Abstract
Degeneration of the intervertebral disc (IVD) is a major underlying contributor to back pain—the single leading cause of disability worldwide. However, we possess a limited understanding of the etiology underlying IVD degeneration. To date, there are a limited number of mouse models that have been used to target proteins in specific compartments of the IVD to explore their functions in disc development, homeostasis and disease. Furthermore, the majority of reports exploring the composition and function of the outer encapsulating annulus fibrosus (AF) of the IVD have considered it as one tissue, without considering the numerous structural and functional differences existing between the inner and outer AF. In addition, no mouse models have yet been reported that enable specific targeting of genes within the outer AF. In the current report, we discuss these issues and demonstrate the localized activity of Cre recombinase in the IVD of Col1a2-Cre(ER)T;ROSA26mTmG mice possessing a tamoxifen-dependent Cre recombinase driven by a Cola2 promoter and distal enhancer and the mTmG fluorescent reporter. Following tamoxifen injection of 3-week-old Col1a2-Cre(ER)T;ROSA26mTmG mice, we show Cre activity specifically in the outer AF of the IVD, as indicated by expression of the GFP reporter. Thus, Col1a2-Cre(ER)T;ROSA26mTmG mice may prove to be a valuable tool in delineating the function of proteins in this unique compartment of the IVD, and in further exploring the compositional differences between the inner and outer AF in disc homeostasis, aging and disease.
Keywords: Intervertebral disc, Outer annulus fibrosus, Inner annulus fibrosus, Tissue-specific gene deletion, Col1a2
Introduction
Back pain, the leading specific cause of disability in non-fatal health outcomes of disease and injury (Lim et al. 2012), has a lifetime incidence as high as 84 % (Walker 2000) and a point prevalence of 11.9 % (Hoy et al. 2012). Of the numerous spine-related pathologies associated with pain in the lumbar spine, intervertebral disc (IVD) herniation and degeneration are the most significant contributors and hence are targets for intervention (Kuslich et al. 1991; Deyo and Weinstein 2001). Yet, despite the large burden that IVD disease places on health care systems worldwide, the molecular mechanisms underlying IVD disease remain largely unknown; indeed, there are no clinical therapies targeting its pathophysiology. Thus, identifying the relative roles and function of proteins within the unique compartments of the IVD represents an important step towards understanding the pathogenesis of IVD disease and identifying therapeutic targets for this crippling condition.
The IVD, the connective tissue structure that lies between vertebrae of the spine, is essential for spine stabilization and movement (Adams and Roughley 2006). The disc consists of three distinct, highly specialized yet interdependent tissues: the nucleus pulposus (NP), the annulus fibrosus [AF; being further divided into the inner annulus fibrosus (IAF) and outer annulus fibrosus (OAF)] and the cartilage endplates. Precise regulation of matrix synthesis by distinct IVD cell types is essential for the IVD to fulfill its physiological function of absorbing compressive loading and conferring flexibility to the spine. Each tissue type of the IVD contains a unique protein composition that is hypothesized to play an important role in maintaining tissue health.
Many proteins that play crucial roles in functionally and compositionally related tissues (such as cartilage, ligament and tendon) have been identified as therapeutic targets in pathological conditions afflicting these tissues. However, we possess a limited understanding of the roles of many of these proteins in IVD health and dysfunction. Due in part to the limited number of genetic tools that specifically target distinct cell types within the disc, only a relatively small number of studies have used a targeted approach to assess the relative contribution of individual subpopulations of cell types to IVD function. Specifically, the inner and outer AF show distinct biochemical and structural properties that underlie differences in their respective biochemical and physiological functions (Antoniou et al. 1996; Hayes et al. 2001; Bruehlmann et al. 2002; Han et al. 2012; Li et al. 2014) (discussed below). Despite these observations, the vast majority of studies have examined AF structure and function in its entirety. An increasing body of evidence has shown that the OAF shows enriched expression of matricellular proteins including thrombospondin-2 (TSP-2) (Gruber et al. 2006), periostin (POSTN) (Gruber et al. 2011), tenascin (TN) (Gruber et al. 2002) and Secreted Protein, Acidic, and Rich in Cysteine (SPARC) (Gruber et al. 2004). Although they possess important roles in related tissues, the contribution of many such proteins to OAF function has not been rigorously assessed (Bedore et al. 2014).
The above reports suggest that tools to specifically genetically target proteins in the OAF would be extremely useful. One such model might be the Col1a2-Cre(ER)T transgenic mouse which drives the expression of a tamoxifen-inducible Cre recombinase under the control of a specific distal enhancer element localized ~20 kb upstream of the endogenous Col1a2 promoter (Bou-Gharios et al. 1996; Ponticos et al. 2004). In these mice, Cre is expressed in all Col1a2-expressing cells within many tissues, with the exception of osteoblasts derived from endochondral ossification. Although annulus fibrosus cells of intervertebral disc are known to express high levels of type I collagen (Eyre and Muir 1976; Antoniou et al. 1996; Clouet et al. 2009; Li et al. 2014), whether Cre is active in the IVD of Col1a2-Cre(ER)T mice has not been previously examined. In this report, we examined the activity of this Col1a2 promoter and distal enhancer element following Cre induction in 3-week old mice.
Methods
Mice hemizygous for a tamoxifen-dependent Cre recombinase under the control of the Col1a2 promoter and far upstream enhancer elements (Ponticos et al. 2004) were bred with mice harboring a double-fluorescent reporter transgene (mTmG) integrated into the Gt(ROSA)26Sor locus (Muzumdar et al. 2007) to generate Col1a2-Cre(ER)T;ROSA26mTmG mice, as previously described (Parapuram et al. 2015). The mTmG transgene confers expression of membrane-targeted tandem timer (td)Tomato prior to Cre-mediated excision and membrane-targeted green fluorescent protein (GFP) after excision. In this model, cells expressing Cre at the time of tamoxifen induction are permanently labeled by expression of GFP, while all other cells are labeled by expression of (td)Tomato (Muzumdar et al. 2007).
To induce Cre recombinase, 3-week-old littermate Col1a2-Cre(ER)T;ROSA26mTmG mice were given 0.1 ml intraperitoneal injections of a 10 mg/ml tamoxifen solution in corn oil, or corn oil alone as a vehicle control, for five consecutive days. One hundred days later, mice were sacrificed and individual IVDs were dissected out of the lumbar spine. IVDs were fixed in formalin overnight, incubated at 4 °C in 30 % (v/v) sucrose, embedded in OCT mounting medium (VWR, Mississauga, ON) and sectioned at a thickness of 8 μm. Mounting was performed with VECTASHIELD Mounting Medium with DAPI (Burlingame, CA), and images were acquired using a Leica Microsystems DM16000B fluorescent microscope and DFC360FX camera. Images of whole IVDs were produced from 100x magnification images using AutoStitch image reconstruction software (Brown and Lowe 2006). All aspects of this study were conducted in accordance with the policies and guidelines set forth by the Canadian Council on Animal Care and were approved by the Animal Use Subcommittee of the University of Western Ontario, London, ON.
Results
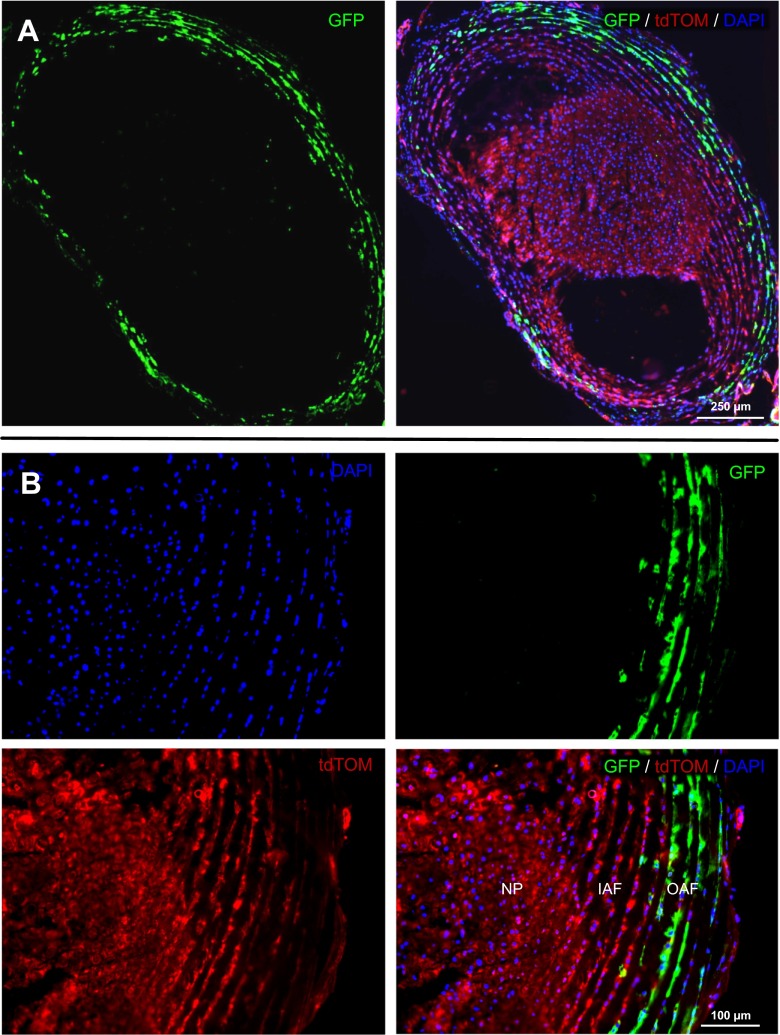
To localize the activity of the Col1a2 promoter/enhancer in the IVD, 3-week-old Col1a2-Cre(ER)T;ROSA26mTmG mice were injected with tamoxifen, and 100 days later localization of reporter expression was detected by visualization of tdTomato and GFP fluorescence from cryosectioned lumbar discs. Examination of lumbar intervertebral discs from tamoxifen-treated Col1a2-Cre(ER)T;ROSA26mTmG mice (Fig. 1) showed GFP expression, indicating Cre-mediated recombination, specifically in cells of the outer annulus fibrosus (OAF), but not inner annulus fibrosus (IAF) or nucleus pulposus (NP).
Fig. 1.
Cre recombinase activity in the intervertebral disc of Col1a2-Cre(ER)T;ROSA26mTmG reporter mice. Reporter mice expressing a tamoxifen-inducible cre recombinase under the control of a Col1a2 promoter/enhancer (Ponticos et al. 2004) show expression of membrane-targeted tdTomato (tdTom) prior to Cre-mediated recombination and membrane-targeted GFP following Cre-mediated recombination. Three-week-old mice were injected with tamoxifen, and 100 days later localization of reporter expression was detected by visualization of tdTom and GFP fluorescence in cryosectioned lumbar discs. Tissue was counterstained with DAPI to allow visualization of nuclei. a Using image reconstruction software, visualization of an entire lumbar intervertebral disc of tamoxifen-treated Col1a2-Cre(ER)T;ROSA26mTmG mice shows GFP expression indicating Cre-mediated recombination specifically in the outer annulus fibrosus (OAF), but not in the inner annulus fibrosus (IAF) or nucleus pulposus (NP). TdTom expression is detected in untargeted cells (i.e., cells not expressing Cre under the control of the Col1a2 promoter/enhancer at the time of tamoxifen injection). b 200x magnification of NP/IAF/OAF boundary. Scale bars indicate 250 and 50 μm, respectively
Discussion
Whole-body knockout mice provide useful tools to characterize the functions of specific proteins in cellular and physiological processes in vivo. However, the use of such mice to study pathologies is particularly challenging as candidate regulators of IVD function may also be involved broadly in embryogenesis and/or tissue homeostasis. As a consequence, deletion of these genes is often embryonically lethal. To circumvent such problems, tissue-specific and inducible Cre systems have been developed to drive or delete a gene of interest in the desired tissue in a time- and or tissue-specific manner in the IVD (Table 1). To target genes of interest specifically in the NP, Shh (Choi et al. 2012; Maier et al. 2013), Foxa2 (Frank et al. 2007; Merceron et al. 2014) and Noto (McCann et al. 2012; Bedore et al. 2013) Cre transgenic mice have been used, whereas Agc Cre transgenic mice have been used to target genes of the IAF and NP (Henry et al. 2009). In addition, the Col2a1 Cre (Hsieh et al. 2009; Chen et al. 2014) shows specific activity and has been used to target genes in the IAF. Lastly, the Gdf5 Cre shows activity in both the IAF and OAF (Mundy et al. 2011). Importantly, no transgenic mice had been identified that enable the analysis of protein function specifically in the OAF.
Table 1.
Identified tissue-specific transgenic mouse models with Cre recombinase activity in the IVD
| Cre | NP | IAF | OAF | Reference |
|---|---|---|---|---|
| Gdf5 | - | + | + | (Mundy et al. 2011) |
| Acn | + | + | - | (Henry et al. 2009) |
| Noto | + | - | - | (McCann et al. 2012) |
| Shh | + | - | - | (Choi et al. 2012) |
| Col2al | - | + | - | (Chen et al. 2014) |
| Foxa2 | + | - | - | (Frank et al. 2007) |
+ Cre activity detected; -Cre activity not detected
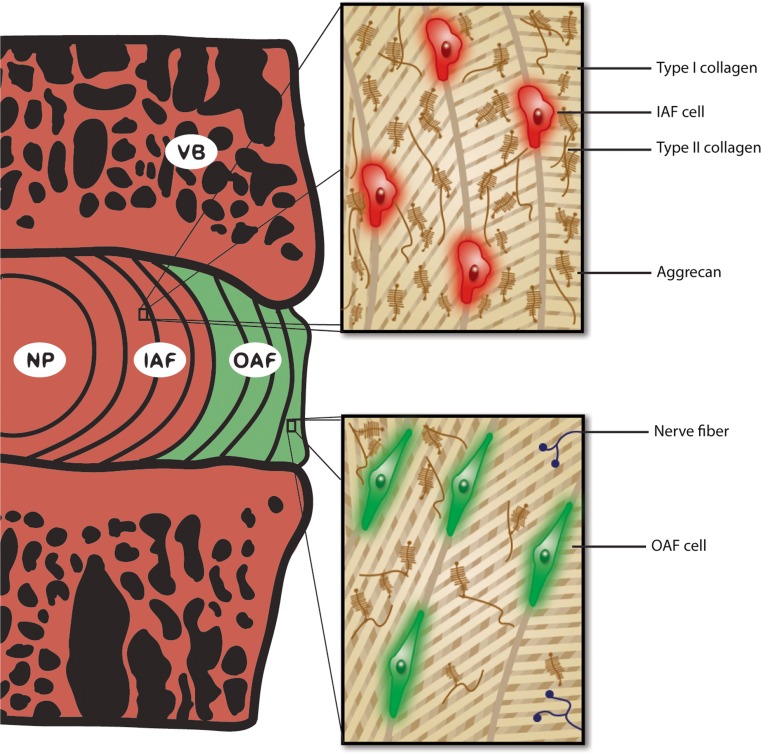
Salient differences have been reported between the IAF and OAF (Fig. 2) with respect to cellular morphology and matrix composition (Bruehlmann et al. 2002), biochemical, structural and biomechanical properties (Hayes et al. 2001; Han et al. 2012; Li et al. 2014), as well as relative protein composition changes during degeneration (Antoniou et al. 1996). Type I collagen is more abundant and collagen fibrils are larger in diameter and more tightly packed in the OAF than IAF, which collectively confers a higher stiffness to the tissue (Han et al. 2012). Conversely, the IAF shows a higher aggrecan and type II collagen content (Li et al. 2014), narrower fibril diameter and larger extrafibrillar spacing (Han et al. 2012), which together allows the IAF to more readily conform its shape in response to changes in osmotic pressure within the NP. Thus, differences in structure and protein composition of the inner and outer annulus are complementary in conferring mechanical properties to the entire AF that allow it to fulfill both of its major functions: to effectively absorb osmotic pressure from the NP, and prevent structural failure leading to pain (Aprill and Bogduk 1992). Another notable difference between IAF and OAF relevant to pathophysiological studies is that in a healthy IVD the OAF is innervated, whereas nerve fibers do not reach into the IAF (Bogduk et al. 1981). In addition, within the OAF cells show fusiform (spindle-like) morphology, whereas IAF cells are more spherical in shape (Bruehlmann et al. 2002). This evidence suggests that delineation of the IAF and OAF is critical in the study of the pathogenesis of IVD degeneration, particularly when considering the major compositional and structural changes that occur in the AF during ageing and disc degeneration (Antoniou et al. 1996; Ye et al. 2015). Thus Col1a2-Cre(ER)T mice may serve as a valuable tool for identifying the function of proteins that reside in this unique tissue, and hence will allow us to gain a better understanding of the processes and pathways regulating OAF health and function in tissue homeostasis and disease. Given that the expression of type I collagen is dynamic in development, homoestasis and aging, further assessment of the potential utility of Col1a2-Cre(ER)T;ROSA26mTmG mice will require a more detailed temporal analysis of the activity of the Col1a2 promoter/enhancer activity in these mice.
Fig. 2.
Schematic representation illustrating key differences between inner and outer annulus fibrosus tissues of a healthy intervertebral disc. The inner annulus fibrosus (IAF) and outer annulus fibrosus (OAF) show marked yet complimentary distinctions in their biochemical and structural properties. OAF cells have a fusiform (spindle-like) morphology, whereas IAF cells are more spherical in shape. Type I collagen is more abundant in the OAF than the IAF, whereas the IAF shows higher aggrecan and type II collagen content. In addition, collagen fibrils are larger in diameter and more tightly packed in the OAF than the IAF. The OAF is also innervated, whereas nerve fibres do not reach into the IAF in a healthy IVD. The pattern of fluorescent protein expression in IVDs from the Col1a2-Cre(ER)T;ROSA26mTmG mouse is depicted in this schematic. NP nucleus pulposus, VB vertebral body
Acknowledgments
We would like to thank Min Kyu Mark Kim for his artistic input and assistance with schematic design.
Compliance with ethical standards
Role of funding source
This work was funded by the Canadian Institutes of Health Research (CIHR) [MOP-115718] to CAS, and [MOP-77603] to AL. JB has been supported by the Joint Motion Program – A CIHR Training Program in Musculoskeletal Health Research and Leadership, a fellowship from the Arthritis Society and a CIHR Doctoral Research Award. JB is also the recipient of a Springer Scholarship from the International CCN Society. KQ has been supported by undergraduate research awards from the CIHR and the Joint Motion Program. CAS is supported by a New Investigator Award from CIHR and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
Competing interests
The authors declare that they have no competing interests.
References
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361–369. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- Bedore J, Sha W, McCann MR, Liu S, Leask A, Seguin CA. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 2013;65(10):2634–2644. doi: 10.1002/art.38075. [DOI] [PubMed] [Google Scholar]
- Bedore J, Leask A, Seguin CA. Targeting the extracellular matrix: matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014;37:124–130. doi: 10.1016/j.matbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(Pt 1):39–56. [PMC free article] [PubMed] [Google Scholar]
- Bou-Gharios G, Garrett LA, Rossert J, Niederreither K, Eberspaecher H, Smith C, Black C, Crombrugghe B. A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol. 1996;134(5):1333–1344. doi: 10.1083/jcb.134.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Lowe DG. Automatic panoramic image stitching using invariant features. Int J Comput Vis. 2006;74(1):59–73. doi: 10.1007/s11263-006-0002-3. [DOI] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201(2):159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li S, Xie W, Wang B, Chen D. Col2CreER(T2), a mouse model for a chondrocyte-specific and inducible gene deletion. Eur Cell Mater. 2014;28:236–245. doi: 10.22203/ecm.v028a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev. 2012;129(9–12):255–262. doi: 10.1016/j.mod.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouet J, Grimandi G, Pot-Vaucel M, Masson M, Fellah HB, Guigand L, Cherel Y, Bord E, Rannou F, Weiss P, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford) 2009;48(11):1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157(1):267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Elliott SA, Park EJ, Hammond J, Saijoh Y, Moon AM. System for inducible expression of cre-recombinase from the Foxa2 locus in endoderm, notochord, and floor plate. Dev Dyn. 2007;236(4):1085–1092. doi: 10.1002/dvdy.21093. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Hanley EN., Jr Tenascin in the human intervertebral disc: alterations with aging and disc degeneration. Biotech Histochem. 2002;77(1):37–41. doi: 10.1080/bih.77.1.37.41. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Leslie K, Hanley EN., Jr Cellular, but not matrix, immunolocalization of SPARC in the human intervertebral disc: decreasing localization with aging and disc degeneration. Spine (Phila Pa 1976) 2004;29(20):2223–2228. doi: 10.1097/01.brs.0000142225.07927.29. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Hanley EN., Jr Immunolocalization of thrombospondin in the human and sand rat intervertebral disc. Spine (Phila Pa 1976) 2006;31(22):2556–2561. doi: 10.1097/01.brs.0000241117.31510.e3. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Norris RA, Kern MJ, Hoelscher GL, Ingram JA, Zinchenko N, Hanley EN., Jr Periostin is expressed by cells of the human and sand rat intervertebral discs. Biotech Histochem. 2011;86(3):199–206. doi: 10.3109/10520291003722774. [DOI] [PubMed] [Google Scholar]
- Han WM, Nerurkar NL, Smith LJ, Jacobs NT, Mauck RL, Elliott DM. Multi-scale structural and tensile mechanical response of annulus fibrosus to osmotic loading. Ann Biomed Eng. 2012;40(7):1610–1621. doi: 10.1007/s10439-012-0525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20(2):107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47(12):805–814. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Chen NT, Lo SH. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Mol Carcinog. 2009;48(6):545–552. doi: 10.1002/mc.20491. [DOI] [PubMed] [Google Scholar]
- Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22(2):181–187. [PubMed] [Google Scholar]
- Li J, Liu C, Guo Q, Yang H, Li B. Regional variations in the cellular, biochemical, and biomechanical characteristics of rabbit annulus fibrosus. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Lo Y, Harfe BD. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0055528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MR, Tamplin OJ, Rossant J, Seguin CA. Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5(1):73–82. doi: 10.1242/dmm.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merceron C, Mangiavini L, Robling A, Wilson TL, Giaccia AJ, Shapiro IM, Schipani E, Risbud MV. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, Koyama E, Pacifici M. Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol. 2011;351(1):70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Parapuram SK, Thompson K, Tsang M, Hutchenreuther J, Bekking C, Liu S, Leask A. Loss of PTEN expression by mouse fibroblasts results in lung fibrosis through a CCN2-dependent mechanism. Matrix Biol. 2015;43:35–41. doi: 10.1016/j.matbio.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Abraham D, Alexakis C, Lu QL, Black C, Partridge T, Bou-Gharios G. Col1a2 enhancer regulates collagen activity during development and in adult tissue repair. Matrix Biol. 2004;22(8):619–628. doi: 10.1016/j.matbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205–217. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Ye D, Liang W, Dai L, Zhou L, Yao Y, Zhong X, Chen H, Xu J. Comparative and quantitative proteomic analysis of normal and degenerated human annulus fibrosus cells. Clin Exp Pharmacol Physiol. 2015;42(5):530–536. doi: 10.1111/1440-1681.12386. [DOI] [PubMed] [Google Scholar]