Abstract
There is an inevitable association between cell signaling pathways and tumorigenesis. Wnt and notch pathways play important roles during development and self-renewal. Beside the independent role of such pathways on tumor progression, different cross talks between these pathways through tumorigenesis are emphasized. In this study, we analyzed cross talk between Wnt and NOTCH signaling pathways through assessment of probable correlation between MAML1 and PYGO2 as the main transcription factors of these pathways, respectively in esophageal squamous cell carcinoma (ESCC) patients. Levels of MAML1 and PYGO2 mRNA expression in 48 ESCC patients were compared to the correlated margin normal tissues using real-time polymerase chain reaction (PCR). Eleven out of 48 patients (22.9 %) have shown the concomitant MAML1/PYGO2 over expression in significant correlation with tumor size (p = 0.046) and depth of tumor invasion (p = 0.050). We showed that there is a significant correlation and feedback between these markers during the ESCC progression and metastasis.
Keywords: Cancer stem cell, Signaling pathway, Crosstalk, Feedback, Gene expression, Real time PCR
Introduction
Northeastern Iran is one the most prevalent areas for esophageal squamous cell carcinoma (ESCC), (Gholamin et al. 2009). Despite new advances in prognosis, diagnosis, and therapeutic methods, majority of patients are diagnosed usually in advanced pathological tumor stage (III/IV) having extended metastasis of tumor cell throughout the body which prevents an efficient surgery (Headrick et al. 2002; Hulscher et al. 2002). Regarding the importance of early diagnosis in patients’ survival, elucidation of molecular mechanisms involved in ESCC progression and metastasis is essential to introduce new prognostic and therapeutic targets.
Developmental cell signaling pathways are key players of both embryonic development and tumorigenesis. Among these different pathways, Wnt and NOTCH signaling cascades are truly involved in cancer progression. Wnt signaling pathway is activated via the binding of secreted Wnt ligands to the cysteine-rich domain of Frizzled (Fzd) family receptors. Subsequently, signal transduction continues through ‘canonical’ or ‘non-canonical’ pathways. In Canonical pathway, Wnt/Fzd binding activates Dishevelled (Dvl), which prevents the β-catenin phosphorylation and ubiquitination, leading to β-catenin stabilization and its cytoplasmic accumulation. Then β -catenin enters to the nucleus, where it binds to the transcriptional complex comprising the TCF-1, BCL-9, and Pygopus (PYGO2) (Klaus and Birchmeier 2008). PYGO2 is one of the essential components in canonical Wnt/β-catenin transcriptional complex (Belenkaya et al. 2002) which is involved also in tumorigenesis (Popadiuk et al. 2006) and development (Song et al. 2007). PYGO2 has a C-terminal plant homeo-domain (PHD) which can bind to the histone methylated lysines (H3K4me) and activate transcription through the recruitment of β-catenin/BCL9 on the methylated histones in promoter sequences of Wnt target genes (Fiedler et al. 2008; Gu et al. 2009). It has been shown that malfunction of canonical pathway is associated with tumorigenesis (Fukui et al. 2005; Winn et al. 2005; You et al. 2004). PYGO2 directly or indirectly regulates critical cell cycle genes such as cyclin D1 and p21, leading cells to G1–S phase transition (Gu et al. 2009). Moreover, it has been shown that the PYGO2 expression in HeLa cells is involved in anti-apoptotic activity through DNA fragmentation, caspase-9/3 activation, and indirectly blocking of BCL-2 as an anti-apoptotic factor (De et al. 2009). In Wnt pathway the PYGO2 functions as a mediator between the chromatin remodeling complex and transcriptional machinery through its evolutionarily conserved PHD domain (Kessler et al. 2009).
NOTCH signaling is a cell–cell contact dependent pathway which influences cell fate decision through a family of four transmembrane receptors including Notch1–Notch4 (Luo et al. 2005). These receptors are activated by cell surface ligands of neighboring cells. After activation, the Notch intracellular domain (NICD) releases into the cytoplasm via a proteolytic process. Subsequently, the NICD enters to the nucleus and activates CSL (CBF/RBP-Jk, suppressor of Hairless, LAG-1) transcription factors which are the main factors of the NOTCH signaling transcription machinery. Homologous mammalian mastermind- like (MAML) proteins, especially MAML1, are also included in this transcriptional machinery as coactivators (Lin et al. 2002; Wu et al. 2002). In the absence of NICD, CSL suppresses the transcription of NOTCH target genes through binding to the regulatory cis-acting elements of promoter and recruitment of SMRT (silencing mediator of retinoid and thyroid receptors) co-repressors (Kao et al. 1998; Oswald et al. 2002). Furthermore, it has been shown that MAML1 is associated with a variety of key proteins such as p53, β-catenin, and NF-kB (Jin et al. 2010; Zhao et al. 2007). MAML1 recruits different co regulators, such as histone acetyl transferase (HAT) p300, which acetylates histone H3 and H4 tails in chromatin leading to formation of an active transcriptional region (Saint Just Ribeiro et al. 2007).
Considering the importance of different cell signaling pathways in ESCC progression and development (Moghbeli et al. 2013a, b, 2014), in the present study we assessed the probable correlation between the NOTCH and Wnt signaling pathways in ESCC samples. Although there are several reports about the Wnt/NOTCH associations in different cancers, there is not any report describing their association in ESCC. Therefore, to investigate the probable involvement of Wnt/NOTCH associations in ESCC tumorigenesis, we compared the levels of MAML1 and PYGO2 mRNA expressions in tumor with corresponding normal esophageal tissues and evaluated their probable correlations with the clinicopathological features of the patients.
Materials and methods
Tissue samples
Forty eight ESCC patients who were undergone the tumor resection were gathered from Omid, Qaem, and Imamreza Hospitals of Mashhad University of Medical Sciences (MUMS). All the cases have not received any chemo radiotherapeutic treatments before the surgery and the tumor specimens involved at least 70 % of tumor cells. Fresh tissues (tumor and margin normal) were preserved in RNA later solution (Qiagen, Hilden, Germany) and stored at −20 °C before the mRNA extraction. The study was approved by ethic committee of Mashhad University of Medical Sciences and all patients declared their informed consent.
RNA extraction, cDNA synthesis, comparative RT-PCR and statistical analysis
Total RNA extraction and cDNA synthesis were performed as described before (Moghbeli et al. 2013a, b, 2014). Levels of MAML1/PYGO2 mRNA expression were evaluated in duplicate reactions via the comparative relative RT-PCR (SYBR Green method, GENETBIO, Korea/Stratagene Mx-3000P, La Jolla, CA) (Forghanifard et al. 2012; Moghbeli et al. 2013a, b, 2014). Correlation between the levels of MAML1/PYGO2 mRNA expression and continuous and qualitative clinicopathological features of tumors were assessed by the Pearson’s/Spearman and ANOVA/t-tests, respectively (significant p value of <0.05, SPSS 16.0, Chicago, IL).
Results
Study population
Sample selection was restricted based on specific criteria in which the samples should not receive any chemo-radio therapeutic modalities prior the tumor resection and at least 70 % of tumor tissue should be comprised of tumor cells. Therefore the cases that were deprived of such prerequisites were excluded and 48 cases were finally enrolled in the present study. Age of patients at the time of diagnosis was ranged from 30 to 83 years (mean ± SD: 61.85 ± 12.33 years). Tumor size was ranged between 1.5 and 12 cm (mean ± SD: 4.23 ± 1.91 cm). All the Clinicopathological features of patients are summarized in Table 1.
Table 1.
Correlation between level of MAML1/PYGO2 mRNA expression and clinicopathological features of ESCC patients
| Total | PYGO2 overexpression | MAML1 overexpression | PYGO2/MAML1 overexpression | P Value | |
|---|---|---|---|---|---|
| Patients | 48 | 17(35.4 %) | 21(43.8 %) | 11(22.9 %) | |
| Mean age (mean ± SD) | 61.85 ± 12.33 | 63.41 ± 2.49 | 62.05 ± 2.16 | 61.55 ± 3.47 | |
| Size (mean ± SD) | 4.23 ± 1.91 | 3.49 ± 0.40 | 3.79 ± 0.32 | 3.55 ± 0.54 | |
| Sex | 0.270 | ||||
| Male | 28(58.3 %) | 11(64.7 %) | 15(71.4 %) | 8(72.7 %) | |
| Female | 20(41.7 %) | 6(35.3) | 6(28.6 %) | 3(27.3 %) | |
| Location | 0.411 | ||||
| Lower | 21(43.8 %) | 9(52.9) | 8(38.1 %) | 6(54.5 %) | |
| Middle | 27(56.2 %) | 8(47.1) | 13(61.9 %) | 5(45.5 %) | |
| Grade | 0.433 | ||||
| P.D | 8(16.7 %) | 4(23.5 %) | 5(23.8 %) | 3(27.3 %) | |
| M.D | 31(64.6 %) | 10(58.8 %) | 12(57.1 %) | 7(63.6 %) | |
| W.D | 9(18.8 %) | 3(17.6 %) | 4(19.0 %) | 1(9.1 %) | |
| Lymph node | 0.473 | ||||
| Yes | 22(45.8 %) | 7(41.2 %) | 9(42.9 %) | 4(36.4 %) | |
| No | 26(54.2 %) | 10(58.8 %) | 12(57.1 %) | 7(63.6 %) | |
| Stage | 0.804 | ||||
| I/II | 29(60.4 %) | 10(58.8 %) | 14(66.7 %) | 7(63.6 %) | |
| III/IV | 19(39.6 %) | 7(41.2 %) | 7(33.3 %) | 4(36.4 %) | |
| Depth of tumor invasion (T) | 0 .050 | ||||
| T1 | 1(2.1 %) | 1(5.9 %) | 1(4.8 %) | 1(9.1 %) | |
| T2 | 7(14.6 %) | 2(11.8 %) | 2(9.5 %) | – | |
| T3 | 40(83.3 %) | 14(85.4 %) | 18(85.7 %) | 10(90.9 %) | |
| Age | 0.428 | ||||
| <60 | 17(35.4 %) | 6(35.3 %) | 8(38.1 %) | 5(45.5 %) | |
| ≥60 | 31(64.6 %) | 11(64.7 %) | 13(61.9 %) | 6(54.5 %) | |
| Size | 0.046 | ||||
| <3 | 8(16.7 %) | 6(35.3 %) | 4(19.0 %) | 4(36.4 %) | |
| ≥3 | 40(83.3 %) | 11(64.7 %) | 17(81.0 %) | 7(63.6 %) | |
Bold values indicate significant correlation between mRNA expression and clinicopathological feature
Levels of MAML1/PYGO2 mRNA expression in ESCC patients
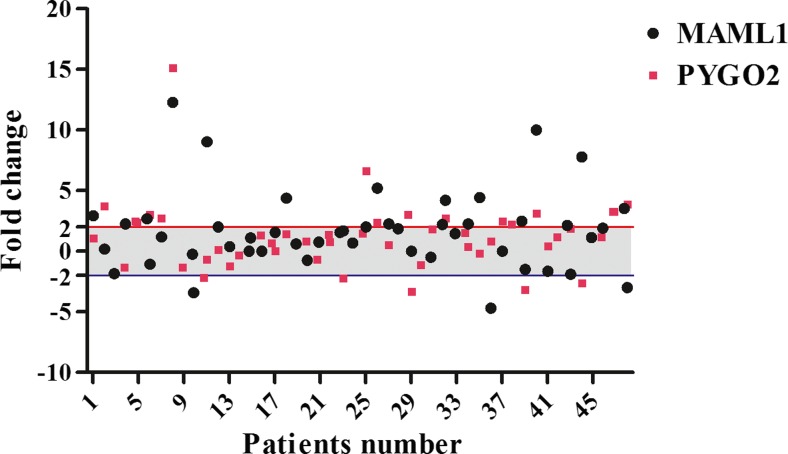
Levels of MAML1/PYGO2 mRNA expressions were assessed through the comparative relative real time PCR in tumor specimens in comparison with their corresponding margin normal tissues. Seventeen out of 48 cases (35.4 %) and 21 out of 48 samples (43.8 %) showed PYGO2 and MAML1 over expression, respectively. Concomitant overexpression of Maml1/Pygo2 was detected in eleven out of 48 patients (22.9 %). Over and underexpression of the genes were defined with more than +2 fold and less than −2 fold in level of mRNA expression, respectively. The normal expression was considered for the cases between these two thresholds. The minimum and maximum fold changes of gene expression were −4.70 and 12.30 (mean ± SD: 1.66 ± 3.23) and −3.31 and 15.10 (mean ± SD: 1.27 ± 2.86) for the MAML1 and PYGO2, respectively (Fig. 1).
Fig. 1.
Scatter plot represent descriptive analysis of relative gene expression of MAML1 and PYGO2 in ESCC patients. The thresholds for the over and under expressed cases are shown by the red and blue lines, respectively. The grey area mentions to the cases with normal levels of MAML1 and PYGO2 mRNA expression
Clinicopathological features and PYGO2/MAML1 mRNA expression
Previously we have reported the clinicophatological relevance of Pygo2 and Maml1 mRNA expression in ESCC patients in separate reports (Forghanifard et al. 2012; Moghbeli et al. 2013a, b, 2014). In the present study we assessed their concomitant expression in ESCC samples and evaluated their correlations with clinicopathological features. Overexpression of both genes was detected in eleven out of 48 (22.9 %) ESCC tissues. There was a significant correlation between the Pygo2/Maml1 overexpression and depth of tumor cell invasion especially in invaded tumor cells to the adventitia (T3, 10/11, 90.9 %) (p = 0.040). There was not any significant correlation between the concomitant overexpression of Pygo2/Maml1 and the other clinicopathological features of patients such as grade, tumor stage, lymph node status and location. Having analyzed the samples with concomitant overexpression of the genes, we found that most cases were observed among the male patients 8/11(72.7 %). Furthermore, the majority of these tumor samples were moderately differentiated (7/11, 63.6 %) in advanced stages of tumor progression (ΙΙΙ/ΙV, 7/11, 63.6 %). In addition, these tumors were distributed in lower and middle parts of esophagus with 6/11(54.5 %) and 5/11(45.5 %), respectively. Moreover, the minority of such cases showed lymph node metastasis (4 out of 11, 36.5 %). Although there were not any significant correlation between PYGO2/MAML1 overexpression and continuous clinicopathological features such as age and tumor size, the Pygo2/Maml1 over expressed patients had noticeably lower ages in comparison with the only Maml1 and Pygo2 up regulated cases (62.60 ± 2.67 vs. 66.83 ± 2.96 years old, respectively). Patients with ≤60 years old had higher levels of Pygo2 and Maml1 mRNA expression in comparison with others who were ˃60 years old (mean ± SD of fold changes: 2.14 ± 0.94 vs. 0.79 ± 0.36 and 2.44 ± 0.90 vs. 1.23 ± 0.52, respectively). In the case of tumor sizes, it seems that overexpression of these genes is inversely correlated to the size of tumors. Indeed, the tumors without any overexpression of the genes were interestingly bigger in size compared to the Pygo2/Maml1 overexpressed tumors (mean ± SD: 4.90 ± 0.49 cm vs. 3.55 ± 0.54 cm). There was a significant correlation between tumor size and Pygo2/Maml1 mRNA expression in which 19 out of 21 (90.5 %) cases without any overexpression in these markers were bigger than 3 cm (p = 0.046). The Maml1 over expressed tumors were also bigger than the Pygo2 up regulated cases (4.05 ± 0.32 cm vs. 3.38 ± 0.61 cm). It was observed that, levels of Maml1 and Pygo2 mRNA expressions was correlated together significantly (P = 0.030). The level of Maml1 mRNA expression in tumors with only Maml1 overexpression was lower than that in the Pygo2/Maml1 overexpressed cases (mean ± SD: 3.94 ± 0.80 vs. 4.41 ± 1.06 fold changes). The levels of Pygo2 mRNA expression in the Pygo2/Maml1 over expressed cases were also interestingly higher than that in the only Pygo2 overexpressed tumors (mean ± SD: 4.24 ± 1.5 vs. 2.95 ± 0.31 fold changes). Levels of Maml1 mRNA expression in males were higher than females (mean ± SD: 1.83 ± 0.67 vs. 1.37 ± 0.62 fold changes). In contrast, levels of Pygo2 mRNA expression in females were higher than males (mean ± SD: 1.34 ± 0.48 vs. 1.22 ± 0.63). Interestingly, we observed that Pygo2 expression is higher in tumor cells without any lymph node metastasis in comparison with the lymph node metastatic tumors (mean ± SD: 1.70 ± 0.68 vs. 0.76 ± 0.41 fold changes). In contrast, the levels of Maml1 mRNA expression in lymph nodes metastatic tumors were higher than the cases without lymph nodes metastasis (mean ± SD: 2.01 ± 0.71 vs. 1.37 ± 0.63 fold changes). In the case of tumor location, levels of Pygo2 and Maml1 mRNA expression in tumors at middle part of esophagus were higher than the lower part (mean ± SD: 1.69 ± 0.63 vs. 0.73 ± 0.48) and (mean ± SD: 1.95 ± 0.70 vs. 1.29 ± 0.59), respectively.
Discussion
Transcriptional co activators are one of the most important components of transcriptional machineries which mediate gene transcription process (Spiegelman and Heinrich 2004). In the present study we assessed the probable correlation between MAML1 and PYGO2 as the major co activators of NOTCH and Wnt cell signaling pathways, respectively, through expressional analysis in tumors and correlated margin normal esophageal tissues. We showed significant correlation between these markers. There are also significant correlations between the MAML/PYGO2 over expression and some of the clinicopathological features including depth of tumor invasion and tumor size. Regarding the concomitant Maml1 and Pygo2 over expression in ESCC cases, we hypothesized that these factors may regulate a cross-talk between NOTCH/Wnt pathways during the ESCC progression. Crosstalk between cell signaling pathways can be categorized into several types including cooperative, separated, and direct crosstalk (Collu et al. 2014). In cooperative mechanism, all of the pathways are united to regulate a specific target while in separated crosstalk, one pathway has a regulatory role on the other one. Direct crosstalk is referred to mutual interaction of different cell signaling pathway transcriptional machineries. The independent crosstalk between NOTCH and Wnt signaling has been shown in self-renewal process of mammary stem cells. In such cells, Wnt pathway inhibits the notch signaling through chromatin remodeling of Notch3 promoter region using the β-catenin/PYGO2 complex. In this process, chromatin remodeling of Notch3 promoter region suppresses its expression (Gu et al. 2013). Dishevelled also inhibits NOTCH through a direct protein-protein interaction (Hayward et al. 2005; Munoz-Descalzo et al. 2010). In line with presented nuclear correlations of these transcription cofactors, It has been reported that the correlation between NOTCH and Wnt pathways is also observed in the cytoplasmic level where the GSK3b as a kinase is involved in several signaling pathways such as β-catenin and NOTCH (Espinosa et al. 2003; Watcharasit et al. 2003). Having applied GSK-3β, Wnt signaling can induce the notch pathway through phosphorylation of NICD and its stabilization (Foltz et al. 2002). GSK-3β is negative regulator of Wnt pathway through phosphorylation and degradation of β-catenin. MAML1 is also negatively regulated by GSK3b. GSK3b inhibits MAML1 through direct interaction with the MAML1 N terminus which is reported to interact with NOTCH and p53 to plays its role in the transcription activity of these factors (Saint Just Ribeiro et al. 2007). This may introduces the regulatory mechanisms for these pathways through cytoplasmic interaction versus nuclear cooperation of their main coactivators.
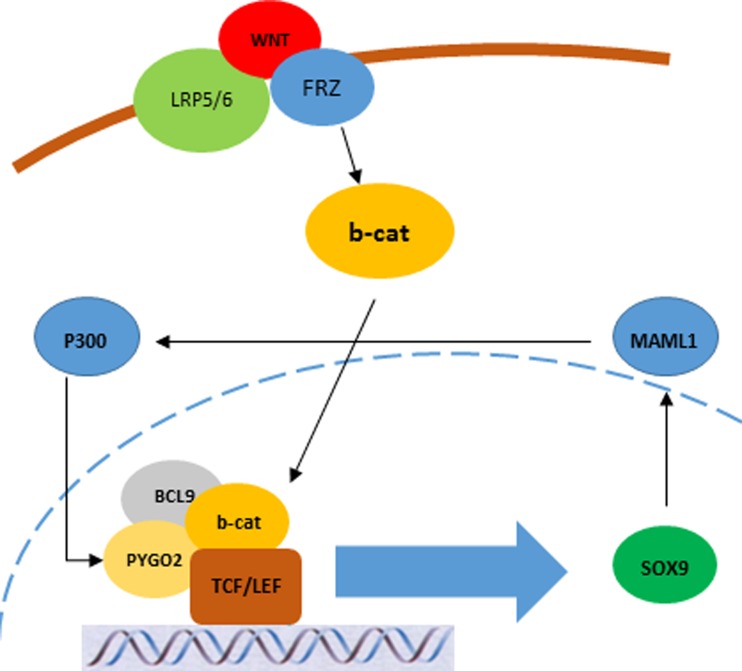
In this study, the Pygo2/Maml1 overexpressed cells were mostly invaded to the adventitia showing T3 depth of tumor invasion, interestingly. It may rely to the role of cooperation between the concomitant expression of these genes and tumor invasiveness and metastasis. It was observed that, the levels of either Pygo2 or Maml1 mRNA expression in tumors with only one gene overexpression were lower than those with concomitant overexpression of the genes. Therefore, it seems that there is a probable feedback between these proteins in ESCC. There is a binding site for the histone acetyltransferase P300 in promoter sequence of Pygo2 and it has been shown that MAML1 recruits p300 to NOTCH-targeted genes through a direct interaction with the C/H3 domain of p300. Therefore, p300-MAML1 complex may be able to activate the PYGO2 gene transcription through histone acetylation of related chromatin. This acetylation may result in chromatin remodeling of Pygo2 promoter region leads to activate its expression. On the other hand, Sox9 which is regulated by Wnt pathway has a binding site in the Maml1 promoter sequence. It seems that these pathways may have a mutual feedback through MAML1 and PYGO2 using the P300 and Sox9 as mediators during the ESCC progression and development (Fig. 2).
Fig. 2.
Probable crosstalk between Wnt and NOTCH pathways through the SOX9 and P300 as the mediators for the PYGO2 and MAML1, respectively
Given appropriate knowledge about the interactions between such pathways paves the way for the targeted therapy regarding the cell context. For example, if tumor initiation is due to Wnt activation and NOTCH suppression, using a Wnt ligand inhibitor may be advantageous as a disruptor for the Wnt–NOTCH crosstalk (Chen et al. 2009; Gonsalves et al. 2011). While, targeted therapy for the NOTCH transcription machinery will not result to the proficient consequences because the crosstalk is remained intact.
In conclusion, evaluating of Maml1 and Pygo2 concomitant mRNA expression in ESCC patients clarified that their concomitant expression may have important role in ESCC progression and development. This may show a probable cross talk between Wnt and NOTCH pathways through their mastermind transcription factors which can be introduced for ESCC diagnosis and targeted therapy, especially in advanced stages of ESCC. To the best of our knowledge, this is the first report describing clinical relevance of concomitant expression of Maml1 and Pygo2 in ESCC and evaluating probable crosstalk between related cell signaling pathways. Although, it needs further studies to find more detailed information about this crosstalk in ESCC patients, concomitant evaluation of several pathways shows a better view of molecular mechanisms involved in ESCC system biology.
Acknowledgment
This work was supported by a grant from the Vice Chancellor for Research at Mashhad University of Medical Sciences, and was part of a Ph.D. student’s dissertation, No. 921202.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71:3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De D, Chen A, Wu Z, Lv S, He G, Qi Y. Overexpression of Pygopus2 protects HeLa cells from vinblastine-induced apoptosis. Biol Chem. 2009;390:157–165. doi: 10.1515/BC.2009.014. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- Fiedler M, Sanchez-Barrena MJ, Nekrasov M, Mieszczanek J, Rybin V, Muller J, Evans P, Bienz M. Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Mol Cell. 2008;30:507–518. doi: 10.1016/j.molcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3beta modulates notch signaling and stability. Curr Biol. 2002;12:1006–1011. doi: 10.1016/S0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Moaven O, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, Moghbeli M, Nejadsattari T, Parivar K, Abbaszadegan MR. Expression analysis elucidates the roles of MAML1 and Twist1 in esophageal squamous cell carcinoma aggressiveness and metastasis. Ann Surg Oncol. 2012;19:743–749. doi: 10.1245/s10434-011-2074-8. [DOI] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, Sotoudeh M, Rajabi-Mashhadi MT, Forghani MN, Farrokhi F, Abbaszadegan MR. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg. 2009;33:1439–1445. doi: 10.1007/s00268-009-0070-y. [DOI] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rheaume C, Bilanchone V, Veltmaat JM, Takemaru K, Millar S, Lee EY, Lewis MT, Li B, Dai X. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–826. doi: 10.1083/jcb.200810133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Watanabe K, Sun P, Fallahi M, Dai X. Chromatin effector Pygo2 mediates Wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell. 2013;13:48–61. doi: 10.1016/j.stem.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A. Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headrick JR, Nichols FC, 3rd, Miller DL, Allen MS, Trastek VF, Deschamps C, Schleck CD, Thompson AM, Pairolero PC. High-grade esophageal dysplasia: long-term survival and quality of life after esophagectomy. Ann Thorac Surg. 2002;73:1697–1702. doi: 10.1016/S0003-4975(02)03496-3. [DOI] [PubMed] [Google Scholar]
- Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- Jin B, Shen H, Lin S, Li JL, Chen Z, Griffin JD, Wu L. The mastermind-like 1 (MAML1) co-activator regulates constitutive NF-kappaB signaling and cell survival. J Biol Chem. 2010;285:14356–14365. doi: 10.1074/jbc.M109.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Hausmann G, Basler K. The PHD domain is required to link Drosophila Pygopus to Legless/beta-catenin and not to histone H3. Mech Dev. 2009;126:752–759. doi: 10.1016/j.mod.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Lin SE, Oyama T, Nagase T, Harigaya K, Kitagawa M. Identification of new human mastermind proteins defines a family that consists of positive regulators for notch signaling. J Biol Chem. 2002;277:50612–50620. doi: 10.1074/jbc.M209529200. [DOI] [PubMed] [Google Scholar]
- Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Moghbeli M, Abbaszadegan MR, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, Forghanifard MM. Association of PYGO2 and EGFR in esophageal squamous cell carcinoma. Med Oncol. 2013;30:516. doi: 10.1007/s12032-013-0516-9. [DOI] [PubMed] [Google Scholar]
- Moghbeli M, Moghbeli F, Forghanifard MM, Garayali A, Abbaszadegan MR. Cancer stem cell markers in esophageal cancer. Am J Cancer Sci. 2013;2:37–50. [Google Scholar]
- Moghbeli M, Forghanifard MM, Aarabi A, Mansourian A, Abbaszadegan MR. Clinicopathological sex- related relevance of Musashi1 mRNA expression in esophageal squamous cell carcinoma patients. Pathol Oncol Res. 2014;20:427–433. doi: 10.1007/s12253-013-9712-3. [DOI] [PubMed] [Google Scholar]
- Munoz-Descalzo S, Sanders PG, Montagne C, Johnson RI, Balayo T, Arias AM. Wingless modulates the ligand independent traffic of Notch through Dishevelled. Fly (Austin) 2010;4:182–193. doi: 10.4161/fly.4.3.11998. [DOI] [PubMed] [Google Scholar]
- Oswald F, Kostezka U, Astrahantseff K, Bourteele S, Dillinger K, Zechner U, Ludwig L, Wilda M, Hameister H, Knochel W, Liptay S, Schmid RM. SHARP is a novel component of the Notch/RBP-Jkappa signalling pathway. Embo J. 2002;21:5417–5426. doi: 10.1093/emboj/cdf549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popadiuk CM, Xiong J, Wells MG, Andrews PG, Dankwa K, Hirasawa K, Lake BB, Kao KR. Antisense suppression of pygopus2 results in growth arrest of epithelial ovarian cancer. Clin Cancer Res. 2006;12:2216–2223. doi: 10.1158/1078-0432.CCR-05-2433. [DOI] [PubMed] [Google Scholar]
- Saint Just Ribeiro M, Hansson ML, Wallberg AE. A proline repeat domain in the Notch co-activator MAML1 is important for the p300-mediated acetylation of MAML1. Biochem J. 2007;404:289–298. doi: 10.1042/BJ20061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA. pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004;119:157–167. doi: 10.1016/j.cell.2004.09.037. [DOI] [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- Wu L, Sun T, Kobayashi K, Gao P, Griffin JD. Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol. 2002;22:7688–7700. doi: 10.1128/MCB.22.21.7688-7700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, Jablons DM. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Katzman RB, Delmolino LM, Bhat I, Zhang Y, Gurumurthy CB, Germaniuk-Kurowska A, Reddi HV, Solomon A, Zeng MS, Kung A, Ma H, Gao Q, Dimri G, Stanculescu A, Miele L, Wu L, Griffin JD, Wazer DE, Band H, Band V. The notch regulator MAML1 interacts with p53 and functions as a coactivator. J Biol Chem. 2007;282:11969–11981. doi: 10.1074/jbc.M608974200. [DOI] [PubMed] [Google Scholar]