Abstract
The binding of antigen to the B cell receptor (BCR) results in a cascade of signalling events that ultimately drive B cell activation. Uncontrolled B cell activation is regulated by negative feedback loops that involve inhibitory co-receptors such as CD22 and CD32B that exert their functions following phosphorylation of immunoreceptor tyrosine-based inhibition motifs (ITIMs). The CD22-targeted antibody epratuzumab has previously been shown to inhibit BCR-driven signalling events, but its effects on ITIM phosphorylation of CD22 and CD32B have not been properly evaluated. The present study therefore employed both immunoprecipitation and flow cytometry approaches to elucidate the effects of epratuzumab on direct phosphorylation of key tyrosine (Tyr) residues on both these proteins, using both transformed B cell lines and primary human B cells. Epratuzumab induced the phosphorylation of Tyr822 on CD22 and enhanced its co-localisation with SHP-1. Additionally, in spite of high basal phosphorylation of other key ITIMs on CD22, in primary human B cells epratuzumab also enhanced phosphorylation of Tyr807, a residue involved in the recruitment of Grb2. Such initiation events could explain the effects of epratuzumab on downstream signalling in B cells. Finally, we were able to demonstrate that epratuzumab stimulated the phosphorylation of Tyr292 on the low affinity inhibitory Fc receptor CD32B which would further attenuate BCR-induced signalling. Together, these data demonstrate that engagement of CD22 with epratuzumab leads to the direct phosphorylation of key upstream inhibitory receptors of BCR signalling and may help to explain how this antibody modulates B cell function.
Keywords: CD22, Phosphorylation, CD32B, B cell receptor, Epratuzumab
Introduction
The B cell receptor (BCR) not only imparts specific antigen-binding capacity to B cells but also plays a key role in pathways of B cell development, activation and survival (Grimaldi et al 2005; Keren and Melamed 2005). The binding of antigen to the BCR results in a well-characterised cascade of phosphorylation events and calcium flux changes that ultimately drive B cell activation (reviewed in Dörner et al 2012). However, such events require fine balancing and therefore inhibitory co-receptors of the BCR complex, such as CD22 and the Fc receptor CD32B (FcγRIIb), play a role in preventing overstimulation of B cells. Such inhibitory proteins are known to function by signaling through immunoreceptor tyrosine-based inhibition motifs (ITIMs) (Nitschke 2005; Tsubata 2012) and CD22, a B cell-restricted protein, possesses six tyrosine (Tyr) residues on its intracellular tail of which three are located within ITIMs. Following BCR activation, phosphorylation of these specific Tyr residues on CD22 results in the recruitment of adaptor and inhibitory signaling molecules (Otipoby et al 2001; Poe et al 2000). On human CD22 for example, Tyr822 phosphorylation recruits SHP-1 which dephosphorylates Syk whilst Tyr807 binds to the intracellular signalling adaptor protein Grb2, with subsequent broad down-modulation of B cell activation. In contrast, CD32B contains a single cytoplasmic ITIM motif (Tyr292) that down-regulates activating signals primarily through the recruitment of SH2 domain-containing phosphatases such as SHIP-1 (Coggeshall 1998; Muta et al 1994).
Epratuzumab is a humanized monoclonal antibody targeting CD22 that is being evaluated in the clinic for both autoimmune diseases and in cancer (Goldenberg 2006). Previous work has shown that epratuzumab down-modulates BCR-activated signalling of B cells in culture e.g., reduced phosphorylation of spleen tyrosine kinase (Syk) and phospholipase γ2 (PLCγ2) and inhibition of calcium flux (Sieger et al 2013; Maloney et al 2014). This leads to functional inhibition of B cells such as removal of the BCR complex from the B cell surface, reduced proliferation and diminished production of pro-inflammatory cytokines (Rossi et al 2013; Fleischer et al. 2015a; Jacobi et al 2008), arguing that epratuzumab essentially works by enhancing the normal inhibitory function of CD22 on the BCR (Dörner et al 2015). In vitro studies using transformed B cell lines have shown that epratuzumab can enhance global Tyr phosphorylation on CD22 (Carnahan et al 2003; Qu et al 2008), suggesting that this antibody may be capable of directly triggering this upstream inhibitory component of BCR activation. However, to date there has not been a comprehensive and systematic analysis of specific CD22 ITIMs and this has therefore been explored in the current study using both transformed B cell lines and primary B cells. Importantly, we have also assessed the capacity of epratuzumab to engage and enhance Tyr phosphorylation of the low affinity inhibitory Fc receptor, CD32B which is the only Fc receptor expressed on mature B cells.
Materials and methods
Epratuzumab formats and isotype control antibodies
Epratuzumab IgG was clinical grade material (lot number 105766). The F(ab’)2 and Fab stocks were prepared from epratuzumab IgG by pepsin digestion at UCB Pharma (Slough, UK). The human IgG1 isotype control antibody was obtained from Sigma (Poole, UK) and the F(ab’)2 (CDP850) and Fab (A33) isotype controls were made at UCB Pharma (Slough, UK).
B cell purification and cell culture
Human peripheral blood mononuclear cells (PBMC) from healthy donors (HD) were isolated by Ficoll density-gradient centrifugation and B cells purified using the human B-cell isolation Kit-II (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. Some experiments were also conducted with an enriched B cell fraction, obtained from whole blood using RosetteSep® Human B-cell Enrichment Cocktail according to the manufacturer’s protocol (StemcellTechnologies, Grenoble, France). Human blood samples were obtained based on local ethics approval (Charité Universitätsmedizin Berlin) and after written informed consent was obtained from volunteers. Human tonsils were sourced with ethical approval from the Human Tissue Resource Centre (London, UK) and B cells were purified using a modified single-step rosetting method (Saxon et al 1976) using 2-aminoethyl-isothiouronium bromide (AET)-treated sheep red blood cells (TCS Biosciences, Buckingham, UK). Daudi cells (a human B cell lymphoma cell line) were purchased from Sigma (Poole, UK). The culture medium used for all experiments was RPMI 1640/10 % fetal calf serum/2 mM glutamine.
Immunoprecipitation and Western blotting
Daudi or tonsil-derived B cells (1 × 107) were incubated for 5 or 15 min periods at 37 °C with 10 μg/mL of either epratuzumab, epratuzumab F(ab’)2, anti-human IgM F(ab’)2 (Jackson Labs, Pennsylvania, USA) or human IgG1 isotype control. Cells without antibody treatment were used as a negative control. Cells were harvested and lysed in RIPA buffer supplemented with phosphatase and protease inhibitor cocktails (Thermo Scientific, Lutterworth, UK). CD22 and CD32B proteins were immunoprecipitated by preincubating the cell lysates overnight at 4 °C with either 5 μg of anti-CD22 (epratuzumab) or 5 μg anti-CD32B (Abcam, Cambridge, UK) antibodies. Human and rabbit IgG whole molecule antibodies (5 μg each) were used as negative controls for the anti CD22 and anti CD32B antibodies respectively. The antigen-antibody complexes were subsequently captured by incubation for an additional 12 h at 4 °C using pre-washed protein G magnetic beads (Cell Signalling Technology, Hitchin, UK). The beads were then washed 3 times in lysis buffer and resuspended in sample loading buffer containing dithiothreitol (DTT) reducing reagent. Bound protein was eluted from the beads by heating at 95 °C for 5 min. Following SDS-PAGE and immunoblotting, immunoprecipitated proteins were analysed by probing with specific anti-phospho antibodies (CD32B Tyr292, CD22 Tyr822, Tyr842 and Tyr807, all from Abcam, Cambridge, UK). Total protein blots were carried out to confirm equal protein loading. The immunoprecipitation control antibody ChromoPure human IgG, whole molecule was obtained from Jackson Labs, Pennsylvania, USA.
Flow cytometry
PBMC (1 × 106 cells) from HD were equilibrated for 30 min at 37 °C and subsequently stimulated with 12 μg/mL goat F(ab’)2 anti-IgM/IgG (Jackson ImmunoResearch, Newmarket, UK) or 10 μg/mL epratuzumab (in IgG, F(ab’)2 and Fab formats) for different time periods. After stimulation, PBMC were immediately fixed with Lyse/Fix Buffer (Becton-Dickinson GmbH, Heidelberg, Germany) and permeabilized (Becton-Dickinson GmbH, Heidelberg, Germany) according to the manufacturer’s protocol. Fluorescently-tagged antibodies against cell surface markers were used to identify B cells (CD3-/CD14-/CD19+/CD20+) and CD27+ memory and CD27- naïve B cells. Antibodies against CD3 (clone UCHT1), CD14 (clone M5E2), CD19 (clone SJ25C1), CD20 (clone H1 (FB1)) and CD27 (clone L128) were all obtained from Becton-Dickinson GmbH, Heidelberg, Germany. CD22 phosphorylation was monitored intracellularly using an antibody specific for CD22 Tyr822 (clone 12A/CD22; Becton-Dickinson GmbH, Heidelberg, Germany).
Confocal microscopy
Purified B cells (1 × 105 cells) or an enriched B cell population (0.5 × 105 cells) from HD were equilibrated for 60 min at 37 °C and subsequently stimulated with RPMI (control) or F(ab’)2 epratuzumab-A488 (10 μg/mL) for 60 min and immediately fixed with Lyse/Fix Buffer (Becton-Dickinson GmbH, Heidelberg, Germany) and permeabilized (Becton-Dickinson GmbH, Heidelberg, Germany) according to the manufacturer’s protocol. Cells were stained for 40 min at room temperature with mouse anti-human SHP-1 (clone 52/PTP1C; Becton-Dickinson GmbH, Heidelberg, Germany), with goat anti-human IgM/IgG (Jackson ImmunoResearch, PA, USA) and, for the RPMI control, with F(ab’)2 epratuzumab-A488 and washed with PBS/1 % FCS and stained for 40 min at room temperature with the secondary antibody donkey anti-mouse-RRX and donkey anti-goat-A647. Finally, cells were centrifuged at 250 g for 1 min on a slide, covered with Vectashield Hard mounting medium containing DAPI (blue) (Vector Laboratories, CA, USA) to stain the nucleus. The co-localization coefficient (overlay, white/yellow) of SHP-1 (red), BCR (green) and CD22 (purple) was assessed with Zen 2011 light edition software (Carl Zeiss, Jena, Germany; original magnification: X630).
Results
Immunoprecipitation studies demonstrate that epratuzumab directly induces the phosphorylation of ITIMs on CD22
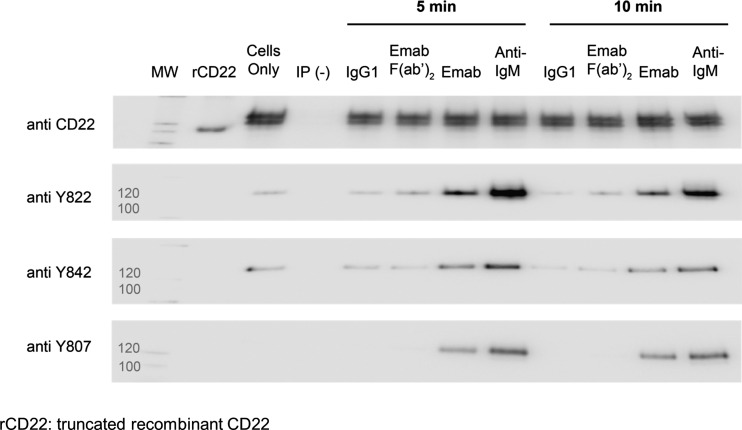
Immunoprecipitation experiments confirmed that BCR activation by anti-IgM F(ab’)2 in Daudi cells strongly induced CD22 tyrosine phosphorylation (Carnahan et al 2003; Qu et al 2008), and we were able to show that this occurred on all three regulatory tyrosine residues (Tyr822, Tyr842 and Tyr807), with the strongest signals appearing after 5 min (Fig. 1). Epratuzumab IgG treatment of Daudi cells under the same conditions also resulted in increased phosphorylation of all three sites, although generally weaker than that seen after BCR activation and the signal was strongest at the 5 min time point (Fig. 1). A F(ab’)2 fragment of epratuzumab was unable to induce phosphorylation of Tyr842 and Tyr807 and less able to induce phosphorylation of Tyr822, although the signal was greater than that observed with the isotype control or background.
Fig. 1.
Engagement of CD22 with epratuzumab directly stimulates increased tyrosine phosphorylation at key regulatory sites on CD22. Daudi cells were stimulated in a BCR-dependent manner (Lanes 7 and 11) with 10 μg/mL anti IgM (Fab’)2 or in a BCR-independent manner with 10 μg/mL epratuzumab IgG (Lanes 6 and 10) or 10 μg/mL epratuzumab F(ab’)2 (Lanes 5 and 9) for the time periods indicated. Untreated cells (Lane 2) were used as a control to assess the level of basal phosphorylation. Cells were also treated with 10 μg/mL IgG1 isotype negative control (Lanes 4 and 8). Following cell lysis, CD22 was immunoprecipitated as described in the “Materials and Methods” section. Human IgG whole molecule was used as a negative control antibody for immunoprecipations (Lane 3). The samples were immunoblotted and probed using specific anti-phospho Tyr antibodies to assess the relative changes at these sites. To ensure equal protein loading, an anti-CD22 blot was also carried out
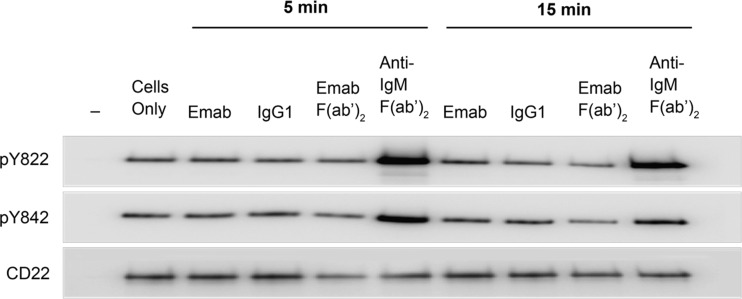
Experiments performed with tonsil-derived purified B cells were hampered by the fact that there was always a constitutively high basal phosphorylation of Tyr842 and Tyr822 on CD22 (Fig. 2; note the high signal in the “cells only” lane). Although this signal was modestly elevated after BCR activation with anti-IgM F(ab’)2, no effect with epratuzumab could be observed.
Fig. 2.
Engagement of CD22 with epratuzumab has no effect on CD22 Tyr822 and Tyr842 phosphorylation in tonsil-derived B cells. CD22 was immunoprecipitated from tonsil derived B cells following the treatment and methods described in the legend to Fig. 1. The samples were immunoblotted and probed using specific anti-phospho Tyr antibodies. To ensure equal protein loading an anti-CD22 blot was also carried out
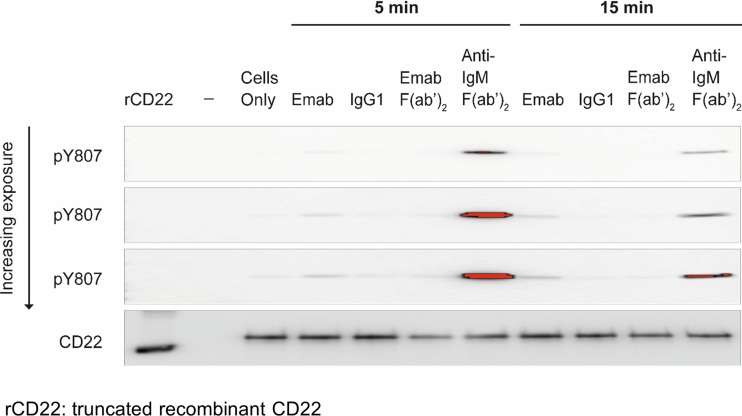
However, epratuzumab did modestly but reproducibly increase CD22 Tyr807 phosphorylation in primary tonsil-derived B cells (Fig. 3) with signals observed at later time points. No increase in phosphorylation was observed using epratuzumab F(ab’)2, in keeping with our data in Daudi B cells.
Fig. 3.
Engagement of CD22 with epratuzumab directly stimulates an increase in CD22 Tyr807 phosphorylation in tonsil-derived B cells. CD22 was immunoprecipitated from tonsil derived B cells following the treatment conditions described in the legend to Fig. 1. The samples were immunoblotted and probed using a specific anti-phospho Tyr 807 antibody as described in “Materials and Methods” section. To ensure equal protein loading an anti-CD22 blot was also carried out. The figure shown is a representative of three independent experiments and shows increasing, incremental (10 s/high resolution) exposure times following immunoblot with the anti-Tyr 807 antibody on an ImageQuant LAS 400 instrument (GE Healthcare, Amersham,UK)
Flow cytometry studies demonstrate that epratuzumab directly induces the phosphorylation of Tyr822 on CD22
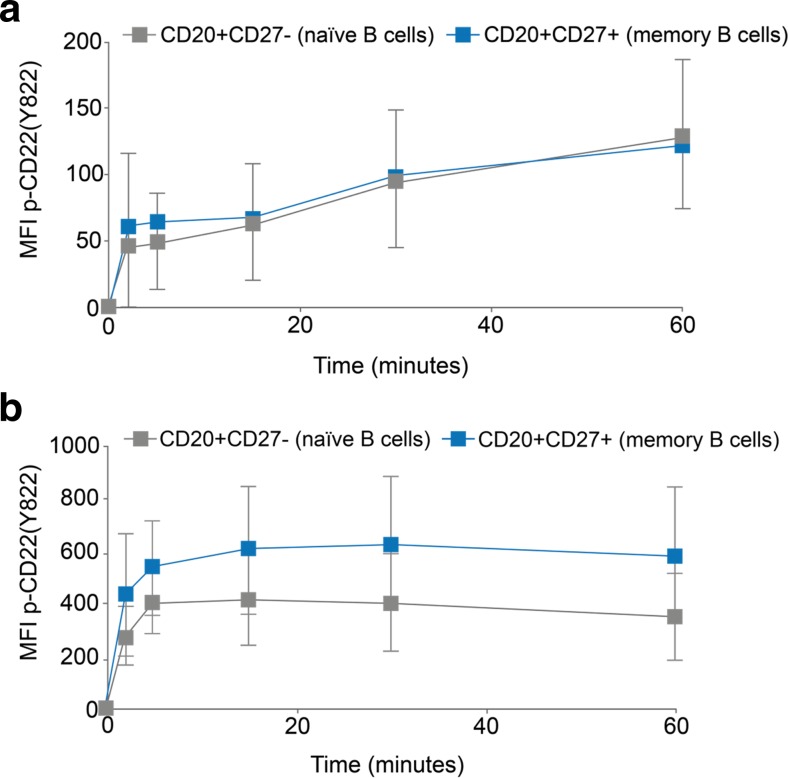
Experiments to explore epratuzumab-induced changes in CD22 phosphorylation were also performed by flow cytometry, although reagents suitable for use by this method are only available for Tyr822. PBMC from HD were cultured with epratuzumab (in IgG, F(ab’)2 and Fab formats) or appropriate isotype controls over time and, after fixation and permeabilisation, stained for both B cell lineage markers and with anti-CD22 Tyr822. B cells stimulated with either epratuzumab F(ab’)2 (Fig. 4a) or anti-IgM/IgG (anti-BCR) (Fig. 4b) showed a rapid increase in CD22 Tyr822 phosphorylation in flow cytometry experiments. The response was stronger with anti-IgM/IgG than epratuzumab but both were maximal by around 5–10 min. Both CD27+ memory and CD27- naïve B cells responded, although following stimulation with anti-IgM/IgG, memory B cells showed a greater response than naïve B cells.
Fig. 4.
Engagement of CD22 with epratuzumab directly stimulates CD22 Tyr822 phosphorylation in HD B cells assessed by flow cytometry. PBMC from HD were incubated over time with epratuzumab (a; n = 7) or anti-IgM/IgG (b; n = 8), fixed and permeabilised and stained with B cell subset markers and anti-CD22 Tyr822. Data show CD22 Tyr822 signal on CD20+/CD27- naïve B cells (grey symbols) and CD20+/CD27+ memory B cells (blue symbols). MFI = mean fluorescence intensity after subtraction of time zero values
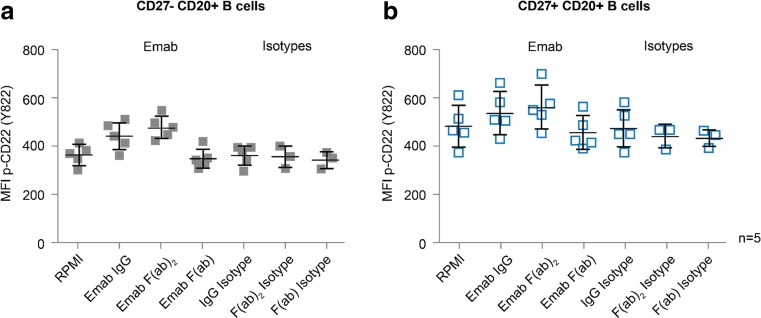
In experiments carried out to assess various formats of epratuzumab (Fig. 5), CD22 Tyr822 phosphorylation was shown to be induced with both IgG and F(ab’)2 formats to a similar extent above the relevant isotype controls, but not with the monovalent Fab fragment of this antibody, although the enhanced effects observed with the bivalent formats were not statistically significant in this experiment. The effect was seen in both CD27- naïve and CD27+ memory B cells and, whilst modest, these data do support the result obtained in immunoprecipitation experiments (Fig. 1) where the Tyr822 ITIM was investigated.
Fig. 5.
Enhanced CD22 Tyr822 phosphorylation in HD B cells with epratuzumab requires bivalency but not the Fc portion of the antibody. PBMC from HD (n = 5) were incubated with epratuzumab in IgG, F(ab’)2 or Fab formats (or appropriate isotype matched controls) for 1 h at 37 °C, fixed and permeabilised and stained with B cell subset markers and anti-CD22 Tyr822 prior to flow cytometric analysis. Data are shown for CD20+/CD27- naïve B cells (a) and CD20+/CD27+ memory B cells (b). MFI = mean fluorescence intensity
Epratuzumab induces co-localization of CD22 and SHP-1
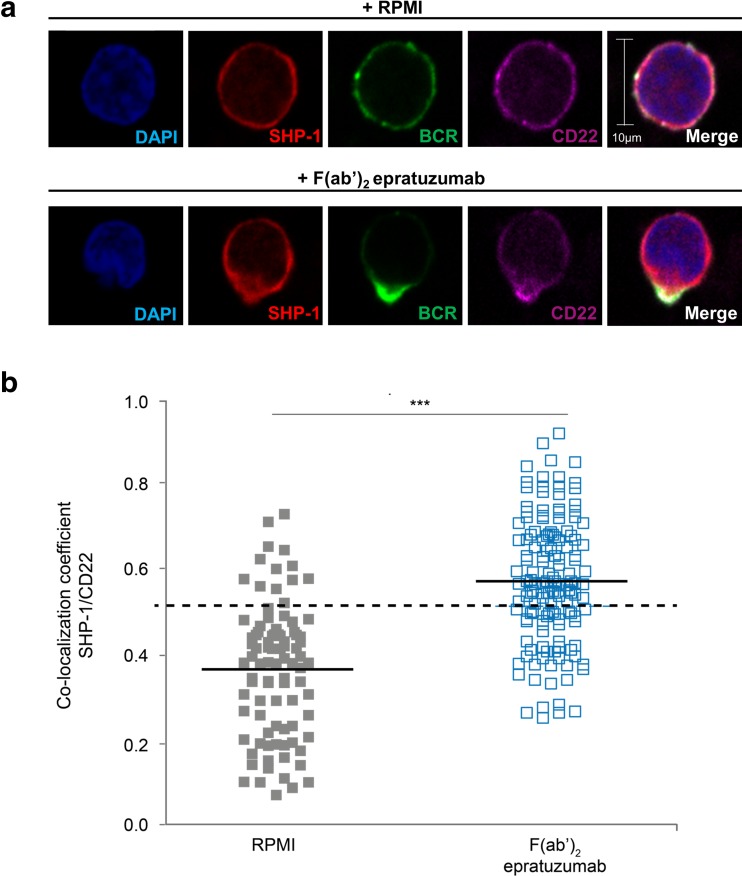
The most consistent and robust changes observed with epratuzumab were found with the Tyr822 ITIM on CD22 and, since SHP-1 is recruited after phosphorylation of this Tyr residue, confocal microscopy experiments were performed to investigate the association of CD22 with SHP-1. It has previously been shown that epratuzumab induces the co-localization of CD22 with the BCR component CD79α (Sieger et al 2013) and we therefore also investigated BCR localization. As shown in Fig. 6a, treatment of enriched HD B cells with F(ab’)2 epratuzumab-A488 induced co-localization of CD22 and SHP-1 together with the BCR since there was increased merging of red (SHP-1), green (BCR) and purple (CD22) staining on the B cells in culture. Of interest, the co-localization frequently occurred in a single pole (or bud) on the B cell surface. In other experiments where only CD22 and SHP-1 were labelled, the co-localization co-efficients of these two proteins was assessed quantitatively and, as shown in Fig. 6b, a statistically significant difference was shown between untreated and epratuzumab-treated purified B cells.
Fig. 6.
Stimulation with epratuzumab leads to recruitment of the phosphatase SHP-1 to the activated CD22 complex in B cells. a Co-localization was visualized by confocal microscopy. An enriched pool of HD B cells were incubated with RPMI (control) or F(ab’)2 epratuzumab-A488 for 60 min and stained for SHP-1 (red), BCR (green), CD22 (purple) and nuclei stained with DAPI (blue); b The co-localization coefficient of SHP-1 with CD22 was assessed with Zen 2011 light edition software. Each symbol represents an individual B cell of 3 healthy donors. Horizontal lines indicate the mean; co-localization coefficient >0.5 (dotted line) indicates co-localization. ***p < 0.001 by Wilcoxon’s signed rank test
Immunoprecipitation studies demonstrate that epratuzumab directly induces the phosphorylation of Tyr292 on CD32B
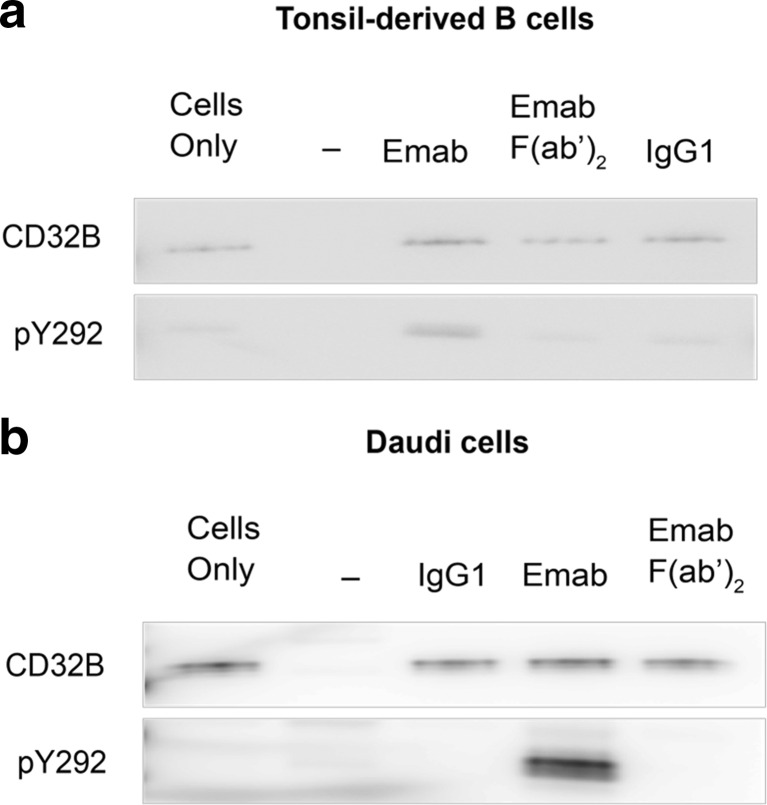
Epratuzumab IgG also induced an increase in CD32B Tyr292 phosphorylation in both tonsil-derived B cells (Fig. 7a) and Daudi cells (Fig. 7b). Although the signal was stronger in Daudi cells, there was a consistent induction of the ITIM site on CD32B in both cell types. As expected, no increase in CD32B Tyr292 was observed using epratuzumab F(ab’)2 which lacks the Fc portion.
Fig. 7.
Engagement of CD22 with epratuzumab directly stimulates the phosphorylation of Tyr292 on CD32B. Tonsil-derived B cells (a) and Daudi (b) cells were stimulated with 10 μg/mL of either epratuzumab IgG or epratuzumab F(ab’)2 for the time periods indicated. Untreated cells were used as a control to assess the level of basal phosphorylation. Cells were also treated with 10 μg/mL human IgG1 isotype as a negative control. Following cell lysis, CD32B was immunoprecipitated using an anti-CD32B antibody as described in “Materials and Methods” section. Rabbit IgG whole molecule was used as a negative control antibody for immunoprecipations. The samples were immunoblotted and probed using an anti-CD32B (phospho Tyr292) antibody to assess the relative phosphorylation changes. To ensure equal protein loading an anti-CD22 blot was also carried out. The figure shown is a representative image from three independent experiments
Discussion
Our results reveal that the engagement of CD22 with epratuzumab on the Daudi B cell lymphoma line and on primary human B cells directly enhances the phosphorylation of key regulatory Tyr residues on the CD22 intracellular tail and builds on previously reported data with B cell lines showing increased global CD22 phosphorylation with this antibody (Carnahan et al 2003; Qu et al 2008). Such events would be expected to lead to the recruitment of phosphatases such as SHP-1, which was also demonstrated in the present study. Finally, epratuzumab directly stimulated the phosphorylation of a key regulatory Tyr residue on the low affinity inhibitory Fc receptor, CD32B. The results are summarised schematically in Fig. 8.
Fig. 8.
Summary of the effects of epratuzumab on CD22 and CD32B phosphorylation events in B cells. 1: In resting B cells, the BCR, CD22 and CD32B are not in close association; 2: Epratuzumab directly induces the phosphorylation of Tyr822 and, modestly, Tyr807 on CD22, leading to the recruitment of SHP-1 and Grb2, respectively, and co-localisation of CD22 and the BCR (see also Sieger et al 2013); 3: Phosphorylation of Tyr292 on CD32B also supports diminished B cell activation
Whilst there were technical problems associated with measuring some of the individual phosphorylation events in all cell types, increased phosphorylation of Tyr822 was most robustly demonstrated using both methods. The demonstration using confocal microscopy for increased association of CD22 with SHP-1 with epratuzumab would be an expected consequence of the increased phosphorylation of this Tyr residue. These data concerning direct effects in the absence of BCR activation complement data by our group showing a similar down-modulation by epratuzumab of signals induced by activation through the BCR (Sieger et al 2013; Maloney et al 2014), where the antibody also increased association of CD22 with the BCR complex itself. Overall, it would appear that such events represent the “initiating signal” that leads to subsequent inhibition of signalling and downstream immunoregulatory changes such as inhibition of cytokine production or B cell proliferation (Fleischer et al. 2015a; Jacobi et al 2008). Interestingly, the basal phosphorylation signal for Tyr822 (and Tyr842) was constitutively high in tonsil-derived B cells which has also been previously reported in terms of total CD22 phosphorylation (Carnahan et al 2003) and may be a natural consequence of an increased activation state of tonsil B cells, since tonsils tend to be surgically removed during inflammatory episodes. The data from tonsils suggest that CD22 ligation is not able to increase the phosphorylation of some Tyr residues, indicating that preactivated B cells may be less responsive to these inhibitory pathways. Interestingly, recent data indicates that a similar mechanism may also exist in systemic lupus erythematosus (SLE), where the B cells from patients have an intrinsically disturbed balance of BCR-initiated signalling pathways including enhanced Tyr phosphatase activity coupled with increased Akt phosphorylation (Fleischer et al. 2015b).
We were however able to show that in tonsil-derived B cells, epratuzumab can enhance the phosphorylation of Tyr807, another key regulatory residue on CD22 that is reported to recruit the SH2 adaptor protein Grb2. Whilst the key effect was modest in magnitude, it has been known for some time that CD22 forms a quaternary complex with Grb2, SHIP and Shc to negatively regulate BCR-driven events such as Ca2+ flux (Poe et al 2000). Indeed, Grb2-deficient mice show hyperactivated Ca2+ flux responses in response to BCR activation (Ackermann et al. 2011; Jang et al 2009). Additionally, Grb2 may also be involved in translating inhibitory signals driven through activation of CD32B (Isnardi et al 2004; Neumann et al 2011), which is of interest given our data showing induced phosphorylation of CD32B driven by epratuzumab.
In our studies, we found that a F(ab’)2 fragment of the epratuzumab antibody was not as active as the full length IgG and, in fact, we could only demonstrate an effect with the F(ab’)2 fragment when Tyr822 was investigated. Many other functional responses induced with epratuzumab such as CD22 internalisation and inhibition of downstream BCR signalling appear to require the antibody to be bivalent but not dependent on the presence of the Fc moiety (Sieger et al 2013; Shock et al. 2014). It is hypothesized that the cross-linking event directly pulls CD22 into association with the BCR which then cluster in (or in the vicinity of) lipid rafts leading to subsequent internalisation and downstream functional effects (discussed in Dörner et al 2015). Indeed, our confocal microscopy data showed that epratuzumab tends to pull proteins together in a single ‘bud’ on the B cell surface. There is no apparent role for Fc-FcR-mediated events and, indeed, experiments are often conducted with a F(ab’)2 fragment to eliminate any confounding role of FcR’s in mixed cell cultures. The limited functional effect seen with F(ab’)2 epratuzumab on some readouts in the current study suggests that there may be a threshold for the direct induction of specific Tyr phosphorylation events that is more easily achieved with the co-engagement of FcR’s. Alternatively, some effects with epratuzumab such as the loss of B cell surface proteins onto neighbouring cells (aka trogocytosis) have been shown to be dependent on the Fc (Rossi et al 2013) and this may indicate a differential mechanism for the phosphorylation of some of the regulatory Tyr residues on CD22.
Although CD16 is expressed on B cell progenitor cells in the bone marrow, CD32B is the only FcγR expressed on mature B cells. When B cells are activated with immune complexes or anti-BCR, CD32B is known to co-aggregate with the BCR to deliver inhibitory BCR signals. Specifically, phosphorylation of the ITIM motif at Tyr292 by Src family tyrosine kinase members leads to the recruitment of SH2-domain containing phosphatases, in particular SHIP-1 but to a lesser extent SHP-1. Some of the functional consequences of this include inhibition of Ca2+ mobilization and proliferation (Ravetch and Bolland 2001). In the context of the present experiments, epratuzumab could trigger the phosphorylation of CD32B directly via its Fc interaction but this is unlikely due to the low affinity of CD32B for monomeric IgG and is more likely to be due to concomitant binding to CD22 and CD32B, thereby inducing the clustering of all three molecules (CD32B, CD22 and BCR) together on the B cell surface. Another consequence of CD32B/BCR co-ligation is the dephosphorylation of CD19 which further attenuates BCR signalling and epratuzumab has been shown to down-regulate CD19 function on B cells (Rossi et al 2013). Bispecific antibodies that co-engage CD32B with CD79β, a component of the BCR complex, have been shown to inhibit signalling and functional activation of B cells and such an approach has been proposed as a potential therapy in autoimmune diseases (Veri et al 2010). Interestingly, the B cells from patients with autoimmune diseases such as SLE (Mackay et al 2006) and RA (Catalán et al 2010) express CD32B at lower levels relative to healthy individuals and, in this context, it would be relevant to explore the effects of epratuzumab on CD22 and CD32B phosphorylation events in B cells from patients with autoimmune diseases.
Conclusions
Epratuzumab directly induced phosphorylation of inhibitory ITIM motifs within key negative regulatory B cell molecules. On CD22, these included Tyr822, with a concomitant increase in SHP-1 co-localization and Tyr807, both of which would inhibit BCR-driven signaling. Finally, epratuzumab induced Tyr292 phosphorylation on the inhibitory Fc receptor CD32B, potentially further dampening a hyper-reactive B cell response. Overall, the data provide further evidence that epratuzumab down-modulates B cell activation events in normal B cells extending also to CD32B.
Acknowledgments
The authors acknowledge Jennifer Timoshanko, PhD, UCB Pharma, UK, for publication coordination and Helen Chambers, DPhil, Costello Medical Consulting, UK, for editorial assistance, which was funded by UCB Pharma. The authors also thank Helen Brand, BSc, UCB Pharma, UK, for preparing epratuzumab F(ab’)2 and Fab batches.
Author contributions
SL designed the study, performed the experiments, analyzed the data, wrote the manuscript and was involved in the interpretation of the data. SJF and AW designed the study, performed the experiments, analyzed the data and were involved in the interpretation of the data. AS designed the study, analyzed the data, wrote the manuscript and was involved in the interpretation of the data. AM, CD and TD designed the study, analyzed the data and were involved in the interpretation of the data. All authors critically reviewed the manuscript for important intellectual content and approved the final version.
Abbreviations
- BCR
B cell receptor
- HD
Healthy donor
- FcR
Fc receptor
- ITIM
Immunoreceptor tyrosine-based inhibition motif
- PBMC
Peripheral blood mononuclear cells
- SLE
Systemic lupus erythematosus
- Tyr
Tyrosine
References
- Ackermann JA, Radtke D, Maurberger A, Winkler TH, Nitschke L. Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 2011;30:1621–1633. doi: 10.1038/emboj.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T, Juan T, Talvenheimo J, Montestruque S, Sun J. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9:3982s–3990s. [PubMed] [Google Scholar]
- Catalán D, Aravena O, Sabugo F, Wumann P, Soto L, Kalergis AM, Cuchacovich M, Aguillón JC. B cells from rheumatoid arthritis patients show important alterations in the expression of CD86 and FcγRIIb, which are modulated by anti-tumor necrosis factor therapy. Arth Res Ther. 2010;12:R68. doi: 10.1186/ar2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall KM. Inhibitory signals by B cell FcγRIIb. Curr Opin Immunol. 1998;10:306–312. doi: 10.1016/S0952-7915(98)80169-6. [DOI] [PubMed] [Google Scholar]
- Dörner T, Shock A, Smith KG. CD22 and autoimmune disease. Int Revs Immunol. 2012;31:363–378. doi: 10.3109/08830185.2012.709890. [DOI] [PubMed] [Google Scholar]
- Dörner T, Shock A, Goldenberg DM, Lipsky PE. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: implications for the treatment of systemic lupus erythematosus. Autoimmun Rev. 2015;14:1079–1086. doi: 10.1016/j.autrev.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Fleischer V, Sieber J, Fleischer SJ, Shock A, Heine G, Daridon C, Doerner T. Epratuzumab inhibits the production of proinflammatory but not regulatory cytokines by B cells from healthy donors and SLE-patients. Arth Res Ther. 2015;17:185. doi: 10.1186/s13075-015-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer SJ, Daridon C, Fleischer V, Lipsky PE, Dörner T. Enhanced tyrosine phosphatase activity underlies dysregulated B-cell receptor signaling and promotes survival of human lupus B-cells. Arthritis Rheumatol Accept Article. 2015 doi: 10.1002/art.39559. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6:1341–1353. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J Immunol. 2005;174:1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- Isnardi I, Lesourne R, Bruhns P, Fridman WH, Cambier JC, Daëron M. Two distinct tyrosine-based motifs enable the inhibitory receptor FcRIIB to cooperatively recruit the inositol phosphatases SHIP1/2 and the adapters Grb2/Grap. J Biol Chem. 2004;279:51931–51938. doi: 10.1074/jbc.M410261200. [DOI] [PubMed] [Google Scholar]
- Jacobi AM, Goldenberg DM, Hiepe F, Radbruch A, Burmester GR, Dörner T. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Ann Rheum Dis. 2008;67:450–457. doi: 10.1136/ard.2007.075762. [DOI] [PubMed] [Google Scholar]
- Jang IK, Zhang J, Gu H. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol Rev. 2009;232:150–159. doi: 10.1111/j.1600-065X.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- Keren Z, Melamed D. Antigen receptor signaling competence and the determination of B cell fate in B-lymphopoiesis. Histol Histopathol. 2005;20:187–196. doi: 10.14670/HH-20.187. [DOI] [PubMed] [Google Scholar]
- Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcγRIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney A, Hotson D, Rapecki S, Fossati G, Lumb S, Rosen D, Putta S, Wale N, Spellmeyer D, Cesano A, Hawtin R, Shock A. Epratuzumab induces broad inhibition of B cell receptor proximal signaling but has opposing effects on distal signaling in B cell subsets: a profile of effects on functional immune signaling by single cell network profiling. Arthritis Rheum. 2014;66:S1255. [Google Scholar]
- Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- Neumann K, Oellerich T, Heine I, Urlaub H, Engelke M. Fc gamma receptor IIb modulates the molecular Grb2 interaction network in activated B cells. Cell Signal. 2011;23:893–900. doi: 10.1016/j.cellsig.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem. 2001;276:44315–44322. doi: 10.1074/jbc.M105446200. [DOI] [PubMed] [Google Scholar]
- Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF. CD22 Forms a quaternary complex with SHIP, Grb2, and Shc: a pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J Biol Chem. 2000;275:17420–17427. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- Qu Z, Goldenberg DM, Cardillo TM, Shi V, Hansen HJ, Chang C-H. Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood. 2008;111:2211–2219. doi: 10.1182/blood-2007-08-110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Ann Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang C-H. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. 2013;122:3020–3029. doi: 10.1182/blood-2012-12-473744. [DOI] [PubMed] [Google Scholar]
- Saxon A, Feldhaus J, Robins RA. Single step separation of human T and B cells using AET treated SRBC rosettes. J Immunol Methods. 1976;12:285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Shock A, Kilgallen B, Koetse W, Stach C, Bongardt S, Galateanu C. Pharmacodynamic effects of the CD22-targeted monoclonal antibody epratuzumab on B cells in patients with systemic lupus erythematosus. Arthritis Rheum. 2014;66:S856. [Google Scholar]
- Sieger N, Fleischer S, Mei H, Reiter K, Shock A, Burmester G, Daridon C, Dörner T. CD22 ligation inhibits downstream B cell receptor signaling and Ca2+ flux upon activation. Arthritis Rheum. 2013;65:770–779. doi: 10.1002/art.37818. [DOI] [PubMed] [Google Scholar]
- Tsubata T. Role of inhibitory BCR co-receptors in immunity. Infect Disord Drug Targets. 2012;12:181–190. doi: 10.2174/187152612800564455. [DOI] [PubMed] [Google Scholar]
- Veri M-C, Burke S, Huang L, Li H, Gorlatov S, Tuaillon N, Rainey GJ, Ciccarone V, Zhang T, Shah K, Jin L, Ning L, Minor T, Moore PA, Koenig S, Hohnson S, Bonvini E. Therapeutic control of B cell activation via recruitment of Fcγ receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 2010;62:1933–1943. doi: 10.1002/art.27477. [DOI] [PubMed] [Google Scholar]