Abstract
Purpose
The clinical use of kurtosis imaging is impeded by long acquisitions and post-processing. Recently, estimation of mean kurtosis tensor W̅ and mean diffusivity (D̅) was made possible from 13 distinct DWI acquisitions (the 1-3-9 protocol) with simple post-processing. Here, we analyze the effects of noise and nonideal diffusion encoding, and propose a new correction strategy. We also present a 1-9-9 protocol with increased robustness to experimental imperfections and minimal additional scan time. This refinement does not affect computation time and also provides a fast estimate of fractional anisotropy (FA).
Methods
1-3-9/1-9-9 data are acquired in rat and human brains, and estimates of D̅, FA, W̅ from human brains are compared to traditional estimates from an extensive DKI data set. Simulations are used to evaluate the influence of noise and diffusion encodings deviating from the scheme, and the performance of the correction strategy. Optimal b-values are determined from simulations and data.
Results
Accuracy and precision in D̅ and W̅ are comparable to nonlinear least squares estimation, and is improved with the 1-9-9 protocol. The compensation strategy vastly improves parameter estimation in non-ideal data.
Conclusion
The framework offers a robust and compact method for estimating several diffusion metrics. The protocol is easily implemented.
Keywords: Diffusion, kurtosis, higher-order tensors, orientational sampling, fractional anisotropy
Introduction
Diffusion weighted MRI (DWI) is uniquely sensitive to tissue pathology and is widely used to investigate tissue microstructure. Standard clinical DWI approximates the water diffusion profile by a Gaussian distribution (accurate for free diffusion), when estimating the apparent diffusivity D(n̂) along a direction n̂, or the full diffusion tensor D (1,2). In tissue however, diffusion is generally non-gaussian. Therefore, diffusion kurtosis imaging (DKI), which improves on DWI by including the leading deviation from Gaussianity, is an increasingly popular method for analysis of DWI data. Along each direction DKI obtains apparent kurtosis, K(n̂) in addition to D(n̂). When K(n̂) is estimated along many directions, various metrics can be computed: mean kurtosis (MK), kurtosis anisotropy (3,4), fractional kurtosis anisotropy (5–7), and axial and radial kurtosis (3,8,9).
Numerous reports find DKI to be a valuable supplement to standard DWI. In stroked animals, MK improved infarct visualization over mean diffusivity D̅ (10), and informed early assessment of stroke due to different time-course than D̅ (11,12). In (13), histology supported DKI's enhanced sensitivity compared to DTI in detecting stroke-induced microstructural changes in gray matter (GM) and white matter (WM). Elevated MK was reported in stroke patients in (14–17). MK’s reflection of cellular changes was also seen in traumatic brain injury (TBI) (18,19) with early increases in FA (fractional anisotropy) and MK. When D̅ and FA had normalized, hyper-normal MK persisted and was associated with increased reactive astrogliosis identified by immunohistochemistry (c.f. review in (20)). MK may hold value for other neurological diseases, e.g. epilepsy (21), attention deficit hyperactivity disorder (22), chronic mild stress (23,24), gliomas (25,26), Parkinson’s disease (27,28), multiple sclerosis (29), and for the study of brain maturation (30,31). In summary, the literature suggests that DKI is a sensitive, albeit unspecific, microstructural biomarker (32) of clinical value, improving sensitivity to microstructural features resolvable with MR microscopy (33–36).
Clinically, DWI is typically used alongside other MRI techniques leading to comprehensive examination protocols. A key challenge for DKI, therefore, is to collect the required data in a clinically feasible time - for acute MRI, mere minutes. Often, DKI data analysis estimates both the diffusion tensor, D, and the rank-4 kurtosis tensor, W, corresponding to a total of 22 free parameters. This requires a substantial amount of data: hence, a typical DKI protocol acquires 30 uniformly distributed directions at 3–6 b-values from 0–3 ms/µm2 (4,37). Thus, neither scan time nor computational load is negligible, limiting exploration and clinical use of DKI.
Recently, we formulated an apparent mean kurtosis with a direct relation to the diffusion signal and the kurtosis tensor (6,38). In this framework MK is the average over all directions of the kurtosis tensor, W, and we will refer to this MK variant as the mean (of the) kurtosis tensor, MKT, or W̅. Our analysis of data from human and animal brain demonstrated strong similarity of W̅ and MK, which was also reported in (7). We further showed that W̅ is simply related to W through its trace. This allowed a rotationally invariant way of estimating W̅ from 13 images: 1 at b=0, 3 at b1=1 ms/µm2 and 9 at b2=2.5 ms/µm2 acquired using well-defined directions. We termed this the 1-3-9 scheme. Recently, 1-3-9 estimates of diffusion and kurtosis lesion size and severity were found to agree very well with conventional DKI in acute stroke in rats (39). Recent clinical findings show the method’s utility in glioma patients (40).
The 1-3-9 scheme rests on a number of approximations. These include exact realization of the prescribed directions and constant effective b-values over each shell, as well as the Gaussian description of diffusion at low b-value. Furthermore, with an estimation based on summation of a low number of log signals, there is a need to characterize the effects of Rician noise on bias and precision. Here we address all of these issues. Beginning with a determination of optimal b-values for fixed tissue and in vivo applications, we proceed by evaluating the influence of Rician noise on parameter estimation. Next, we consider errors in W̅ and D̅ originating from deviations in diffusion encoding from the prescribed b-values and directions. The nominal directions are typically unfulfilled in practice, e.g. due to interaction of imaging and diffusion gradients, so effective b-matrices deviate from the nominal ones in a directionally dependent manner. Even though this can be alleviated in principle, and b-matrices can be calculated accurately (even for advanced sequences (41,42)) hardware imperfections may still play a role so that actual encoding deviates from that prescribed by the protocol (43,44). The result is that the signal is probed on two “rugged b-shells”. Another source may be co-registration, necessitated by subject motion, requiring post hoc rotations of each volume’s b-matrix. We propose and demonstrate postprocessing methods to mitigate effects of b-matrix deviations on the accuracy of metric estimates. To circumvent the approximation of Gaussian diffusion, a strategy was employed already in (6) based on (45). Here the 1-3-9 protocol is extended so all nine directions are acquired at both non-zero b-values. This 1-9-9 method inherently estimates D̅ without presuming the Gaussian approximation at the low b-value with the benefits shown in (46), and robustly estimates DKI parameters with better accuracy and precision. Moreover, the 1-9-9 scheme enables simultaneous estimation of FA in addition to D̅ and W̅ without fitting, and the entire diffusion tensor can be estimated with e.g. constrained linear least squares.
Theory
We first recapitulate the theoretical background of the 1-3-9 method leading naturally to the derivation of the 1-9-9 scheme. A method to compensate for b-matrix imperfections is then introduced based on the 1-9-9 scheme.
In the DKI formulation, the signal reads (8):
| (1) |
where b is the diffusion weighting applied along n̂ = (nx, ny, nz) and subscripts label Cartesian components (e.g. i = x,y,z). The kurtosis tensor Wijkl is defined as in (8), W̅ is its average over all directions (S2 is the sphere):
| (2) |
Here 𝕀 is the fully symmetric isotropic tensor (47), and Tr is the trace. On this basis it was shown that W̅ may be obtained from W(n̂) along nine distinct directions, n̂(i), n̂(i+) and n̂(i−) (i=1,2,3)(6,38):
| (3) |
The nine directions are given explicitly as normalized vectors in the Supporting Materials (SM), (Supporting Table 1). In this manner the 1-3-9 method estimates W̅ from one b=0 ms/µm2 image, three images at low b1~1 ms/µm2 and the nine prescribed directions at b2~2.5 ms/µm2. Using three orthogonal b1 directions also included in the b2 shell allows a good estimation of D̅ (45). Then Eq. (3) yields W̅ from the nine directions acquired at b2. If instead all nine directions are acquired at b1 and b2, one can use Eq. (3) to form a set of two equations with unknowns D̅ and W̅ :
| (4) |
and obtain estimates for D̅ :
| (5) |
and for W̅ :
| (6) |
Because the 1-9-9 method uses more data and accounts for the kurtosis at lower b-values, it could be expected to be more robust than 1-3-9. Eqs. (5) and (6) enable a straightforward numerical analysis of the dependence on b-values and SNR, and will be used to estimate optimal b-values (see Methods). Throughout SNR is calculated as the mean signal in a homogenous region in the object imaged divided by the standard deviation of the signal in a background region, corrected for Rayleigh distribution in a standard manner.
The 1-9-9 strategy yields D(n̂) and W(n̂) along nine directions, which is sufficient to estimate FA without fitting. The log-signal with diffusion weighting b along a direction n̂ is:
| (7) |
where W(n̂j) =K(n̂j)D(n̂j)2/D̅2. With measurements at two different b-values, b1 and b2, we find
| (8) |
and
| (9) |
The fractional anisotropy (FA) (48) is defined as:
| (10) |
where λi are eigenvalues of D, overbar denotes averaging, and std and rms abbreviate standard deviation and root mean square, respectively. An FA estimate is obtained from the variance (var) of D(n̂) over the 9 sampling directions:
| (11) |
which is exact in the limit where the variance is over all directions on the sphere, and hence FA199 would approximate FA to arbitrary precision with increasingly complete sampling of the sphere. Setting D̅ = D̅199 from Eq. (5) keeps the method self-contained.
Alternatively, all elements Dij of D can be estimated by the linear least squares solution of Dijninj = D(n̂) for all nine directions n̂i. This approach would provide all of the metrics from diffusion tensor imaging, but will not be considered in depth here. However, a comparison of the FA obtained with this method is included in the results section.
If the directions or b-values deviate from the nominal ones prescribed by the 1-9-9 protocol, the basic Eq. (3) does not hold. However, if we can find weights wa (a=1…9) such that
| (12) |
with b̅ the shell-average of effective b-values and (a = 1…9) the experimental b-matrices, it is straightforward to establish the generalization of (3)
| (13) |
In practice, one cannot expect an exact solution to Eq. (12), so we approximate the weights by the least squares solution, i.e. choosing weights that minimize the squared difference between the left and right hand sides of Eq. (12). This optimization is performed independently for b1 and b2. Since Eq. (12) is a linear system of 21 equations (one for each of the independent components of bij and bijbkl) with 9 unknowns (wa), the least squares solution is found effectively with standard linear routines.
Methods
Fixed rat brain data acquisition
A rat brain was fixed as in (10). Prior to imaging, one hemisphere was washed in PBS for 24 hours to improve signal by removing excess fixative. Data was acquired using a 9.4T MRI system (Bruker Biospin, Germany) equipped with a 15 mm quadrature coil using a standard DW spin echo sequence. 15 b-values (0–3 ms/µm2 in steps of 0.2 ms/µm2) were acquired along 33 gradient directions each. These directions were a combination of a 3-dimensional 24-point spherical 7-design (11) and the nine directions identified in (6). Imaging parameters were: TE=23.3 ms, TR=4 s, δ/Δ=4/14 ms, 2 averages, SNR at b=0 >65. Fifteen image slices were acquired at a resolution of 100 µm × 100 µm × 500 µm.
Human MRI data
Human data was acquired in normal volunteers on a Siemens Trio 3T using a 32 channel head coil and a double spin echo DW EPI sequence. Separate data sets were acquired for b-value optimization and for investigation of the fast parameter estimation method. Padding was used to avoid head motion during the acquisitions.
Data set A: b-value optimization
Data consisted of 1 b=0 image and 14 shells with 33 directions at b-values of 0.2–3.0 ms/µm2. We employed the same 33 direction sampling scheme as in rat brain, except an additional 10 b=0 images were acquired for the analysis and to assess temporal stability of SNR. Image resolution was kept at 3 mm isotropic to provide optimal b-values relevant for typical acute imaging protocols. A total of 32 slices were acquired, with TR=4300 ms, TE=103 ms. The SNR was 51 at b=0 and varied approximately 5% in both GM and WM across 10 b=0 acquisitions.
Data protocol B: investigation of the fast protocols
Three subjects were scanned at a resolution of 2.5 mm isotropic. CSF suppression (FLAIR) was employed as recommended in (49). The DWI sampling was identical to data set A. Imaging parameters were TR = 7200 ms, TE = 116 ms, TI = 2100 ms, 19 consecutive slices, and all data had SNR > 39 at b=0. When showing data from protocol B we use the same subject throughout, but analysis results are reported based on whole-brain data from all three subjects.
Postprocessing
All data sets were evaluated visually for quality (artifacts and subject movement). Due to the padding around the subjects head, image registration was found to be unnecessary in all cases.
Data processing
Identical analysis was performed in human and rat brain data: Eq. (1) was fitted to the normalized data (S(b)/S(b=0)) from b=0–3.0 ms/µm2 using non-linear (and unweighted) least squares fitting implemented in Matlab®. We have previously observed a low sensitivity to initial values, which were here the same for all voxels and chosen to be neutral (e.g. zero-valued Euler angles) or non-extreme physically relevant values. The fit yielded tensors D and W providing ground truth estimates of D̅, FA, and W̅ for comparison to the rapid protocols. A method relying on only nine encoding directions may be expected to perform differently in tissues displaying different degrees of anisotropy. Therefore, our analysis investigates performance in areas of low anisotropy (mostly GM) and high anisotropy (WM). The low anisotropy range was 0.1<FA<0.3, corresponding to FA for GM in FLAIR data (50). The high anisotropy region was defined by 0.6<FA<1 corresponding to regions of uniformly ordered WM. In the 1-9-9 analysis, we calculated the metrics using Eqs. (5), (6), (11) with b-values b1 = 1 ms/µm2 and b2 = 2.4 ms/µm2.
b-value optimization
In mapping the optimal b-value pair, D̅199 and W̅199 was calculated from all combinations of b1 and b2 in data set A. Parameter estimates at each b-value pair were compared to true values and the absolute %-error on each parameter summed over the entire image plane. A penalty term of 20 %-points was added for each pixel where no estimate was obtainable (producing NaNs or Infs in matlab) with the 1-9-9 method. This served as a way to take into account overall quality of the parameter map. This was performed on both human data set A and the fixed rat brain data.
Numerical optimization of b-values
For numerical estimation of optimal b-values we use numerically generated 1-9-9 signals: for each b1,b2 combination, the voxel signal S is computed using Eq. (7). We use as input DTI and DKI tensors obtained from the fit of Eq. (1) to the human data set. Subsequently Gaussian noise with an SNR=51 as in the experiments (data set A), was added to the real and imaginary channels, the modulus operation applied, and estimates of D̅ and W̅ obtained using Eqs. (5) and (6). This operation is repeated 500 times, and mean absolute error as well as variance over noise realizations is determined and averaged over 100 voxels selected randomly from brain tissue on a single slice. The entire procedure is repeated on a 60 × 60 grid of values of (b1,b2).
Simulations of the robustness of the 1-3-9 and 1-9-9 methods to experimental inaccuracies
The ground truth tensors from one slice of a data set acquired with protocol B were used as input to the DKI model to generate 1-3-9 and 1-9-9 data sets with varying degrees of Rician noise and b-matrix perturbations.
In a first set of simulations, Rician noise propagation was investigated in one typical WM and one typical GM voxel. After generating the signal from the ground truth D and W, various levels of complex Gaussian noise was added before the modulus operation. Subsequently, the average accuracy and precision of diffusion and kurtosis metrics were assessed using 500 noise realizations. For comparison, estimation with nonlinear least squares (NLS) was also applied to signals generated from five b-values from 0 to 2.5 µm2/ms and 30 directions.
In a second set of simulations, the consequences of imperfect diffusion encoding on the rapid diffusion and kurtosis estimates were investigated. 150 pixels were chosen randomly in each tissue type, and from the underlying ground truth tensors, 1-3-9/1-9-9 diffusion signal sets were generated with varying degrees of deviation from the ideal experiment. First, gradient directions alone were allowed to deviate from the nominal directions. A range of deviations from 0–10° were simulated with 50 random phases for each direction and each deviation level. The absolute %-error from ground truth in 1-3-9 and 1-9-9 estimates was calculated for each realization and averaged over phases. The mean absolute error was then calculated over pixels. To address b-value errors, a similar simulation was performed with unperturbed directions and b-value deviations that varied randomly between the nine directions producing non-spherical ("rugged") b-value shells. Here, b-value deviation for each direction was coupled between the high and low b-value to mimic gradient hardware performance. Fifty realizations of each noise level was performed. We also evaluated the absolute %-error in the presence of both directional deviation and b-value variation. In all of these simulations, we also evaluated the efficiency of the weighted average method described in the theory section in correcting non-ideal b-matrices. The correction scheme was implemented in Matlab using the function lsqlin.
Results
Investigation of optimal b-value combination
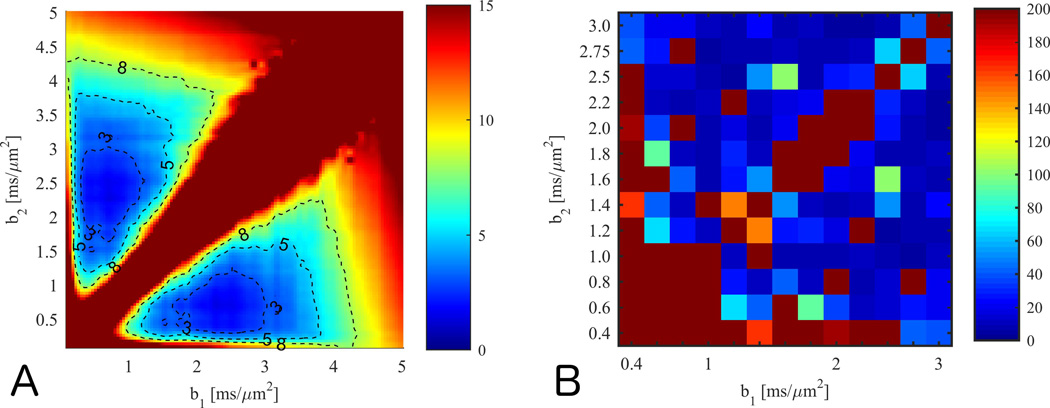
Simulation results of the sum of mean absolute error in estimates of D̅ and W̅ is shown in Fig. 1A. As expected, a high error ridge exists along the b1=b2 diagonal, but an extended region centred at (0.7,2.3) ms/µm2 has a sum of errors below 3%. The average variability (precision) in this region is about 10%, but this drops to about 4% when 10 outlier pixels are removed. The experimental b-value optimization spans a smaller b-value range and indicates the lower b-value to be larger than 1 ms/µm2, but otherwise shows similar qualitative behaviors with some outliers present (Fig. 1B). The error maps suggest that many b-value combinations produce reasonable estimates. Evidently, b1 = 1 ms/µm2 produces good estimates over an extended range of b2 values, and this is also common choice for routine estimation of D̅. Hence we use this value in the following, and recommend a b2 larger than 2.4 ms/µm2.
Fig. 1.
Panel A (B) shows simulation results of the mean absolute error on estimation of D̅ and W̅ as function of b1 and b2 in the 1-9-9 scheme. Two outlier pixels dominating the mean were removed. In panel C, combined experimental error (absolute %-error) on estimates of D̅ and W̅ using the 1-9-9 protocol compared to the values from a fit to a large data set. The minimum error occurs at high values for b2 but similar minima are present at lower b-values.
Comparing W̅ from data set A to W̅139 and W̅199 at these optimal b-values shows the fast estimates to be virtually identical and with equally strong linear correlation to W̅ (0.8 for both methods in data set A and 0.97 for both methods in the smoothed data used in (6)). We therefore recommend these b-values for both the 1-3-9 and the 1-9-9 methods. Optimization on fixed rat brain data yielded ideal b-values b1,b2 = 0.8–1.0 ms/µm2, 2.4–2.6 ms/µm2. Parameter estimates from a fit to the full rat brain data set are compared to 1-3-9 and 1-9-9 estimates at these b-values in Supporting materials Figs 1–3. The 20 %-point penalty for lost pixels used in our optimization was applied in only 1% of the pixels at the optimal b-value combination.
Robustness of parameter estimation
Figure 2 illustrates precision and accuracy in the fast protocol estimates of D̅ and W̅. Two sets of ground truth inputs are used: in the upper row (A, B), a typical WM voxel corresponding to D̅ = 0.74 µm2/ms, W̅ = 1.12 FA=0.72, and in the lower row (C, D), a typical GM voxel with D̅ = 0.86 µm2/ms, W̅ = 0.5 FA=0.04. Both estimates of D̅ (A, C) have a low bias for all SNR values and perform quite comparably to NLS. Above an SNR of 20, D̅139 is relatively robust and typically deviates less than ~10%. However, D̅199 estimates are substantially more precise than the D̅139 estimates and remain below 5% for SNR > 20, and the NLS are even more precise. The performance in terms of bias and precision is relatively similar in GM and WM. Both fast estimates of W̅ (B, D) tend to underestimate mean kurtosis, and the 1-3-9 scheme markedly more so than the 1-9-9 scheme, but in both cases this bias tends rapidly toward zero with increasing SNR: for 1-9-9, it is less than 5% when SNR is larger than 20. The bias of NLS is slightly larger than 1-9-9, and has an opposite sign. Regarding precision, the 1-9-9 scheme for kurtosis estimation is comparable to NLS and also vastly outperforms the 1-3-9 scheme with a precision better than 4% for WM when SNR>20. For low kurtosis values, such as in the GM voxel for the lower row of Fig. 2, the relative precision becomes poorer, and drops below 5% for both NLS and 1-9-9 only when the SNR surpasses 40.
Fig. 2.
Precision and bias for the fast estimates of 1-3-9 (red) and 1-9-9 (green) estimates of D̅ (A,C) and W̅ (B,D) quantified by standard deviation (filled area) and mean values (markers) over 500 noise realizations. For comparison, nonlinear least squares (blue) are shown also. The top row (A, B) corresponds to a WM voxel with ground truth values D̅ = 0.74 µm2/ms, W̅ = 1.12 FA=0.72, and in lower row (C, D) to a GM voxel with ground truth values D̅ = 0.86 µm2/ms, W̅ = 0.5 FA=0.04.
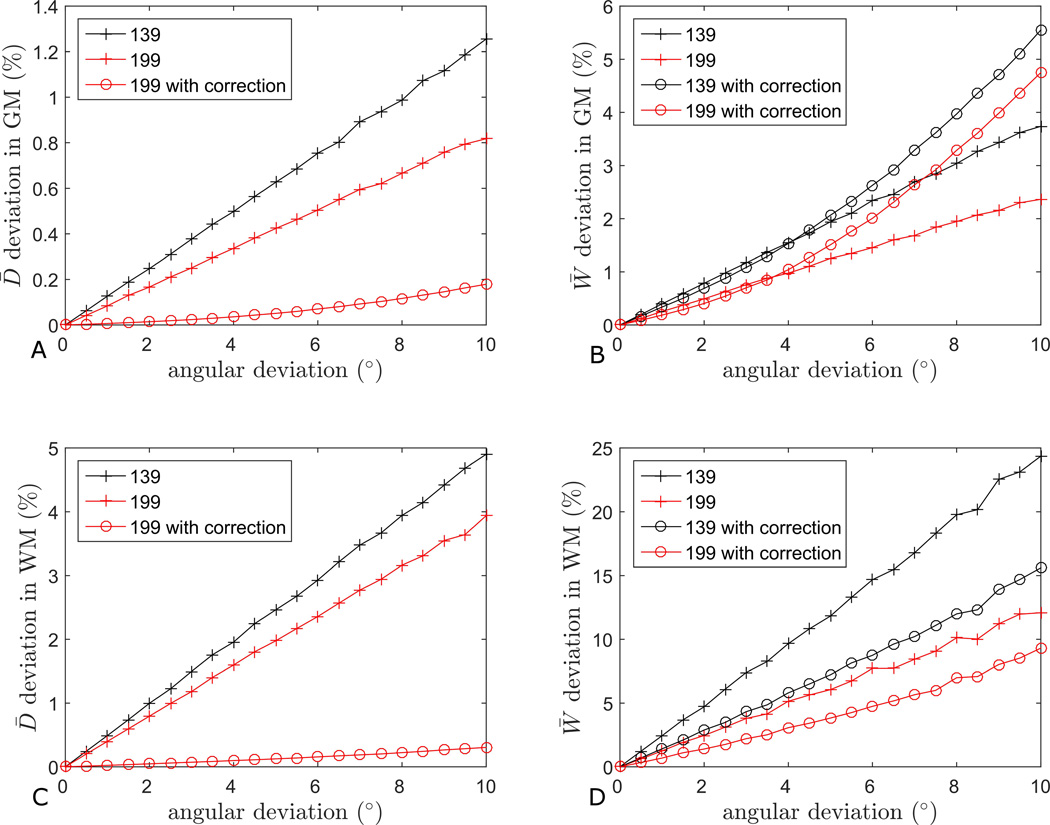
Figure 3 shows the effects of imperfect diffusion directions on the estimates of D̅ and W̅ in GM (panels A and B) and WM (C and D). As expected, the deviation from ideal estimates is a steadily increasing function of deviation in diffusion directions. However, the overall magnitude of the error in the estimations is quite small in all cases, except for a maximum of about 25%, attained in the case of 1-3-9 estimation of W̅ in WM (Fig. 3D) for an angular deviation of about 10°. Note that the deviation of the 1-9-9 estimates are consistently well below the 1-3-9 estimates, indicating that the 1-9-9 scheme is more robust. Estimating D̅ in GM (Fig. 3A) with either scheme is the least sensitive to imperfect directions, followed by D̅ in WM (Fig. 3C), W̅ in GM (Fig. 3B), and W̅ in WM (Fig. 3D). The effects of applying the correction scheme are also shown for all parameters except D̅139 where the correction method does not apply as it requires two nine direction shells to be present for correction of the D̅ estimate. For D̅199 the correction scheme reduces the estimation error to less than 1% in both GM and WM (Fig. 3A and C). As an extreme case, combining 1-9-9 with the compensation strategy improves estimation of D̅ by a factor of 20 in WM (Fig. 3C), and the estimate of W̅ by more than a factor of two in WM (Fig. 3D), compared to the 1-3-9 scheme without compensation. In the case of W̅, the correction scheme is seen to improve the estimates in WM (Fig. 3D) but the effect is negligible or even negative in GM (Fig. 3B).
Fig. 3.
Comparison of robustness to angular inaccuracy in the 1-3-9 and 1-9-9 estimates of D̅ and W̅. The effect on D̅ estimation in gray matter is shown in panel A and in white matter in panel C. The effect on W̅ estimation in gray matter is shown in panel B and in white matter in panel D.
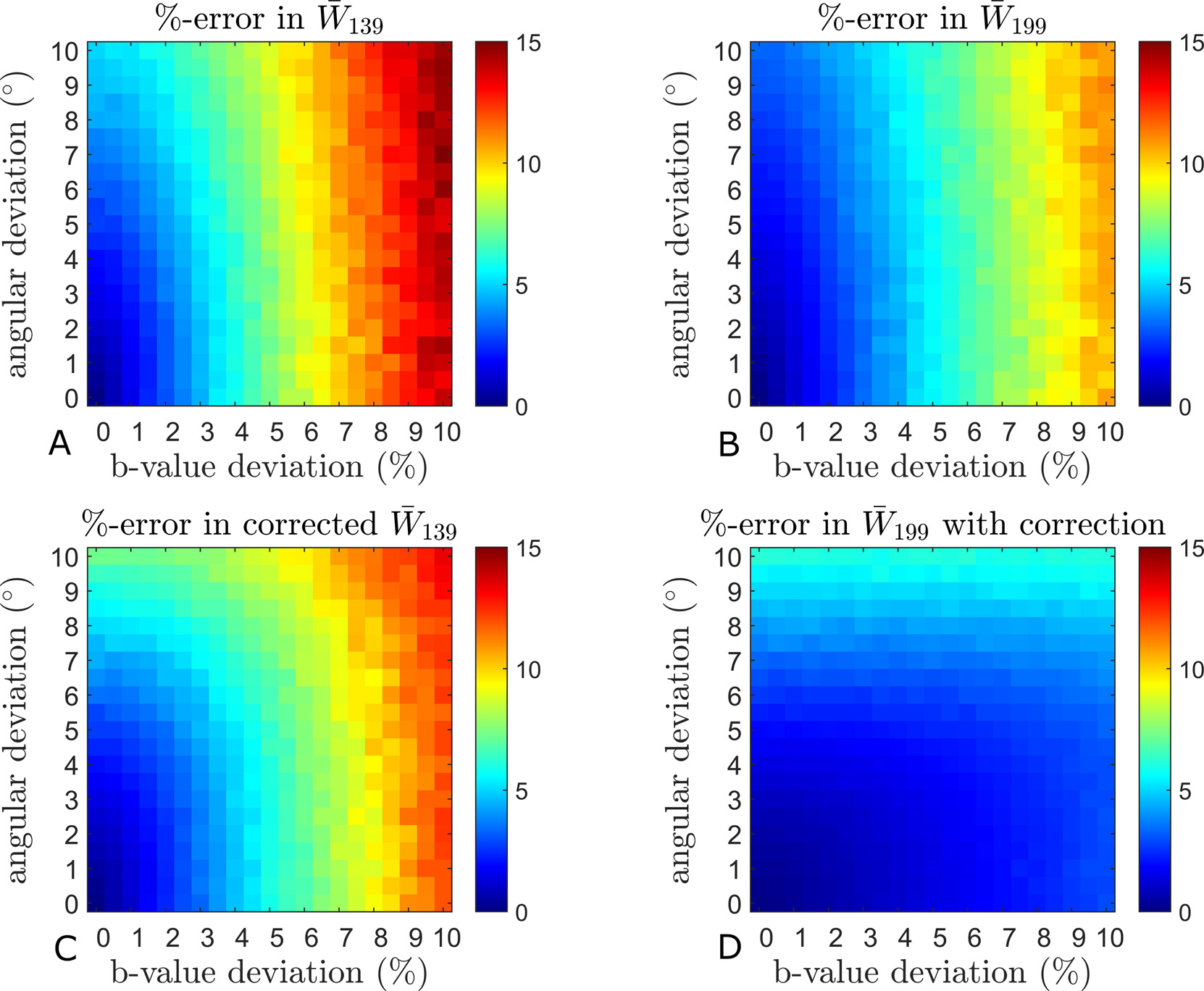
Figure 4 evaluates the influence of b-values varying across directions. Estimation errors monotonically increase to a maximum of about 20% for W̅ in GM (Fig. 4B). This time, the deviations for D̅ in GM (Fig. 4A) and WM (Fig. 4C) are the smallest, followed by W̅ in WM (Fig. 4D), and maximum for W̅ in GM (Fig. 4B). Again, the 1-9-9 scheme consistently outperforms the 1-3-9 scheme. The combination of 1-9-9 and the correction scheme improves estimation substantially, reaching a factor of >10 for D̅ (Fig. 4A) and ~5 for W̅ in GM (Fig. 4B). The compensation is most effective in the case of the 1-9-9 scheme, where the error drops below 4% in all cases. The graphs also demonstrate the positive effect of using the shell-average of the varying b-values in the 1-3-9 estimation of D̅ (Fig. 4A and C). When both b-values and diffusion directions depart from the nominal values, the deviations in the estimates are conveniently shown as color maps with the b-value deviations and angular deviations indicated along the horizontal and vertical axis, respectively. Figures 5 and 6 show the effects on W̅139 and W̅199 in GM and WM, respectively. The top rows (A, B) show the uncorrected estimates and the bottom rows (C, D) show the effect of the correction scheme. In both tissues, one readily appreciates the increased robustness of the 1-9-9 protocol compared to the 1-3-9 protocol. Even so, the influence of imperfections in the diffusion encoding on the estimated metrics can be just over 14% in W̅139 in both GM and WM (Fig. 5A and 6A)). The most robust estimate is W̅199 in WM (Fig. 6B), which stays below 10%. In GM (Fig. 5), the influence of angular deviations is seen to be weaker than the influence of b-value deviations, which could be expected due to the more isotropic nature of GM. Indeed, the behavior is different in WM where angular deviations are more important than b-value deviations for W̅139 (Fig. 6A).
Fig. 4.
Evaluation of the effect of b-value deviations among true directions on parameter estimates. The graphs also show the effect of using the correction scheme in the estimation of D̅ and W̅ in gray (A and B, respectively) and white matter (C and D) as well as the effect of using the mean b-values in the estimation of D̅139 (A and C).
Fig. 5.
Simulation results from 75 random pixels of GM. The combined effect of rugged b-value shells and angular deviations in 1-3-9 (A) and 1-9-9 (B) are mapped. The bottom row shows the effect of using the correction scheme in 1-3-9 (C) and 1-9-9 (D). See the methods section for simulation details.
Fig. 6.
Simulation results from 75 random pixels of WM. The maps show the combined effect of rugged b-value shells and angular deviations in 1-3-9 (A) and 1-9-9 (B). The bottom row shows the effect of the correction scheme in 1-3-9 (C) and 1-9-9 (D). See the methods section for simulation details.
Panels C and D of Figs. 5 and 6 show the effect of applying the compensation scheme before estimation of W̅. This generally decreases the estimation errors, with the most significant effect on W̅199 in GM (Fig. 5B and D) where the maximum error is almost halved and the sensitivity to b-value deviation is reduced and for GM almost completely removed. Results from identical simulations based on the data from fixed rat brain are provided in SM (Supporting Figs 4–7). The same overall behavior is found.
Figure 7 is similar to Figs. 5 and 6, but considers in detail a high FA region in the corpus callosum (average FA = 0.8). Here the influence of b-value and direction errors are of almost equal strength (Fig. 7A) and quite significant. Even in this case, the compensation strategy manages to improve the estimates (Fig. 7B) and substantially increases the window with low error estimates.
Fig. 7.
Correction example in high FA region (Corpus callosum, average FA in ROI=0.8). Panel A shows the effect without correction. In B the correction scheme is seen to extend the low error region to include even large deviations in both b and angles.
Evaluation of the 1-9-9 method
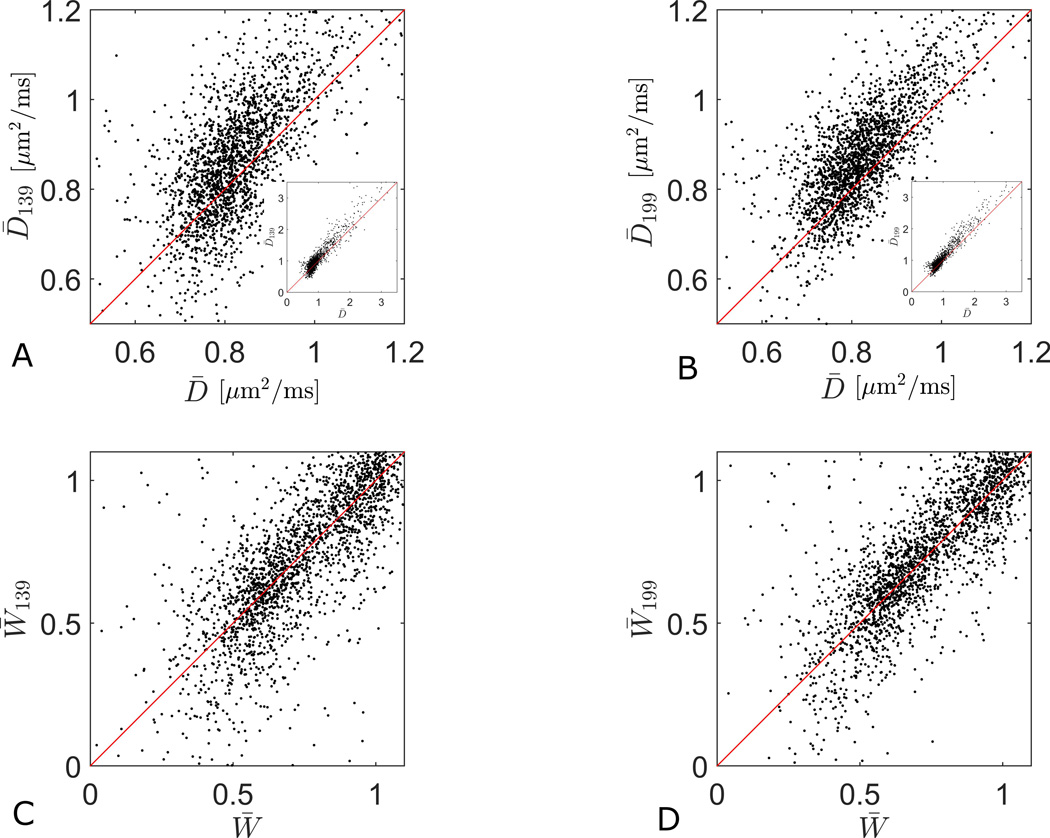
Figure 8 compares ground truth values from protocol B to the 1-3-9 and 1-9-9 estimates of D̅ (Fig. 8A–C) and W̅ (Fig. 8D–F) in a representative slice. Note that the D̅199 map (Fig. 8C) is slightly more noisy than D̅ (Fig. 8A), and D̅139 (Fig. 8B) noisier still. A similar tendency is noticeable in the W̅ estimates. The scatter plots in Fig. 9 show the correlations between the estimates shown in Fig. 8. The linear correlation coefficients for D̅ and W̅ from 1-3-9 are 0.94 (Fig. 9A), 0.79 (Fig. 9C) and for 1-9-9, 0.96 (Fig. 9B) and 0.83 (Fig. 9D), confirming that the association to the full estimates are stronger with the 1-9-9 protocol. For whole brain the correlations for this subject for D̅ and W̅ from 1-3-9 are 0.84 and 0.71, and for 1-9-9, 0.86 and 0.75. Over all three subjects the whole brain correlations are: for D̅139 0.84+/−0.02, for D̅199 0.87+/−0.02, for W̅139 0.67+/−0.04, and for W̅199 0.72+/−0.03. The SM provides more information, including whole brain maps of full estimates, 1-3-9 estimates, and 1-9-9 estimates and their correlation in three subjects (Supporting Figs. 8,12,16 (D̅) and 10,14,18 (W̅), panels A–C in each).
Fig. 8.
Parameter maps of D̅ (A) and W̅ (D) obtained from tensor model fit to the full data set. The corresponding estimates obtained with the 1-3-9 method are seen in B and E, respectively. The 1-9-9 estimates are shown in C and F. Note that the 1-9-9 estimates generally appear less noisy. This is also seen in a similar comparison based on the rat brain data (see SM).
Fig. 9.
Scatterplots comparing the 1-3-9 and 1-9-9 estimates to the true parameter values. The plots correspond to the maps shown in fig 1. The insets in the D̅ correlation plots (A and B) show the entire range of values, whereas the main graphs focus on the densest part of the data cloud. The linear correlation coefficients for D̅ (A) and W̅ (C) from 1-3-9 are 0.94, 0.76 and for 1-9-9 0.96 (B) and 0.81 (D). In each plot the red line is the identity line.
The supplementary parameter maps show that all three estimations fail to produce credible parameter values in some pixels. In the subject shown throughout the manuscript we find that this loss is largest for the 1-3-9 method (5.2%), while the loss of pixels is significantly lower for the 1-9-9 method (3.5%), which is very similar to the loss seen in the fit (3%). All percentages are for whole brain. This trend is consistent in all three subjects.
Evaluation of the 1-9-9 estimate of FA
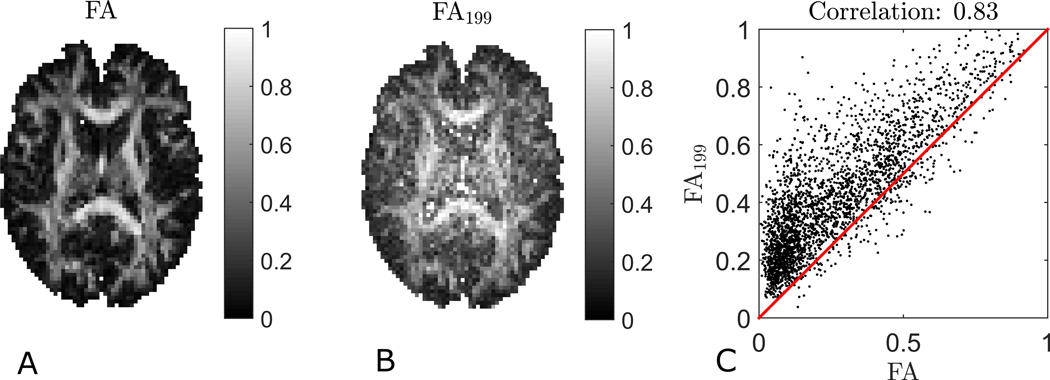
Figure 10 compares an FA map (Fig. 10A) to the FA199 (Fig. 10B) map. The contrast is qualitatively quite similar in the two maps, but the FA199 map presents as somewhat more noisy. Moreover, it tends to overestimate FA, especially in GM, resulting in less overall contrast. These observations are supported by the scatterplot shown in Fig. 10C, which nevertheless correspond to a linear correlation coefficient of 0.83 (0.81 for whole-brain for this subject and on average 0.77+/− 0.04 for whole brain across the three subjects). Using linear least squares to estimate D from the 1-9-9 data yields a slightly better estimate of FA with a correlation coefficient of 0.85 when compared to true FA (data not shown).
Fig. 10.
Figures of true FA (A), FA from the 1-9-9 method (B) and a scatterplot showing the correlation between the two (C). The red line is the identity line. The fast estimate is seen to generally overestimate FA. This effect is largest for low FA regions causing some loss of contrast in the FA map obtained by 1-9-9. The linear correlation coefficient is 0.83.
Discussion
We extended the 1-3-9 fast kurtosis imaging protocol (6) by acquiring all nine diffusion directions at both nonzero b-values. This 1-9-9 method allowed fast estimation of D̅, FA, and W̅ from 19 DWIs. We compared the performance of the fast protocols to nonlinear least squares, and characterized the impact of a number of practical factors on the accuracy of 1-3-9 and 1-9-9 estimates, specifically departures of b-values and diffusion directions from nominal directions. These effects are unavoidable or even quite typical in experiments. We devised a practical and straightforward procedure to compensate for some of these imperfections, and performed a b-value optimization on both fixed rat brain and normal human brain.
We examined the b-value dependency of parameter fidelity using experimental data and numerical simulations. The data-based b-value optimization was performed on data where no registration or smoothing was employed. This is important for online reconstruction in acute settings where preprocessing is unfeasible. The analysis showed behavior in qualitative agreement with predictions by simulations using realistic noise characteristics and levels. The error was low over an extended b-value range, and a good b-value combination for the 1-9-9 scheme in vivo is b1 ≤ 1 ms/µm2 and b2 ≈ 2.5 ms/µm2, but also that precise selection of these two b-values was not critical. The selected minimum is a realistic choice considering gradient performance available on most systems, and enables a reasonably low TE and sufficient SNR. These values also agree with typical recommendations, so that b1 = 1 ms/µm2 ensures bD̅ ≈ 1 and in most areas, while b2 = 2.5 ms/µm2 corresponds to in areas of low W̅ (e.g. GM). The W̅ estimates produced by the 1-3-9 and 1-9-9 methods at these b-values were found to be nearly identical, and so the b-value recommendation extends to the 1-3-9 method (11). Note that if a substantially different gradient profile (e.g. diffusion time) is used, the underlying “true” diffusion and kurtosis tensors may be different. This in turn will imply that the optimal b-values could be different. However, we believe this will have only a minor effect on our results due to the slow variation of the error as function of b-values, and since diffusion parameters are not likely to show a strong diffusion time dependence in the most commonly probed regimes.
In fixed rat brain an optimal b-value range of b1 = 0.8–1 ms/µm2, b2 = 2.4–2.6 ms/µm2 was found. Considering lower diffusivity in fixed tissue it was surprising to find similar optimal b-values. This might be due to the interplay of shorter restriction distances in fixed rat brain and differences in sequence timing between pre-clinical and clinical MR-systems. Further work is needed to elucidate this. In simulations of scheme robustness and effectiveness of the compensation strategy based on rat data, the same overall behavior was observed but effect sizes were different. This is not surprising considering the different characteristic structural length scales and measurement conditions available in microimaging experiments.
The 1-9-9 method was shown to offer improved robustness against non-optimal experimental conditions compared to the originally proposed 1-3-9 method (6). This was observed in numerical simulations of the effects of Rician noise, and of angular deviations in diffusion directions and b-value inaccuracies alone and in combination.
With Rician noise present, the accuracy and precision with ideal diffusion encoding was already quite high for both protocols, and the 199 protocol performed as good or better than the NLS estimation in terms of W̅ estimation. This is remarkable as the NLS method employed substantially more data and required advanced and time-consuming postprocessing. For D̅ estimation, the NLS estimator was more precise, but the 1-9-9 D̅ estimate is comparable to the NLS estimate in terms of bias.
The performance of all three estimation strategies applied will be affected by low SNR. This was also observed in our analysis where fewer pixels produced unrealistic W̅ values (defined here as outside of the 0–4 range) in the b-value optimization based on lower resolution data (higher SNR) than in the evaluation of 1-9-9 based on the higher resolution (lower SNR) protocol B. In all cases the 1-9-9 estimation was more robust than 1-3-9 and had pixel loss close to or comparable to the loss seen in the fit. We again note that our analysis is performed on data where no preprocessing has been applied, and the examples should therefore be taken as a raw performance demonstration. The number of pixels lost in the analysis will possibly be reduced by preprocessing strategies. Our analysis of the improvements offered by the 1-9-9 strategy over 1-3-9 do not in general indicate improvements to be limited to particular brain regions, although a slight improvement in problematic deep structures is seen (SM Figs. 8 and 10, panels D–F).
In large studies, data preprocessing (image registration, eddy current compensation etc) is standard. Based on registration results from FSL (51) in seven patients with substantial head motion, we found that no image plane was rotated more than 3°. This is well within the region where the 1-9-9 method is stable to directional deviations and therefore in principle, the analysis could be performed on the registered data directly with estimation errors on the level of noise induced errors.
For more severe cases a compensation strategy using redistribution of directional weights in parameter calculation was demonstrated. Implementation of the correction scheme is straightforward when effective b-matrices are known. When only effective b-values are known, using shell-averaged b-values in the analysis already improves parameter estimates (only shown for D̅139). Although the deteriorating effects of inaccurate encoding were not large to begin with, the compensation strategy offered substantial relative improvement.
FA estimation was also demonstrated with the 1-9-9 protocol, and displayed high correlation with true FA, although a slight systematic overestimation was observed. This likely stems from the method’s low number of diffusion directions compared to the recommendations for state-of-the-art diffusion tensor estimation (49). Nevertheless, when time is a concern, the FA199 estimate is a reasonable estimate, obtained in addition to robust estimates of D̅ and W̅. Even the full diffusion tensor, D, could be obtained with linear least squares, facilitating maps of radial and axial diffusivities, and direction encoded color (DEC) FA (52). To increase the robustness of these estimates, including FA, more directions could be acquired with an accompanying increase in scan time but negligible effect on postprocessing time.
Since the 1-9-9 method uses data from a standard diffusion sequence, it is readily implemented. It may be simpler to implement than the 1-3-9 method, since 1-9-9 requires the same encoding directions for both non-zero b-values, which most platforms assume when specifying gradient tables. Like 1-3-9, 1-9-9 post-processing does not require fitting procedures and all post-processing can therefore be implemented on the scanner. Both fast methods have been implemented for Siemens systems with online post-processing by one of the authors (JF), and are available as c2p. In principle, both schemes could be incorporated in single shot multiecho sequences, with b-values and directions encoded in different echo pathways, c.f. the recent MEDITATE framework (53). Likewise, the correction strategy suggested here for nonideal diffusion weighting could also be useful for such sequences, as well as isotropic diffusion weighting schemes (54).
While recent advances in simultaneous multi-slice EPI (55) give access to 2–10 fold reduction of acquisition times, reconstruction is still time consuming on all but the newest generation of reconstruction computers. Our technique can easily, and without loss, be combined with simultaneous multi-slice EPI resulting in further acceleration. These gains in speed can be traded for SNR or higher resolution and allow for improved data quality through the use of e.g. cardiac gating, navigator echoes, CSF nulling, blip up/down EPI, and readout segmented EPI.
In conclusion, the 1-9-9 estimates of D̅, FA, and W̅ correlate strongly with their true values. Accuracy and precision is better than for 1-3-9 and comparable to advanced estimations based on larger data sets. Scanner implementations of 1-9-9 with online post-processing enable simultaneous, real-time estimation of the three metrics. This might be useful when scan time is a constraint, particularly in the acute setting.
Supplementary Material
Acknowledgments
The authors were supported by the Danish Ministry of Science, Technology and Innovation’s University Investment Grant (MINDLab). We are grateful to Louise Rydtoft (LR) for preparing the rat brain. LR and BH (in part) were supported by NIH 1R01EB012874-01. SNJ acknowledges support from the Lundbeck Foundation R83-A7548 and Simon Fougner Hartmans Familiefond. The authors wish to thank Lippert’s Foundation and Korning’s Foundation for financial support. The 9.4T lab was made possible by funding from the Danish Research Counsil's Infrastructure program, the Velux Foundations, and the Department of Clinical Medicine, AU. The 3T Magnetom Tim Trio was funded by a grant from the Danish Agency for Science, Technology and Innovation.
References
- 1.Wesbey GE, Moseley ME, Ehman RL. Translational Molecular Self-Diffusion in Magnetic Resonance Imaging: II. Measurement of the Self-Diffusion Coefficient. Investigative Radiology. 1984;19(6):491–498. doi: 10.1097/00004424-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui ES, Cheung MM, Qi L, Wu EX. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage. 2008;42(1):122–134. doi: 10.1016/j.neuroimage.2008.04.237. [DOI] [PubMed] [Google Scholar]
- 4.Poot DH, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE transactions on medical imaging. 2010;29(3):819–829. doi: 10.1109/TMI.2009.2037915. [DOI] [PubMed] [Google Scholar]
- 5.Jespersen SN. Equivalence of double and single wave vector diffusion contrast at low diffusion weighting. NMR Biomed. 2012;25(6):813–818. doi: 10.1002/nbm.1808. [DOI] [PubMed] [Google Scholar]
- 6.Hansen B, Lund TE, Sangill R, Jespersen SN. Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med. 2013;69(6):1754–1760. doi: 10.1002/mrm.24743. [DOI] [PubMed] [Google Scholar]
- 7.Glenn GR, Helpern JA, Tabesh A, Jensen JH. Quantitative assessment of diffusional kurtosis anisotropy. NMR Biomed. 2015;28(4):448–459. doi: 10.1002/nbm.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen JH, Helpern JA, Ramani A, Lu HZ, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 9.Hui ES, Cheung MM, Qi L, Wu EX. Advanced MR diffusion characterization of neural tissue using directional diffusion kurtosis analysis. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2008;2008:3941–3944. doi: 10.1109/IEMBS.2008.4650072. [DOI] [PubMed] [Google Scholar]
- 10.Grinberg F, Ciobanu L, Farrher E, Shah NJ. Diffusion kurtosis imaging and log-normal distribution function imaging enhance the visualisation of lesions in animal stroke models. NMR Biomed. 2012;25(11):1295–1304. doi: 10.1002/nbm.2802. [DOI] [PubMed] [Google Scholar]
- 11.Hui ES, Du F, Huang S, Shen Q, Duong TQ. Spatiotemporal dynamics of diffusional kurtosis, mean diffusivity and perfusion changes in experimental stroke. Brain Res. 2012;1451:100–109. doi: 10.1016/j.brainres.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung JS, Wang E, Lo EH, Sun PZ. Stratification of heterogeneous diffusion MRI ischemic lesion with kurtosis imaging: evaluation of mean diffusion and kurtosis MRI mismatch in an animal model of transient focal ischemia. Stroke. 2012;43(8):2252–2254. doi: 10.1161/STROKEAHA.112.661926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudrapatna SU, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, Leemans A, van der Toorn A, Dijkhuizen RM. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. Neuroimage. 2014;97:363–373. doi: 10.1016/j.neuroimage.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JH, Falangola MF, Hu C, Tabesh A, Rapalino O, Lo C, Helpern JA. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2011;24(5):452–457. doi: 10.1002/nbm.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui ES, Fieremans E, Jensen JH, Tabesh A, Feng W, Bonilha L, Spampinato MV, Adams R, Helpern JA. Stroke assessment with diffusional kurtosis imaging. Stroke. 2012;43(11):2968–2973. doi: 10.1161/STROKEAHA.112.657742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helpern JA, Lo C, Hu C, Falangola MF, Rapalino O, Jensen JH. Diffusional kurtosis imaging in acute human stroke. Proceedings of the 17th Annual Meeting of ISMRM; Honolulu, Hawaii. 2009. p. 3493. [Google Scholar]
- 17.Latt J, van Westen D, Nilsson M, Wirestam R, Stahlberg F, Holtas S, Brockstedt S. Diffusion time dependent kurtosis maps are visualized ischemic lesions in stroke patients. Proceedings of the 17th Annual Meeting of ISMRM; Honolulu, Hawaii. 2009. p. 40. [Google Scholar]
- 18.Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59(1):467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, Silver JM, Grossman RI, Inglese M. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. Journal of neurotrauma. 2012;29(13):2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostergaard L, Engedal TS, Aamand R, Mikkelsen R, Iversen NK, Anzabi M, Naess-Schmidt ET, Drasbek KR, Bay V, Blicher JU, Tietze A, Mikkelsen IK, Hansen B, Jespersen SN, Juul N, Sorensen JC, Rasmussen M. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(10):1585–1598. doi: 10.1038/jcbfm.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Zhang Y, Wong CS, Wu PM, Zhang Z, Gao J, Qiu D, Huang B. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR Biomed. 2012;25(12):1369–1377. doi: 10.1002/nbm.2809. [DOI] [PubMed] [Google Scholar]
- 22.Helpern JA, Adisetiyo V, Falangola MF, Hu C, Di Martino A, Williams K, Castellanos FX, Jensen JH. Preliminary evidence of altered gray and white matter microstructural development in the frontal lobe of adolescents with attention-deficit hyperactivity disorder: a diffusional kurtosis imaging study. J Magn Reson Imaging. 2011;33(1):17–23. doi: 10.1002/jmri.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado y Palacios R, Campo A, Henningsen K, Verhoye M, Poot D, Dijkstra J, Van Audekerke J, Benveniste H, Sijbers J, Wiborg O, Van der Linden A. Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biol Psychiatry. 2011;70(5):449–457. doi: 10.1016/j.biopsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Delgado y Palacios R, Verhoye M, Henningsen K, Wiborg O, Van der Linden A. Diffusion Kurtosis Imaging and High-Resolution MRI Demonstrate Structural Aberrations of Caudate Putamen and Amygdala after Chronic Mild Stress. PloS one. 2014;9(4):e95077. doi: 10.1371/journal.pone.0095077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254(3):876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 26.Van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, De Keyzer F, Van Gool SW, Van Calenbergh F, De Vleeschouwer S, Van Hecke W, Sunaert S. Gliomas: diffusion kurtosis MR imaging in grading. Radiology. 2012;263(2):492–501. doi: 10.1148/radiol.12110927. [DOI] [PubMed] [Google Scholar]
- 27.Wang JJ, Lin WY, Lu CS, Weng YH, Ng SH, Wang CH, Liu HL, Hsieh RH, Wan YL, Wai YY. Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology. 2011;261(1):210–217. doi: 10.1148/radiol.11102277. [DOI] [PubMed] [Google Scholar]
- 28.Kamagata K, Tomiyama H, Motoi Y, Kano M, Abe O, Ito K, Shimoji K, Suzuki M, Hori M, Nakanishi A, Kuwatsuru R, Sasai K, Aoki S, Hattori N. Diffusional kurtosis imaging of cingulate fibers in Parkinson disease: comparison with conventional diffusion tensor imaging. Magn Reson Imaging. 2013;31(9):1501–1506. doi: 10.1016/j.mri.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Raz E, Bester M, Sigmund EE, Tabesh A, Babb JS, Jaggi H, Helpern J, Mitnick RJ, Inglese M. A better characterization of spinal cord damage in multiple sclerosis: a diffusional kurtosis imaging study. AJNR American journal of neuroradiology. 2013;34(9):1846–1852. doi: 10.3174/ajnr.A3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A, Ferris SH, Helpern JA. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 2008;28(6):1345–1350. doi: 10.1002/jmri.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization?: A rodent brain maturation study. Neuroimage. 2009;45(2):386–392. doi: 10.1016/j.neuroimage.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23(7):836–848. doi: 10.1002/nbm.1506. [DOI] [PubMed] [Google Scholar]
- 33.Flint JJ, Lee CH, Hansen B, Fey M, Schmidig D, Bui JD, King MA, Vestergaard-Poulsen P, Blackband SJ. Magnetic resonance microscopy of mammalian neurons. Neuroimage. 2009;46(4):1037–1040. doi: 10.1016/j.neuroimage.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint JJ, Hansen B, Portnoy S, Lee CH, King MA, Fey M, Vincent F, Stanisz GJ, Vestergaard-Poulsen P, Blackband SJ. Magnetic resonance microscopy of human and porcine neurons and cellular processes. Neuroimage. 2012;60(2):1404–1411. doi: 10.1016/j.neuroimage.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flint JJ, Hansen B, Fey M, Schmidig D, King MA, Vestergaard-Poulsen P, Blackband SJ. Cellular-level diffusion tensor microscopy and fiber tracking in mammalian nervous tissue with direct histological correlation. Neuroimage. 2010;52(2):556–561. doi: 10.1016/j.neuroimage.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen B, Flint JJ, Heon-Lee C, Fey M, Vincent F, King MA, Vestergaard-Poulsen P, Blackband SJ. Diffusion tensor microscopy in human nervous tissue with quantitative correlation based on direct histological comparison. NeuroImage. 2011;57(4):1458–1465. doi: 10.1016/j.neuroimage.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen B, Lund TE, Sangill R, Jespersen SN. Erratum: Hansen, Lund, Sangill, and Jespersen. Experimentally and Computationally Fast Method for Estimation of a Mean Kurtosis. Magnetic Resonance in Medicine. 2013;69:1754–1760. doi: 10.1002/mrm.24743. Magnetic Resonance in Medicine 2014;71(6):2250-2250. [DOI] [PubMed] [Google Scholar]
- 39.Sun PZ, Wang Y, Mandeville E, Chan ST, Lo EH, Ji X. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR Biomed. 2014;27(11):1413–1418. doi: 10.1002/nbm.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tietze A, Hansen MB, Ostergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean Diffusional Kurtosis in Patients with Glioma: Initial Results with a Fast Imaging Method in a Clinical Setting. Am J Neuroradiol. 2015;36(8):1472–1478. doi: 10.3174/ajnr.A4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiselev VG. Calculation of diffusion effect for arbitrary pulse sequences. Journal of magnetic resonance. 2003;164(2):205–211. doi: 10.1016/s1090-7807(03)00241-6. [DOI] [PubMed] [Google Scholar]
- 42.Solomon E, Shemesh N, Frydman L. Diffusion weighted MRI by spatiotemporal encoding: analytical description and in vivo validations. Journal of magnetic resonance. 2013;232:76–86. doi: 10.1016/j.jmr.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi S, Nagy Z, Moller HE, Symms MR, Carmichael DW, Josephs O, Weiskopf N. The effect of local perturbation fields on human DTI: characterisation, measurement and correction. NeuroImage. 2012;60(1):562–570. doi: 10.1016/j.neuroimage.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy Z, Weiskopf N, Alexander DC, Deichmann R. A method for improving the performance of gradient systems for diffusion-weighted MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58(4):763–768. doi: 10.1002/mrm.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen JH, Hu C, Helpern JA. Rapid data acquisition and postprocessing for diffusional kurtosis imaging. Proceedings of the 17th Annual Meeting of ISMRM; Honolulu, Hawaii. 2009. p. 1403. [Google Scholar]
- 46.Veraart J, Poot DH, Van Hecke W, Blockx I, Van der Linden A, Verhoye M, Sijbers J. More accurate estimation of diffusion tensor parameters using diffusion Kurtosis imaging. Magn Reson Med. 2011;65(1):138–145. doi: 10.1002/mrm.22603. [DOI] [PubMed] [Google Scholar]
- 47.Jeffreys H. Isotropic Tensors. P Camb Philos Soc. 1973 Jan;73:173–176. [Google Scholar]
- 48.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 49.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 50.Ma X, Kadah YM, LaConte SM, Hu X. Enhancing measured diffusion anisotropy in gray matter by eliminating CSF contamination with FLAIR. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;51(2):423–427. doi: 10.1002/mrm.10703. [DOI] [PubMed] [Google Scholar]
- 51.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1999;42(3):526–540. [PubMed] [Google Scholar]
- 53.Baete SH, Cho G, Sigmund EE. Multiple-echo diffusion tensor acquisition technique (MEDITATE) on a 3T clinical scanner. NMR Biomed. 2013;26(11):1471–1483. doi: 10.1002/nbm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong EC, Cox RW, Song AW. Optimized isotropic diffusion weighting. Magn Reson Med. 1995;34(2):139–143. doi: 10.1002/mrm.1910340202. [DOI] [PubMed] [Google Scholar]
- 55.Setsompop K, Cohen-Adad J, Gagoski BA, Raij T, Yendiki A, Keil B, Wedeen VJ, Wald LL. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. NeuroImage. 2012;63(1):569–580. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.