Phenotyping Resource of Standardised Screens http://empress.har.mrc.ac.uk/). Mice were put on a rod rotating at 4rpm. The speed of rotation was gradually increased up to 40rpm in a 5min interval and the time until mice fall to the floor was measured. Each mouse was tested three times with 20min between each trial. Coordinated motor activity was also measured using the beam-walking test [25]. Each mouse was put on an end of 12mm and 6mm wide (square or round) and 1m long beams with 50cm elevation from the floor. The other end of beam was attached to a dark box (20×20×20cm) and the time for a mouse to finish walking along the beam to the dark box was measured. The test took place during three consecutive days. During the first two days mice were trained three times per day using the 12mm square beam. The last day the animals were tested three times in all the beams (12mm square and round and 6mm square and round). Additionally, the total of successful crossing for the 6mm round beam were counted. Only male mice were used. The number of mice tested per experimental group was P30: WT 20, golli-KO 23; P60: WT 20, golli-KO 21.
Open field
For adaptation to the new environment before the test mice were placed in the procedure room for at least 2h. The open field apparatus consisted of a round box (1m diameter) with opaque walls (50cm high) and was placed in indirect light (50lx). The mice attached to the swivel unit were gently placed in the open field facing the opaque walls and allowed to freely explore the apparatus for 10min. The total distance travelled and the time spent in the center area (60cm diameter) were automatically recorded using a video camera system. The apparatus was cleaned with 70% ethanol between subjects. Only male mice were used. The number of mice tested per experimental group was P30: WT 20, golli-KO 22; P60: WT 20, golli-KO 22.
Elevated plus-maze
The elevated plus maze tests were carried out as previously described [26, 27]. Mice were tested on an elevated plus-maze (95cm elevation from the floor) consisting of two open arms (30×5cm, 50lx) and two closed arms made of dark plexiglass (30×5×15cm, 35lx) extending from a central (5×5cm) platform. Briefly, mice were placed individually on the central platform facing an open arm and allowed to traverse the maze freely for 5min. Open or closed arm entries were defined as all four paws in an arm and a center entry was defined as both forepaws placed in the center. The time spent in each arm and the number of arm entries (70% of mouse in an arm) was tracked and scored. The maze was cleaned with 70% ethanol between subjects. Only male mice were used. The number of mice tested per experimental group was P30: WT 20, golli-KO 25; P60: WT 20, golli-KO 25.
Novel object recognition test
This was carried out following the procedures described previously [28] with minor modifications. In brief, mice were trained over three sessions separated by 24h to discriminate a novel object from a familiar one in a plastic round box. In the first trial, animals were placed in the empty arena and allowed to explore and habituate to the environment for 10min. Twenty-four hours later, animals were put back in the chamber for the training session and exposed to two identical objects located in two opposite corners of the box. The exploration time of each object was recorded to control for object and side bias. After 24h, mice were returned to the box to start the testing trial, during which they were exposed to a familiar object and a novel object, which was different from the familiar one in color and shape and placed in one of the same positions as the objects during the training. The exploration time for each object familiar (Tf) and novel object (Tn) was recorded for determination of the exploration index [Tn/(Tn+Tf)]. Exploration was defined as facing (within 2cm of the object) or touching the object. The chamber and objects were thoroughly cleaned with 70% alcohol after every session to ensure olfactory cues did not play a role in the test. Only male mice were used. The number of mice tested per experimental group was P30: WT 15, golli-KO 17; P60: WT 15, golli-KO 17.
Social novelty test
The testing apparatus consisted of a rectangular, three-chambered box and a lid with a video camera. Each chamber was 20×40×22cm and the dividing walls were made from clear Plexiglas, with small square openings (5×3cm) allowing access into each chamber. An unfamiliar C57BL/6 J male (stranger 1), that had had no prior contact with the subject mice, was placed in one of the side chambers. The location of stranger 1 in the left vs. right side chamber was systematically alternated between trials. The stranger mouse was enclosed in a small, round wire cage, which allowed nose contact between the bars, but prevented fighting. The cage was 11cm in height, with a bottom diameter of 9cm, vertical bars 0.5cm apart. The subject mouse was first placed in the middle chamber and allowed to explore the entire test box for a 10min session. The amount of time spent in the empty chamber (E) and the chamber were the stranger 1 was placed (S1) were measured with the aid of a camera fitted on top of the box. Each mouse was tested in a 10min session to quantify the social affiliation index S1/(S1+E). After the first 10min session, a second unfamiliar mouse was placed in the chamber that had been empty during the first 10min session. This second stranger was also enclosed in an identical small wire cage. The test mouse thus had a choice between the first, already-investigated unfamiliar mouse (stranger 1), and the novel unfamiliar mouse (stranger 2). The amount of time spent in the strange 1 chamber (S1′) and the strange 2 chamber (S2) was measured for determination of the social novelty index S2/(S2+S1′). Only male mice were used. The number of mice tested per experimental group was P60: WT 15, golli-KO 15.
Statistical Analysis
Normal distributions were tested in each data set using Kolmogorov-Smirnov tests. For data with normal distributions, single between-group comparisons were made by the Student paired t-test, and multiple comparisons were investigated by one-way ANOVA followed by Bonferroni’s multiple comparison tests to detect pair-wise between-group differences. All statistical tests were performed in Graphpad Prism (Graphpad Software). A fixed value of p<0.05 for one tailed tests was the criterion for reliable differences between groups. Data are presented as mean ± SEM unless otherwise noted.
RESULTS
Golli is essential for normal neuronal maturation in vitro
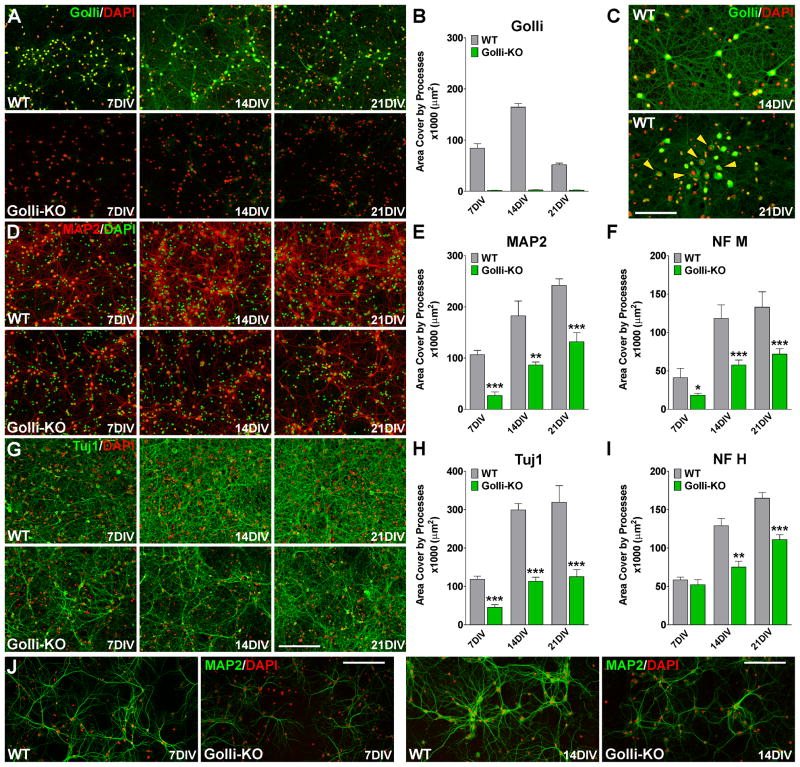
We started our immunocytochemical studies of cortical neurons analyzing the in vitro expression of golli. As it was expected, no golli staining was found in primary cultures of golli knock-out (KO) neurons (Figure 1A and B), however the level of golli expression was high in wild type (WT) neurons at 14 days in vitro (14DIV) (Figure 1A and B). In agreement with previous publications [7], the main subcellular location of golli proteins in neurons was in axonal and dendritic processes and the cell nuclei (Figure 1C), but at 21DIV, golli expression decreased and there was a shift in subcellular localization from nuclei and cell processes to the cell soma (Figure 1C, yellow arrows). Our findings indicate that the absence of golli attenuated the extension of dendrites and the expression of key cytoskeletal proteins in primary cultures of cortical neurons (Figure 1D–I). Analysis of the morphological complexity of cortical neurons at different time points showed that, although the golli-KO neurons elaborated processes and dendrites, these were not as extensive as those elaborated by WT neurons; interestingly these changes persist and become more evident after three weeks in culture (Figure 1D–I). High magnification pictures of MAP2 positive cells presented similar results with golli-KO neurons displaying reduced dendrite production (Figure 1J). Concurrently, we have found a significant reduction in the expression of several neural specific cytoskeletal proteins in golli-KO neurons including the microtubule-associated protein 2 (MAP2) (Figure 1D and E), neurofilament M and H (Figure 1F and I) and the neuron-specific class III beta-tubulin (Tuj1) (Figure 1G and H). Additionally, golli-KO neurons express less synapsin 1 (Figure 2A and B), a protein implicated in synaptogenesis and axonogenesis and GAP43, a protein that regulates neurite formation, regeneration, and synaptic plasticity (Figure 2C and D). Doublecortin (DCX) is a microtubule-associated protein expressed by neuronal precursor cells and immature neurons in embryonic and adult cortical structures. Downregulation of DCX occurs at the same time that these cells begin to express NeuN, a marker for mature neurons [29]. More DCX positive cells were found in the golli-KO cell population than in the WT cultures, for example ~50% of the golli-KO neurons express high levels of DCX after three weeks in vitro when less than 25% of the WT cells remain positive for this immature neuronal marker (Figure 2E and F). Furthermore, the number of NeuN positive cells was significantly lower in golli-KO cultures at all the time points analyzed (Figure 2G and H). Similar results were obtained by western blot techniques (Figure 2I). In summary, our in vitro data showed decreased process extension and maturation in postnatal cortical neurons from the golli-KO mice.
FIGURE 1. Golli-KO neurons failed to morphologically mature.
WT and golli-KO neurons were stained with antibodies against golli (A–C), MAP2 (D, E and J), neurofilament M (F), Tuj1 (G and H) and neurofilament H (I) and the total area cover by processes in a 1mm2 was examined by confocal microscopy at 7, 14 and 21 days in vitro (DIV). Scale bar = 120μm (A, D and G); 80μm (C and J). Values are expressed as mean ± SEM of 5 independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls.
FIGURE 2. The absence of golli interrupts the maturation of cortical neurons.
WT and golli-KO neurons were stained with antibodies against synapsin1 (A and B), GAP43 (C and D), DCX (E and F) and NeuN (G and H). For synapsin1 and GAP43 the total area cover by processes in a 1mm2 was examined by confocal microscopy and the percentage of positive cells in each experimental condition was determined for DCX and NeuN. Scale bar = 120μm. Values are expressed as mean ± SEM of 5 independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls. (I) Western blot analysis of synapsin1, GAP43, DCX, NeuN and Tuj1 expression in cortical neurons was performed using P84 and β-actin as internal standard. Data from 4 independent experiments are summarized based on the relative spot intensities and plotted as percent of controls. Values are expressed as mean ± SEM, ***p<0.001 vs. respective controls.
To determine the role of golli on neuron proliferation we calculated the percentage of proliferating cells at different time points in vitro. We labeled proliferating neurons from WT and golli-KO mice with an anti Ki67 antibody, a marker of mitotic cells. After 14 and 21DIV, there were more Ki67/MAP2 double positive neurons in the golli-KO cell population than in the WT cultures (Figure 3A and B). Importantly, no significant differences between groups were found at 7DIV (Figure 3B). We also examined the role of golli on neuron apoptotic cell death. For these studies we used a real-time caspase-3 assay, which detects caspase-3 activity within individual living cells. This assay is bi-functional in that it is able to detect both intracellular caspase-3 activity and also stain the cell nucleus, which undergoes morphological changes during the apoptotic process. Examples of such measurements are shown in Figure 3C. The combined use of real time confocal microscopy and the caspase-3 indicator revealed that neurons lacking golli displayed a significant increase in the percentage of caspase-3 positive cells (Figure 3C and D). Parallel experiments using a specific antibody for activated caspase-3 showed similar results at 7, 14 and 21DIV (Figure 3E and F).
FIGURE 3. Golli expression affects neuronal survival and proliferation.
(A) Microphotographs showing Ki67+/MAP2+ neurons grown at 14 and 21DIV. (B) The percentage of Ki67+/MAP2+ cells in each experimental condition was compared with respective controls. (C) Real-time caspase-3 assay, using NucView 488 caspase-3 substrate, was performed as described in Materials and Methods. Fluorescent field images were obtained with a specific GFP filter at 6min intervals for a period of 24h beginning 14 days after plating. Bright field images were superimposed to show the cell morphology. Yellow arrowheads designate some apoptotic cells (caspase-3+ cells). Time is denoted in hours in the bottom right corner. (D) Cell death was evaluated by measuring the percentage of caspase-3+ cells in each experimental group for a period of 24h. (E) Microphotographs showing caspase-3+/MAP2+ neurons grown at 7 and 21DIV. (F) The percentage of caspase-3+/MAP2+ cells in each experimental condition was compared with respective controls. Values are expressed as mean ± SEM of at least 5 independent experiments. Scale bar = 100μm. **p<0.01, ***p<0.001 vs. respective controls.
Golli modulates voltage-operated calcium channels in isolated cortical neurons
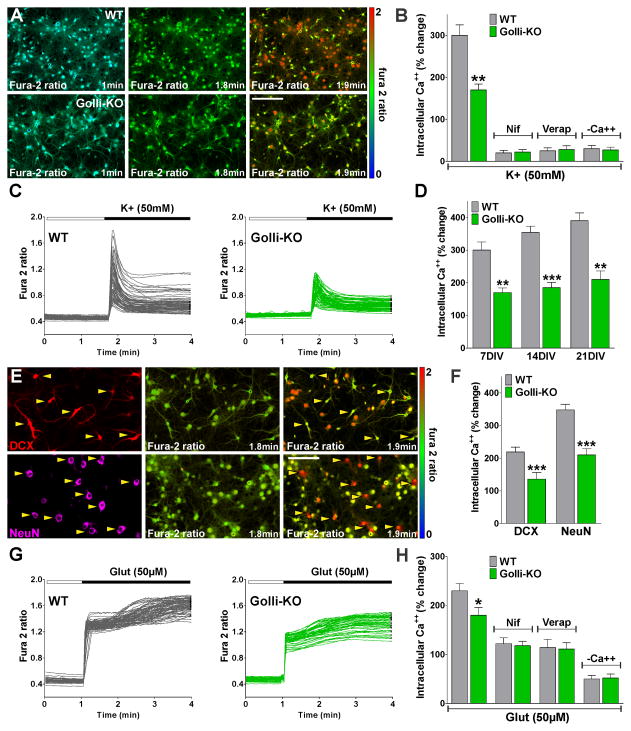
To determine whether Ca++ homeostatic mechanisms might be altered in neurons from the golli-KO mice, primary cultures of purified KO and WT cortical neurons were loaded with a membrane-permeable form of the Ca++ indicator dye fura-2. Whole cell intracellular Ca++ concentrations were measured in neurons using an Olympus spinning disc confocal microscope equipped with calcium-imaging software. Although resting Ca++ levels did not differ between the two genotypes, the magnitude of the Ca++ response after plasma membrane depolarization was significantly blunted in the golli-KO neurons at 7DIV (Figure 4A). For example, in WT neurons, bath application of a solution containing high K+ caused an average Ca++ increase of ~300% (Figure 4B and C). In golli-KO neurons, the Ca++ transient induced by high K+ was significantly smaller (~170%) (Figure 4B and C). In control experiments, fura-2 signals were abolished in zero Ca++ and were blocked by nifedipine and verapamil, confirming that these changes in intracellular Ca++ results from Ca++ influx through L-type VOCCs (Figure 4B). Additional experiments were made at 14 and 21DIV allowing analysis of the developmental regulation of voltage-operated Ca++ influx in cortical neurons. In agreement with our previous findings, Ca++ uptake after plasma membrane depolarization was significantly lower in golli-KO cells at 14DIV as well as at 21DIV (Figure 4D). Since no culture is perfectly synchronous, we performed Ca++ imaging and immunostaining for stage-specific markers in the same cells to determine the phenotype of the neurons from which we had just obtained Ca++ entry data. This involved relocating the cells with the computer driven stage after storing the coordinates of the cells. Such an experiment is shown in Figure 4E and F where we performed immunocytochemical staining for DCX and NeuN after confocal Ca++ imaging of cortical neurons at 14DIV. NeuN positive neurons responded to depolarization with large increases in intracellular Ca++ (Figure 4E and F). In contrast, the more immature DCX+ cell population responded with a significantly smaller increase in the fura-2 signal under the same high K+ stimulus (Figure 4E and F). Importantly, both cell populations were below control levels in golli-KO cultures (Figure 4F), suggesting that VOCC activity is reduced in golli-KO neurons at different maturational stages.
FIGURE 4. Golli-KO neurons exhibit decreased intracellular Ca++ concentrations after plasma membrane depolarization.
Primary cultures of cortical neurons were incubated in a chamber with 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal microscope. (A) VOCC activity was examined in cortical neurons from golli-KO and WT mice using high K+ (50mM). Fura-2 images were obtained with specific filters at 2s intervals for a total of 4min. Each frame represents a single section of a fura-2 time-lapse experiment. An increased fura-2 fluorescence ratio is indicated by warmer colors. Time is denoted in minutes in the bottom right corner. Scale bar = 80μm. (B and H) Ca++ uptake was stimulated in WT and golli-KO neurons using high K+ (50mM) and glutamate (50μM) in the presence of nifedipine (15μM), verapamil (15μM) and zero Ca++ medium (−Ca++). (C and G) Fura-2 imaging of Ca++ responses to 50mM K+ and 50μM glutamate in WT and golli-KO neurons. Note that each trace corresponds to a single cell and the horizontal bar indicates the time of high K+ and glutamate addition. (D) Averaged Ca++ response to high K+ (50mM) stimulation in WT and golli-KO neurons at 7, 14 and 21DIV. (E) Immunocytochemical staining for DCX and NeuN was used after confocal calcium imaging to determine the developmental stage at which neurons responded to high K+. Scale bar = 60μm. (F) Averaged Ca++ response to high K+ (50mM) stimulation in DCX and NeuN positive neurons from WT and golli-KO cultures at 14DIV. The graphs show the average amplitude calculated from the responding cells, expressed as percentage of change of the emission intensities. Each agonist was applied by a fast and local perfusion system. Values are expressed as mean ± SEM of at least 5 independent experiments. **p<0.01, **p<0.001 versus control.
In order to analyze the effect of golli on ligand-gated Ca++ channels, Ca++ influx was stimulated in cortical neurons using glutamate (Figure 4G). Our results show a substantial decrease of glutamate-induced Ca++ influx in golli-KO neurons at 14DIV (Figure 4G and H). Glutamate receptor-mediated Ca++ signaling in neurons is initiated by increased influx of Na+ and Ca++ through the activated NMDA and AMPA/Kainate receptors. Subsequently, Na+ influx can depolarize the plasma membrane and trigger a secondary increase in intracellular Ca++ through VOCCs. Therefore, to exclude the contribution of VOCCs, glutamate stimulation was performed in the presence of VOCC inhibitors. A significant reduction in glutamate-induced Ca++ influx was found after pre-treating the cells with verapamil or nifedipine (Figure 4H), but more importantly, no differences between genotypes were detected under this experimental condition (Figure 4H). Furthermore, no differences among WT and golli-KO neurons were found in the absence of external Ca++ (Figure 4H). In summary, these results indicate that genotype changes in Ca++ entry after glutamate treatment were mediated by VOCCs and that ionotropic (NMDA and AMPA/Kainate) and metabotropic glutamate receptors are not affected by golli.
Next, we transfected WT and golli-KO neurons with two of the main golli isoforms J37 and BG21 and examined the effect of depolarization with high K+ to induce Ca++ uptake in the cells. We found a significant increase in intracellular Ca++ concentrations in both WT and golli-KO neurons overexpressing either golli J37 or BG21 relative to control cells transfected with the empty GFP vector (Figure 5A–C). To determine if the influx pathway could be inhibited, golli transfected neurons were exposed to nifedipine and verapamil. These specific VOCCs inhibitors had a strong effect on the amplitude of the Ca++ influx in both WT and golli-KO neurons overexpressing either golli J37 or BG21 (Figure 5C). Furthermore, we found that golli overexpression causes the cells to elaborate dendrites and processes. Confocal microscopy of WT and golli-KO neurons overexpressing J37 and BG21 golli-GPP show an augmented extension of neurites and processes relative to cells transfected with the empty GFP plasmid (Figure 5D and E). Importantly, in the presence of verapamil, there was strong inhibition of the elaboration of processes induced by golli in neuronal cells (Figure 5D and E).
FIGURE 5. Increased expression of golli proteins enhance calcium influx and processes outgrowth in cortical neurons.
(A) The fura-2-based video-imaging approach was employed in order to evaluate the ability of high K+ (50mM) to elicit a Ca++ response in cortical neurons overexpressing J37 and BG21 golli-GFP. An increased fura-2 fluorescence ratio is indicated by warmer colors. Time is denoted in minutes in the lower right corner. Yellow arrowheads show WT cortical neurons overexpressing J37 and BG21 golli-GFP that were selected for the analysis. Scale bar = 80μm. Intracellular Ca++ concentrations in selected cells are plotted with respect to the time of stimulation in (B). (C) Ca++ uptake was stimulated with 50mM K+ in WT and golli-KO neurons overexpressing J37 and BG21. The graphs show the average amplitude calculated from the responding cells, expressed as percentage of change of the emission intensities. Nifedipine and verapamil were applied at 15μM. (D) Fluorescent images of WT cortical neurons transfected with J37-golli-GFP, BG21-golli-GFP and with the no modified GFP vector (GFP-Vector). Cells were transfected at 3DIV and grown for 4 days in the presence or in the absence of verapamil (15μM). Scale bar = 100μm (E) The total area cover by GFP positive processes was assessed by confocal microscopy in a 1mm2. (F) Diagrammatic scheme of the golli-GFP constructs designed to examined the regions on golli protein that might be responsible for increase in Ca++ imflux in neurons. The golli protein was divided into the MBP and golli domain. (G) Ca++ uptake was stimulated in WT and golli-KO neurons overexpressing different golli-GPP constructs using high K+ (50mM). The graphs shows the average amplitude calculated from the responding cells, expressed as percentage of change of the emission intensities. Values are expressed as mean ± SEM of 5 independent experiments. **p<0.01, ***p<0.001 vs. respective controls.
In order to identify any motifs on the golli protein that might be important in the effects of golli on VOCC activity, site-directed mutagenesis and deletion analyses were used to generate a series of golli-green fluorescent protein (GFP) DNA constructs. The mutated golli-GFP plasmids were transfected into WT and golli-KO neurons and Ca++ transients measured to determine what sites on the molecule might be important for Ca++ regulation. Figure 5F shows a cartoon of the mutations/deletions generated for analysis. The golli-MBPs consist of two domains, a golli domain of 133 amino acids and an MBP domain of variable length (Figure 5F). In J37 Del1 the first 45 amino acids from the N-terminus of the golli domain were removed where in J37 Del2 the first 110 amino acids of the golli domain were eliminated (Figure 5F). We found that elimination of the first 45 or 110 amino acids from the N-terminus of J37 (J37 Del1 and J37 Del2 respectively) abolished the Ca++ entry increase (Figure 5G). Feng and co-workers [30] found that myristoylation of the glycine residue at the N-terminus of golli BG21 was important for targeting golli to the plasma membrane in the Jurkat T-cell line. In the present study, we confirmed that mutation of the myristoylation sites (gly->ala at position 2) of either golli J37 or BG21 (J37 and BG21 Myr) entirely reversed the Ca++ effect in WT and golli-KO neurons, indicating that membrane association is essential for golli action on the enhancement of Ca++ entry in cortical neurons (Figure 5G). Additionally, deleting a calmodulin binding-like site (J37 NoCalm) within the J37 golli domain reduced the effect by ~15% in WT neurons and by 25% in golli-KO cells (Figure 5G). Note that these studies on Ca++ influx after high K+ stimulation correlate completely with the results obtained on the effects of transfected golli on cell morphology (Figure 5D, E and G). This correlation implies a clear relationship between golli, Ca++ uptake and neurites extension.
To determine the influence of L-type mediated Ca++ influx on neuronal maturation, WT and golli-KO cells were cultured with Bay K 8644, an L-type Ca++ channel agonist that prolongs single channel open time without affecting the close time. Neurons were treated with Bay K 8644 (10μM) for 7 days starting at 7DIV. As it was expected, Bay K treatment enhanced the amplitudes of Ca++ transients after membrane depolarization in WT neurons as well as in golli-KO cells (Figure 6A and D). In WT neurons, Bay K increased L-type Ca++ influx by approximately 26% whereas in golli-KO cultures the rise was significantly higher (~51%) (Figure 6A and D). More importantly, 7 days of Bay K treatment stimulated the morphological complexity of MAP2 and Tuj1 positive neurons (Figure 6B and C), increased the percentage of NeuN positive cells and decreased the proportion of neurons expressing DCX in golli-KO cultures (Figure 6E and F). Examples of such morphological and immunohistochemical changes are shown in Figure 6B and E. Moreover, in golli-KO cultures, Bay K treatment was able to prevent apoptotic cell death and drop cell proliferation to normal levels (Figure 6G). Similar results were obtained in experiments in which WT neurons were treated with Bay K during 7DIV (Figure 6C, F and G). Bay K treatment had no significant effect on either apoptotic cell dead or cell proliferation in WT neurons (Figure 6G). But we did find a significant increase in the morphological complexity of MAP2 and Tuj1 expressing neurons in WT cultures treated with Bay K (Figure 6C). Furthermore, WT neurons responded to Bay K treatment with an increase in the percentage of NeuN positive cells and with a substantial reduction in DCX immunolabeling (Figure 6F). These data suggest that golli effect on neuronal differentiation is strongly correlated with the activity of voltage-operated Ca++ channels, especially the L-type, and that Ca++ influx through these channels plays a key role in promoting neuronal differentiation.
FIGURE 6. Pharmacological activation of L-type Ca++ channels stimulates maturation and prevent cell dead in golli-KO neurons.
WT and golli-KO neurons were treated with Bay K 8644 (10μM) for 7 days starting at 7DIV. (A) Fura-2 imaging of Ca++ responses to 50mM K+ in golli-KO neurons treated with Bay K. Note that each trace corresponds to a single cell and the horizontal bar indicates the time of high K+ addition. (B) MAP2 and Tuj1 immunostaining in golli-KO neurons treated with Bay K for 7 days. Scale bar = 120μm. (C) The total area cover by MAP2 and Tuj1 positive processes was assessed by confocal microscopy in a 1mm2. (D) Averaged Ca++ response to high K+ (50mM) stimulation in WT and golli-KO neurons treated with Bay K for 7 days. The graphs show the average amplitude calculated from the responding cells, expressed as percentage of change of the emission intensities. (E) NeuN and DCX immunostaining in golli-KO neurons treated with Bay K for 7 days. Scale bar = 120μm. (F and G) The percentage of NeuN, DCX, caspase-3/MAP2 and Ki67/MAP2 positive neurons was evaluated after 7 days of Bay K treatment in WT and golli-KO neurons. Values are expressed as mean ± SEM of 5 independent experiments. **p<0.01, ***p<0.001 vs. respective controls.
Morphological and Ca++ signaling abnormalities in the cortex and hippocampus of golli-KO mice
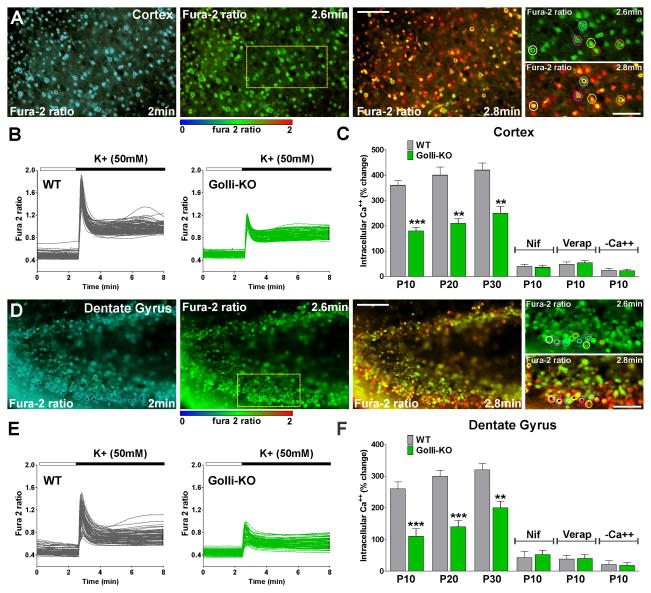
We extended our analysis of golli regulation of Ca++ channels and neuronal maturation to a more intact context by performing similar experiments in acute tissue slices obtained from golli-KO animals. Through in situ fura-2 ratiometric Ca++ imaging measurements [16, 31] we measured VOCCs activity in subpopulations of neurons in selected brain regions. Initially, we focused our in situ Ca++ measurements of neurons in slice preparations containing the primary somatosensory cortex. Our goal in these experiments was to confirm the in vitro data in live preparations with respect to VOCCs activity in neurons lacking golli. Recordings were made at postnatal day ten (P10). To examine in situ VOCC Ca++ entry fura-2 loaded tissue slices were perfused with 50mM K+ by a fast and local perfusion system. Exposure to high K+ containing medium triggered a significant increase in the intracellular Ca++ concentration in cortical neurons (Figure 7A). As expected, golli-KO neurons exhibited Ca++ signaling following stimulation with high K+, but these Ca++ signals were significantly weaker than that of the control cells (Figure 7B). Pharmacological experiments demonstrated that this Ca++ response was abolished in the presence of verapamil and nifedipine, specific VOCCs blockers and in the absence of external Ca++ (Figure 7C). Furthermore, Ca++ uptake after plasma membrane depolarization was significantly lower in golli-KO cells at P20 as well as at P30 (Figure 7C). Similar results were found in hippocampal neurons. A decrease in the activity of VOCCs in golli-KO neurons located in the upper and lower blade of the dentate gyrus was found at all the time points tested (Figure 7D–F).
FIGURE 7. Reduced in situ VOCC activity in cortical and hippocampal neurons from the golli-KO mice.
Brain slices were incubated in a chamber with 95% O2 and 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal inverted microscope. Fura-2 images were obtained for brain slices with specific filters at 2s intervals for a total of 8min. (A and D) Time-lapse series of WT cortical and hippocampal neurons at P10 located in the somatosensory cortex and the upper and lower blade of the dentate gyrus respectively. Scale bar = 100μm. Colored circles in high magnification insets show neurons that were selected for the analysis. Scale bar = 60μm. An increased fura-2 fluorescence ratio is indicated by warmer colors and time is denoted in minutes in the lower right corner. (B and E) VOCC activity was examined in cortical and hippocampal neurons from WT and golli-KO mice at P10. Note that each trace corresponds to a single cell and the horizontal bar indicates the time of high K+ addition. (C and F) VOCC activity was examined in cortical and hippocampal neurons of WT and golli-KO mice at P10, P20 and P30 using high K+ (50mM). Cortical and hippocampal neurons from WT and golli-KO mice at P10 were also stimulated with high K+ (50mM) in the presence of nifedipine (15μM), verapamil (15μM) and zero Ca++ medium (−Ca++). The graphs show the average amplitude calculated from the responding cells expressed as percentage of change of the emission intensities. Values are expressed as mean ± SEM of at least 4 independent experiments. **p<0.01, ***p<0.001 vs. respective controls.
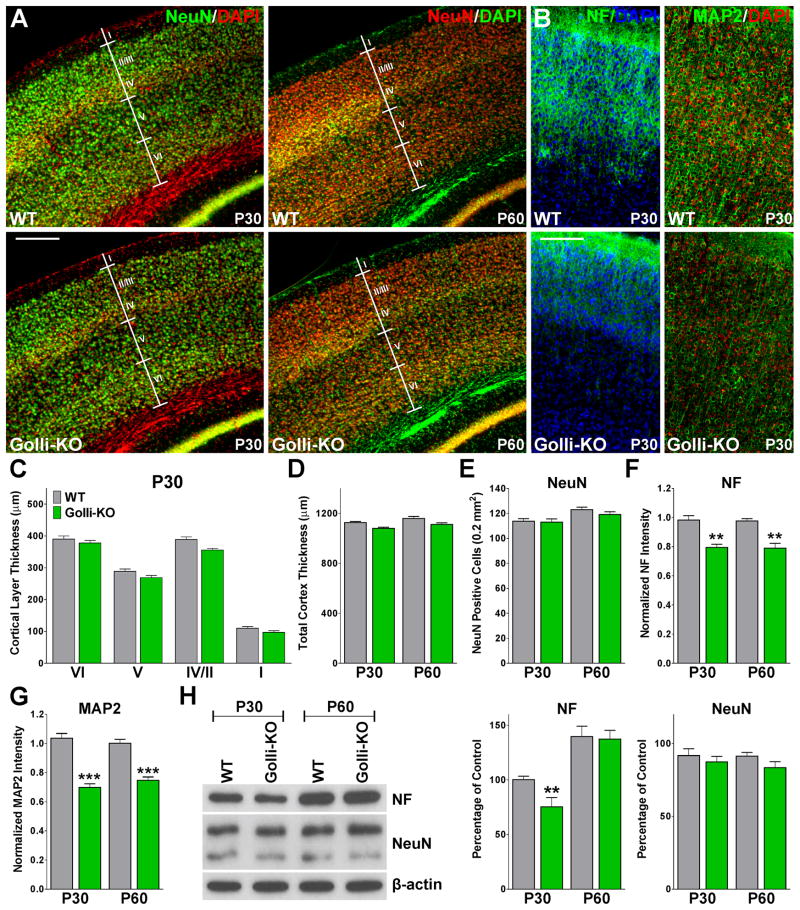
These Ca++ imaging experiments were correlated with morphological changes in the same regions of the brain. The primary somatosensory cortex of golli-KO animals displayed a normal thickness and a regular density of NeuN positive cells (Figure 8A, C, D and E), however we found a significant decrease in the density of neurofilament L and MAP2 positive dendrites in the golli-KO cortex at P30 and P60 (Figure 8B, F and G). In the same line, the CA1/3 sections of the hippocampus, the upper blade and the granule cell layer of the dentate gyrus were reduced in the golli-KO mice at P30 and P60 (Figure 9A, C, D and E). Likewise, we found a reduction in the concentration of neurofilament L and MAP2 dendrites in the CA1/3 sections of the hippocampus (Figure 9B and F, all data not shown). These morphological measurements and immunohistochemical results were completed by western blot experiments (Figure 8H and 9G). Western blot performed with protein samples obtained from the golli-KO somatosensory cortex display reduced expression of neurofilament L at P30 and normal levels of NeuN at P30 and P60 (Figure 8H). On the other hand, decreased expression of neurofilament L and NeuN was found in protein extracts from the golli-KO hippocampus at both ages (Figure 9G).
FIGURE 8. Lower density of neurofilament and MAP2 positive dendrites in the golli-KO cortex.
NeuN (A), neurofilament L and MAP2 (B) immunohistochemistry in coronal cortical sections of P30 and P60 WT and golli-KO mice. The cortical layers thickness (C), the total cortex thickness (D), the total number of NeuN positive cells (E) and the normalized neurofilament L and MAP2 fluorescent intensity (F and G) was measured in the somatosensory cortex of P30 and P60 WT and golli-KO mice. Scale bar = 300μm (A); 200μm (B). Values are expressed as mean ± SEM of 5 independent experiments. **p<0.01, ***p<0.001 vs. respective controls. (H) Western blot analysis of neurofilament L and NeuN expression in the somatosensory cortex of P30 and P60 WT and golli-KO mice was performed using β-actin as internal standard. Data from 4 independent experiments are summarized based on the relative spot intensities and plotted as percent of controls. Values are expressed as mean ± SEM, **p<0.01 vs. respective controls.
FIGURE 9. Abnormal hippocampal morphology in postnatal golli-KO mice.
NeuN (A) and MAP2 (B) immunohistochemistry in coronal hippocampal sections of WT and golli-KO mice at P30. Scale bar = 300μm (A and B upper panel); 180μm (A lower panel); 80μm (B lower panel). The thickness of the CA1/3 sections (C), the length of the upper and lower blade of the dentate gyrus (D), the granule cell layer thickness (E) and the normalized MAP2 fluorescent intensity were measured in coronal hippocampal sections of P30 and P60 WT and golli-KO mice. Values are expressed as mean ± SEM of 5 independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls. (G) Western blot analysis of neurofilament L and NeuN expression in the hippocampus of P30 and P60 WT and golli-KO mice was performed using β-actin as internal standard. Data from 4 independent experiments are summarized based on the relative spot intensities and plotted as percent of controls. Values are expressed as mean ± SEM, *p<0.05, **p<0.01, vs. respective controls.
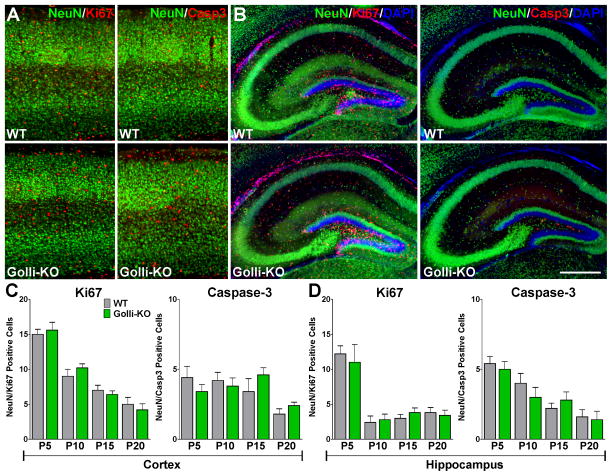
The majority of neurons are born embryonically, however neurogenesis continues in the postnatal brain throughout life. New neurons are produced from neural stem and progenitor cells in the subgranular zone of the dentate gyrus and the ventricular zone of the lateral ventricles [32]. To assess the impact of golli on neural progenitor cells proliferation and survival in vivo we performed immunohistochemical studies using neuronal lineage markers in combination with indicators of cell proliferation and death such as Ki67 and caspase-3. We have found that the number of proliferating neural progenitor cells (DCX+/Ki67+) and the number of apoptotic immature neurons (DCX+/caspase-3+) was increased in the dentate gyrus of the hippocampus as well as in the somatosensory cortex of the golli-KO brain at P5 and P10 (Figure 10A–C and E–G). Furthermore, during the first postnatal week the total number of DCX+/Sox2+ and DCX+/Sox9+ neural progenitor cells was decreased in the same areas of the golli-KO brain (Figure 10A, D, E and H). Interestingly, no changes in neural progenitor cell proliferation or death were detected at later developmental stages (P15 and P20) (Figure 10B–C and F–G). In contrast with the DCX positive cell population, no significant differences in cell proliferation or apoptosis were observed when the more mature NeuN marker was used to identify the neurons (Figure 11C and D). Despite the fact that a rise in the number of Ki67 and caspase-3 positive cells is clearly visible in the golli-KO somatosensory cortex and hippocampus at P10, very few of these cells were positive for NeuN (Figure 11A and B). Taken together, our in situ results uncover a key role for golli protein in the regulation of VOCC mediated Ca++ influx in postnatal cortical and hippocampal neurons and suggest that the absence of golli affects the proliferation and survival of young neurons throughout early postnatal development.
FIGURE 10. Irregular postnatal neurogenesis in the golli-KO brain.
(A and E) DCX/Ki67, DCX/caspase-3, DCX/Sox2 and DCX/Sox9 immunohistochemistry in coronal cortical and hippocampal sections of WT and golli-KO mice at P5. Scale bar = 200μm. The total number of double positive cells was stereologically quantified in the somatosensory cortex (0.6mm2, including all the cortical layers) (B, C and D), and in the dentate gyrus of the hippocampus (0.4mm2, including upper and lower blades and the subgranular zone) (F, G and H) in P5, P10, P15 and P20 WT and golli-KO animals. Values are expressed as mean ± SEM of 5 independent experiments. *p<0.05, **p<0.01 vs. respective controls.
FIGURE 11. NeuN positive neurons showed normal proliferation and viability in the perinatal golli-KO brain.
(A and B) NeuN/Ki67 and NeuN/Caspase-3 immunohistochemistry in coronal cortical and hippocampal sections of WT and golli-KO mice at P10. Scale bar = 250μm. The total number of double positive cells was stereologically evaluated in the somatosensory cortex (0.6mm2, including all the cortical layers) (C), and in the dentate gyrus of the hippocampus (0.4mm2, including upper and lower blades and the subgranular zone) (D) in P5, P10, P15 and P20 WT and golli-KO animals. Values are expressed as mean ± SEM of 5 independent experiments.
Behavioral abnormalities in the golli-KO mice
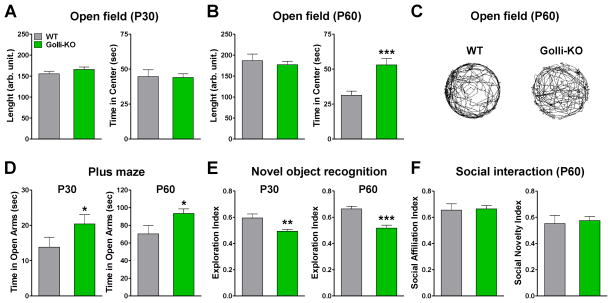
We have found that golli-KO mice exhibit behavioral abnormalities. No significant differences were found between golli-KO and WT mice in the modified-SHIRPA protocol (data not shown). Measures of neuromuscular strength and motor coordination learning did not differ between the genotypes. Specifically, grip strength, latency to fall in the wire hang test, and latency to fall off the rotarod did not differ significantly between golli-KO and WT mice (Figure 12A, all data not shown). The beam-walking assay can assess fine motor coordination and balance. The goal of this test is for the mouse to stay upright and walk across an elevated narrow beam to a safe platform. Performance on the beam is quantified by measuring the time it takes for the mouse to traverse the beam and the number of paw slips that occur in the process. This task is particularly useful for detecting subtle deficits in motor skills and balance that may not be detected by other motor tests, such as the rotarod. No significant differences were observed between WT and golli-KO mice when 12mm square or round beams were used (Figure 12B and C). But our data showed that golli-KO mice performed significantly better than the WT animals in 6mm square beam at both P30 as well as P60 (Figure 12B and C). Interestingly, the percentage of mice that crossed the entire 6mm round beam was much higher in the golli-KO than in the WT group (Figure 12D) suggesting normal fine motor coordination and reduced anxiety and fear in these animals.
FIGURE 12. Normal motor coordination and reduced anxiety and fear in golli-KO mice.
(A) WT and golli-KO mice were evaluated in the rotarod test. The latency to fall off the rotarod was measured in P30 and P60 mice. (B and C) 12mm square or round beams and 6mm square beams were used to assess fine motor coordination in WT and golli-KO mice at P30 and P60. (D) Percentage of mice that crossed the entire 6mm round beam in the different experimental groups. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls.
To determine the effect of golli on locomotor activity, animals were placed into a open field chamber and the total distance travelled and the time spent in the center area were automatically recorded for 10min. Golli-KO mice and controls displayed no significant difference in the total distance travelled, but we did find a significant increase in the time spend in the center of the field in P60 golli-KO animals (Figure 13A–C). Similarly, golli-KO mice spent significantly more time in the open arms of the elevated plus maze when compared with controls at both ages tested (Figure 13D). These data indicate that, while golli-KO animals did not show lethargy as interpreted by no changes in the total distance travelled, there was an increase in the time spend in the center of the open field and in the open arms of the elevated plus maze which could be indicative of an anxiolytic-like phenotype.
FIGURE 13. Anxiolytic-like behavior and reduced episodic memory in the golli-KO mice.
(A and B) WT and golli-KO mice were evaluated in the open field test. The total distance travelled and the time spent in the center area were measured at P30 and P60. (C) Representative path plots of the complete path traveled during an open field session in P60 WT and golli-KO mice. (D) WT and golli-KO mice were evaluated in the elevated plus-maze test. The total time spent in the open arms was assessed in P30 and P60 animals. (E) WT and golli-KO mice were evaluated in the novel object recognition test. The exploration index was determined in P30 and P60 animals. (F) Evaluation of social interaction in P60 WT and golli-KO mice. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls.
Next we examined the golli-KO mouse for learning deficits using the novel object recognition test. Our data show a clear distinction among the genotypes with golli-KO mice performing significantly worse than the WT mice with respect to discriminate a novel object from a familiar one. We found that both, adolescents (P30) and young adults (P60) golli-KO mice displayed a significant reduction in the novel object recognition index relative to controls (Figure 13E). Finally, social interaction was tested using the Crawley’s three-chamber social approach test in which the social preference of mice can be quantified by comparing the time spent around a wire cage containing a stranger mouse versus the time spent around an empty cage [33]. This test allows for both sociability and a social novelty preference test. No significant genotype effect was found in the social affiliation index as well as in the social novelty index (Figure 13F). In summary, no deficits in locomotor activity, motor coordination, balance and social behavior were found in the golli-KO mouse. However, we have determined that the golli-KO mouse exhibit an anxiolytic phenotype and a reduction in the episodic memory. These effects of the ablation of golli-MBP products on behavior and memory suggest an important role for these proteins in neuronal function.
DISCUSSION
Golli modulation of VOCCs affects neuronal development
Voltage-operated Ca++ channels (VOCCs) serve as the principal routes of Ca++ entry into electrically excitable cells such as neurons. The nervous system expresses VOCCs with unique cellular and subcellular distribution and specific functions. L-type VOCCs are distributed at neuronal cell bodies, dendrites and spines, and the postsynaptic L-type VOCCs regulate neuronal excitability and gene expression [34–37]. For example, in the postnatal hippocampus, Ca++ influx through L-type VOCCs is essential for expression of the transcription factor NeuroD, and inhibition of pro-glial genes HES1 and Id2 [38]. In this work we have identified an important relationship between L-type Ca++ influx and golli proteins in neuronal maturation. We have found that voltage-mediated Ca++ influx is reduced in postnatal neurons from the golli-KO mice and that increased expression of golli proteins enhances Ca++ entry into neuronal cells. Golli proteins can modulate Ca++ influx through L-type VOCCs in the plasma membranes of cortical and hippocampal neurons and this intracellular Ca++ modulation affect Ca++ dependent functions such as neurites extension, proliferation and cell survival. In vitro, golli-KO neurons display a simple morphology and reduced levels of key cytoskeletal proteins such as MAP2, neurofilament and Tuj1. Also, golli-KO neurons express less synapsin1 and GAP43, two critical proteins for normal synaptogenesis/axonogenesis, and retain immature neuronal markers such as DCX. All these changes affect the survival of golli-KO neurons, which shows a significant increase in apoptotic cell death. Importantly, undeveloped DCX positive neurons as well as mature NeuN expressing cells from golli-KO cultures show decreased voltage-operated Ca++ entry, suggesting that VOCC activity is reduce in golli-KO neurons at different maturational stages. Confirming the involvement of voltage-gated Ca++ influx in this phenomenon, these alterations in golli-KO neurons were reversed by the L-type VOCC activator Bay K 8644. Bay K treatment enhances voltage-mediated Ca++ influx and promotes the expression of mature neuronal markers in golli-KO cultures. More importantly, activation of L-type VOCCs in golli-KO neurons prevent cell death by apoptosis and consistent with neuronal maturation, reduce cell proliferation. These data suggest that golli effect on neuronal differentiation is strongly correlated with the activity of L-type VOCCs, and that Ca++ influx through these channels plays a key role in promoting neuronal maturation.
Several studies have shown that intracellular Ca++ regulates axon outgrowth and growth cone motility in different ways [39]. Depolarization-induced Ca++ entry through VOCCs has been shown to maintain neurites and cell survival after nerve growth factor deprivation [40, 41]. Likewise, Ca++ has been implicated in directing growth cone guidance [42, 43]. The responses of growth cones to many guidance molecules, such as netrin-1 and BDNF, are dependent on an influx of extracellular Ca++ through L-type VOCCs [44, 45]. One property of golli proteins is their ability to induce extension of processes when overexpressed in oligodendrocyte cell lines [17]. In this report we have shown that golli overexpression cause neurite outgrowth in cultured cortical neurons. Confocal microscopy of WT and golli-KO cells overexpressing BG21 and J37 golli-GPP show an increased extension of neurites and processes. Interestingly, this phenomenon disappeared in the presence of VOCC blockers, indicating that the morphological changes induced by golli overexpression could be mediated by VOCCs. The principal subcellular location of golli proteins in neurons was in axonal and dendritic processes. With development and neuronal maturation, golli expression decreased and/or there was a striking shift in subcellular localization from nuclei and cell processes to the cell soma in isolated cortical neurons. We hypothesize that golli proteins are needed along neurites to regulate the activity of VOCCs. The principal role of golli may be to maintain locally high levels of Ca++ within the neurites and thereby contribute to neurites extension and maintenance throughout neuronal maturation.
Golli proteins can bind several molecules important in Ca++ signaling; Kaur and collaborators [46] and Feng and co-workers [30] found that myristoylation of BG21 is essential for the interaction of golli with the plasma membrane in T-cells. In this study we found that removing the first 45 or 110 amino acids from the N-terminus of the golli domain completely obliterated the Ca++ response. More importantly, the sole mutation of the myristoylation site located at position 2 have the same effect suggesting that binding of golli to the plasma membrane is essential for modulating Ca++ homeostasis. By what mechanism could golli-MBPs modulate the activity of Ca++ channels? It is possible that golli serves as an adaptor protein in a complex that may directly or indirectly modulate the activity of Ca++ channels at the plasma membrane. By virtue of its high hydrophilicity and net charge, golli has the appropriate physical properties to be an adaptor protein. Harauz and colleagues [47] have shown that golli proteins form extended structures in solution that would be ideal for binding other molecules in a signaling assembly [47]. This notion of golli proteins serving as potential adaptor proteins in signaling complexes is supported by other studies showing that they can bind calmodulin in a Ca++ dependent fashion, and that they are also able to bind phosphoinositides [46]. Calmodulin is required for the modulation of several important enzymes and ion channels involved in synaptic efficiency and neuronal plasticity [48]. In this work we found that deleting portions of the golli domain in J37 containing a putative calmodulin binding-like site reduced the Ca++ response, suggesting that the interaction of golli with calmodulin could be important in the effects of this protein on VOCC activity and neuron development.
Behavioral abnormalities and irregular cortical and hippocampal maturation in the golli-KO mice
Our experiments demonstrate that the expression of golli in neurons is essential for brain development and suggest that an abnormal cortical and hippocampal maturation could be responsible for some of the behavioral changes found in the golli-KO mouse. The golli-KO animals display significant morphological changes in the cortex and hippocampus. The density of dendrites in the somatosensory cortex of young golli-KO mice was significantly decreased. In the hippocampus, the CA1/3 sections, the dentate gyrus and the granule cell layer were reduced in the golli-KO mice compare to control animals. During the first two postnatal weeks, immature cortical and hippocampal neurons from the golli-KO brain proliferate faster than control cells and show a higher susceptibility to die by apoptosis. All these changes in the cortex and hippocampus of young golli-KO animals were correlated with an abnormal activity of L-type VOCCs in the same neuronal populations, suggesting that golli modulation of VOCCs in neurons during the postnatal development is responsible for these alterations. As a consequence of this irregular cortical and hippocampal maturation, the golli-KO mouse exhibit alterations of executive-level processes such as memory and anxiety. We have determined that the golli-KO mouse display an anxiolytic phenotype and a reduction in the episodic memory. Measures of neuromuscular strength and motor coordination did not differ between the genotypes. However, the golli-KO mice spent significantly more time in the central area of the open field and in the open arms of the plus maze when compared with controls and displayed a substantial reduction in the novel object recognition index relative to controls. Most evident in adolescent life, these alterations subside in young adults.
The proximal golli promoter of the MBP gene was used as a molecular marker for specific subpopulation of neurons because of its early and restricted expression in cerebral cortex and olfactory bulb in mice. For instance, it was used to drive the expression of various reporters to precisely identify the principal preplate neurons and their neuroblasts in the embryonic brain [49, 50]. It has been shown that targeted genetic ablation of golli-expressing principal preplate neurons of the neocortex during prenatal development delayed organization of these neurons, desynchronizes and isolates the developing neocortex from the rest of the brain, and permanently impairs its connectivity [49]. Golli may promote selective survival of cortical and hippocampal neurons, perhaps through positive impact on neurite arborization and establishment of stable synaptic connectivity. The positive feedback loop between golli, neuronal differentiation and L-type VOCC function highlighted by our findings may be involved in the functional integration of newly generated neurons into pre-existing neural circuits of the postnatal brain, a process that is reinforced by establishment of synaptic connections and the consequent membrane depolarization-induced Ca++ influx.
Specific genetic ablation of the golli proteins in mice produces a phenotype of defective myelination in both white and gray matter structures [12]. Although gross myelination appeared normal, electron microscopic and immunohistochemical studies indicated that myelination is delayed in the golli-KO mouse [12]. Since myelination is important for normal CNS maturation and function we cannot completely exclude the possible contribution of abnormal oligodendrocyte maturation in some of the behavioral and anatomical alterations found in the brain of golli-KO animals. However, our in vitro experiments showed that perinatal neurons isolated from the cortex of golli-KO mice fail to mature in vitro and in situ Ca++ imaging experiments, performed during the first postnatal week, revealed decreased L-type VOCC activity in different subpopulations of golli-KO neurons. Since all these experiments were performed before the initiation of the myelination process our results suggest that the absence of golli is affecting neuron development in a cell autonomous manner.
Golli-MBP and psychiatric disorders
The myelin-related gene group has been repeatedly reported to be dysregulated (mostly under-expressed) in schizophrenia patients [51–55]. The chromosomal region 18q, where the golli-MBP gene is located, has been linked to both schizophrenia and bipolar disorder. There is evidence that genes in this region are involved in psychiatric and cognitive disorders [56, 57]. For example, a genome wide scan suggested the golli-MBP locus (18q23) is a susceptibility locus for bipolar disorder [58]. Several studies have shown changes in classic MBP gene expression in schizophrenia and bipolar disorder in different brain regions [59, 53, 55]. Baruch and co-workers [60] have performed a case-control association analysis of golli specifically in two separate Jewish Ashkenazi cohorts. They also performed an expression analysis of golli mRNA in post-mortem dorsolateral prefrontal cortex samples of schizophrenia patients. They found a strong association between single nucleotide polymorphisms and risk haplotypes with schizophrenia. Although the expression analysis found no significant differences in golli mRNA levels, these findings suggest that golli is a possible susceptibility gene for schizophrenia.
Dysregulation of the Ca++ signaling pathway has been implicated in the development of some of the major psychiatric diseases such as schizophrenia. [61]. It has been demonstrated that the proper formation and maintenance of dendritic trees and synaptic contacts requires optimal levels of Ca++ signaling [62]. Thus, imbalance in Ca++ signaling may be the underlying cause of a reduction in dendritic volume and/or rearrangement in synaptic connectivity postulated for schizophrenia [63, 64, 65]. Moreover, the central role of Ca++ signaling in cell death [66] suggests that abnormality in this signaling may cause a reduction in neuronal number described in cortical and subcortical regions of schizophrenic patients [67]. Based on the above, Lidow hypothesized that altered Ca++ signaling is a central unifying element of multiple molecular and protein changes reported in schizophrenia [61]. In this work we showed that Ca++ homeostasis is altered in postnatal neurons from the golli-KO mice and that these animals display abnormal behavior including deficits in episodic memory and reduced anxiety. Therefore, this work has great implication in understanding the molecular events governing psychiatric and cognitive disorders and golli-MBPs can be added to the growing list of different proteins underlying Ca++ signaling in the etiology of schizophrenia.
Acknowledgments
National Institute of Neurological Disorders and Stroke grant 1R01NS078041-01A1 and National Multiple Sclerosis Society Grant RG4554-A-2.
References
- 1.Campagnoni AT, Pribyl TM, Campagnoni CW, Kampf K, Amur-Umarjee S, Landry CF, Handley VW, Newman SL, Garbay B, Kitamura K. Structure and developmental regulation of Golli-mbp, a 105kb gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 1993;268:4930–4938. [PubMed] [Google Scholar]
- 2.Givogri MI, Bongarzone ER, Campagnoni AT. New insights on the biology of myelin basic protein gene: the neural-immune connection. J Neurosci Res. 2000;59:153–159. [PubMed] [Google Scholar]
- 3.Campagnoni AT, Skoff RP. The pathobiology of myelin mutants reveal novel biological functions of the MBP and PLP genes. Brain Pathology. 2001;11:74–91. doi: 10.1111/j.1750-3639.2001.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritz RB, Kalvakolanu I. Thymic expression of the golli-myelin basic protein gene in the SJL/J mouse. J Neuroimmunol. 1995;57:93–99. doi: 10.1016/0165-5728(94)00167-m. [DOI] [PubMed] [Google Scholar]
- 5.Pribyl TM, Campagnoni C, Kampf K, Handley VW, Campagnoni AT. The major myelin protein genes are expressed in the human thymus. J Neurosci Res. 1996;45:812–819. doi: 10.1002/(SICI)1097-4547(19960915)45:6<812::AID-JNR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Pribyl TM, Campagnoni CW, Kampf K, Ellison JA, Landry CF, Kashima T, McMahon J, Campagnoni AT. Expression of the myelin basic protein gene locus in neurons and oligodendrocytes in the human fetal central nervous system. J Comp Neurol. 1996;374:342–353. doi: 10.1002/(SICI)1096-9861(19961021)374:3<342::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Landry CF, Ellison JA, Pribyl TM, Campagnoni C, Kampf K, Campagnoni AT. Myelin basic protein gene expression in neurons: developmental and regional changes in protein targeting within neuronal nuclei, cell bodies, and processes. J Neurosci. 1996;16:2452–2462. doi: 10.1523/JNEUROSCI.16-08-02452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landry CF, Ellison J, Skinner E, Campagnoni AT. Golli-mbp proteins mark the earliest stages of fiber extension and terminal arboration in the mouse peripheral nervous system. J Neurosci Res. 1997;50:265–271. doi: 10.1002/(SICI)1097-4547(19971015)50:2<265::AID-JNR15>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Tosic M, Rakic S, Matthieu J, Zecevic N. Identification of Golli and myelin basic proteins in human brain during early development. Glia. 2002;37:219–228. doi: 10.1002/glia.10028. [DOI] [PubMed] [Google Scholar]
- 10.Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Macklin WB, Colwell C, Campagnoni AT. Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca++ influx. J Neurosci. 2009;29:6663–6676. doi: 10.1523/JNEUROSCI.5806-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paez PM, Fulton DJ, Spreur V, Handley V, Campagnoni CW, Campagnoni AT. Regulation of store-operated and voltage-operated Ca++ channels in the proliferation and death of oligodendrocyte precursor cells by Golli proteins. ASN Neuro. 2009;1:e00003. doi: 10.1042/AN20090003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs EC, Pribyl TM, Feng JM, Kampf K, Spreuer V, Campagnoni C, Colwell CS, Reyes SD, Martin M, Handley V, Hiltner TD, Readhead C, Jacobs RE, Messing A, Fisher RS, Campagnoni AT. Region-specific myelin pathology in mice lacking the golli products of the myelin basic protein gene. J Neurosci. 2005;25:7004–7013. doi: 10.1523/JNEUROSCI.0288-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs EC, Reyes SD, Campagnoni CW, Irene Givogri M, Kampf K, Handley V, Spreuer V, Fisher R, Macklin W, Campagnoni AT. Targeted overexpression of a golli-myelin basic protein isoform to oligodendrocytes results in aberrant oligodendrocyte maturation and myelination. ASN Neuro. 2009;1:e00017. doi: 10.1042/AN20090029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paez PM, Cheli VT, Ghiani CA, Spreuer V, Handley VW, Campagnoni AT. Golli myelin basic proteins stimulate oligodendrocyte progenitor cell proliferation and differentiation in remyelinating adult mouse brain. Glia. 2012;60:1078–1093. doi: 10.1002/glia.22336. [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, Paez PM, Campagnoni AT. The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN Neuro. 2010;2:e00027. doi: 10.1042/AN20090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paez PM, Fulton DJ, Spreur V, Handley V, Campagnoni AT. Modulation of Canonical Transient Receptor Potential Channel 1 in the Proliferation of Oligodendrocyte Precursor Cells by the Golli Products of the Myelin Basic Protein Gene. J Neurosci. 2011;31:3625–3637. doi: 10.1523/JNEUROSCI.4424-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paez PM, Spreuer V, Handley V, Feng JM, Campagnoni C, Campagnoni AT. Increased expression of golli myelin basic proteins enhances calcium influx into oligodendroglial cells. J Neurosci. 2007;27:12690–12699. doi: 10.1523/JNEUROSCI.2381-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echeverria V, Greenberg D, Dore S. Expression of Prostaglandin E2 Synthases in Mouse Postnatal Cortical Neurons. Ann NY Acad Sci. 2005;1053:460–471. doi: 10.1111/j.1749-6632.2005.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 19.Reyes SD, Campagnoni AT. Two separate domains in the golli myelin basic proteins are responsible for nuclear targeting and process extension in transfected cells. J Neurosci Res. 2002;69:587–596. doi: 10.1002/jnr.10319. [DOI] [PubMed] [Google Scholar]
- 20.Hof P, Youn WG, Bloom FE, Belichenko PV, Celio ZMR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. Elsevier; Amsterdam, Netherlands: 2000. [Google Scholar]
- 21.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15:204–215. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawitz J, Kroon T, Hjorth JJ, Meredith RM. Functional calcium imaging in developing cortical networks. J Vis Exp. 2011;56:3550. doi: 10.3791/3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Masuya H, Inoue M, Wada Y, Shimizu A, Nagano J, Kawai A, Inoue A, Kagami T, Hirayama T, Yamaga A, Kaneda H, Kobayashi K, Minowa O, Miura I, Gondo Y, Noda T, Wakana S, Shiroishi T. Implementation of the modified-SHIRPA protocol for screening of dominant phenotypes in a large-scale ENU mutagenesis program. Mamm Genome. 2005;16:829–837. doi: 10.1007/s00335-005-2430-8. [DOI] [PubMed] [Google Scholar]
- 25.Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8):12. doi: 10.1002/0471142301.ns0812s15. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Cha HY, Seo JJ, Hong JT, Han K, Oh KW. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O’Donnell JM, Zhang HT. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 30.Feng JM, Hu YK, Xie LH, Colwell CS, Shao XM, Sun XP, Chen B, Tang H, Campagnoni AT. Golli protein negatively regulates store depletion-induced calcium influx in T cells. Immunity. 2006;24:717–727. doi: 10.1016/j.immuni.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Paez PM, Fulton DJ, Spreur V, Handley V, Campagnoni AT. Multiple kinase pathways regulate voltage-dependent Ca++ influx and migration in oligodendrocyte precursor cells. J Neurosci. 2010;30:6422–6433. doi: 10.1523/JNEUROSCI.5086-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ming GL, Song HJ. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 34.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Cav1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca++ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- 37.Yagami T, Kohma H, Yamamoto Y. L-type voltage-dependent calcium channels as therapeutic targets for neurodegenerative diseases. Curr Med Chem. 2012;19:4816–4827. doi: 10.2174/092986712803341430. [DOI] [PubMed] [Google Scholar]
- 38.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 39.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 40.Teng KK, Greene LA. Depolarization maintains neurites and priming of PC12 cells after nerve growth factor withdrawal. J Neurosci. 1993;13:3124–3135. doi: 10.1523/JNEUROSCI.13-07-03124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin JL, Sanz-Rodriguez C, Juhasz A, Deckwerth TL, Johnson EM., Jr Chronic depolarization prevents programmed death of sympathetic neurons in vitro but does not support growth: requirement for Ca++ influx but not Trk activation. J Neurosci. 1995;15:643–664. doi: 10.1523/JNEUROSCI.15-01-00643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 43.Gu X, Spitzer NC. Distinct aspects of neuronal differentiationencoded by frequency of spontaneous Ca++ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 44.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 45.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 46.Kaur J, Libich DS, Campagnoni CW, Wood DD, Moscarello MA, Campagnoni AT, Harauz G. Expression and properties of the recombinant murine Golli-myelin basic protein isoform J37. J Neurosci Res. 2003;71:777–784. doi: 10.1002/jnr.10547. [DOI] [PubMed] [Google Scholar]
- 47.Harauz G, Ishiyama N, Bates IR. Analogous structural motifs in myelin basic protein and in MARCKS. Mol Cell Biochem. 2000;209:155–163. doi: 10.1023/a:1007176216360. [DOI] [PubMed] [Google Scholar]
- 48.Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie YY, Jacobs E, Fisher R. Targeted ablation and reorganization of the principal preplate neurons and their neuroblasts identified by golli promoter transgene expression in the neocortex of mice. ASN Neuro. 2009;1:e00018. doi: 10.1042/AN20090038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piñon MC, Jethwa A, Jacobs E, Campagnoni A, Molnár Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 2009;587:1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 52.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dys-regulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10:565–573. doi: 10.1017/S1461145706007310. [DOI] [PubMed] [Google Scholar]
- 54.Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neuro-trophic gene expression. Ann NY Acad Sci. 2004;1025:84–91. doi: 10.1196/annals.1316.011. [DOI] [PubMed] [Google Scholar]
- 55.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 56.Craddock N, Jones I. Genetics of bipolar disorder. J Med Genet. 1999;36:585–594. doi: 10.1136/jmg.36.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampson RM, Malloy MP, Mors O, Ewald H, Flannery AV, Morten J, Porteous DJ, Muir WJ, Blackwood DH. Mapping studies on a pericentric inversion (18) (p11.31 q21.1) in a family with both schizophrenia and learning disability. Psychiatric Genetics. 1999;9:161–163. doi: 10.1097/00041444-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Van Broeckhoven C, Verheyen G. Report of the chromosome 18 workshop. Am J Med Genet. 1999;88:263–270. [PubMed] [Google Scholar]
- 59.Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004;29:2293–2302. doi: 10.1007/s11064-004-7039-x. [DOI] [PubMed] [Google Scholar]
- 60.Baruch K, Silberberg G, Aviv A, Shamir E, Bening-Abu-Shach U, Baruch Y, Darvasi A, Navon R. Association between golli-MBP and schizophrenia in the Jewish Ashkenazi population: are regulatory regions involved? Int J of Neuropsychopharmacology. 2009;12:885–894. doi: 10.1017/S1461145708009887. [DOI] [PubMed] [Google Scholar]
- 61.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Research Reviews. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 64.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 65.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–48. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 66.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]