Abstract
Objectives
Chronic patellofemoral pain (PFP) is a common orthopedic condition for which little is understood of the alterations in pain processing such as hyperalgesia, hypoesthesia, and the relationship of altered knee mechanics to hyperalgesia. We assessed pain, pressure pain thresholds (PPT), detection to light touch, and the relationship of pain and PPT’s to knee abduction angle during a stair step down task between females with and without PFP.
Methods
Twenty females diagnosed with PFP and 20 age matched healthy females participated in this study. Individuals underwent an instrumented assessment of knee mechanics during a stair step down task, PPT and detection of light touch over the center of the patella and lateral retinaculum, and PPT outside painful area over the right elbow.
Results
The PFP group had significantly lower PPT values at the patella (p = 0.02) lateral retinaculum (p = 0.001), and at the elbow (p = 0.03). There was an elevated threshold to detect light touch over the center of their patella (p = 0.04). A significant relationship between both pain (r = −0.49, p = 0.03) and PPT values (r = 0.65, p = 0.004) to the frontal plane knee angle in the PFP group which was not present in the control group (r = −0.17,p = 0.49) or in the elbow (r = −0.009, p = 0.972).
Discussion
These results suggest that PFP is characterized by an increase in both localized and centralized pain sensitivity which is related to movement mechanics. Thus, PFP has both biomechanical, nociceptive components as well as inferred aspects of altered central sensitization.
Keywords: pressure pain thresholds, hyperalgesia, knee abduction, anterior knee pain, females
Introduction
Patellofemoral pain (PFP) is a common orthopedic condition that accounts for up to 15 – 43% of injuries that military recruits suffer from and 25 – 40% of all knee injuries reported to sports medicine clinics per year [1–4]. The condition is characterized by diffuse pain over the peripatellar and retropatellar regions of the patella that is provoked with activities such as squatting, climbing and descending stairs, walking, and running. Unfortunately, PFP often becomes chronic with up to 91% of patients reporting continued pain at 4–18 year follow ups and is associated with a reduction in functional abilities [5–7]. Interestingly, women are two times more likely than men to develop PFP with pain commonly first reported in adolescence [4, 8, 9]. While PFP is typically associated with younger patients, emerging evidence suggests that having PFP earlier in life is linked to the later development of patellofemoral osteoarthritis [10]. Collectively, conservative estimates put the annual cost to treat PFP in the United States alone at 8.3 billion dollars [11]. Despite the high incidence, chronicity, and cost of treating PFP, little is still understood of the pain physiology associated with the condition.
The prevailing theory is that PFP originates from excessive patellofemoral contact stress and/or maltracking of the patella [12]. For example, a cadaveric simulation showed increasing the Q-angle or knee abduction angle resulted in more focal patellofemoral contact stress over the lateral aspect of the patella [13]. Similar mechanics of a greater knee abduction angle (tibia moving away from the body resulting in a more valgus knee position) during stair step down task have been observed in females with PFP [14]. This may stretch the lateral retinaculum attached to the patella compressing the patellofemoral joint irritating nociceptors in both the subchondral bone and retinaculum, which over time may result in pain, sensitization and subsequent hyperalgesia [9]. While speculated upon, the link between greater knee abduction angles, pain and hyperalgesia, is completely untested in patients with PFP. Further, to date, only one paper has assessed localized hyperalgesia in patients with PFP, finding lower pressure to pain thresholds (PPT) as compared to healthy participants at the patellofemoral joint [9]. While informative, this study did not test the degree of generalized hyperalgesia which has been reported in other chronic orthopedic conditions such as osteoarthritis [15, 16]. In addition, hypoesthesia as measured by an elevated threshold to detect light touch has been reported in several pain conditions including PFP, neuropathic pain, knee osteoarthritis, and capsaicin-induced pain [17–19]. Hypoesthesia likely reflects altered spinal processing of sensory input [15, 19]. Whether both hypoesthesia and hyperalgesia are both localized to site of pain (knee) and more generally outside the site of pain (elbow) extends to conditions such as PFP is unknown. Thus, we hypothesized that individuals with PFP would have lower PPT measurements both at the knee and elbow, and that their threshold to detect light touch would be increased as compared to healthy participants. We also hypothesized that frontal plane knee angles during a single leg step down task would be correlated with pressure pain thresholds and self-reported greater pain in the PFP group but not the control group.
Materials and Methods
Female between the ages of 18–45 were recruited for this study. The study was approved by the universities institutional review board and before inclusion into the study all subjects provided written informed consent. Individuals were recruited from local medical practices and from advertisements in the local community. Potential subjects with PFP were evaluated by a licensed physical therapist to determine if they qualified for the study. Participants were included in the PFP group if they reported pain of at least 3 out of 10 on the numeric pain rating scale. During the evaluation the participant had to report pain either during retropatellar\peripatellar palpation or pain during the patellar compression test. In addition, the subject could not report pain with palpation along the infrapatellar fat pad, patellar tendon/ligament, nor could have pain emanating from these structures during resisted knee extension. Also the examiner ruled out possible ligamentous, tendon and internal derangement during the evaluation through such tests as the Lachman test, McMurray test, and anterior drawer. Participants from the PFP group were also excluded if they had any previous elbow, back or lower extremity surgeries. Subjects in the control group were excluded if they reported any of the above conditions. In addition, at the time of evaluation the participant recorded their maximum pain level within the past week on a 10 point numeric pain scale as well as the duration of their symptoms. Subjects in the PFP group also filled out the lower extremity functional index which has been shown to be a valid and reliable measure in those with PFP [20]. The maximum score is 100 (no limitations) with lower scores indicting greater impairments [20]. On the day of testing subjects reported any medication to control their pain that they were taking. Lastly, if the participant had bilateral symptoms the more painful patellofemoral joint was assessed.
Quantitative sensory testing
First, participants were seated in a quiet room in semi reclined position with their knees flexed to 40°. The patient’s patella was marked on the center of patella, and the lateral retinaculum. The bilateral patellofemoral joints were assessed and data from the contra-lateral limb reported among those subjects who did not have bilateral symptoms. The location of the center of the patella was determined by measuring the midpoint in the medial-lateral and superior to inferior direction and determining the center. From the center location we then measured laterally to the border of the patella and placed a second mark 2 cm lateral to this position to assess the lateral retinaculum. The center of the patella and lateral retinaculum were chosen based off previous work showing little regional differences in pressure pain thresholds across the patella [9]. The two locations represented an assessment directly over the patella and one of the peripatellar regions. To help minimize the risk of the subject guessing the application of the stimuli for either PPT or sensory testing, the inter-stimulus interval was varied randomly by the tester in a range of 4–20 seconds. Following previously established procedures light touch threshold detection was then performed [17]. We used a standard set of Semmes-Weinstein monofilaments (1.65g – 6.65 g) (North Coast Medical, Inc, Gilroy, CA USA). Participants were instructed to close their eyes and reply “Yes” when they could detect the light touch. Monofilaments were tested in ascending order starting at 2.36g with testing continuing until they were able to detect three out of four trials at a given monofilament level. In our pilot testing (n = 12) we found good reliability with this method of testing (interclass correlation coefficient = 0.88). Next, using the same previously marked areas the participants PPT levels were then assessed. The PPT testing included a remote site that was located 3 cm inferior from the right lateral epicondyle of the elbow. Using a 1cm2 probe pressure was applied at 40 kPa/s Medoc (Medoc, Durham, NC, USA) perpendicular to the skin. Participants were instructed to press a stop button when the sensation changed from pressure to the first instance of pain. Two trials per site were taken and then averaged. These techniques have been previously shown to have good interrater reliability with interclass correlation coefficients ranging from 0.70 – 0.94 [21, 22].
Motion Analysis
Participants then completed the instrumented gait analysis. First, markers were placed bilaterally on the acromion process, iliac crest, L5S1, anterior superior iliac spines, and greater trochanter. Markers were also placed unilaterally on the medial and lateral femoral condyles, head of fibula, tibial tuberosity, medial and lateral malleolus, as well as the 1st and 5th metatarsal heads and bases. Rigid shells containing a cluster of four tracking markers were also secured to the posterior aspect of the thigh and shank. We have previously reported excellent intra-rater reliability (interclass correlation coefficient = 0.91, stand error of measurement = 0.97) in measuring frontal plane knee motion using this configuration of markers [23]. Knee abduction is defined as the tibia abducting relative to the femur resulting in a valgus position of the knee. All participants wore laboratory shoes, New Balance WR662 (New Balance, Brighton, MA, USA). A standing calibration trial was collected after which a hip motion trial was performed for the purposes of establishing the hip joint center [24]. The subjects then performed 3 single leg step downs off of a 20.32cm riser. The individuals kept the limb with PFP on the box flexing the knee till the heel of the non-involved limb touched the floor. The control subjects used the same leg as the PFP subjects they were matched too. Three dimensional marker trajectories were recorded at 200Hz with a 10 camera motion analysis system (Motion Analysis Corp, Santa Rosa, USA).
Post processing of the data was done with Visual 3D software (C-motion, Germantown, MD, USA) to filter the data, identify the functional hip joint center and calculate the joint angles. The marker trajectory data was filtered at 8Hz and force data at 35Hz using a 4th order Butterworth low pass filter. Joint angles were calculated as the distal segment relative to the proximal segment with an x-y-z (medio-lateral, antero-posterior, vertical) Cardan angle sequence. After which custom Labview code (National instruments, Austin, Texas) was used to extract the frontal plane knee angle (tibia relative to the femur) from the involved limb from when the opposite heel touched the ground. In this coordinate system knee abduction is negative and knee adduction is positive. Each subject’s joint angular values were calculated as the average of the three trials. Outcomes were summarized (mean ± sd) by group. Comparisons of outcomes were performed using two-sample t-tests for unequal variances; a two-sided significance level of 0.05 was used. Correlation analyses with scatterplots were used to investigate relationships between outcomes; these were completed by individual groups. Pearson product moment correlation coefficients were then determined.
Results
There were a total of 20 individuals in the PFP group (23.2 ± 5.6 yrs, 67.2 ± 9.3 Kg, 1.64 ± 0.09 M) and 20 in the control group (22.7 ± 5.0 yrs, 60.5 ± 8.0 Kg, 1.65 ± 0.06 M). Subjects in the PFP group were significantly heavier (p = 0.03), but were similar in height (p = 0.61 and age (p = 0.85). However, the greater weight was not correlated to pain (r = 0.055, p = 0.82) within the PFP group. Also, none of the subjects reported any current pain medication beyond the occasional use of non-steroidal anti-inflammatory drugs. Participants in the PFP group reported pain for 3.4 ± 4.4 years with a maximum pain intensity of 5.8 ± 2.0 on the numeric pain rating scale. The PFP subjects also reported a pain rating of 54.4 ± 11.4 on the lower extremity functional index. Due to equipment malfunction PPT values were not recorded on two participants in the PFP group.
To evaluate if there was hyperalgesia at the site of pain we tested PPT over the patella and lateral retinaculum of the knee. We show that the PFP group had significantly lower PPT over both the center of the patella (p = 0.02) and over the lateral retinaculum (p < 0.00) when compared to healthy controls (Table 1). We also report that there was not a statistically significant difference between the involved limb to the non-involved limb in those subjects with only unilateral symptoms (n = 15) at either the lateral retinaculum (p = 0.17, 243.1 ± 114.4 involved vs. 296.6 ± 156.8 kPa un-involved) or the center of the patella (p = 0.68, 321.3 ± 185.4 involved, vs 322.5 ± 176.0 kPa uninvolved).
Table 1.
Comparison of variables of interest: Pressure pain thresholds, threshold to detect light touch (monofilament) and knee adduction angle between the PFP group and healthy group. Knee adduction angles are positive and knee abduction angles are negative.
| Variables | PFP group | Normal group | p-value |
|---|---|---|---|
| Pressure Pain Threshold Center of Patella (kPa) | 321.3 ± 185.4 | 478.3± 226.8 | 0.02 |
| Pressure Pain Threshold Lateral Retinaculum (kPa) | 243.1 ± 114.4 | 462.3± 284.8 | 0.00 |
| Pressure Pain Threshold Right Elbow (kPa) | 179.7 ± 68.1 | 304.0± 232.0 | 0.03 |
| Monofilament Center of Patella (g/mm2) | 3.9 ± 0.6 | 3.6± 0.4 | 0.04 |
| Monofilament Lateral Retinaculum (g/mm2) | 3.9± 0.55 | 3.7± 0.33 | 0.27 |
| Knee Adduction Angle (Degrees) | 7.7± 4.2 | 12.36± 5.8 | 0.00 |
To determine if there was a widespread increase in pain sensitivity in those with PFP we tested PPTs over the forearm on the right side. We found a significant reduction in the PPT in the PFP group as compared to the control subjects (p = 0.03).
To test if there were changes in sensation, we tested thresholds to light touch. We found that the PFP group had significantly higher thresholds to light touch over the center of the patella (p = 0.04) but not the lateral retinaculum (p = 0.27) (Table 1). Additionally, there was no difference between the involved and un-involved knee at either the lateral retinaculum (p = 0.82, 3.9 ± 0.55 involved, vs 4.0 ± 0.74 g/mm2 uninvolved) or at the center of the patella (p = 0.82, 3.9 ± 0.6 involved vs 3.9 ± 0.75 g/mm2 uninvolved).
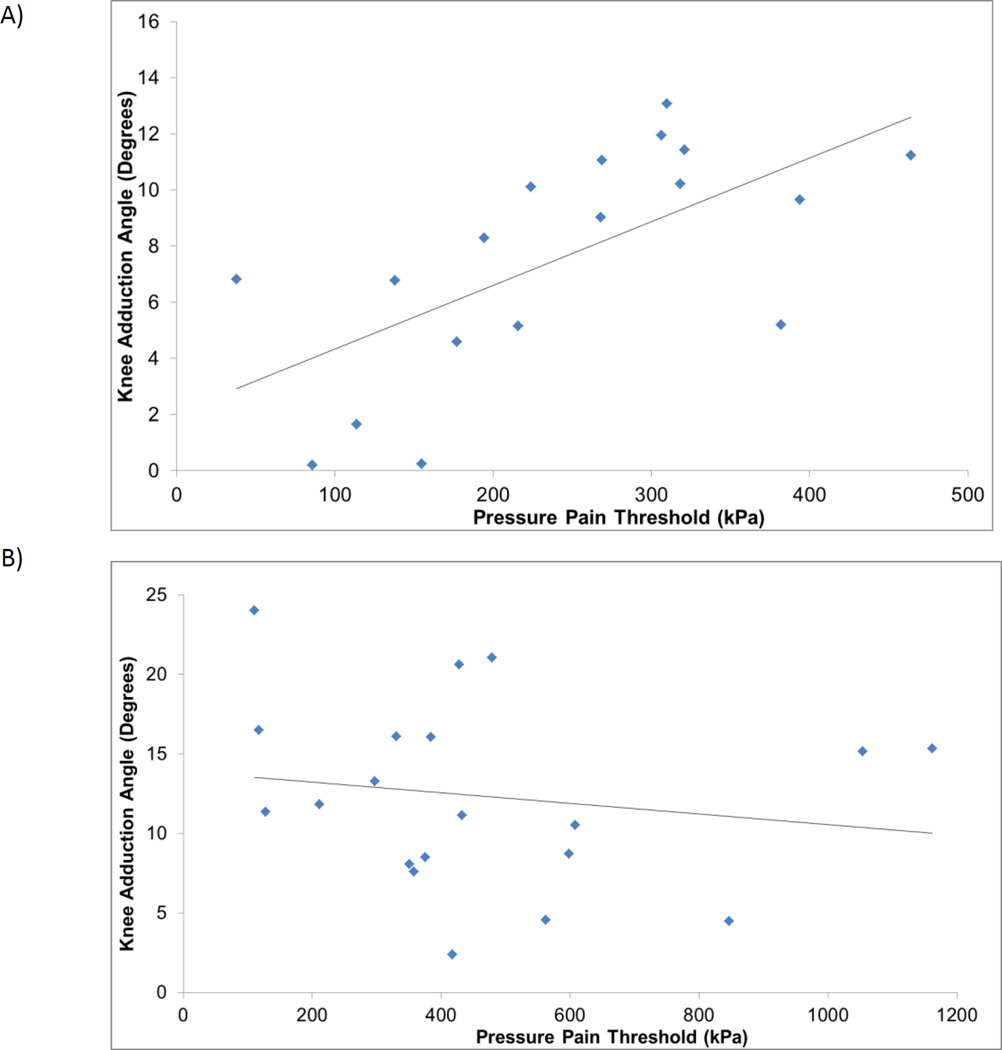
To assess if those in the PFP cohort had altered biomechanics at the knee joint we examined the knee frontal plane angle during a function task. We show that PFP group squatted in a significantly less adducted position (Table 1). We then examined the relationship between PPT, pain, and the frontal plane knee angle to determine if there was a relationship between altered biomechanics and pain. The maximum reported pain levels in the past week was associated with greater knee abduction angle in the PFP cohort (r = −0.49, p = 0.03). Similarly, greater relative knee abduction was associated with lower lateral retinaculum PPT values in the PFP group (r = 0.65, p = 0.004) but not the control group (r = −0.17,p = 0.49) (Figure 1). Similar results were found when comparing the center of the patella to knee adduction\abduction angle with a significant relationship in the PFP group (r = 0.53, p = 0.023) that was not found in the control group (r = −0.250,p = 0.288). Lastly, we found no association between PPT of the lateral elbow to the knee adduction\abduction angle in either the PFP (r = −0.009, p = 0.972) or control group (r = −0.47, p = 0.844)
Figure 1.
The relationship between pressure pain threshold to frontal plane knee angle in the PFP group (A) and the control group (B) greater knee adduction is positive and greater knee abduction is negative.
Discussion
The purpose of this study was to define the differences in localized and generalized hyperalgesia, the degree of hypoesthesia and the relationship of hyperalgesia and pain to knee frontal plane angle in females with PFP. We found that compared to the control group females with PFP had lower PPT’s both locally at the patellofemoral joint and at a remote location (elbow). They also had a significantly impaired ability to detect light touch over the center of the patella. Lastly, a smaller knee adduction angle during a single leg step down in the PFP group was related to a lower PPT at the knee and greater self-reported pain intensity. This relationship did not exist in the control group or to the PPT at the elbow. These results suggest that the pain response in PFP extends beyond a localized increase in pain and that a relationship exists between altered movement mechanics and increased nociceptive input.
Females with PFP in this study had lower PPT values at the knee. The lower PPT at the knee are in agreement with a previous study that focused on adolescents with PFP as well as several studies in patients with knee osteoarthritis [9, 15, 16]. We have also shown for the first time a significant relationship of lower PPT values to frontal plane knee angles during a step down task in the PFP group which was not observed in the control participants (Figure 1). The observed localized hyperalgesia may be due to activation and sensitization of group III and IV afferent nociceptors as the result of either greater compression of the subchondral bone or strain on the lateral retinaculum, potentially as the result of the knee moving towards knee abduction [25, 26]. Alternatively, greater localized pain and pain sensitivity may contribute to altered movement patterns. Further prospective, longitudinal studies are needed to identify causality in these reported relationships.
The lower PPT values over the right forearm suggest that the PFP group may also be experiencing more generalized hyperalgesia. To date, there have been no reports of this phenomenon among patients with PFP but our results are similar to other conditions that have inferred enhanced central excitability from generalized hyperalgesia such as knee osteoarthritis, chronic lower back pain and whiplash injuries [16, 28, 29]. Alternatively, due to the cross sectional nature of this study, we cannot rule out that some individuals had a heightened predisposition towards greater central sensitivity to the painful response. The greater generalized hyperalgesia could be due to a barrage of the nociceptive input from the peripheral tissues such as an inflamed or damaged joint capsule or subchondral bone resulting in increased excitability of neurons in the nociceptive pathways in the central nervous system [25, 29–33]. However, we found no relationship between PPT at the elbow and the frontal plane knee angle during the squat. This would suggest that alterations in knee mechanics most likely not a significant contributor to generalized hyperalgesia. Additional research is needed isolating possible factors of the generalized hyperalgesia within this population. The generalized hyperalgesia seen within this cohort would suggest that they are more sensitive to painful stimuli and may explain why in part many individuals continue to experience pain up to 14 years after completing initial course of therapy [7].
Our finding of hypoesthesia within the PFP group agrees with a previous paper and provides further support of altered sensory pathways in the PFP cohort [34, 35]. Hypoesthesia, is believed to be due to altered central processing in either the spinal cord through inhibition of the Aβ fibers or at the cortical level through suppression non-nociceptive sensory neurons [19, 25]. The end result may be manifested as both hyperalgesia and hypoesthesia over the injured region [19, 25]. Interestingly, we did not find hypoesthesia along the lateral retinaculum only at the center of the patella. A previous report has highlighted the potential for local adaptations in the nerves innervating the patella in chronic PFP [36]. However, whether the previously reported adaptations match the affected sensory distribution pathways over the patella has yet to be determined.
This study is not without several design constraints and limitations. First we choose to focus only on females with PFP since the incidence and prevalence is higher and as such these results may not generalize to males [4]. Due to study constraints and time, we were unable to assess if distal hyperalgesia was present as has been reported in a past paper focusing on females with PFP, or assess, additional body segments [9]. In addition, we were not able to control for whether individuals had taken pain medications on the day of their evaluation (although, none of the subjects reported any medications beyond occasional use of non-steroidal anti-inflammatory medication.) Several other studies on other chronic knee pain conditions have reported similar design constraints with the use of pain medication [15, 17, 18, 34]. Also, while we were able to define a difference in knee angles between groups, we are unable to directly measure the patellofemoral joint mechanics due to soft tissue artifact inherent at this joint. While the relationship between frontal plane knee angle and lower PPT values is intriguing, due to the cross sectional design we are unable to establish a cause and effect relationship. Also, measurements of pain during repeated single leg step downs would have led to a more direct comparison of the relationship of pain during activity than subjects reporting worst pain in the past week. Due to study design constraints we were not able to blind the assessor of PPT and sensation from the group assignment. The assessor was, however, blinded as to the results of the kinematic collection and the research participant made the determination of their PPT and detection to light touch. These initial results support the need for larger randomized studies. Lastly, we observed greater variability within the control group’s response than the PFP (Table 1). The larger standard deviation in the control group may be attributed to a higher tolerance to pressure among some subjects that was not present in the PFP group.
Conclusion
We found that females with PFP had significantly lower PPT both locally at the knee and remote location (the right forearm), suggesting both localized and generalized hyperalgesia. In addition, they had reduced capacity to detect light touch at the patella. Lastly, we found a significant relationship between frontal plane knee motion and PPT values in the PFP group but not the control group. These results provide some of the first evidence that alterations in biomechanics directly relate to hyperalgesia, and pain. These intriguing findings suggest that potentially females with PFP have greater centralization of their pain sensitivity. These results suggest the need for additional larger prospective observational studies to define the time course for these changes and better delineation of what alterations are occurring first.
Supplementary Material
Acknowledgements
This work was supported by a research grant from the Orthopedic Section of the American Physical Therapy Association. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000117 as well as the National Institute of Arthritis and Musculoskeletal and Skin Diseases K23AR062069. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors have no conflict of interests to report.
References
- 1.Kannus P, Aho H, Jarvinen M, Niittymaki S. Computerized recording of visits to an outpatient sports clinic. Am J Sports Med. 1987;15:79–85. doi: 10.1177/036354658701500112. [DOI] [PubMed] [Google Scholar]
- 2.Thijs Y, Van Tiggelen D, Roosen P, De Clercq D, Witvrouw E. A prospective study on gait-related intrinsic risk factors for patellofemoral pain. Clin J Sport Med. 2007;17:437–445. doi: 10.1097/JSM.0b013e31815ac44f. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a Sports Injury Clinic. Br J Sports Med. 1984;18:18–21. doi: 10.1136/bjsm.18.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2009:725–730. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7-year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64:393–400. [PubMed] [Google Scholar]
- 6.Kannus P, Natri A, Paakkala T, Jarvinen M. An outcome study of chronic patellofemoral pain syndrome. Seven-year follow-up of patients in a randomized, controlled trial. J Bone Joint Surg Am. 1999;81:355–363. doi: 10.2106/00004623-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford) 2003;42:380–382. doi: 10.1093/rheumatology/keg093. [DOI] [PubMed] [Google Scholar]
- 8.Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66:685–693. doi: 10.1302/0301-620X.66B5.6501361. [DOI] [PubMed] [Google Scholar]
- 9.Rathleff MS, Roos EM, Olesen JL, Rasmussen S, Arendt-Nielsen L. Lower mechanical pressure pain thresholds in female adolescents with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2013;43:414–421. doi: 10.2519/jospt.2013.4383. [DOI] [PubMed] [Google Scholar]
- 10.Utting MR, Davies G, Newman JH. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12:362–365. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Pal S, Besier TF, Draper CE, Fredericson M, Gold GE, Beaupre GS, Delp SL. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30:927–933. doi: 10.1002/jor.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, Earl-Boehm JE, Davis IS, Powers CM, McConnell J, Crossley KM. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014;48:411–414. doi: 10.1136/bjsports-2014-093450. [DOI] [PubMed] [Google Scholar]
- 13.Huberti HH, Hayes WC. Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66:715–724. [PubMed] [Google Scholar]
- 14.Nakagawa TH, Moriya ET, Maciel CD, Serrao AF. Frontal plane biomechanics in males and females with and without patellofemoral pain. Med Sci Sports Exerc. 2012;44:1747–1755. doi: 10.1249/MSS.0b013e318256903a. [DOI] [PubMed] [Google Scholar]
- 15.Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology (Oxford) 2012;51:535–543. doi: 10.1093/rheumatology/ker343. [DOI] [PubMed] [Google Scholar]
- 16.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. The Journal of Pain. 2003;4:203–211. doi: 10.1016/s1526-5900(03)00557-1. [DOI] [PubMed] [Google Scholar]
- 18.Jensen R, Hystad T, Kvale A, Baerheim A. Quantitative sensory testing of patients with long lasting Patellofemoral pain syndrome. Eur J Pain. 2007;11:665–676. doi: 10.1016/j.ejpain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Geber C, Magerl W, Fondel R, Fechir M, Rolke R, Vogt T, Treede RD, Birklein F. Numbness in clinical and experimental pain--a cross-sectional study exploring the mechanisms of reduced tactile function. Pain. 2008;139:73–81. doi: 10.1016/j.pain.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79:371–383. [PubMed] [Google Scholar]
- 21.Wylde V, Palmer S, Learmonth ID, Dieppe P. Test–retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage. 2011;19:655–658. doi: 10.1016/j.joca.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Persson ALBCBH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. Journal of Rehabilitation Medicine (Taylor & Francis Ltd) 2004;36:17–27. doi: 10.1080/16501970310015218. [DOI] [PubMed] [Google Scholar]
- 23.Noehren B, Manal K, Davis I. Improving between-day kinematic reliability using a marker placement device. J Orthop Res. 2010;28:1405–1410. doi: 10.1002/jor.21172. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz MH, Rozumalski A. A new method for estimating joint parameters from motion data. J Biomech. 2005;38:107–116. doi: 10.1016/j.jbiomech.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Courtney CA, Kavchak AE, Lowry CD, O'Hearn MA. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther. 2010;40:818–825. doi: 10.2519/jospt.2010.3314. [DOI] [PubMed] [Google Scholar]
- 26.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33:639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 27.Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2011;45:691–696. doi: 10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- 28.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden AM, Radanov BP. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17:306–315. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di Stefano G, Radanov BP, Curatolo M. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med. 2004;5:366–376. doi: 10.1111/j.1526-4637.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 30.Dieppe PA. Relationship between symptoms and structural change in osteoarthritis: what are the important targets for therapy? The Journal of Rheumatology. 2005;32:1147–1149. [PubMed] [Google Scholar]
- 31.Martindale JC, Wilson AW, Reeve AJ, Chessell IP, Headley PM. Chronic secondary hypersensitivity of dorsal horn neurones following inflammation of the knee joint. Pain. 2007;133:79–86. doi: 10.1016/j.pain.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Neugebauer V, Lucke T, Schaible HG. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J Neurophysiol. 1993;70:1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- 33.O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11:415–420. doi: 10.1016/j.ejpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Jensen R, Kvale A, Baerheim A. Is pain in patellofemoral pain syndrome neuropathic? Clin J Pain. 2008;24:384–394. doi: 10.1097/AJP.0b013e3181658170. [DOI] [PubMed] [Google Scholar]
- 35.Voerman VF, van Egmond J, Crul BJP. Elevated detection thresholds for mechanical stimuli in chronic pain patients: Support for a central mechanism. Arch Phys Med Rehabil. 2000;81:430–435. doi: 10.1053/mr.2000.3777. [DOI] [PubMed] [Google Scholar]
- 36.Wojtys EM, Beaman DN, Glover RA, Janda D. Innervation of the human knee joint by substance-P fibers. Arthroscopy. 1990;6:254–263. doi: 10.1016/0749-8063(90)90054-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.