Abstract
Purpose
In murine and human hyperoxaluric conditions, macrophages can be seen surrounding renal calcium oxalate (CaOx) crystal deposits. We hypothesize that macrophages play a role in degrading and destroying these deposits and investigated inflammatory response and phagocytic mechanisms when macrophages are exposed to human kidney stones and inorganic crystals.
Materials and Methods
Human monocytes were differentiated into resting, fully-differentiated macrophages by treating with recombinant human M-CSF or GM-CSF for 6 days. After confirming phenotype by flow cytometry, macrophages were exposed for 20 hours to fragments of sterile human CaOx stones or CaOx crystals. Crystal uptake was determined, and supernatant cytokine and chemokine profiles were analyzed using antibody arrays. qRT-PCR was used to validate mRNA profile expression.
Results
Under direct-vision fluorescent microscopy, activated human macrophages were noted to surround both stone fragments and synthesized crystals and destroy them in a step-by-step process that involved clathrin-mediated endocytosis and phagocytosis. An inflammatory cascade was released by macrophages, including chemokines CCL2, CCL3, interleukin-1 receptor antagonist (IL-1ra), complement component C5/C5a and IL-8. The response patterns to stone and crystal material was dependent on macrophage phenotype and activation status.
Conclusions
In our in vitro study, macrophages differentiated with M-CSF displayed a greater ability to phagocytize crystal deposits than those treated with GM-CSF. Following clathrin-mediated endocytosis, macrophages released a number of cytokines crucial for inflammatory immune response, suggesting that tissue macrophages play an important role in preventing kidney stone disease by removing and digesting interstitial renal crystal deposits.
Keywords: Nephrolithiasis, Macrophage, Calcium oxalate, Monocyte chemoattractant protein-1
INTRODUCTION
Nephrolithiasis is a common disease with recurrence rates up to 50% within the first five years after initial stone episode.1 Calcium-containing stones represent about 80% of all cases, occurring as pure calcium oxalate (CaOx) or mixed with calcium phosphate (CaP). Inorganic crystalline material such as CaOx can induce strong immune responses in human macrophages – responses that are directly controlled by crystal chemistry.2 Macrophages including renal tissue macrophages scavenge dead cells through phagocytosis and are critical to maintaining healthy kidneys.3–5 In addition, experimental studies in hyperoxaluric rodents show that renal macrophages frequently surround CaOx crystals that have migrated into the interstitium.6–9 Studies in a mice model of hyperoxaluria and CaOx nephrolithiasis led to the conclusion that the migration of macrophages is associated with crystal phagocytosis which is accomplished via inflammation related genes.10
When tissue is damaged due to injury or infection, inflammatory CCR2+ monocytes are recruited from the circulatory system and differentiate into macrophages while migrating into the affected tissue. Local production of chemokines is critically important for attraction of these monocytes, in particular CCL2 (or MCP1) which serves as a natural ligand for CCR2. When these responses are not well controlled (i.e. over-recruitment of neutrophils), inflammatory macrophages and neutrophils produce reactive oxygen species and bioactive lipids (prostanoids, leukotrienes) that can cause significant local tissue damage. Therefore, maintaining the balance between pro-inflammatory and anti-inflammatory macrophages in affected tissue has a major impact on the progression and resolution of chronic diseases. We explored their role in crystal inflammatory response and phagocytosis by exposing human macrophages to kidney stones and inorganic crystals.
MATERIALS AND METHODS
Stones and Crystals
Kidney stones, 3–5 mm in size, were obtained from six patients undergoing kidney stone surgery after Institutional Review Board approval from University of Florida, Gainesville, FL. (IRB # 431-06). All participants provided written informed consent. Stones were verified to be >95% (CaOx) monohydrate using Fourier transform-infrared spectroscopy. Whole kidney stones were decontaminated in 70% ethanol, crushed into small pieces using ethanol-pretreated ceramic pestle and mortar in biosafety cabinet under sterile conditions. Crushed stones were kept overnight under UV light before using in experiments. Calcium oxalate monohydrate was purchased from ACROS Organics. In some experiments, inorganic crystals of CaOx were synthesized in our laboratory as previously described.11 Briefly, 0.5 mM Na2C2O4 5 was mixed with 5 mM CaCl2·2H20 in a buffer containing 90 mM Tris-HCl (pH 7.4) and 10 mM NaCl (all from Aldrich-Sigma). The solution was incubated at 25° C overnight, and CaOx crystals were re-suspended in methanol. After second centrifugation at 2000 g for five minutes, methanol was discarded and crystals were air-dried in sterile conditions in a biosafety cabinet. CaOx crystals were labeled according to manufacturer’s protocol with fluorescent dye Q-Dot using Qdot® 655 ITK™ Carboxyl Quantum Dots (Life Technologies).
Preparation of Human Macrophages
Human buffy coat samples were obtained from LifeSouth with the approval of Institutional Review Board at the University of Florida. Peripheral blood mononuclear cells (PBMC) from blood samples were separated by Lymphoprep (Accu-Prep, 1.077 g/ml, Oslo, Norway) gradient density centrifugation described previously.12 The PBMCs were washed twice with 10 ml of PBS, and red blood cells were lysed using ACK lysing buffer (BioWhittaker, Walkersville, MD). Monocytes were purified from PBMC using the MACS method (Miltenyi Biotec) according to the manufacturer’s instructions. Briefly, cells were incubated with beads conjugated with anti-CD14 beads and positively selected using LS columns (Miltenyi Biotec). 95% of recovered cells expressed monocyte marker CD14. Monocytes were differentiated into macrophages over a 7 day period as described before.13 Cells were seeded in 24- or 6-well culture plates (1× 106 cell/ml) in complete RPMI 1640 culture medium and treated either with 20 ng/ml of recombinant human M-CSF or GM-CSF (R&D Systems, Minneapolis, MN) at day 0 and day 3.
Preparation of Human Dendritic Cells
Purified blood monocytes were cultured in RPM-1640 medium supplemented with 10 & FBS, antibiotics, HEPES, recombinant human GM-CSF (20 ng/ml, R&D Systems) and IL-4 (20 ng/ml, R&D Systems) in 24-well tissue culture plates (Corning-Costar, Corning, NY) at 1.0 × 106 cells/ml in a 2 ml final volume. Cells were incubated for 6–7 days at 37 °C, 5% CO2 in a fully humidified incubator. On days 3 and 5 fresh culture medium and cytokines were added to the cultures.
Cytospins
One hundred microliters of each sample were added to the sample port of a cytospin slow absorption-pad chamber, with 100 ml PBS with 20% FBS added to Shandon EZ single cytofunnels. Cell suspensions were centrifuged at 500 or 1000 rpm for 5 minutes in a Shandon cytocentrifuge. Slides were immediately fixed and stained with Wright-Giemsa solution (Fisher Scientific).
Macrophage Exposure to Stone Fragments and Crystals
Macrophages were exposed to stone fragments, and CaOx crystals for up to 72 hours. Stone fragments ranged in size from 10 microns to 400 microns. CaOx crystals were 5–20 microns in size. Macrophages were regularly examined. Supernatants were collected after 24 hours of exposure and processed as described previously.
Proteome Antibody Array profiling
Macrophages were cultured in the presence of human kidney stone pieces, calcium oxalate, and calcium phosphate or in plain culture medium (control) at humidified CO2 incubator at 37° C for twenty hours. Then cell culture supernatants were collected, filtered, stored at −80° C. The human cytokine and chemokines proteome arrays were used to measure 36 cytokines (C5/C5a, CD154, G-CSF, GM-CSF, CXCL1, CCL1, CD54, IFN-γ, IL-1α, IL-1β, IL1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-16, IL-17, IL-17E, IL-23, IL-27, IL-32α, CXCL10, CXCL11, CCL2, MIF, CCL3, CCL4, PAI-1, CCL5, CXCL12, TNFα, and sTREM-1) and 32 chemokines (CCL21, CCL28, CXCL16, Chemerin, CXCL5, CCL26, CX3CL1, CXCL1, CCL14, CCL1, IL-8, IL-16, CXCL10, CXCL11, lyphotactin, CCL2, CCL7, CCL22, midkine, CXCL9, CCL3, CCL4, CCL15, CCL20, CCL19, CSCL7, CCL18, CSCL4, CCL5, CXCL12, CCL17, and CXCL17) respectively (R&D Systems, Minneapolis, MN).
mRNA qRT-PCR
Total RNA was collected from the cell pellet and extracted with TRI reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. CCL2, CCL3, CCL4, CCL22, IL-1Rα, IL-6, IL-8, S100a9, and GAPDH levels were analyzed by qRT-PCR. mRNA qRT-PCR was performed using the TaqMan High-Capacity cDNA Reverse Transcription Kit, TaqMan Fast Advance PCR Master Mix, and TaqMan mRNA assay primers (Applied Biosystems, ?city state?). All reactions were analyzed using 7900HT Fast Real-Time PCR System (Applied Biosystems). Relative expression of mRNA were determined by the ΔΔCT method where the cycle threshold (CT) values, corresponding to the PCR cycle number at which fluorescence emission reaches a threshold above baseline emission, were determined for the mRNA expression relative to untreated controls. Analyses were performed using JMP pro version 10 (SAS, Cary, NC). P-test was used to evaluate significance. P values less than 0.05 were considered significant.
Flow Cytometry and Imaging Analysis
Flow cytometric analysis of macrophages was performed as follows. To block Fc receptors, 106 cells were incubated for 15 min at 4° C with anti-CD16/CD32 mAbs. Cells were then incubated for 30 min on ice in 50 μl of PBS with 1 μg of relevant fluorochrome-conjugated or matched isotype control antibodies. The expression of macrophage markers was assessed using the fluorochrome-conjugated monoclonal antibodies from Biolegend. Cytometry data were acquired with a FACS Calibur flow cytometer (BD Biosciences) and analyzed with CXP software (Beckman Coulter). Results were expressed as the percentage of positive cells and mean fluorescence intensity. Macrophage uptake of stones/crystals was evaluated using EVOS Fluorescent imaging microscope (Life Technology).
Statistical Analysis
The statistical significance between values was determined by the Student t test. All data were expressed as the mean ± SD. Probability values ≥ 0.05 were considered non-significant. Flow cytometry data and microscope pictures shown are representative of at least two separate determinations.
RESULTS
Human Macrophages Internalize the Calcium Oxalate Crystals
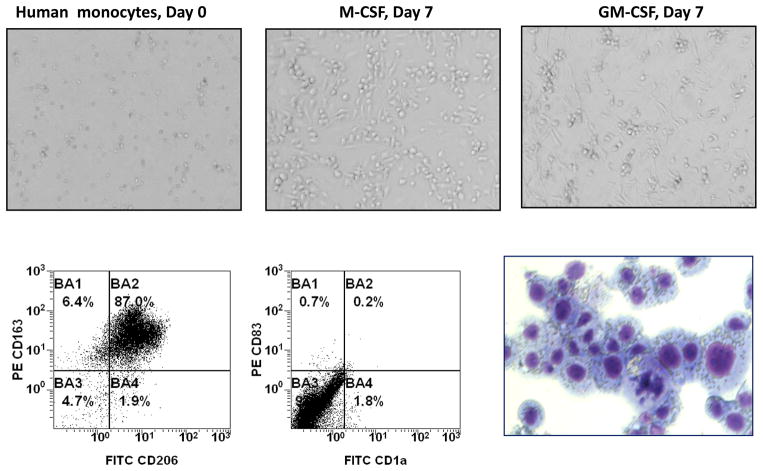
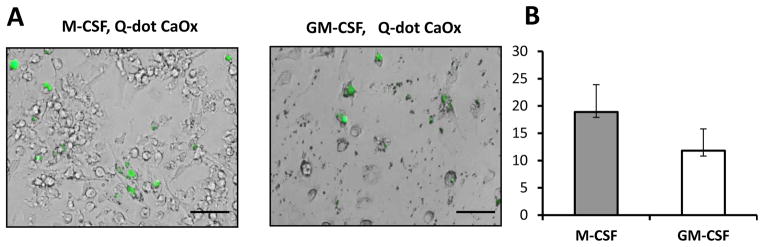
Mature macrophages are easily recognized under microscope because of their typical elongated shape and high adhesiveness to the plastic (Figure 1, upper panel). Macrophage immune phenotype was confirmed using flow cytometry (Figure 1, lower panel). Differentiated macrophages acquired macrophage markers CD163 and CD206, but did not express dendritic cell markers CD1a or CD83. In order to examine the ability of mature human macrophages to uptake CaOx crystals, we prepared the CaOx crystals, labeled them with fluorescent dye q-DOT and then added fluorescent-labeled CaOx to the macrophages in various concentrations: 0.1 mg/ml; 0.2 mg/ml; 0.5 mg/ml and 1 mg/ml. The presence of crystals in macrophages was evaluated using fluorescent imaging system. As shown in Figure 2, both M-CSF and GM-CSF-induced macrophages were able to internalize CaOx crystals.
Figure 1. Monocyte to macrophage differentiation.
A. Blood monocytes were isolated from healthy donors using magnetic beads and, subsequently, differentiated toward macrophages by culturing in the presence of M-CSF or GM-CSF for six days. Macrophage morphology was confirmed by microphotographs (upper panel, magnification 20x), flow cytometry and cytospin (lower panel, magnification 100x). Specifically, M-CSF-differentiated macrophages were collected on day seven, stained with fluorochrome-conjugated monoclonal antibodies against CD206, CD163, CD83, CD1a markers and analyzed by flow cytometry. Portion of cells was used for preparation of cytospins following by fixation and staining with hematoxylin-eosin.
Figure 2. Both M-CSF and GM-CSF- induced macrophages are able to uptake fluorescein-labeled calcium oxalate crystals.
Calcium Oxalate (CaOx) crystals were prepared and chemically conjugated with fluorescent q- Dot in our laboratory as described in Materials and Methods. Human macrophages were cultured in complete culture medium in the presence of q-Dot-labeled CaOx (0.2 mg/ml) for twenty four hours and then were analyzed by fluorescent microscopy. Representative images (A) and quantification (B) are shown. Percentage of macrophages containing internalized fluorescein-labeled CaOx is shown as average means ± SD (n=3). Scale bar = 100 micron.
Human Macrophages Are Able to Internalize Small Fragments of Natural Kidney Stones
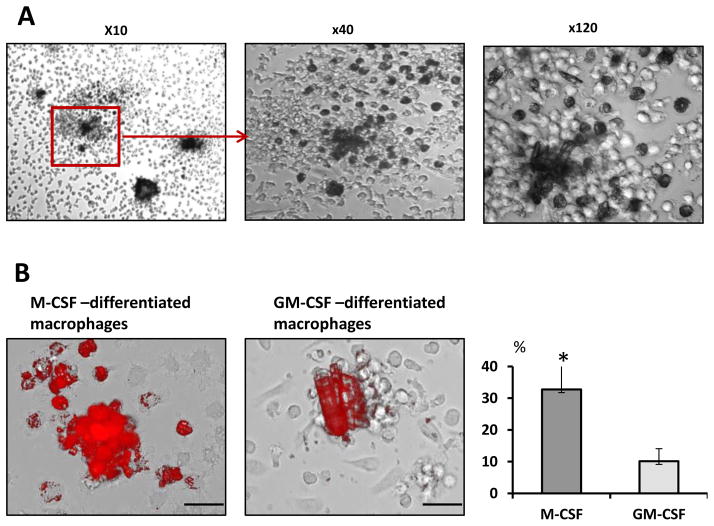
In order to examine the ability of human macrophages to internalize naturally formed calcium oxalate kidney stones obtained from patients, we added to the M-CSF-induced macrophages the crushed and decontaminated kidney stone fragments. Within 24 hours kidney stone fragments were surrounded by macrophages. After 72 hours of exposure stone fragments up to 200 μm across were eventually disintegrated. These stones/crystals were visibly being internalized by the macrophages leading to their gradual destruction while larger than 200 μm stones were more resistant to the macrophage-mediated clearance. Internalized stones/crystals appeared as dark spots (Figure 3A). Moreover, the intracellular presence of engulfed stone pieces in macrophages was easily visualized by fluorescent microscopy. Macrophage uptake of human kidney stone fragments could be observed with fluorescent light microscope due to autofluorescence of the kidney stone fragments (Figure 3B, upper panel). Indeed, some calcium salts well known to be visibly fluorescent under exposure to the fluorescent light.14 We next investigated whether monocytes that differentiated in the presence of GM-CSF also could similarly to M-CSF-differentiated macrophages exhibit the efficient kidney stone clearance. Results presented in Figure 3B (lower panel) demonstrate that GM-CSF-differentiated macrophages display much weaker ability to disintegrate and uptake natural kidney stones as compared to the M-CSF-differentiated macrophages.
Figure 3. M-CSF but not GM-CSF stimulates in vitro kidney stones clearance by human macrophages.
A. Blood monocytes were isolated from healthy donors using magnetic beads and, subsequently, differentiated toward macrophages by culturing in the presence of M-CSF or GM-CSF for six days. On day six natural kidney stone pieces were added to the macrophage cultures. Forty eight hours later macrophage uptake of stones/crystals was evaluated using fluorescent imaging microscope.
B. Mature human macrophages (day 6) were exposed to the kidney stones. Light microscope photographs were taken forty eight hours after addition of crystals. Dark spots in macrophage’s cytoplasm indicate mineral uptake. Representative images and quantification are shown. Percentage of macrophages containing phagocytized fluorescent material is shown as average means ± SD. Scale bar = 50 micron. All experiments were repeated three times. Student t-test was performed between treated and untreated. P-values less than 0.05 considered significant.
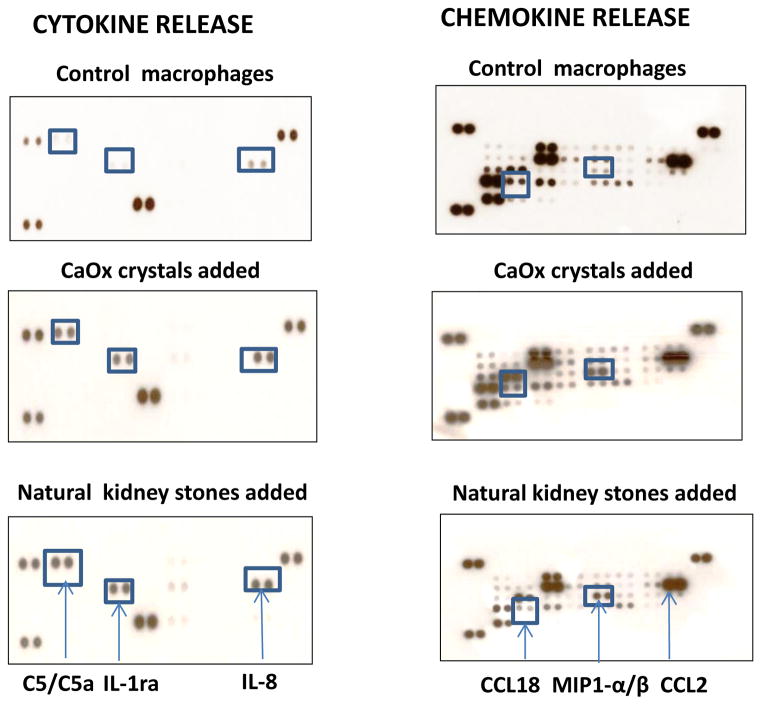
Kidney Stone/Crystal Exposure And Internalization Stimulates Cytokine And Chemokine Production In Macrophages
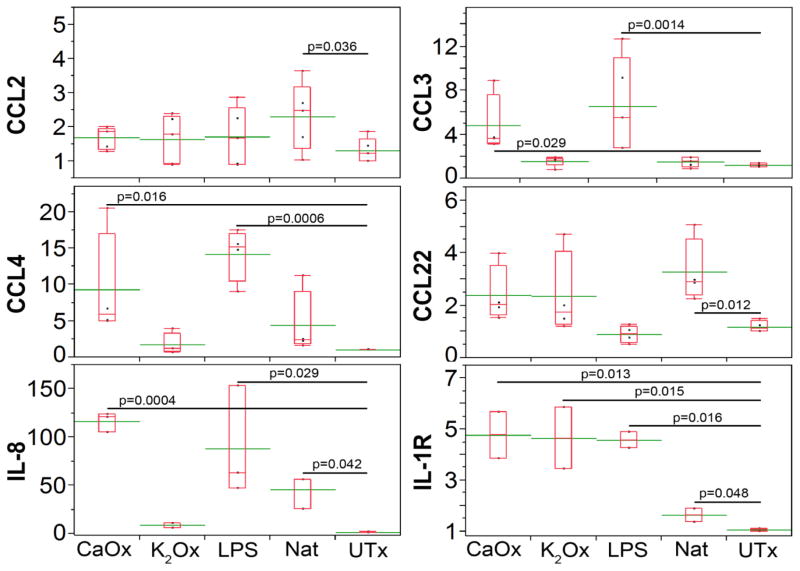
A recent study demonstrated that inorganic crystalline materials are able to stimulate cytokine response in human macrophages and dendritic cells.2 The magnitude of the response and range of secreted cytokines depends on crystal’s chemistry. To assess the ability of natural human kidney stones to induce secretion of cytokines and chemokines in human macrophages, we employed the Proteome Profiler Antibody Arrays that allow detecting multiple proteins simultaneously. As shown in Figure 4 (left panel), 24-hour exposure of macrophages to the natural kidney stones stimulated production of interleukin-1 receptor antagonist (IL-1ra), complement component C5/C5a and IL-8. Similar cytokine response pattern was detected when synthetic CaOx crystals were added to the macrophage cultures. These results were confirmed by qRT-PCR (Figure 5). Using the same approach, we also assayed chemokines secreted in response to stones/crystals. As can be seen in Figure 4 (right panel), both natural kidney stones and CaOx crystals stimulated production of chemokines CCL3/CCL4 (MIP 1α/β) and CCL2 (MCP), but secretion reduced CCL18.
Figure 4. Macrophages release of chemokines and cytokines in response to kidney stones and calcium oxalate crystals.
Monocytes were isolated from blood of “healthy” donor using magnetic beads and differentiated into macrophages by culturing with M-CSF for 6 days in six-well cell culture plates. On day six, macrophages were exposed to natural kidney stones or calcium oxalate crystals or were left untreated (control). Cells were cultured in complete culture medium at 37° C in humidified CO2 incubator for twenty four hours. Cell-free culture supernatants were collected and stored at −80° C. The presence of cytokines and chemokines in supernatants was evaluated using human Chemokine and Cytokine Proteome Arrays obtained from R&D Systems. Experiments were repeated twice.
Figure 5. Natural stones and crystals stimulate cytokine and chemokine gene expression in macrophages.
On the sixth day, macrophages were exposed for 18 hours to natural kidney stones (Nat), calcium oxalate (CaOx, 0.78 mM), potassium oxalate (K2Ox, 0.78 mM), E. Coli O55:B5 lipopolysaccharide (LPS, 1 μg/ml), and untreated (UTx). Taqman Real-time PCR (Applied Biosystems) was used to determine expression of CCL2, CCL3, CCL4, IL-8, and IL-1ra. Relative fold change was calculated using ddCt method relative to UTx. All genes of interest were normalized to GAPDH. Real time PCR was performed on ABI 7900HT Fast Realtime PCR system. Experiments were done in triplicates and repeated twice. Green line through box plots represents the mean. Student t-test was performed between treated and untreated. P-values less than 0.05 considered significant.
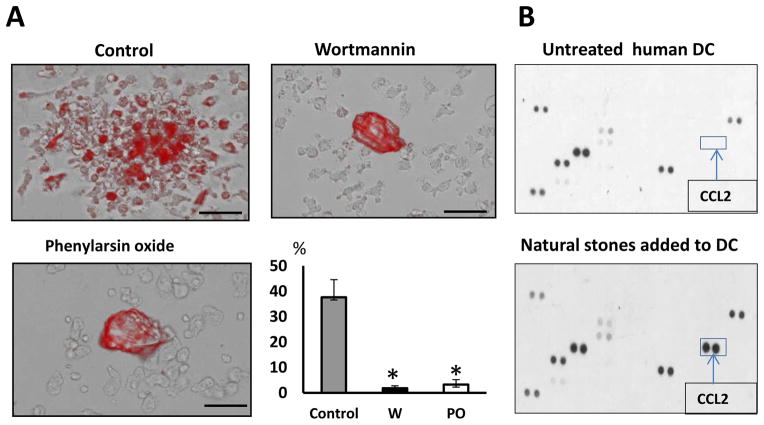
Both Clathrin-Dependent Endocytosis And Phagocytosis Are Involved In Macrophage-Mediated Clearance Of Kidney Stones. We next investigated the mechanism responsible for internalization of kidney stones in human macrophages. Macrophages are professional phagocytes that able to engulf the large structures such as apoptotic cells and pieces of cell debris through phagocytosis. Nevertheless, the major route for endocytosis in most mammalian cells is clathrin-mediated endocytosis.15 Therefore, to clarify the roles of these endocytic pathways in kidney stones uptake by macrophages, we first pre-treated the M-CSF-differentiated macrophages with specific inhibitors: phenylarsine oxide and wortmannin (Sigma-Aldrich), which inhibit clathrin-dependent endocytosis and phagocytosis, respectively 16 and then added the natural kidney stones to the pre-treated cells. The presence of internalized stone/crystal material in macrophage’s cytoplasm of was examined forty eight hours later using fluorescent microscopy (Figure 6A). Interestingly that cytokine response of human dendritic cells to the kidney stones is much weaker (Figure 6B) as these cells exhibit poor kidney stone- clearing activity (data not shown). Collectively, obtained results indicate that both inhibitors of endocytosis affect the engulfment of naturally-produced kidney stones by macrophages.
Figure 6. Kidney stones clearance by macrophages is mediated through phagocytosis and clathrin-dependent endocytosis.
A. Monocyte to macrophage differentiation was facilitated by the addition of M-CSF. On day 6, inhibitors of phagocytosis and clathrin-dependent endocytosis were added to macrophages one hour before adding of naturally-produced kidney stones. Images were taken 48 hours after addition of stones/crystals to the macrophages. Representative images and quantification are shown. Percentage of macrophages containing phagocytized fluorescent material is shown as average means ± SD (n=3). Scale bar = 100 micron. Student t-test was performed between treated and untreated. P-values less than 0.05 considered significant.
B. Human dendritic cells were generated from blood monocytes as described in section Materials and Methods. On day 7, dendritic cells were exposed to natural kidney stones or were left untreated (control). Cells were cultured in complete culture medium at 37° C in humidified CO2 incubator for twenty four hours. Cell-free cell culture supernatants were collected and stored at −80° C. The presence of chemokines in cell supernatants was evaluated using human Chemokine and Cytokine Proteome Array.
DISCUSSION
Calcium oxalate (CaOx) crystals and apatitic calcium phosphate (aCaP) are frequently seen in human urine as bi-products of normal mineral excretion.17 Interstitial CaOx depositions are typically seen in patients with primary or enteric hyperoxaluria. This type of CaOx is associated with extensive inflammation,18 while interstitial aCaP deposition alone is not.19
Animal model studies have shown that interstitial CaOx deposits are frequently surrounded by macrophages and multinucleated giant cells.8, 9, 20 In our experimentally induced hyperoxaluria studies, rapid CaOx induction results in crystals that are typically moved through the proximal and distal tubules with the filtrate.6, 7 However, some CaOx crystals stop moving and become deposited within the tubular lumens. Over time, these crystals could be seen within the renal interstitium surrounded by the macrophages, and eventually, the kidneys become completely crystal free.7 This CaOx crystal deposition appears to start with crystal binding to the tubular epithelial cells and/or growth too large to pass the tubular system. Crystals are then either endocytosed by the renal epithelial cells and transferred to the interstitium or the overlying epithelium is destroyed, causing the retained crystals to be overgrown by neighboring epithelial cells and become incorporated in the renal interstitium.21–23 Exposure of renal epithelial cells to CaOx crystals results in the increased synthesis of osteopontin, bikunin, heparin sulfate, monocyte chemoattractant protein 1, and prostaglandin E2, which are known modulators of biomineralization and calcification.24–26
In the present study, we demonstrate that human macrophages are capable of phagocytizing and eroding naturally-produced calcium oxalate crystals. Macrophages surround stones/crystals and then step-by-step engulf kidney stone fragments and degenerate them. Uptake of kidney stone fragments or crystals by macrophages stimulates the release of specific array of chemokines and cytokines, including chemokines CCL2 (monocyte chemotactic protein-1, MCP-1), CCL3 (MIP-1α), interleukin-1 receptor antagonist (IL-1ra), complement component C5/C5a and IL-8 (CXCL-8). It is interesting to note that the cytokine/chemokine response of human dendritic cells to the kidney stones differs from macrophage’s response and only CCL2 could be detected in DC’s supernatant using proteome array. Importantly, dendritic cells were unable to provide clearance of naturally-formed kidney stones.
Local production of chemokines, and particularly CCL2, is critically important for attraction of inflammatory CCR2+ monocytes to the site of inflammation or injury, as CCL2 serves as a natural ligand for chemokine receptor 2 (CCR2). In addition to the inflammatory/immune cells such as macrophages and dendritic cells, CCL2 also can be produced by epithelial kidney cells in response to CaOx or excess of oxalate ions.24 Since catalase treatment reduced CCL2 expression in epithelial cells, oxidative stress induced by CaOx may be involved in the regulation of CCL2 production. Interestingly that CCL2 is also implicated in other renal diseases such as glomerulonephritis. Thus, administration of anti-CCL2 antibodies in a model of glomerulonephritis reduces infiltration of macrophages and T cells, reduces crescent formation, as well as scarring and renal impairment.
It is well known that the local production of chemokines and cytokines in response to specific stimuli leads to shaping of inflammatory/immune response and promotes recruitment of certain inflammatory or immune cells to the site of inflammation. Obtained results suggest that digesting of kidney stones by the macrophages prompts them to recruit more phagocytes such as macrophages, neutrophils and immature dendritic cells (CCL2, CCL3, IL-8) and, perhaps, shape up the “healing”, anti-inflammatory response through neutralizing pro-inflammatory cytokine IL-1 alpha/beta (via IL-1ra).
Depending upon the phenotype and activation status macrophages may exhibit both pro-inflammatory and anti-inflammatory functions. Our data indicate that anti-inflammatory M-CSF-induced human macrophages (in presence or absence of IL-4) are able to disintegrate and uptake, (at least in vitro), kidney stones. On the contrary, pro-inflammatory GM-CSF-induced macrophages successfully internalized calcium oxalate crystals but at the same time exhibited much weaker activity against larger pieces of natural kidney stones. Such discrepancy in GM-CSF and M-CSF-induced macrophages could be explained by the differential expression of receptors in these cell subsets required for efficient phagocytosis. Furthermore, we suggest that kidney stone-clearing activity of M-CSF-induced macrophages may represent one of the “healing” functions of anti-inflammatory macrophages. Gene array profiling studies of the kidneys of CSF-1 deficient hyperoxluric mice showed disordered expression of anti- inflammatory genes and increase in CaOx crystal deposition indicating a critical role for CSF-1 signaling.27
It was previously hypothesized,9, 10, 27, 28 the kidney may actively remove interstitial crystal deposits through a cellular mechanism that includes a “sterile inflammation”. Our present and previous data suggest that enhanced production of certain cytokines/chemokines such as CCL2 by macrophages and epithelial cells in response to CaOx is an important part of such mechanism that involves several steps: a) recruitment of CCR2+ monocytes; b) in situ differentiation of recruited blood monocytes into “healing” anti-inflammatory macrophages and c) subsequent macrophage-mediated clearance of CaOx deposits. However, the increased recruitment of monocytes from peripheral blood to the site of CaOx-induced inflammation in kidney won’t guarantee the macrophage-mediated clearance of CaOx deposition, because it requires specific local cytokine milieu that promotes differentiation of recruited monocytes into “healing” but not pro-inflammatory macrophages. In addition, recruited blood monocytes could differentiate in situ into dendritic cells, which are not able to remove CaOx deposits and might further accelerate the kidney damage through CaOx-induced NLRP3 inflammasome activation and enhanced IL-1beta production.29 The third scenario that could potentially prevent the macrophage-mediated removal of interstitial crystal deposits and promote accumulation of crystals and development of nidus would involve a lack of local inflammatory signals, i.e. chemokines such as CCL2 required for recruitment of blood monocytes.
CONCLUSION
Data obtained in our study clearly indicate that mature human macrophages are able to up-take and digest the kidney stone pieces and CaOx crystals suggesting that these inflammatory cells could play an important physiologic role in preventing kidney stone disease by removing and digesting stones in kidney tissue. During this process macrophages release inflammatory proteins that include specific set of cytokines and chemokines. We show also that macrophage colony-stimulating factor appears to be important for formation and function of kidney stone-clearing macrophages and phagocytosis and clathrin-mediated endocytosis involved in this process.
Acknowledgments
Funding: This study was supported by NIH Grant T32 DK 094789 (J.V., S.R.K.) and by AUA Care Foundation Rising Star in Urology Research Award in conjunction with Astellas Global Development, Inc. (B.K.C.)
Key of Definitions for Abbreviations
- CaOx
Calcium oxalate
- M-CSF
Macrophage colony stimulating factor
- GM-CSF
Granulocyte macrophage colony stimulating factor
- CCL
Chemokine ligand
- CXCL
Chemokine (C-X-C) motif) ligand
- CCR
Chemokine receptor
- CD
Cluster of differentiation
- IL
Interlukin
- IL-R
Interleukin receptor
- MCP
Monocyte chemoattractant protein
- mRNA
messenger RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an american college of physicians clinical guideline. Ann Intern Med. 2013;158:535. doi: 10.7326/0003-4819-158-7-201304020-00005. [DOI] [PubMed] [Google Scholar]
- 2.Williams GR, Fierens K, Preston SG, et al. Immunity induced by a broad class of inorganic crystalline materials is directly controlled by their chemistry. J Exp Med. 2014;211:1019. doi: 10.1084/jem.20131768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Q, Wang Y, Harris DC. Pathogenic and protective role of macrophages in kidney disease. Am J Physiol Renal Physiol. 2013;305:F3. doi: 10.1152/ajprenal.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SR, Finlayson B, Hackett RL. Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol. 1982;107:59. [PMC free article] [PubMed] [Google Scholar]
- 7.Khan SR, Shevock PN, Hackett RL. Acute hyperoxaluria, renal injury and calcium oxalate urolithiasis. J Urol. 1992;147:226. doi: 10.1016/s0022-5347(17)37202-6. [DOI] [PubMed] [Google Scholar]
- 8.McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- 9.de Water R, Noordermeer C, van der Kwast TH, et al. Calcium oxalate nephrolithiasis: effect of renal crystal deposition on the cellular composition of the renal interstitium. Am J Kidney Dis. 1999;33:761. doi: 10.1016/s0272-6386(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 10.Okada A, Yasui T, Fujii Y, et al. Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. J Bone Miner Res. 2010;25:2701. doi: 10.1002/jbmr.158. [DOI] [PubMed] [Google Scholar]
- 11.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 12.Kusmartsev S, Su Z, Heiser A, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 13.Sierra-Filardi E, Nieto C, Dominguez-Soto A, et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- 14.Glasser J, Fonda GR. The fluorescence of calcium phosphate double salts. J Am Chem Soc. 1938;60:722. [Google Scholar]
- 15.Rappoport JZ. Focusing on clathrin-mediated endocytosis. Biochem J. 2008;412:415. doi: 10.1042/BJ20080474. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- 17.Tiselius HG, Lindback B, Fornander AM, et al. Studies on the role of calcium phosphate in the process of calcium oxalate crystal formation. Urol Res. 2009;37:181. doi: 10.1007/s00240-009-0191-7. [DOI] [PubMed] [Google Scholar]
- 18.Khan SR, Finlayson B, Hackett R. Renal papillary changes in patient with calcium oxalate lithiasis. Urology. 1984;23:194. doi: 10.1016/0090-4295(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 19.Evan AP, Coe FL, Lingeman JE, et al. Insights on the pathology of kidney stone formation. Urol Res. 2005;33:383. doi: 10.1007/s00240-005-0488-0. [DOI] [PubMed] [Google Scholar]
- 20.de Water R, Noordermeer C, Houtsmuller AB, et al. Role of macrophages in nephrolithiasis in rats: an analysis of the renal interstitium. Am J Kidney Dis. 2000;36:615. doi: 10.1053/ajkd.2000.16203. [DOI] [PubMed] [Google Scholar]
- 21.Verkoelen CF, Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007;72:13. doi: 10.1038/sj.ki.5002272. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Mo L, Goldfarb DS, et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol. 2010;299:F469. doi: 10.1152/ajprenal.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan SR, Canales BK. Ultrastructural investigation of crystal deposits in Npt2a knockout mice: are they similar to human Randall’s plaques? J Urol. 2011;186:1107. doi: 10.1016/j.juro.2011.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant. 2003;18:664. doi: 10.1093/ndt/gfg140. [DOI] [PubMed] [Google Scholar]
- 25.Umekawa T, Hatanaka Y, Kurita T, et al. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol. 2004;15:635. doi: 10.1097/01.asn.0000113321.49771.2d. [DOI] [PubMed] [Google Scholar]
- 26.Khan SR, Joshi S, Wang W, et al. Regulation of macromolecular modulators of urinary stone formation by reactive oxygen species: transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol. 2014;306:F1285. doi: 10.1152/ajprenal.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi K, Okada A, Kitamura H, et al. Colony-stimulating factor-1 signaling suppresses renal crystal formation. J Am Soc Nephrol. 2014;25:1680. doi: 10.1681/ASN.2013060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Water R, Leenen PJ, Noordermeer C, et al. Cytokine production induced by binding and processing of calcium oxalate crystals in cultured macrophages. Am J Kidney Dis. 2001;38:331. doi: 10.1053/ajkd.2001.26098. [DOI] [PubMed] [Google Scholar]
- 29.Joshi S, Wang W, Peck AB, et al. Activation of the NLRP3 Inflammasome in Association with Calcium Oxalate Crystal Induced Reactive Oxygen Species in Kidneys. J Urol. 2015;193:1684. doi: 10.1016/j.juro.2014.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]