Abstract
Objective
To describe vitamin D levels and prevalence of vitamin D sufficiency, insufficiency and deficiency in a large, ethnically/racially diverse population of youth with type 1 diabetes (T1D) and type 2 diabetes (T2D) in comparison to national data and examine the associations between clinical/demographic factors and vitamin D levels.
Methods
25-hydroxy vitamin D (25OHD) levels were measured in 215 youth with T1D and 326 youth with T2D enrolled in the Pediatric Diabetes Consortium (PDC). These levels were compared with those of youth of the same age without diabetes from the 2005–2006 NHANES Survey.
Results
Vitamin D deficiency (<21 ng/mL) was present in 36% of PDC participants, and insufficiency (21–29 ng/mL) was present in an additional 34%.Thirty-six percent of age-matched youth in the NHANES Survey were vitamin D deficient and an additional 41% were insufficient. Deficiency or insufficiency varied by race/ethnicity, being highest in African-Americans (86%), intermediate in Hispanics (77%), and lowest in non-Hispanic whites (47%). Lower 25OHD levels were observed in African American and Hispanic youth, during fall and winter, and at sites in the northern United States (all p-values<0.001). Youth with T2D had significantly lower 25OHD levels than youth with T1D (p<0.001), but this difference was largely eliminated after adjusting for race/ethnicity and socio-economic status.
Conclusions
Vitamin D deficiency/insufficiency is present in a substantial proportion of youth with diabetes, particularly minorities, but the prevalence appears similar to that in youth without diabetes. Further studies are needed to examine whether youth with diabetes would benefit from vitamin D supplementation.
Keywords: vitamin D deficiency, type 1 diabetes, type 2 diabetes, pediatrics
Introduction
The classic role of vitamin D is in calcium-phosphate metabolism and bone formation and the non-classic role is as a potential anti-inflammatory and immune-modulating hormone. Vitamin D insufficiency and deficiency are common in youth (1); especially in youth with type 1 diabetes (T1D) (2–4) and type 2 diabetes (T2D) (5; 6). Proposed mechanisms of an increased prevalence of vitamin D insufficiency and deficiency include genetic inheritance (7; 8) increased BMI (9), and concurrent albuminuria with enhanced excretion of vitamin D binding protein (10) and less healthy diets in youth with diabetes. In addition to bone health, youth with diabetes may have additional benefits from being vitamin D sufficient. Past research has demonstrated that vitamin D insufficiency/deficiency in pediatric diabetes may contribute to insulin resistance (3; 5; 11; 12), poor glycemic control (13), and the development of microvascular and macrovascular complications (14–16).
In 2009, the Pediatric Diabetes Consortium (PDC) was formed with the objective of improving the care of children with diabetes through sharing of best practices, collecting outcomes data with a common database, and collectively advocating for improvements in pediatric diabetes care focused on evidence-based education. A cohort of youth with newly diagnosed T1D was enrolled in the PDC T1D NeOn registry by 7 U.S. pediatric diabetes centers between 2009 and 2011 and a cohort of youth with T2D was enrolled by 8 pediatric diabetes centers between 2012 and 2014. The large size and broad geographic distribution of patients within the two registries, as well as the differences in racial/ethnic and socio-economic characteristics between the two registries, prompted us to examine the prevalence of vitamin D sufficiency, insufficiency, and deficiency in these two groups of youth with diabetes.
Methods
Patients
This study of the PDC T1D and T2D cohorts included 215 participants with T1D and 326 with T2D who had 25OHD levels measured between April 2012 and December 2013 and who were ≥10 years old at the time of testing. Participants who were <10 and >22 years of age were excluded from this study to balance age distribution between the two cohorts. The protocols were approved by the Institutional Review Board (IRB) at each of the participating centers. Informed consent was obtained from participants older than 18 years of age and from parents of those less than 18 years of age. Assent was also obtained from participants <18 years of age as required by local IRB regulations.
On enrollment, participants in the PDC T1D NeOn registry had to be managed at one of the participating PDC centers within 3 months of diagnosis. A detailed description of the PDC T1D NeOn registry has been published previously (17). The PDC T2D registry included participants who were diagnosed with T2D using the criteria of the American Diabetes Association including being antibody negative, BMI >85th percentile for age and gender prior to diabetes-associated weight loss and at least one of the following: 1) HbA1c ≥6.5% (≥48 mmol/mol), 2) random glucose >200 mg/dL (11.1 mmol/L), 3) fasting glucose ≥126 mg/dL (7.0 mmol/L), or 4) 2-hour OGTT glucose ≥200 mg/dL (11.1 mmol/L).
Data Collection
Demographic, socioeconomic and clinical characteristics data were collected from medical records and from interviews with the patient and/or parent. Vitamin D (25OHD) levels were measured by the IDS-iSYS analyzer at the Mineral Metabolism Laboratory, Yale University, and New Haven, CT. This assay has been found to be highly accurate (18). Vitamin D deficiency was defined according to the Endocrine Society Clinical Practice Guideline as a 25OHD level < 21 ng/mL and vitamin D insufficiency was defined as 25OHD level between 21 ng/mL and 29 ng/mL (19). One hundred and seven participants were fasting at the time of the blood draw and 434 were not.HbA1clevels were measured by point of care device or local laboratory within 28 days of the collection of the vitamin D sample.
Statistical Analysis
In order to compare our results with the national population, the distribution of 25OHD levels from the NHANES survey 2005–2006 were calculated for the same age range as our sample (10–<22 years). Mean 25OHD levels, 95% confidence interval, percent of vitamin D deficiency and insufficiency were calculated from our diabetes cohort and the NHANES 2005–2006 data, and sampling weights were used to make the NHANES survey reflective of the general US population. (http://wwwn.cdc.gov/nchs/nhanes/search/nhanes13_14.aspx). Race-specific 25OHD levels were also compared.
Least squares regression was used to determine the association of demographic/clinical factors with vitamin D levels. Multivariable models were constructed using stepwise selection methods and included all factors with the p-value <0.10. Due to multiple comparisons, only factors with p-values <0.01 were considered statistically significant while factors with p-value <0.10 were included to adjust for possible confounding. Adjusted means from multivariable analysis were defined to be the least squares means (ls-means) from the regression model. Interactions among all possible pairwise combination of factors remaining in the multiple regression model were also assessed and none were found to be significant (p-value <0.01). Residual plots from the final multivariate model were evaluated for approximate normal distribution, outliers and homogeneity of variance. All reported p-values are two-sided. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
The demographic characteristics of the 215 study participants with T1D and 326 with T2D are shown in Table 1. There were substantial differences between the two cohorts with a larger percentage of racial/ethnic minorities in the T2D cohort: 29% of the T1D cohort were Hispanic or Latino and 8% were African-American compared with 59% Hispanic or Latino and 27% African-American in the T2D cohort; 46% of youth with T1D were female compared with 63% of youth with T2D. Mean age was 14.5±2.7 and 16.1±2.5 years for T1D and T2D respectively.
Table 1.
Demographic and Clinical Characteristics at Testing by Diabetes Type
| Overall | White | Hispanic | Black | |||||
|---|---|---|---|---|---|---|---|---|
| T1D N=215a |
T2D N=326a |
T1D N = 123 |
T2D N=24 |
T1D N=62 |
T2D N=191 |
T1D N=16 |
T2D N=87 |
|
| Age (years) | ||||||||
| Mean ± SD | 14.5 ± 2.7 | 16.1 ± 2.5 | 14.5 ± 2.6 | 16.5 ± 2.3 | 14.4 ± 2.8 | 16.1 ± 2.5 | 15.4 ± 3.0 | 16.2 ± 2.5 |
| Female | 99 (46%) | 206 (63%) | 47 (38%) | 15 (63%) | 31 (50%) | 116 (61%) | 12 (75%) | 61 (70%) |
| BMI (%)b | ||||||||
| Median (quartiles) | 75% (52%, 92%) | 99% (96%, 99%) | 70% (30%, 85%) | 99% (97%, 99%) | 84% (61%, 96%) | 98% (96%, 99%) | 96% (77%, 99%) | 99% (97%, 100%) |
| Diabetes Duration (years) | ||||||||
| Median (quartiles) | 3.2 (2.7, 3.7) | 2.5 (0.8, 4.7) | 3.2 (2.7, 3.7) | 3.1 (1.2, 4.6) | 3.2 (2.7, 3.7) | 2.4 (0.8, 4.6) | 3.2 (2.7, 3.7) | 2.4 (1.0, 5.4) |
| HbA1c (% [mmol/mol]) | ||||||||
| Mean ± SD | 8.5 ± 2.1 (69 ± 23) |
8.1 ± 2.6 (65 ± 28) |
8.3 ± 2.0 (67 ± 22) |
7.0 ± 1.6 (53 ± 17) |
8.5 ± 1.9 (69 ± 20) |
8.3 ± 2.7 (67 ± 29) |
8.6 ±2.6 (71 ± 28) |
8.3 ± 2.7 (67 ± 30) |
| Health Insurance | ||||||||
| Private | 115 (55%) | 86 (26%) | 89 (75%) | 12 (50%) | 13 (21%) | 30 (16%) | 7 (44%) | 30 (34%) |
| Parent Education | ||||||||
| High School or less | 77 (44%) | 222 (73%) | 22 (23%) | 14 (58%) | 45 (78%) | 148 (83%) | 9 (75%) | 50 (63 %) |
| Family Income | ||||||||
| <$25,000 | 31 (22%) | 99 (45%) | 6 (8%) | 6 (30%) | 20 (39%) | 68 (53%) | 3 (30%) | 21 (39%) |
| Site | ||||||||
| Los Angeles, CA | 59 (27%) | 83 (25%) | 9 (7%) | 1 (4%) | 38 (61%) | 75 (39%) | 8 (50%) | 3 (3%) |
| Houston, TX | 1 (<1%) | 35 (11%) | 0 | 1 (4%) | 0 | 24 (13%) | 1 (6%) | 10 (11%) |
| Stanford, CA | 25 (12%) | 26 (8%) | 13 (11%) | 0 | 9 (15%) | 21 (11%) | 0 | 1 (1%) |
| Denver, CO | 81 (38%) | 69 (21%) | 59 (48%) | 6 (25%) | 13 (21%) | 46 (24%) | 3 (19%) | 14 (16%) |
| Gainesville, FL | 20 (9%) | 16 (5%) | 18 (15%) | 6 (25%) | 0 | 2 (1%) | 1 (6%) | 7 (8%) |
| New Haven, CT | 24 (11%) | 58 (18%) | 19 (15%) | 7 (29%) | 2 (3%) | 20 (10%) | 3 (19%) | 26 (30%) |
| Ann Arbor, MI | 5 (2%) | 1 (<1%) | 5 (4%) | 0 | 0 | 0 | 0 | 0 |
| Philadelphia, PA | 0 | 38 (12%) | 0 | 3 (13%) | 0 | 3 (2%) | 0 | 26 (30%) |
| Season | ||||||||
| Winter | 38 (18%) | 66 (20%) | 16 (13%) | 3 (13%) | 15 (24%) | 37 (19%) | 3 (19%) | 23 (26%) |
| Spring | 56 (26%) | 73 (22%) | 36 (29%) | 4 (17%) | 13 (21%) | 41 (21%) | 3 (19%) | 21 (24%) |
| Summer | 55 (26%) | 92 (28%) | 33 (27%) | 12 (50%) | 13 (21%) | 49 (26%) | 8 (50%) | 22 (25%) |
| Fall | 66 (31%) | 95 (29%) | 38 (31%) | 5 (21%) | 21 (34%) | 64 (34%) | 2 (13%) | 21 (24%) |
. Number of participants with missing or unknown data by type (T1D/T2D): race/ethnicity (4/2), health insurance (5/0), parent education (39/21), family income (71/107), BMI% (121/29) and HbA1c (73/41).
The overall mean 25OHD level was 24.8 (99 % confidence interval 23.7to 25.9) ng/mL (Table 2). Vitamin D deficiency occurred in 36% of youth and vitamin D insufficiency occurred in an additional 34%, with the remaining 30% of youth having a 25OHD level above 29 ng/mL. The prevalence of vitamin D deficiency and insufficiency varied according to race/ethnicity. Deficiency was present in 14% of non-Hispanic whites, 38% of Hispanics, and 65% of African-Americans, and insufficiency was present in 33% of non-Hispanic whites, 39% of Hispanics, and 21% of African-Americans. Of note, 9% of type 2 diabetes participants indicated they were taking vitamin D supplements (data on dose were not available), mean 25OHD level in these youth was 27±9 ng/mL; these data were not available for the T1D cohort. A sensitivity analysis excluding the 9% T2D participants who were taking vitamin D supplements generated similar results (data not shown).
Table 2.
Comparing Vitamin D Levels between PDC Sample and US General Populationa
| PDC | NHANES | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (99%C.I.) | %Deficiency (99%C.I.)b |
%Insufficiency (99%C.I.)c |
N | Mean (99%C.I.) | %Deficiency (99%C.I.)b |
%Insufficiency (99%C.I.)c |
|
| All | 541d | 24.8 (23.7, 25.9) | 36 (31, 42) | 34 (28, 39) | 2496e | 24.0 (21.9, 26.1) | 36 (26, 45) | 41 (35, 47) |
| White | 147 | 31.3 (29.0, 33.6) | 14 (6, 21) | 33 (23, 43) | 683 | 27.6 (25.7, 29.4) | 18 (13, 24) | 48 (42, 55) |
| Hispanic | 253 | 23.4 (22.1, 24.7) | 38 (30, 45) | 39 (31, 47) | 880 | 20.0 (18.2, 21.9) | 56 (43, 69) | 37 (27, 46) |
| Black | 103 | 19.3 (17.1, 21.5) | 65 (53, 77) | 21 (11, 32) | 813 | 14.5 (12.3, 16.6) | 83 (74, 92) | 15 (8, 23) |
-Estimated from NHANES 2005–2006 survey results
-Deficiency defined as 25OHD level < 21 ng/mL
-Insufficiency defined as 25OHD 21–29 ng/mL
-Includes 32 subjects with other race/ethnicity and 6 subjects with missing race/ethnicity.
-Includes 120 subjects with other race/ethnicity.
From the NHANES 2005–2006 data, mean age was 15.4 years (99% confidence interval 15.0 to 15.9 years), 48% female, and median (interquartile range) BMI was 71% (40%, 91%).The national mean 25OHD level in the same age range was 24.0 (99% confidence interval 21.9 to 26.1) ng/mL, with vitamin D deficiency in 36% of youth and vitamin D insufficiency in 41%. From the NHANES database, deficiency was present in 18% of non-Hispanic whites, 56% of Hispanics, and 83% of African-Americans, and insufficiency was present in 48% of non-Hispanic whites, 37% of Hispanics, and 15% of African-Americans (Table 2). The 25OHD level from NHANES data was significantly lower than the level from PDC cohort among each race/ethnicity group (p<0.001 for all three groups).
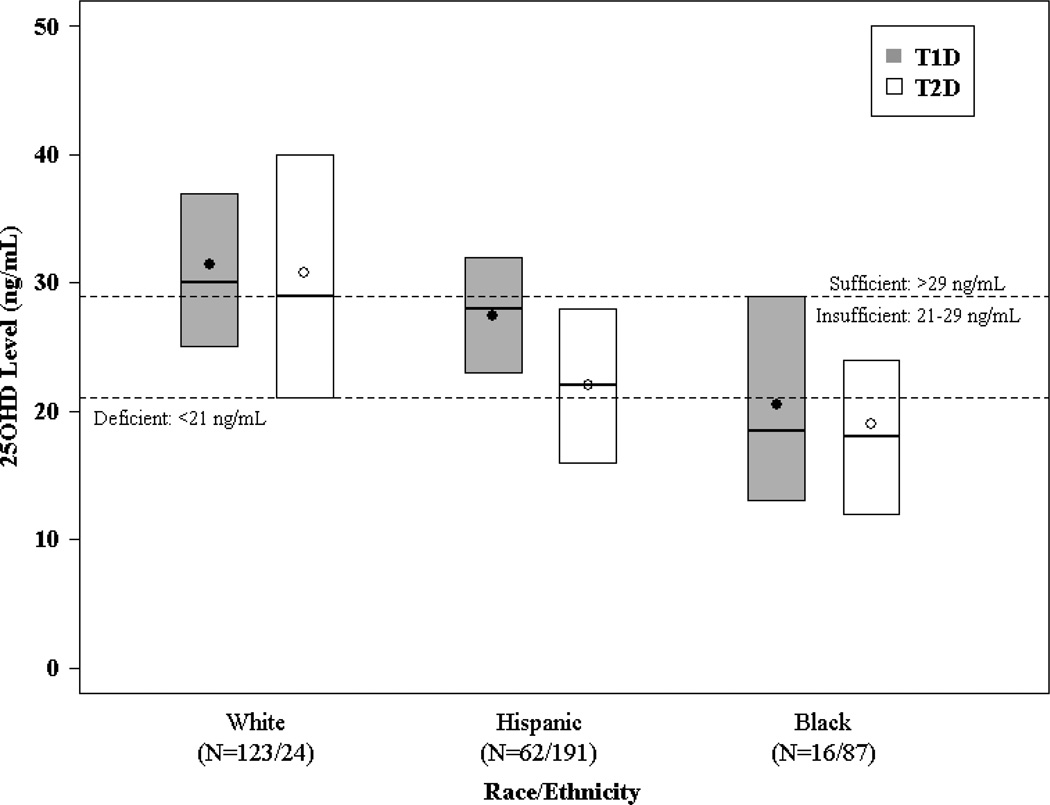
Differences in unadjusted vitamin D levels were present when youth with T1D and T2D were compared (Supplemental Table 1). However, this was largely, though not entirely, explained by differences in race/ethnicity distribution between diabetes types. As seen in Figure 1, the distribution of vitamin D levels was similar comparing non-Hispanic white T1D and T2D youth and comparing African-American T1D and T2D youth, though for both diabetes types the vitamin D levels were substantially lower in the African-American than white participants. For the Hispanic youth, the levels in T1D appeared to be slightly higher than in T2D.
Figure 1. Vitamin D Levels by Race/Ethnicity and Diabetes Type.
The bottom and top of each box denote the 25th and 75th percentiles, the line inside the box denotes the median and the dot is the mean.
In addition to race/ethnicity and diabetes type, in univariable models, lower 25OHD levels were associated with older age, female sex, BMI ≥85%, non-private insurance, lower parental education level and lower household income. In addition, 25OHD levels varied substantially among the eight study sites and by season with the highest levels in summer and lowest levels in winter. HbA1c levels and diabetes duration were not significantly associated with 25OHD levels (Supplemental Table 1). There was no statistically significant interaction between site and race/ethnicity. In multivariable analysis, only race/ethnicity (p<0.001), study site (p<0.001) and season (p<0.001) were significantly associated with 25OHD levels; diabetes type was no longer significant (p = 0.05) (Supplemental Table 1).
Discussion
In this large, multi-center study of a diverse group of youth with diabetes, we found that vitamin D deficiency and insufficiency were common (36% and 34%, respectively). In multivariate analysis lower vitamin D levels were seen in African-American and Hispanic youth, in fall/winter months, and in those living in the northern United States. When other variables were taken into account in a multivariate analysis, diabetes type was no longer significantly associated with 25OHD levels. The lower vitamin D levels seen in the African-American youth when compared to the non-Hispanic white youth in our study may be explained by lower levels of vitamin D binding protein secondary to genetic polymorphisms in the vitamin D binding protein gene (7; 8). In addition, the lower vitamin D levels in the Hispanic and African-American participants also could be explained by increased skin pigmentation since this inhibits the synthesis of cholecalciferol, the metabolic precursor of 25OH vitamin D (20). Individuals with type 1 diabetes have also been demonstrated to have enhanced urinary excretion of vitamin D binding protein when compared to non-diabetic controls (10). In this study by Thrailkill et al., participants with albuminuria had more exaggerated urinary losses of vitamin D binding protein than participants with normoalbuminuria, and therefore, this explanation is unlikely to be a major factor in our study of youth with diabetes of relatively short duration.
The prevalence of vitamin D deficiency in healthy American youth of a similar age varies between 19 and 24% when vitamin D deficiency is defined as <12 ng/mL (1). Using this cut point, only 7% (N=38) in our study had vitamin D deficiency. Although the overall level of vitamin D was similar in the NHANES and PDC groups, differences emerged when vitamin D levels were compared by race/ethnicity. When we compared our results with the 2005–2006 NHANES data from youth of the same age, the vitamin D level among each race/ethnicity group was significantly higher in our study participants (p<0.001 for all three groups). Possible explanations for higher vitamin D levels in our PDC cohort include the fact that samples were drawn in 2012–2013 compared to 2005–2006 for the NHANES cohort. It is possible that the PDC cohorts were getting more nutritional counseling as part of their diabetes education that included dietary and vitamin D supplement recommendations resulting in higher vitamin D levels.
Very few foods naturally have or are fortified with vitamin D, and therefore, the major source of vitamin D for children and adults is sun exposure. With increased sedentary behaviors occurring indoors and increased use of sunscreen to prevent skin cancer, dietary vitamin D intake and vitamin D supplements are very important contributors to the vitamin D status of youth (19). It is a limitation of our study that we did not obtain data on dietary vitamin D intake in our participants or dose of vitamin D supplements being used by our participants with type 2 diabetes. Amount of sun exposure is influenced by season of year and latitude. Indeed, in multivariate analysis of our PDC cohort, season and study site were important predictors of vitamin D levels. While previous reports have suggested that obese individuals have lower vitamin D levels because vitamin D is fat-soluble and is sequestered in body fat (9), when vitamin D levels were adjusted for race/ethnicity and study site in the multivariate analysis of our study results, the influence of differences in BMI was no longer significant. Thus in our cohorts, skin pigmentation and sun exposure appear to have a greater impact on circulating vitamin D levels than either obesity or the type of diabetes.
Prevalence of vitamin D deficiency in American youth with T1D as reported in the literature has varied from 15% – 68.5%. For example, Svoren et al. (4) defined vitamin D deficiency as ≤ 20 ng/mL and reported that 15% of 128 youth with T1D living in Massachusetts were vitamin D deficient. In 110 patients with T1D (5–65.1 years of age) living in Florida, 68.5% had a suboptimal vitamin D level ≤ 30 ng/mL (2), and 49% of youth with T1D (n=1426) followed in the SEARCH study had deficient vitamin D levels < 20 ng/mL (3). Only the Florida study included a non-diabetic control group, and they found that 25OHD levels in a group of controls were similar to children with T1D and their first degree relatives. Much of this variation in prevalence can be explained by the disparate definitions of vitamin D deficiency applied in each study, the racial/ethnic composition of the patient population, the geographic location/latitude of the study sites, and the season of the year when samples were drawn. In our study, the three variables most highly associated with vitamin D levels were race/ethnicity, study site, and season.
Reports in the literature of the prevalence of vitamin D deficiency in youth with T2D are less common. De las Heras et al. reported that 59% of 27 youth with T2D were vitamin D deficient with levels < 20 ng/mL (5). To our knowledge only Di Cesar et al. (6) compared the prevalence of vitamin D deficiency (<20 ng/mL) in T1D (n=50) and T2D (n=63), but the participants were all adults. They found that adults with T2D had more vitamin D deficiency than adults with T1D (63.5% vs. 36%). This difference persisted after adjusting for BMI and was unrelated to age, sex, or insulin treatment. Importantly, race/ethnicity were not examined in this study. In our cohort, we found that youth with T1D and T2D had a comparable prevalence of vitamin D deficiency after adjusting for site, race/ethnicity, season, gender, health insurance and family income.
Strengths of our study include the large sample size, and the racial/ethnic and geographic diversity of the participant population. In particular, this is the largest cohort of youth with T2D that have had vitamin D levels systematically measured. One weakness of our study is the lack of a control group of youth without diabetes, so we are unable to directly compare the prevalence of vitamin D deficiency in youth with and without diabetes. However, we were able to compare the prevalence in our cohorts with an aged matched population from the NHANES Study. Limitations of this comparison are the use of different vitamin D assays for each and the fact that the NHANES data was collected in 2005–2006, while ours were taken in 2012–2013. Our data is also limited by the fact that we do not have complete data on dietary vitamin D intake or dose of vitamin D supplements being used by our T1D participants.
Despite these limitations, our findings demonstrate that youth with T1D and T2D had similar vitamin D levels, after controlling for race/ethnicity. In addition, vitamin D deficiency and insufficiency were equally common in youth with and without diabetes after adjusting for race/ethnicity and socio-economic status. From these data, we conclude that the evaluation of vitamin D status should be considered in pediatric patients, especially in those with diabetes and from a racial/ethnic minority population. Additional research is needed to confirm whether youth with diabetes would have benefits, such as improved insulin resistance (3; 5; 11; 12), glycemic control (13), and prevention of diabetes related complications (14–16), from vitamin D supplementation.
Supplementary Material
Acknowledgements
The Pediatric Diabetes Consortium and its activities are supported by the Jaeb Center for Health Research Foundation through an unrestrictive grant from NovoNordisk. The University of Michigan Consortium center is supported by the Michigan Diabetes Research and Training Center from the National Institute of Diabetes and Digestive and Kidney Diseases (DK020572). J.W., C.C., P.C., and K.R. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. R.B., F.B., D.S., E.C., G.K., B.G., S.W., and W.T. researched data, contributed to discussion, and reviewed/edited manuscript. R.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. G. Klingensmith reports payment for educational consultation to Eli Lilly as the planner and participant in a CME course for the Eli Lilly Company, and also is a paid consultant for Novo Nordisk. W. Tamborlane is a paid consultant for Novo Nordisk, Bristol-Meyers Squibb, Boehringer Ingelheim, Takeda, and Janssen.
The Pediatric Diabetes Consortium Study Group:
Clinical Centers: (Listed clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Baylor College of Medicine, Houston, TX: Fida Bacha, MD (PI); Morey Haymond, MD (I); Maria J. Redondo, MD, PhD (I); Elizabeth Johnson (C); Andrene McDonald (C) (2) Children’s Hospital of Los Angeles, Los Angeles, CA: Jamie Wood, MD (PI); Brian Ichihara, BA (C); Megan Lipton, MA, CCRP (C); Marisa Cohen, MPH (C); (3) Stanford University, Stanford, CA: Bruce Buckingham, MD (PI); Breanne Harris, BS (C); Satya Shanmugham, BS (C); (4) Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Georgeanna J. Klingensmith, MD (PI); Heidi Haro, BA, BS (C); Katherine Manseau (C); (5) University of Florida, Gainesville, FL: Desmond Schatz, MD (PI); Janet Silverstein, MD (I); Michael J. Haller, MD (I); Jamie Thomas, BS (C); (6) Yale University, New Haven, CT: William V. Tamborlane, MD (I); Eda Cengiz, MD (PI); Melody Martin, CCRP (C); Amy Steffen, BA (C); Lori Carria, MS (C); Darryll Cappiello (C); (7) University of Michigan, Ann Arbor, MI: Joyce M. Lee, MD, MPH (PI); Surair Bashir (C); Ashley Eason (C); (8) Children’s Hospital of Philadelphia, Philadelphia, PA: Steven M. Willi, MD (PI); Tammy Mawson (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Crystal G. Connor, MS, MPH; Peiyao Cheng, MPH; Beth Stevens; TJ Mouse.
Footnotes
J. Wood, K. Ruedy, R. Beck, F. Bacha, C. Connor, E. Cengiz, B. Gregg, S. Willi, D. Schatz, and P. Cheng report no conflict of interest.
References
- 1.Looker AC. Vitamin D Status: United States, 2001–2006. Hyattsville, MD: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2011. [Google Scholar]
- 2.Bierschenk L, Alexander J, Wasserfall C, Haller M, Schatz D, Atkinson M. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care. 2009;32:1977–1979. doi: 10.2337/dc09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NS T, Crandell JL, Lawrence JM, King IB, Dabelea D, Marcovina SM, D'Agostino RB, Norris JM, Pihoker C, Mayer-Davis EJ. Vitamin D in youth with Type 1 diabetes: prevalence of insufficiency and association with insulin resistance in the SEARCH Nutrition Ancillary Study. Diabetic Medicine. 2013;30:1324–1332. doi: 10.1111/dme.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svoren BM, Volkening LK, Wood JR, Laffel LMB. Clinical and laboratory observation: Significant Vitamin D Deficiency in Youth with Type 1 Diabetes Mellitus. The Journal of pediatrics. 2009;154:132–134. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de las Heras J, Rajakumar K, Lee S, Bacha F, Holick MF, Arslanian SA. 25-Hydroxyvitamin D in Obese Youth Across the Spectrum of Glucose Tolerance From Normal to Prediabetes to Type 2 Diabetes. Diabetes Care. 2013;36:2048–2053. doi: 10.2337/dc12-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cesar DJ, Ploutz-Snyder R, Weinstock RS, Moses AM. Vitamin D Deficiency Is More Common in Type 2 Than in Type 1 Diabetes. Diabetes Care. 2006 doi: 10.2337/diacare.29.1.174. [DOI] [PubMed] [Google Scholar]
- 7.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England Journal of Medicine. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. The Journal Of Clinical Endocrinology And Metabolism. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [corrected] [published erratum appears in AM J CLIN NUTR 2003 May;77 (5):1342]. [DOI] [PubMed] [Google Scholar]
- 10.Thrailkill KM, Jo C-H, Cockrell GE, Moreau CS, Fowlkes JL. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? The Journal Of Clinical Endocrinology And Metabolism. 2011;96:142–149. doi: 10.1210/jc.2010-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. American Journal of Clinical Nutrition. 2011;94:225–233. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- 12.Tunc O, Cetinkaya S, Kixilgun M, Aycan Z. Vitamin D status and insulin requirements in children and adolescent with type 1 diabetes. Journal of Pediatric Endocrinology and Metabolism. 2011;24:1037–1041. [PubMed] [Google Scholar]
- 13.Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Annals of Saudi Medicine. 2010;30:454–458. doi: 10.4103/0256-4947.72265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joergensen C, Hovind P, Schmedes A, Parving H-H, Rossing P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. American Diabetes Association. 2011:1081. doi: 10.2337/dc10-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur H, Donaghue KC, Chan AK, Benitez-Aguirre P, Hing S, Lloyd M, Cusumano J, Pryke A, Craig ME. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. American Diabetes Association. 2011:1400. doi: 10.2337/dc11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, Garg SK, Rewers M. Vitamin D Deficiency and Coronary Artery Calcification in Subjects With Type 1 Diabetes. Diabetes Care. 2011;34:454–458. doi: 10.2337/dc10-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pediatric Diabetes Consortium. The Pediatric Diabetes Consortium: Improving Care of Children with Type 1 Diabetes Through Collaborative Research. Diabetes Technol Ther. 2010;12:685–688. doi: 10.1089/dia.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cluse ZN, Fudge AN, Whiting MJ, McWhinney B, Parkinson I, O'Loughlin PD. Evaluation of 25-hydroxy vitamin D assay on the immunodiagnostic systems iSYS analyser. Annals of Clinical Biochemistry. 2012;49:159–165. doi: 10.1258/acb.2011.011018. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal Of Clinical Endocrinology And Metabolism. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, Mei Z, Curtin L, Roche A, Johnson C. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 22.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Advance Data. 2004:1–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.