Abstract
The increased incidence of non-healing skin wounds in developed societies has prompted tremendous research efforts on the complex process known as “wound healing”. Unfortunately, the weak relevance of modern wound healing research to human health continues to be a matter of concern. This review summarizes the current knowledge of the cellular mechanisms that mediate wound closure in the skin of humans and laboratory animals. The author highlights the anatomical singularities of human skin vs. the skin of other mammals commonly used for wound healing research (i.e. as mice, rats, rabbits, and pigs), and discusses the roles of stem cells, myofibroblasts, and the matrix environment in the repair process. The majority of this review focuses on reepithelialization and wound closure. Other aspects of wound healing (e.g. inflammation, fibrous healing) are referred to when relevant to the main topic. This review aims at providing the reader with a clear understanding of the similarities and differences that have been reported over the past 100 years between the healing of human wounds and that of other mammals.
Keywords: Skin, Wound healing, Epidermis, Extracellular matrix, Granulation tissue, Myofibroblast, Stem cells, Mechanical force
Introduction
Epidermal healing, or reepithelialization, is the renewal of the epidermis that has been damaged by a wound or a burn. Epidermal healing is a chief concern for wound repair since all wounds are intended to ultimately be covered with an epithelium. The endpoint of epidermal healing is wound closure, i.e. restitution of the epidermal continuum, so that the new differentiated epidermis can act as a physical, chemical, and bacteriological barrier (one of skin’s vital functions).
The increased incidence of non-healing wounds has prompted tremendous research efforts on the complex process known as “wound healing”. Unfortunately however, the clinical realities of non-healing wounds are often found disconnected from the questions addressed by modern research projects. One obvious cause for this divergence is the extraordinary variability in wound presentation (size, depth, history of wound care, co-morbidities, etc.). This makes “wounds” difficult to model. The other obvious cause for inadequate clinical relevance is the poor reliability of in vitro and animal models for human wounds. An evaluation of 25 potential wound therapies revealed 15 years ago that human trials and experimental studies agree in only 57 and 53 % of cases when pre-clinical research was conducted in vitro and in small animals, respectively (Sullivan et al. 2001). The agreement rate reaches 78 % when research was conducted in pigs. Because pig research is cumbersome (due to the animal’s long life span and higher husbandry costs), most research remains conducted in small animals. Meanwhile, the human relevance of wound healing research continues to be a matter of concern (Gordillo et al. 2013).
Here, I will summarize the similarities and differences in the skin anatomy between humans and the animals used for wound healing research (mostly the mouse, rat, rabbit and pig). I will then review the cellular mechanisms of epidermal healing in humans and other mammals. Other aspects of the wound healing process (e.g. inflammation, fibrous healing) will be referred to when relevant to the main topic. Lastly, the majority of the work conducted on human wounds may appear dated to the youngest reader (in fact published well before “the Internet” was first introduced in the ‘60s). Most of these ‘dated’ papers established basic principles of human healing that have not been repeated since. They remain as relevant to the understanding of human wounds as they were when first published, and are therefore worth revisiting.
Mammalian skin anatomy
Mammalian skin is comprised of three layers: the epidermis that comprises the surface of the skin, the dermis, and the deep hypodermis (also called subcutaneous adipose layer). The skin is also populated by appendages (hair follicles, sebaceous and sweat glands), nerves, sensory corpuscles, and vasculature. Among all these structures, the epidermis is the part being restored during the reepithelialization process. This layer will be described in greater detail. Other components of the skin such as the dermis and skin appendages also have important supporting roles in the reepithelialization process: they provide nutritional and mechanical support and supply progenitor cells for the restoration of the epidermis. Species similarities and differences in each of these compartments are likely to greatly impact the entire reepithelialization process.
The epidermis
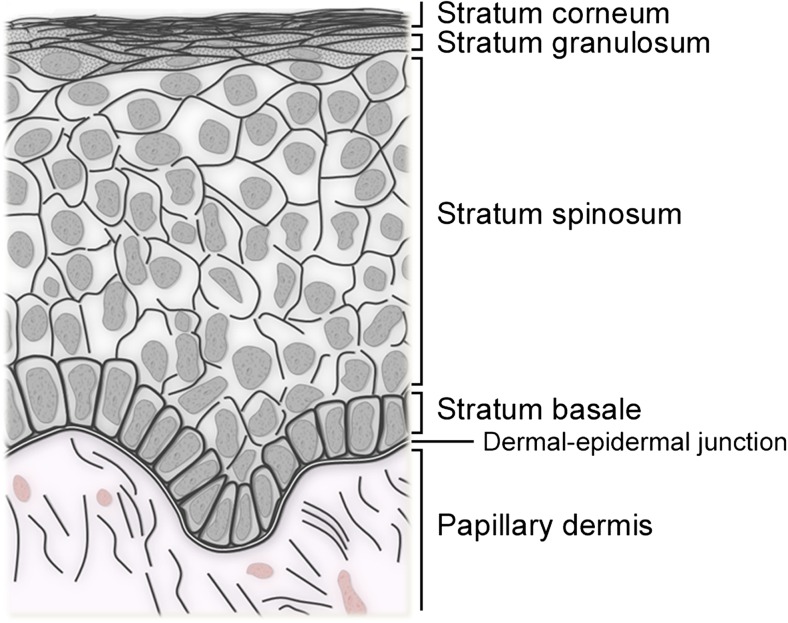
The human epidermis is a stratified epithelium made of keratinocytes organized in layers (Fig. 1). The deepest (basal) layer lies on a basement membrane at the dermal-epidermal junction. Basal keratinocytes can proliferate and give rise to daughter cells that migrate towards the skin surface in supra-basal layers. Keratinocyte upward migration is accompanied by a controlled, approximately 2–4 week-long, maturation program that ultimately leads to the formation of fully differentiated corneocytes. Corneocytes are polygonal squames devoid of nuclei and cytoplasmic organelles, loaded with highly cross-linked proteins, and surrounded by a lipid-rich matrix (Elias et al. 1977). Corneocytes make the outermost layer of the skin (the stratum corneum) highly hydrophobic. The stratum corneum serves as waterproof barrier that prevents water egress and xenobiotic ingress through the skin. Its barrier function makes the skin a vital organ. Restoring this vital barrier is the goal of the reepithelialization process.
Fig. 1.
Organization of the human epidermis. The human skin epidermis is a stratified epithelium comprised of four levels (or strata) on most body sites. These strata are (from deep to superficial): (1) the basal layer (or stratum basale), a unique layer in unwounded skin that contains proliferative keratinocytes and inter-follicular keratinocyte stem cells; (2) the spinous layer (stratum spinosum) is made of several layers of polyhedral keratinocytes that have lost their proliferation potential and initiated differentiation; (3) the granular layer (stratum granulosum). Its granulated appearance in hematoxylin and eosin staining is due to the presence of kerato-hyalin granules, which are filled with proteins highly cross-linked with keratin filaments; and (4) the horny layer (stratum corneum), made of 15–20 layers of cornified (dead) cells devoid of nucleus or cytoplasmic organelles. In addition, a stratum lucidum can be distinguished between stratum granulosum and stratum corneum in the thick skin of the palms and soles. In older publications, the reader might commonly find the description of a Malpighian layer (which comprises stratum spinosum and stratum granulosum), prickle cells (cells of the stratum spinosum in which many desmosomes can be seen), or prickle cell layer (stratum spinosum)
The epidermis of mice, rats, and rabbits is of similar organization but much thinner than the human epidermis (comprises a total of two to three layers) (Gabbiani et al. 1978; Montagna 1984b). The epidermis of hairy animals is also typically smooth at the dermal epidermal junction (Montagna 1972). The epidermis of domestic pigs is closer to that of human skin: both form ridges at the dermal-epidermal junction and have intersecting lines that form a pattern on their external surface. However, the pig’s epidermis contains high levels of alkaline phosphatase in its supra-basal layers (feature solely found in pigs) and a much denser stratum corneum compared to humans (Montagna and Yun 1964).
The evolution of human skin
Many aspects of the anatomy of the skin (pigmentation, dermis, adipose tissue, skin appendages) are unique to humans compared to non-human mammals. The singularity of human skin has developed late during evolution, when the genus Homo (and its only surviving species H. sapiens) developed via its adaptation to endurance running (Bramble and Lieberman 2004). Many energetic, skeletal, and stabilizing features facilitated this evolution. A sine qua non condition to endurance running is the ability to dissipate the metabolic heat generated by prolonged muscle activity. To achieve enduring thermoregulation, drastic changes have been made to the skin. These changes include reduction in body hairs (which typically act as insulators), apparition of eccrine sweat glands on hair-baring skin (to allow evaporative heat loss), extra innervation (which connects sweat glands to the hypothalamus and its heat set-point), and increased vascularization (to feed sweat glands and to regulate skin blood flow). As a result, a human being can generate up to four liters of sweat per hour through his/her skin to maintain constant body temperature (Sato et al. 1989).
Reduced body hair
Human hair follicle density varies upon skin sites. In Caucasians, hair follicle density is the highest on the head and face (~800/cm2), lowest on the lower leg and upper arm (~50/cm2), and similar on chest, abdomen, back, and forearms (65–95/cm2) (Szabo 1967). Since these initial numbers were introduced, several other studies have found slightly lower hair follicle densities on human skin: ~350/cm2 on the head, ~25/cm2 on legs and arms, and ~35/cm2 on chest and forearms (Hwang and Baik 1997; Sinclair et al. 2005; Rittié et al. 2013a).
Hair follicle density is also relatively low in the domestic pig, but only when considering adult animals (~10–20/cm2) as opposed to neonatal pigs (~730/cm2) (Ferry et al. 1995; Mangelsdorf et al. 2014). In contrast, small animals typically used for wound healing research have an abundant fur with follicle density assessed as high as ~1600/cm2 in rat, 1800/cm2 in rabbit, and ~5000/cm2 in mouse skin (Mangelsdorf et al. 2014).
The hair follicle and associated sebaceous and apocrine glands (when present for the latter) form the “hair follicle complex” (“pilosebaceous unit” in humans). Hair follicle complexes are grouped by two or three in all animals and this feature was maintained in humans (Meyer 2009). In humans, pigs, and guinea pigs, each follicle has its own inherent rhythm. In most rodents however, follicles are synchronized by groups and cycle by waves (Stenn and Paus 2001). In rodents, each hair follicle is connected to two sebaceous glands (on opposite sides) whereas there is only one sebaceous gland per hair follicle in pigs and humans (Bigelman and Mertz 2004). The basic morphological organization of hair follicle complexes is similar in all animals although structural details may vary among species (Montagna 1967).
Apparition of eccrine sweat glands on hair-baring skin
All mammals have eccrine sweat glands on their paws (palms and soles in apes and humans). In addition, humans are unique in that they have millions of bodily eccrine sweat glands distributed throughout their hair-baring skin. Although structurally alike and producing a similar water-based sweat, these two types of eccrine sweat glands form two distinct sub-categories based on their function and characteristics. Sweat is made on the palms and soles to increase adherence and grip. Palm and sole sweat glands react primarily to psychogenic signals (such as fear and stress), for they primarily respond to adrenergic stimulation. In contrast, bodily sweat glands produce sweat to regulate body temperature. They are controlled by the hypothalamus and primarily respond to cholinergic effectors (as for salivation and digestion) (Marples 1965; Cui and Schlessinger 2015). Indicative of their different phylogenic origin, paws and body eccrine sweat glands appear at different times during development (16th vs. 22nd week in humans, respectively), both from invagination of the epidermis (Siver et al. 1964; Marples 1965; Montagna 1984b).
Eccrine sweat glands are independent from pilosebaceous units. They are made of a coiled tubular epithelium associated in its coiled secretory portion with myoepithelial cells that allow sweat excretion. Eccrine sweat glands are solitary and evenly distributed throughout the skin, although their density varies upon skin sites (Sato and Sato 1983; Hwang and Baik 1997). Their coil is closely associated in the deep dermis with nerve endings, adipose pockets, and vasculature.
In pigs, so-called “eccrine” sweat glands are histologically distinct (they are branched tubular glands with a secretory epithelium), and are found only on the snout, lips and carpal organ (Montagna and Yun 1964). On the rest of the skin, pigs have another type of gland: the “apocrine” sweat glands. The term “apocrine sweat gland” is a remarkable misnomer for two reasons: 1) these glands have little to do with sweating, and 2) they have a merocrine (not apocrine) type of secretion (i.e. cells remain intact upon secretion). Apocrine glands are coiled tubular epithelial glands associated with hair follicles and are thus part of the hair follicle complex. They have an oily secretion that discharges within or near the tip of the infundibulum (Montagna 1972). They produce lubricant for the fur and are thought to protect skin from rain in animals with otherwise thin stratum corneum [such as in hoofed animals (e.g. horses, camels) and non-human primates (e.g. gorillas, orangutans)]. In humans, apocrine sweat glands develop from virtually all hair follicles between the 20th and the 24th week of development, but regress soon after (Montagna 1984a) except in the axillae, areola of nipple, and genital areas, where they fully develop after puberty (Saga 2002).
Increased vascularization
The skin of human beings is highly vascularized compared to non-human mammals. Blood enters human skin via small arteries that branch to arterioles, which branch again into capillaries (Fagrell 1984). Human skin vascularization supplies a superficial plexus that allows increased skin blood flow (and heat loss by convection), nutritional capillaries that feed the epidermis, and deeper vascularization connected with hair follicles and eccrine sweat glands (which produce sweat from plasma). All are connected through numerous vertical segments and anastomoses (Fagrell 1984). Once blood has passed the capillaries, it exits the skin through one or more systems of venous plexuses (Fagrell 1984). In all, human skin blood supply greatly exceeds what is needed for metabolism (Montagna 1984b).
The vasculature of porcine skin resembles that of humans for the presence of a lower, a mid-dermal and a sub-epidermal plexus (Forbes 1967), although the sub-epidermal vascular plexus is significantly less dense in porcine than in human skin (Vardaxis et al. 1997). Porcine skin also has less vasculature around the hair follicles and sebaceous glands vs. human skin (Montagna and Yun 1964). Comparatively, small rodents have a rudimentary vasculature consisting of two horizontal networks: the primary network is above the panniculus carnosus and below the adipose tissue, while the dermal network encircles the neck of hair follicles. Vertical vessels connecting the two networks run along the regularly-spaced large hair follicles [shown in haired and hairless mice: (Rea 1968; Forbes and Urbach 1969)]. Relative paucity of vertical connection makes the depth at which the blood circulates hardly controllable [shown in hairless mouse: (Argenbright and Forbes 1982)]. Thus, rodent hair-baring skin has low basal and stimulated blood flow [shown in haired mouse: (Forbes and Urbach 1969) and in rat: (Rendell et al. 1993)]. In contrast, hare and rabbit ear skins, which fulfill a thermoregulation function, are also highly vascularized (Montagna 1984b).
Anatomical compensations and compensatory losses
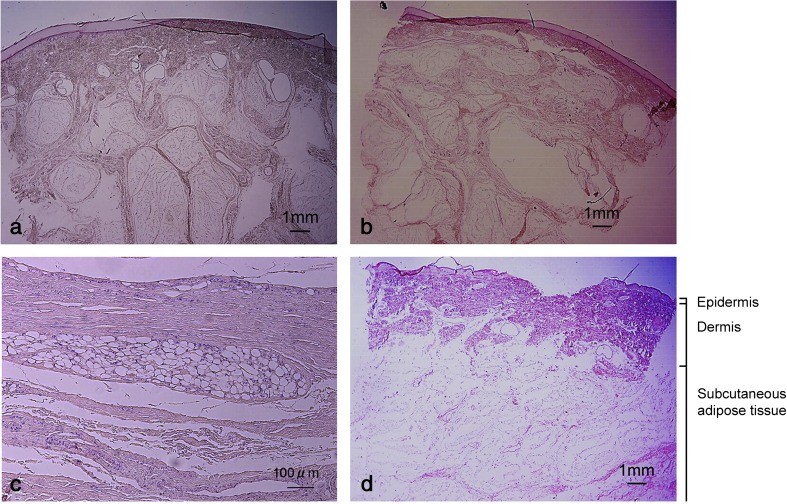
To compensate for the loss of hair (and related insulation), human skin acquired an adipose layer that is much thicker than in smaller mammals, albeit variable among skin sites (Fig. 2). Sensory innervation is also more developed in humans than other mammals (Montagna 1977).
Fig. 2.
Human subcutaneous adipose tissue varies in thickness and organization among skin sites. HE staining of excised skin obtained postmortem from the posterior aspect of heel (a), the plantar aspect of heel (b), the sacrum (c) and the center of the gluteus maximus (d). The three layers that comprise human skin are shown. Note that the adipose layer can be more than 1 cm-thick on some skin sites. Reproduced from (Arao et al. 2013) with permission
Extra skin features have been lost during evolution. Pig and humans are tight-skinned animals, as opposed to most other mammals such as mice, rats or rabbits that are loose-skinned animals. The latter is characterized by the presence of a panniculus carnosus, a layer of striated muscle lying beneath a relatively thin adipose layer. The panniculus carnosus makes the skin independent from deeper muscles and allows large skin movements (the skin thus appears unattached or loose). The panniculus carnosus is particularly important in the context of wound healing: its rich vascular supply constantly irrigates the wound bed [shown in rabbits: (Billingham and Russell 1956)], and it promotes a strong wound contraction that reduces the diameter of a wound by half during the first 24 h of healing [shown in rabbits: (Billingham and Russell 1956; Snowden et al. 1982)].
Implication for wound healing research
Pig and human skin appear anatomically close, or at least closer to each other than to the skin of mice, rats, or rabbits. Indeed, pigs and humans both have an epidermis and dermis thickness combined of ~1–3 mm (Ferry et al. 1995). The hair is sparser in both species when compared to most other mammals, and their dermal connective tissue contains elastic fibers (absent in rodents and rabbits). Epidermis and vascularization are comparable, and they both are devoid of panniculus carnosus (except for some remnants on a few sites (Greenwood 2010)), and thus do not contract their wounds early on. In contrast, humans have pilosebaceous units that are devoid of apocrine glands, and have millions of eccrine sweat glands throughout their bodies.
All things considered, anatomical similarities and differences between species might begin to explain that the porcine model is the best prognosticator of human outcome for testing wound healing treatments (Sullivan et al. 2001). We will see below that this agreement rate is higher for some aspects of the wound healing process (including reepithelialization) and lower for others (dermal healing).
Phases of skin repair
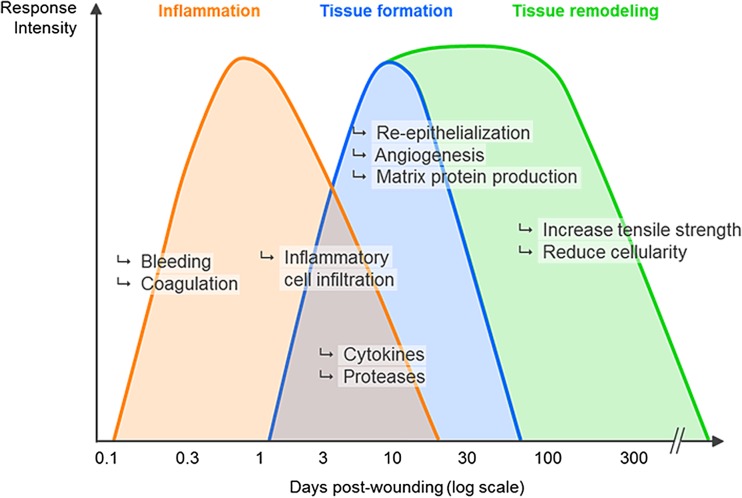
The term “wound healing” designates a complex series of biological processes that are typically described as the succession of three overlapping phases: inflammation, tissue formation (or proliferation phase), and tissue remodeling (Fig. 3). The inflammation phase is sometimes sub-divided into vascular response (hemostasis) and cellular response (inflammation). Overall, the inflammation phase consists in platelet activation that leads to formation of a fibrin clot and secretion of chemotactic agents followed by recruitment of neutrophils (cleanse the wound of foreign particles including bacteria), T-cells (sustain inflammation and recruit monocytes), and activation of macrophages (clear out debris) (Martin and Muir 1990; Clark 1996; Mehendale and Martin 2001; Havran and Jameson 2010; Su and Richmond 2015). The role of the inflammatory phase is to cleanse the wound from foreign cells and molecules before rebuilding the skin.
Fig. 3.
Phases of skin repair. The wound healing process is typically described as the succession of three overlapping phases: inflammation, tissue formation (or proliferation phase), and tissue remodeling. See text for details. This depiction derives mostly from the study of animal wounds and illustrates a wound repair process more segregated and less context-dependent than it is
The tissue formation phase consists in repairing the wound site by restoring the different parts of the skin that have been damaged: epidermis (by a process called reepithelialization, the focus of this review), dermis, and other structures (blood vessels, nerves, pilosebaceous units, and eccrine sweat glands). The nature of the wound will dictate not only the cellular mechanisms by which the tissue formation phase will take place, but also the time required for its completion (see below).
The last stage of the wound healing reaction is the remodeling phase, during which all of the processes that were activated until this point will be gradually turned off. This maturation phase will tentatively normalize epidermal-thickness, cellular content, extra-cellular matrix composition, and blood vessel count as close as possible to a pre-wound stage. Depending on the initial wound, the end-result can be anywhere from very close to the unwounded skin, to severely altered both cosmetically and functionally.
It is conventionally described that one phase needs to wind down before the next phase takes place. For example, the waning of the inflammatory phase is a pre-requisite for the deposition of extracellular matrix in the wound bed (dermal tissue formation). While an arbitrary partition in ‘phases’ is useful for teaching and description purposes, it undermines the fact that the wound healing process is continuous and that all wounds are somewhat heterogeneous in their progression through the wound healing phases. For instance, while studying shallow human wounds, we commonly saw one portion of a wound in the inflammatory phase (evidenced by heavy inflammatory infiltrate) while another area has already progressed in the tissue formation phase (evidenced by type I collagen deposition) (L. Rittié, unpublished observation). Thus, wound healing phases often overlap within a given wound. This phenomenon is probably of bigger impact for large wounds.
Wound phases partitions were also mostly established from studying wounds in laboratory animals. Importantly, the study of deep excisional wounds in humans revealed no correlation between neutrophil counts and the age of the wound, demonstrating that neutrophils persist during the healing of large healthy human wounds (Butterworth 1992). This is not the case in large white pigs (Dyson et al. 1988). Thus, albeit Butterworth's results remain to be confirmed, readers need to bear in mind that the wound healing phases described in Fig. 3: i) derive mostly from studying animal wounds; ii) is probably most useful for didactic purposes; iii) depict a wound repair process more segregated and less context-dependent than it truly is.
The nature of the wound dictates the repair mechanism
Successful wound reepithelialization depends on the effective occurrence of each event of the first two phases of the wound healing process. The amount of time needed for reepithelialization to complete depends on many factors, including specifics of the wound (e.g. location, depth, size, cleanness) and age of the patient (Du Noüy 1916a, b; Gillman et al. 1955).
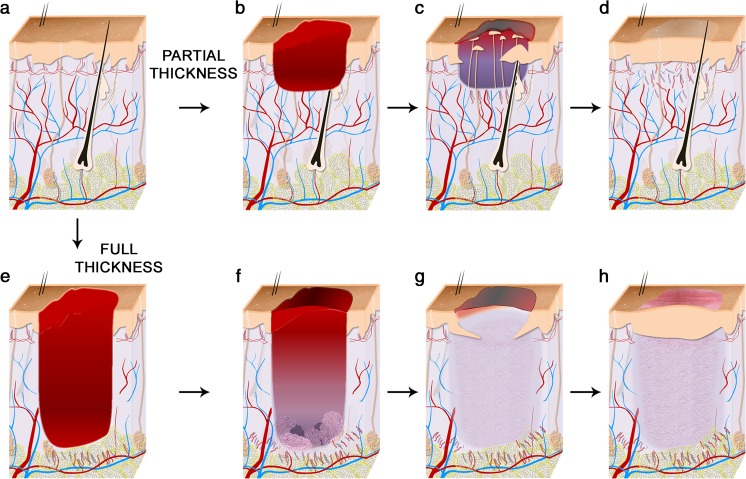
Skin wounds can be of variable depth and thus can affect one or more skin layers. To describe the nature of a lesion, wounds are typically classified as partial- or full-thickness wounds (Fig. 4). Partial-thickness wounds involve the epidermis and may involve a portion of the dermis. These wounds can be further classified in “superficial” and “deep” partial-thickness wounds, depending on the amount of dermis lost. Typically, epithelial-derived adnexal structures such as hair follicles, sebaceous glands, apocrine glands and/or eccrine sweat glands remain partially intact in a partial-thickness wound. Whether superficial or deep, a partial-thickness wound heals primarily by reepithelialization (Van Winkle 1968). In contrast, full-thickness wounds destroy the dermis, and possibly more. They do not heal by reepithelialization alone but require formation of a so-called granulation tissue to fill the void of the wound before epithelial covering.
Fig. 4.
Mechanisms of healing of partial- and full-thickness wounds in human skin. a Unwounded human skin is highly vascularized and contains hair follicles and eccrine sweat glands in a ratio 1:3 on most body sites. b–d Partial-thickness wounds involve the epidermis and may involve a portion of the dermis. They heal primarily by reepithelialization. New epidermis forms from outgrowths of eccrine sweat glands and pilosebaceous units underlying the wound. Keratinocyte migration from the wound edge is minimal. Wounds heal with minimal scarring and skin structure is minimally altered in the repaired site. e–g) Full-thickness wounds destroy the dermis (and possibly more). They do not heal by reepithelialization alone but require formation of a granulation tissue to fill the void of the wound before epithelial covering. After healing, the wound site bares a scar. Skin structure remains greatly modified with disappearance of appendages and altered vasculature
Three main approaches are used to treat a wound. The simplest one, or primary intention, deals with uninfected surgical sites or clean wounds with minimal tissue loss, and consists in closing the wound with sutures. Healing by secondary intention typically allows the repair process to restore larger tissue losses. It lets the wound close itself by a process of reepithelialization, with or without contraction of the underlying tissues (depending on species and wound depth; see below). Third intention healing, which relates to more complex wounds, aims at leaving the wound intentionally open for some time (for observation, disinfection, or debridement) and suture the wound when clean (Dimick 1988). In humans, most clean full-thickness wounds are sutured (with or without grafting) and thus heal by primary intention; epidermal repair then consists in merging the two edges of epidermis brought together. Two common exceptions are the wounds resulting from excision of pilonidal sinuses and hidradenitis suppurativa lesions. Both are frequently treated by excision and healing by secondary intention. The reepithelialization reaction then reaches its full significance, as a larger surface needs to be covered with new keratinocytes.
Reepithelialization of partial-thickness wounds
Partial-thickness wounds are rarely studied in small furred animals: i) their epidermis is about 50 μm-thick, which makes performing partial-thickness wounding a challenge; and ii) the extremely high hair density in small furred animals exaggeratedly intensifies the rate of reepithelialization. Hairless species are generally preferred. Among them, humans and pigs both have a skin thick enough to allow repeatable partial-thickness wounding; hence they are the most commonly studied.
Sequence of events leading to wound closure
In humans and pigs, tissue injury causes the wound healing response to take effect, starting with hemostasis and inflammation (Clark 1996). The nature of a wound is likely to determine the length of the inflammatory phase. Larger wounds with greater blood loss require longer hemostasis, while wounds that can be kept closed with simple pressure will clot faster. Similarly, a sharp injury (e.g. incision, laser wounds) will heal faster than those that generate necrotic tissue. Comparing self-inflicted razor and sodium sulfide (chemical burn) wounds, Bishop observed that chemical wounding kills cells below the level at which dead tissue is produced (Bishop 1945). Similarly, thermal injuries extend beyond the initial burn (Tiwari 2012), and if no adequate measure is taken to improve microcirculation around the initial burn, the adjacent zone of stasis (i.e. where microcirculation has stopped but cells are not quite dead) will undergo necrosis a few days post-burn. In all, necrotic tissue may progressively widen the initial injury and thereby act as an inhibitory factor for the wound healing process. Removal of necrotic tissue (or debridement) is recommended for optimal healing (Bishop 1945; Lu et al. 2002).
Hemostasis produces a blood clot that covers the wound. Thin blood clots will later be dissolved by the serous fluid that oozes through the dermis (termed “exudate”). Exudate is rich in adhesion molecules such as fibronectin, fibrin, and vitronectin that serve as chemoattractants and adhesion substrates to various cell types (Clark 1996). The wound surface begins to dry out and form a scab ten to eighteen hours post-partial-thickness wounding. Sometimes referred to as “slough” in moist wounds, the scab is initially a surface layer of fibrin and dead cells (largely polymorphonuclear neutrophils). The scab is pervious to liquid vapors and thus dries out from the top down until the wound bed equilibrates and is rewetted from below (Winter 1972). Some smaller animals form several scabs that get successively rejected and reformed [e.g. guinea pigs: (Zahir 1965)].
The reepithelialization phase obviously overlaps with the inflammatory phase since migratory activity of epithelial cells has been detected within a few hours of injury [shown in human incisional wounds: (Odland and Ross 1968) and domestic pig partial-thickness wounds: (Winter 1972)]. In pigs, new epidermis originates from hair follicles underlying the wound, apocrine gland ducts, and wound margin (Van Winkle 1968; Winter 1972; Miller et al. 1998). The latter represents only 10 % of the new epidermis, with 90 % originating from appendages within the wound (Winter 1972). In humans, the epidermis mostly originates from the pilosebaceous units and eccrine sweat glands underlying the wound, with also little contribution of the wound margin (Rittié et al. 2013a). In glabrous areas (devoid of hairs) such as palms, the new epidermis originates solely from eccrine sweat glands (Rittié et al. 2013a). In humans, all cells in the outer layer of the sebaceous glands and the outer root sheath of the hair follicle (including the bulge, main stem cell niche) express the proliferation marker Ki-67 for at least 5 days post-wounding, both in young and elderly skin (Rittié et al. 2016). In eccrine sweat glands, all cells of the outer layer of the absorption portion (but almost none in the secretory portion) are proliferative during the reepithelialization process (Rittié et al. 2016). This observation suggests the presence of stem cells in human eccrine sweat glands, but their location and properties have yet to be established (see below).
Overall, the appendage configuration of human and pigs is such that no outgrowth has to migrate farther than half the distance that separates two adnexal structures before meeting another outgrowth moving in the opposite direction. The maximal distance to cover to reepithelialize partial thickness wounds is approximately 1 mm in pigs (Winter 1972), and 500 μm in humans (Rittié et al. 2013a). This feature makes the reepithelialization much more efficient than if keratinocytes had to migrate all the way across the surface of the wound. Accordingly, 200-μm-deep partial-thickness wounds are fully reepithelialized in 6 days in pigs (Winter 1972) and in 8 days in young human adults (Rittié et al. 2013a).
Importance of stem cells for skin repair
The reepithelialization process requires abundant new cells to replace the keratinocytes that have been lost by injury. Adult stem cells, which are cells able to not only self-renew throughout adult life but also generate progeny with differentiation potential, have received exceptional attention. We will briefly review the current knowledge of the involvement of stem cells during wound repair, bearing in mind the following caveat: most of our understanding of adult stem cell physiology comes from studies in mice, which allow lineage tracing (i.e. genetic introduction of a stable marker into a single cell to trace all the progeny of that cell over time). Lineage tracing experiments require genetic manipulation and are thus not compatible with human experimentation. Therefore, mouse observations remain potentially applicable to human healing (until proven irrelevant). They might be only partially pertinent considering what is already known about many species singularities. For instance, stem cell markers often have a pattern of expression that is different in mouse vs. human skin. One example is CD34, a marker of bulge stem cells in mice (Arwert et al. 2012) that is excluded from the bulge region and expressed by non-stem cells in humans (Poblet et al. 1994; Ohyama et al. 2006; Rittié et al. 2009). Conversely, Lrig1-positive cells (which are thought to maintain the interfollicular epidermis in both species) are located in the upper hair follicle (isthmus) in mice (Page et al. 2013) but in the basal layer of the epidermis in humans (Jensen and Watt 2006). These differences are important and might hamper translation of mouse stem cell research to humans.
Skin epithelial adult stem cells
Under normal homeostatic conditions, various epithelial stem cell populations maintain the differentiated lineages that make the epithelial structures of the skin: hair follicle stem cells and sebaceous gland stem cells maintain the hair follicle complexes, and stem cells in the interfollicular epidermis maintain the epidermis (Blanpain and Fuchs 2014; Donati and Watt 2015). Epithelial stem cell populations do not form all at the same time during development. The hair follicle stem cells are specified in the earliest stages of hair follicle morphogenesis and later give rise to sebaceous gland stem cells [shown in mice: (Nowak et al. 2008)]. It is assumed that eccrine sweat glands are maintained by their own stem cell populations as well, although human bodily eccrine sweat glands are remarkably devoid of mitosis in basal conditions, suggesting an extremely low cell turnover [shown in humans: (Holyoke and Lobitz 1952; Lobitz et al. 1954; Rittié et al. 2013a, 2016)]. However, this turnover dramatically increases upon wounding [shown in humans: (Lobitz et al. 1954; Rittié et al. 2013a, 2016)] or in eccrine sweat glands of skin located near surgical sites (Morimoto and Saga 1995). These observations suggest the presence of adult stem cells in bodily eccrine sweat glands, as it is the case of paw stem cells in mice (Lu and Fuchs 2014). Whether the stem cell characteristics of mouse paw eccrine sweat glands can be translated to human bodily eccrine sweat gland cells is an open question that should be careful explored not only based on species differences, but also based on the anatomical differences existing between these two appendageal sub-categories described above.
Stem cell response to wounding
Adult stem cells have an extraordinary plasticity, a term used to describe the functional adaptation they undergo to produce progeny they normally do not maintain. For instance, bulge hair follicle stem cells are activated upon full-thickness wounding and produce progeny in the interfollicular epidermis for reepithelialization [shown in mice: (Taylor et al. 2000)]. This contribution is temporary as hair follicle stem cell tracers disappear from the interfollicular epidermis several weeks post-wounding [shown in mice: (Ito et al. 2005)]. The dramatic increase in mitotic activity in bodily eccrine sweat glands upon wounding noted above suggests that eccrine sweat gland stem cells also contribute progeny for reepithelialization. Eccrine sweat glands are able to repair their acrosyringium (intra-epidermal portion of the sweat gland) upon damage to the stratum corneum [shown in humans: (Lobitz et al. 1954) and mice: (Lu et al. 2012)]. In mice, epidermal scratch wound triggered a very minimal epidermal infiltration of sweat gland-derived cells after wounding (Lu et al. 2012), suggesting that many regenerative cells of the hyper-proliferative epidermis originated from neighboring epidermis. Whether the injury was too mild to clearly establish that eccrine sweat gland could contribute to epidermal repair in these studies remains a possibility. In humans, complete removal of epidermis triggers a proliferation response that is limited to the absorption portion (straight duct) of the gland (Rittié et al. 2016), suggesting that potential sweat gland stem cells are located in the duct and not the coil.
After full-thickness wounding, migrating hair follicle stem cells lose hair follicle markers and adopt an epidermal differentiation program (Blanpain and Fuchs 2014). This is a common trait: stem cell plasticity is accompanied by loss of the corresponding stem cells’ original markers. The mechanism for this switch is unknown. It was proposed that stem cell characteristics are provided by the cell environment as opposed to endogenous cell properties (Rompolas et al. 2013), and that damage to their niche depletes the hair follicle stem cell population (Matsumura et al. 2016). This interesting possibility could have important implications for cutaneous wound healing since damage to the extracellular matrix is a hallmark of skin aging (Rittié and Fisher 2015).
Use of stem cells for wound healing
Stem cells transplantations have been used for wound healing since the early 1800s: split thickness skin grafting is used to treat extensive wounds and burns. The graft is harvested as the top portion of the skin from a donor site. It can be further processed through a mesher that creates small slits in the graft so the graft can be extended to cover large surfaces (Semer and Adler-Lavan 2001). The graft provides new epidermis and its stem cells for reepithelialization, while the donor site heals as partial thickness wound via its appendageal stem cells. While progress in being made on characterizing and harvesting many types of stem cells to treat wounds in a more direct way, clinical use of stem cells remains hampered by cell preparation techniques, delivery techniques, and impact of the micro-environment on cell behavior (Ennis et al. 2013; Duscher et al. 2016).
Importance of the keratinocyte migration substrate
The keratinocytes of appendage outgrowths that form the new epidermis migrate in a plane between viable and non-viable tissue. In a sharp wound, this plane resides between the scab and the dermal surface [e.g. shown in (Rittié et al. 2016)]. In a burn or a dehydrated wound, it is deeper (i.e. beneath the layer of necrotic dermis). Migration within this plane is accompanied by increased collagenase expression in keratinocytes within 24 h of wounding [shown in full-thickness wounds on human buttocks (Inoue et al. 1995)]. Collagenolytic activity is probably a characteristic response of the epidermis to wounding since it was also detected in various types of ulcers and ulcerated pyogenic granulomas [shown on various body sites (Saarialho-Kere et al. 1993)]. In all cases, keratinocytes require a healthy substrate to form viable outgrowths (Hartwell 1929). The importance of the keratinocyte migration substrate is highlighted in the case of ulcers that do not heal and in wounds of elderly subjects. In the former, lack of suitable substrate make cells pile up at the wound margin and form a more differentiated epithelial border that impede further migration (Hartwell 1929; Bishop 1945). In the latter, degraded collagen matrix (a hallmark of aged and photoaged skin (Rittié and Fisher 2015)) is a poor substrate that prevents keratinocytes from forming cohesive outgrowths. Reepithelialization of elderly skin is thereby delayed vs. that of young skin (Rittié et al. 2016). Mechanisms for this observed phenomenon are unclear. It is becoming increasingly accepted that the strength of cell-cell cohesion is dictated by mechanical forces between cells and their substrate (Discher et al. 2005; Maruthamuthu et al. 2011). It is thus plausible that the reduced rigidity of aged skin (Pailler-Mattei et al. 2013), and thus of the migrating keratinocyte environment, would lessen cells’ cohesiveness and slow down reepithelialization of partial thickness wounds. More research is needed to clarify this interesting possibility.
Effects of wound dressings on reepithelialization
Drying of the scab is accompanied by important compaction of underlying tissues and thereby impairs optimal reepithelialization. Indeed, epidermal migration over a moist wound bed is twice faster than over a desiccated one [first described in pigs by (Winter 1962) and later confirmed in many other species including humans, see (Jonkman 1989)]. Removal of the scab and contact with air also slows keratinocyte migration (Hartwell 1929). Occlusive wound dressings can prevent these two phenomena and improve reepithelialization rate [shown in pigs: (Winter 1962) and humans: (Hinman and Maibach 1963), both using polyethylene films].
Importantly, the use of an occlusive wound dressing early on limits tissue dehydration and thereby redirects the course of epithelial migration (Winter 1972; Jonkman 1989). It was suggested that a wound dressing has to be applied within two hours of wounding and for at least twenty-four hours to efficiently promote reepithelialization [shown in pigs: (Eaglstein et al. 1988)]. The mechanism for this effect remains unclear and this observation has yet to be replicated.
Optimal physical properties of a wound dressing include being proof to liquids yet permeable to liquid vapors (original vapor-proof dressings caused wound maceration and increased risk of bacterial growth). The blister roof of a superficial burn fulfills these requirements: if removed, the underlying tissue dries out as a result of exposure to the air and forms a crust. The resulting wound is more painful, heals slower, and has reduced cosmetic outcome at one year than when the blister was drained and the epidermis layer left intact [case-report on lower leg: (Forage 1962)]. The composition of occluding dressings is also important. For instance, polyurethane films appear superior to petroleum jelly in relieving pain, accelerating wound healing and reducing pigmentation and scarring [shown in skin graft donor site (Li et al. 2013)]. The relative efficiency of various plastic films was proposed to be directly linked to the oxygen permeability of these films and to their ability to increase oxygen tension in serous exudates bathing keratinocytes (Winter 1971, 1972). Many wound dressings are now commercially available and careful consideration is needed before selecting a particular wound dressing for a given situation (Dabiri et al. 2016).
Fibrous healing: a pre-requisite for reepithelialization of full-thickness wounds
Full-thickness wounds differ from partial-thickness wounds in that they lack a base for keratinocyte repopulation. Even if traces of epithelial appendage remain in the wound bed, it was perceived early on that “the follicle waits until the derma is ready” (Bishop 1945). Adipose is a poor substrate for keratinocytes and has been categorized as “inimical to epithelial progression” in human wounds (Hartwell 1929). Indeed, the presence of a thin layer of fat is sufficient to prevent two epidermal tongues from merging with each other, even over a period of 73 days (Hartwell 1929). Therefore, a full-thickness wound requires a prior “fibrous” healing (defined as healing that occurs in the dermis, fat, or fascia) prior to reepithelialization.
As mentioned earlier, the presence of a panniculus carnosus provide a tremendous initial advantage to loose-skinned animals in that muscle contraction reduces the wound area by about 50 % within the first twenty-four hours of repair. This feature does not exist in humans or pigs. Interestingly, the panniculus carnosus-mediated initial wound contraction is strongly inhibited by wound dressings [shown in rabbits: (Billingham and Russell 1956; Snowden et al. 1982)]. To potentially increase relevance of wound healing studies, many research experiments in rabbits and rodents are performed removing the panniculus carnosus during wounding. As detailed above however, human skin has many additional singularities, including the thickness of its adipose tissue (Fig. 2). The nature of the tissue underlying the wound is thus likely to differ between species, even when the panniculus carnosus is removed in loose-skinned animals. It is thus not surprising that all studies performed on human wounds revealed important differences in fibrous healing between humans and laboratory animals (Carrel and Hartmann 1916; Hartwell 1930; Butterworth 1992).
Dermal regeneration and fibroblast infiltration
In all cases (after initial contraction or in tight-skinned animals) the wound repair process requires a “filled” wound bed prior to epidermal repair. In humans, this is also true for partial-thickness wounds that reach the reticular dermis (Bishop 1945). Different portions of the dermis have different regeneration capabilities: the papillary (sub-epidermal) dermis regenerates the fastest, followed by the mid-dermis and the lower reticular dermis (Bishop 1945). When granulation do start in the lowest portion of the reticular dermis, Bishop observed that granulation islands start at the site of hair follicles, even when the epithelial component of the hair follicle has been destroyed by the injury. Granulation islands then grow individually until they merge with each other. Thus, the connective tissue sheath surrounding human hair follicles was thought to have distinct cellular and molecular properties that made it important for the repair of human wounds (Bishop 1945). Progress to decipher the details of these properties has been slow but this possibility remains undeniably attractive (Jahoda and Reynolds 2001; Gharzi et al. 2003; Richardson et al. 2005).
Granulation tissue consists in a dense population of macrophages, fibroblasts, and blood vessels embedded in a loose matrix of collagen fibers (primarily made of type I collagen associated with other types of collagen and glycoproteins), fibronectin, and hyaluronic acid (Clark 1985). Granulation tissue matrix is deposited in the wound bed by fibroblasts. When the wound is deep enough so that no dermis or dermal cells remains in the wound bed, granulation tissue must be produced entirely by fibroblasts imported from outside the wound site. The origin of fibroblasts forming the granulation tissue in these cases has yet to be firmly resolved. Several possibilities have been proposed. Early studies in rabbits identified the perivascular sheaths (those surrounding blood-vessels below the panniculus carnosus) and its pericytes as origin for granulation fibroblasts (Hadfield 1963). Stromal elements present in the adipose layer (including vessels) were also proposed [in rabbits: (Hadfield 1963) and pigs: (Miller et al. 1998)]. Another valid possibility is the deposition of blood-circulating hematopoietic cells with mesenchymal characteristics (fibrocytes) in the wound site [mostly shown in mice: (Abe et al. 2001; Wu et al. 2007; Suga et al. 2014; Rinkevich et al. 2015)]. In all, it is possible and likely that multiple sub-populations of fibroblasts contribute together to supplying fibroblasts for the elaboration of granulation tissue. As a matter of fact, the term “fibroblasts” designates a very heterogeneous population of cells. Although all fibroblasts specialize in extracellular matrix deposition in connective tissues, different subtypes do so to various extents (Suga et al. 2014; Rinkevich et al. 2015). Fibroblasts in different skin sites may have different embryonic origin (Driskell and Watt 2015) and no single marker is borne by all fibroblast lineages. This makes the identification of the origin of fibroblasts in granulation tissue both fascinating and challenging. This topic is poised to generate additional important studies.
Granulation tissue formation
Regardless of their origin, new fibroblasts colonize the provisional matrix laid down in the early phases of wound repair and start forming granulation tissue by producing a collagen- and cellular fibronectin-rich matrix. Fibrin clot and early granulation tissue is especially rich in plasma fibronectin. It is interesting to note that fibroblasts in granulation tissue align themselves in the same axis as fibronectin; and collagen is subsequently orientated in the same way (Butterworth 1992). During granulation tissue expansion, collagen fibers and fibroblasts become oriented parallel to the dermal-epidermal junction and along expected lines of stress.
The extracellular matrix composition changes over time and promotes the maturation of resident fibroblasts into myofibroblasts via a feedback mechanism. Myofibroblasts were first identified as differentiated cells that promote dermal wound contraction (Gabbiani et al. 1971). The first step of fibroblast differentiation is its conversion into “proto-myofibroblast”. Proto-myofibroblasts form more mature adhesion complexes with the extracellular matrix than fibroblasts do, and lay down the first collagen bundles (Hinz 2010). Proto-myofibroblasts pre-organize the extracellular matrix by exerting small contraction forces via their stress fibers (containing F-actin but negative for alpha-smooth muscle actin) [shown in rats: (Hinz et al. 2001a)]. Subsequent acquisition of alpha smooth muscle actin in association with contractile actin/myosin-containing stress fibers completes the differentiation into myofibroblasts. Alpha smooth muscle actin-containing stress fibers confer myofibroblasts at least a twofold stronger contractile activity compared with alpha smooth muscle actin-negative proto-myofibroblasts in culture (Hinz et al. 2001b).
Biomechanics is a key controller of the myofibroblast differentiation process: as the composition of the extracellular matrix evolves, so does its stiffness. In rat wounds, the provisional matrix stiffness increases over time: 0.01–10 kPa for the fibrin clot, ~18 kPa in a 7 day-old granulation tissue, and ~50 kPa in a 12 day-old granulation tissue [Young’s modulus values determined by atomic force microscopy: (Hinz 2010)]. These values are in line with the stiffness needed to induce proto-myofibroblast and myofibroblast phenotypes in culture (i.e. 3–5 and 16–20 kPa, respectively) (Hinz 2010). It is expected that a similar increase in granulation tissue stiffness takes place in human wounds (although likely with different kinetics, see below). It is also likely that additional extracellular matrix properties (e.g. biochemical composition) greatly influence fibroblast phenotype and thereby granulation tissue formation and scar tissue deposition (Rittié 2015). Future studies should shed light on these possibilities.
Granulation tissue contraction
Myofibroblasts promote wound contraction [shown in rats: (Gabbiani et al. 1971), and humans: (Ryan et al. 1974)]. Contraction refers to the centripetal movement of the wound margin that aims at reducing the size of the wound bed. Proto-myofibroblast- and myofibroblast-containing granulation tissue generates contractile forces proportional to the granulation tissue volume [shown in rabbits: (Higton and James 1964)]. This observation explains that larger wounds that contain more granulation tissue contract more and close at a faster pace than smaller wounds [shown in humans and dogs: (Carrel and Hartmann 1916) and confirmed in humans: (Butterworth 1992)].
Whether myofibroblasts are necessary to wound contraction has been questioned. Proto-myofibroblasts are indeed nine times more abundant than fully differentiated myofibroblasts in human granulation tissue (Berry et al. 1998). Traction forces generated by proto-myofibroblasts as they migrate on a compliant substrate are also sufficient to contract collagen matrices in 3D cultures (Bell et al. 1979). These observations argue against the need for a specialized contractile cell in granulation tissue. The requirement for differentiated myofibroblasts was also argued against using a granulation tissue excision approach [shown in guinea pigs: (Watts et al. 1958) and pigs: (Gross et al. 1995)]. A species-associated difference is possible based on the observation that myofibroblasts associate with thin collagen fibers in rat granulation tissue while they associate exclusively with thick collagen bundles in humans (Berry et al. 1998). In all, it is plausible that traction forces and contraction forces may be able to at least compensate for each other. In pigs, the appearance of myofibroblasts in granulation tissue coincides with tissue contraction (Welch et al. 1990). In humans however, alpha smooth actin-positive myofibroblasts are detected whether the wound is in a contraction phase or not, and regardless of fibroblast orientation (Butterworth 1992). In addition to their contractibility, myofibroblasts have the particularity to communicate with each other via gap junction (Gabbiani et al. 1978). It is thus plausible that they form multicellular contractile units during wound contraction, thereby ensuring an even shrinkage of the granulation tissue, regardless of the local size of the collagen network. Additional work will be needed to test this interesting possibility.
Wound contraction results from the shortening and compacting of collagen fibers within the granulation tissue (Watts et al. 1958; Hinz et al. 2001a; Tomasek et al. 2002). In vitro studies have shown that myofibroblasts use a ratchet mechanism to contract the surrounding matrix: they pull on the surrounding collagen fibrils to create loops, shorten the fibril, and pull again (Castella et al. 2010). How the collagen matrix gets shortened (to a point of no longer requiring active cell contraction to maintain tissue tension) remains unsolved. Various authors regularly propose that cross-linking of the extracellular matrix mediates the compacting process but the permanent nature of collagen cross-links seems at odds with the dynamic nature of the subsequent remodeling phase (Tomasek et al. 2002).
The concept of matrix shortening and compaction helps understand the following longstanding observation: the greater the contraction, the less residual scar deposited. Human wounds at extremities contract little whereas wounds on the lower back (sacro-coccygeal pilonidal sinus excisions) contract a lot (Berry et al. 1998). As a result, filling is much less in the latter (was estimated at 12.5 % of the wound volume) compared to the former (Berry et al. 1998). The factors that dictate the deposition of scar tissue and amount of contraction remain unclear. The number of soluble factors shown in various models to modulate myofibroblasts properties is virtually endless (Hinz et al. 2012). It is plausible that the origin of fibroblasts initially colonizing the wound bed dictates their ability to deposit extracellular matrix, differentiate into myofibroblasts, and/or contract a wound. More research is needed to test this hypothesis.
Many differences exist in the mechanisms of fibrous healing between humans and animals. So many that Hartwell, comparing humans with rabbits, dogs, pigs, and pigeons, regarded these processes as “extremes of a spectrum” (Hartwell 1930, 1955). These differences include (but are not limited to): i) mitoses were easier detected in animal wounds; ii) while the human fat was considered the main location for fibrous healing, the pattern of subcutaneous fat in animals, including pigs, was very different; iii) animal wounds had larger vessels than human ones; iv) the morphology of cells identified by Hartwell as macrophages was different. In addition, wound contraction occurs after a lag period in dogs, rabbits, and guinea pigs (Carrel and Hartmann 1916; Higton and James 1964). In humans however, this does not seem to be the case and granulation tissue contracts as the wound bed fills (Carrel and Hartmann 1916). These examples make the translation of fibrous healing from small animals to humans difficult.
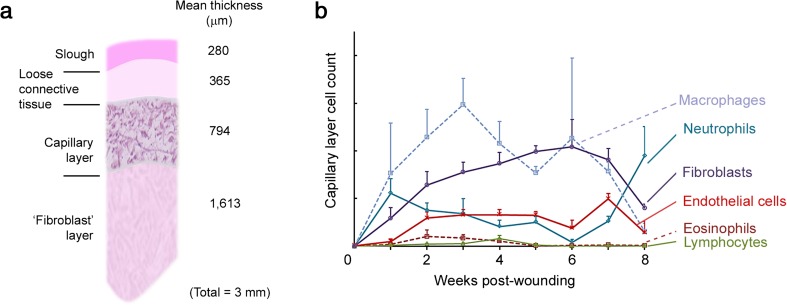
Studying the histology of deep human wounds with repeated biopsies, Butterworth was the first to describe a layered organization of the granulation tissue, as follows and summarized in Fig. 5a. The bottom half is a fibroblast layer (comprised of matrix-embedded proto-myofibroblasts and myofibroblasts oriented with bundles running horizontally to the wound and perpendicularly to the few capillaries present). The fibroblast layer is overlaid by a capillary layer (comprised of longitudinally oriented capillaries, with many inflammatory cells and fibroblasts), itself topped by a loose connective tissue layer (avascular and containing sparse cells of various types), the latter being capped by slough (Butterworth 1992). Although the layers’ thicknesses vary between patients and as healing progresses, each layer represents roughly 50, 25, 15, and 10 % of the depth of the granulation tissue, respectively. As the wound progresses, the thickness of the fibroblast and capillary layers tend to increase while the superficial slough thins out (Butterworth 1992). The cellular composition of the capillary layer established by Butterworth over the course of the healing process is depicted in Fig. 5b. Interestingly, Butterworth also noted a strong correlation between the capillary layer measured thickness and healing progress recorded the week prior to biopsy. The function of the capillary layer is not known and is possibly to serve as a feeding layer, new cell production layer, or both. In any case, it will be interesting to elucidate the potential meaning of this correlation. Importantly, over-granulating (non-healing) wounds did not show a layered organization (Butterworth 1992).
Fig. 5.
Organization of human granulation tissue as deducted from the work of Rosalind Butterworth (Butterworth 1992). a Schematic of the layered organization of the granulation tissue of large full-thickness human wounds healing by second intension. The ‘fibroblast’ layer is mostly populated by proto-myofibroblasts and myofibroblasts, and inflammatory cells are intravascular. Capillaries are present but they are few, large and mature. The capillary layer is the most cellular (see details in b). The loose connective tissue layer contains few, but viable, cells of varying type, and no endothelial cells or formed capillaries. The superficial slough contains fibrin and dead cells. Numbers to the right are average thickness of respective layer in μm. b Cellular composition of the capillary layer of human granulation tissue in the course of healing. Graphs were prepared according to raw data presented in (Butterworth 1992) from 80 biopsies. The cellularity of the capillary layer (the most cellular of all) remains fairly constant throughout healing
The end of contraction either coincides with healing completion, or precedes it by a few days. The granulation tissue then evolves into a scar, which involves a massive decrease in cellularity. It is believed that apoptosis mediates this phenomenon [shown in rats: (Desmoulière et al. 1995)].
Reepithelialization of full-thickness wounds
A second particularity of full-thickness wounds is that when the adnexal structures are completely destroyed, the reepithelialization must proceed entirely from the wound margins. Surprisingly, very few human studies have focused specifically on the cellular mechanisms of reepithelialization of full-thickness wounds. This could be due to the observed tendency of the sides of a human full-thickness wound to flop inward, or to the ethical consideration of sampling large open wounds. It is usually accepted that reepithelialization from the wound margin is similar whether the wound is partial thickness or not. More research is needed to confirm this longstanding belief. Meanwhile, I will describe below the cellular mechanisms of expansion of marginal epidermis as it has been thus far described in the skin of humans compared to other mammals.
Leapfrog mechanism of reepithelialization from the wound edge
Early observations indicated that the bordering epithelium in a one day-old human wound extends slightly over the ends of the incised dermis, creating an “extension membrane” (or epidermal tongue). The epidermal tongue is formed by the supra-basal cells of the spinous layer as opposed to the cells of the basal layer [shown in humans: (Hartwell 1929; Bishop 1945), and pigs: (Winter 1972)]. Basal keratinocytes adhere to the dermal-epidermal junction by focal adhesions and hemidesmosomes (multi-protein complexes that mediate adhesion between the keratinocytes’ actin and keratin filaments, respectively, and the papillary dermis). Disruption of these adhesive structures (by genetic mutation, circulating autoantibodies, or burn) causes blistering of the skin (Turcan and Jonkman 2015). In healthy skin however, these anchors are very strong. No mechanism of detachment after injury has been described, except in the larval amphibians (Weiss 1961). Therefore, that the supra-basal cells of the stratum spinosum are indeed the cells initially forming the epidermal tongue sounds energy-efficient. Presumably, this would imply that spinous keratinocytes, which were initially committed to differentiation while in the intact epidermis, are capable of reverting back to a less differentiated type (since they can eventually produce progeny to form the new epidermis (Van Winkle 1968)). This has yet to be clearly demonstrated.
The formation of the epidermal tongue is accompanied by a keratinocyte “activation” that involves cytoskeleton reorganization through production of specific “activation” keratin proteins, distinct from the keratins present in healthy epidermis. Typically, keratins 6, 16, and 17 are characteristic markers of activated keratinocytes during reepithelialization (Coulombe 1997; Freedberg et al. 2001). Many activated keratinocytes also produce cytoplasmic processes towards the wound site [shown in humans: (Odland and Ross 1968), dogs: (Winstanley 1975), and rats: (Gabbiani et al. 1978)], and supra-basal epidermal keratinocytes within the advancing wound margin express cytoplasmic actin [humans: (Ortonne et al. 1981)]. In rat skin, keratinocytes at the wound edge harbor irregular configuration with more intercellular space and less desmosomes (intercellular adhesion structures that mediate resistance to mechanical stress) (Gabbiani et al. 1978). This observation has yet to be confirmed in human skin.
Epidermal keratinocytes subsequently advance the epidermal tongue and spread across the wound bed. Many studies have revealed that epidermal repair engages single cell migration (Hartwell 1929; Bishop 1945; Weiss 1961; Winter 1972) (see below), as opposed to corneal repair in which cells migrate “in sheets” (Zhao et al. 2003). Activated keratinocytes migrate out of the marginal epidermis and the new epidermis builds-up by successive implantation of cells on the wound surface at the leading edge (Hartwell 1929; Winter 1972), as follows. The first supra-basal cell of the advancing epidermis becomes elongated and stretches over the basal cell underneath it. The supra-basal cell reaches the wound bed substrate and becomes basal. Supra-basal cells from the marginal epidermis are simultaneously renewed by proliferation of basal keratinocytes within the wound margin and the epidermal tongue. The epidermis progresses as the next supra-basal keratinocyte of the wound edge undergoes a similar process. The epidermis is thus said to advance over the wound in a “leapfrog” fashion (Winter 1972; Clark 1988). Increased mitotic activity in basal keratinocytes extends into the adjacent normal epidermis for up to six millimeters [observed in humans after tape stripping: (Williams and Hunter 1957) and partial thickness wounds: (L. Rittié, unpublished observations)]. Differentiation accompanies the advancement of the epithelial tongue (Hartwell 1929; Rittié et al. 2013b). Basal keratinocytes of the advancing tongue (or appendages outgrowths in the case of partial thickness wounds) also harbor characteristics of keratinocyte activation, i.e. mitotic activity [shown in humans: (Williams and Hunter 1957; Rittié et al. 2013a, 2016)], and less desmosomes but more gap junctions than in control epidermis [shown in rats: (Gabbiani et al. 1978) and humans: (Ortonne et al. 1981)].
It is often cited that keratinocyte migration precedes proliferation during reepithelialization. This phenomenon was reported from early studies when mitosis was evidenced by observation of a typical microscopic morphology of dividing cell nuclei, and when authors were assuming epithelial repair was similar across species, from freshwater muscle epithelium to human skin (Arey 1932). These controversial views were clarified by Viziam and collaborators in rabbit wounds using colchicine (arrests cells in mitosis’ metaphase) and tritiated thymidine (labels DNA upon replication). Interestingly, they showed that mitoses were present prior to migration in rabbits although they were not visible in colchicine-untreated wounded animals (Viziam et al. 1964). This description is reminiscent of Bishops’ work, which describes thickening of the peripheral margin in instances when the epidermis had to wait for the granulation tissue to form before migrating (Bishop 1945). Early presence of dividing cells after wounding has since been confirmed with more modern methods including immunohistochemistry [e.g. (Rittié et al. 2013a, 2016)].
Composition of the provisional matrix
The protein composition of the substrate on which reepithelialization occurs is not fully elucidated. When the basement membrane is destroyed by the injury, keratinocytes are believed to progress on a provisional matrix [shown to be made of various proteins in various species and reviewed in (Clark 1996)]. For human wounds, the exact nature of the proteins that promote expansion of the new epidermis remains unclear. In the case of partial thickness wounds, plasma fibronectin and fibrinogen are deposited during hemostasis soon after injury. After a few days, in animal models, cellular fibronectin produced by infiltrating fibroblasts and macrophages still serves as a provisional matrix together with plasma fibronectin [shown in rat xenographs on immunosuppressed mice: (Clark et al. 1983)]. In situ secretion of fibronectin as temporary provisional matrix by the keratinocytes directly present at the front edge of migration has also been suggested by (Jonkman 1989). However, the αvβ6 integrin (fibronectin receptor), although temporarily expressed early on by migrating keratinocytes during reepithelialization of wounded xenographed human skin (Breuss et al. 1995), is not expressed in human wounds until 7 days post-wounding (Haapasalmi et al. 1996). The latter diminishes the likelihood that fibronectin alone would serve as provisional matrix in human wounds. Interestingly, we showed that migrating human appendage outgrowths are surrounded by laminin γ2 secreted by keratinocytes at the margin of outgrowths, as early as the outgrowth forms, and throughout the reepithelialization process [(Rittié et al. 2016) and (L. Rittié, unpublished observations)]. This could be a human-specific trait because laminin is not expressed by migrating keratinocytes in pig skin (Stanley et al. 1981). Whether laminin γ2 serves as substrate for keratinocyte adhesion or is produced in response to contact with type I collagen or scab tissue is not currently known. Other studies have shown that migrating human keratinocytes do not produce the other typical components of the basement membrane (such as type IV and type VII collagens) (Larjava et al. 1993). In all, the exact composition of the provisional matrix that promotes keratinocyte progression over the wound bed remains unclear. More modern analytical techniques such as proteomics should elucidate this important question in the near future.
To what extent the basement membrane is restored after wounding in human skin is also not fully known. The basement membrane appears restored 21 days after linear incision in human forearms (Odland and Ross 1968). However, none of the studies demonstrating complete restoration of the dermal-epidermal junction after excisional wounds in vivo were conducted in humans [reviewed in (Woodley and Briggaman 1988)]. Future studies should elucidate this important point.
Stimuli for epithelial migration and proliferation
Factors enabling and stimulating epidermal regeneration remain ill-defined. A “free edge effect” [by analogy with repair of blood vessels (Clark 1988)] and/or local release of growth factors are often cited (the latter mostly based on in vitro studies). There must also be migration clues contained in regions where reepithelialization occurs since keratinocytes always find their way between dead and healthy tissue. Keratinocyte migration is facilitated by collagenase production [shown in guinea pigs: (Grillo and Gross 1967) and humans: (Saarialho-Kere et al. 1993)] and possibly by enhanced phagocytic activity by keratinocytes at the leading edge [as shown in the healing rat palate: (Gibbins 1968) or human skin after linear incisions: (Odland and Ross 1968)]. Wound dressings reduce keratinocyte mitotic rate [shown after tape stripping in humans: (Van Winkle 1968)], suggesting that surface dehydration may stimulate epidermal mitosis. In full thickness human wounds, epithelial cells proliferate and migrate to cover the surface of each separate granulation, but do not extend beyond its margin before adjacent granulations coalesce (Bishop 1945). Conversely, “over-granulation” frequently appears to inhibit reepithelialization in otherwise healthy looking wounds (Butterworth 1992). Lack of innervation does not prevent the reepithelialization of partial thickness human wounds (Wagner 1964). In all, it appears that the new epithelium heals the wound surface only after granulation tissue has matured to a stage permitting implantation of migrating epithelial cells (Bishop 1945). The nature of this “maturation” remains unclear to this day.
Conclusions
Wound reepithelialization is a complex process that is highly dependent on species and contextual circumstances, even in the absence of impeding factors (that include co-morbid clinical conditions, aging, poor tissue perfusion, malnutrition, unrelieved pressure to the surface of the wound, immune suppression, malignancy, infection, obesity, medications, etc.) In this review, we have detailed several aspects of the wound healing process and highlighted similarities and differences between mammals. Many more studies are needed to answer numerous remaining questions. The selection of an appropriate animal model is a critical decision for any wound-healing study. Anatomical specificities, type of injury, nature of the wound, size of the injury, wound treatments are all important factors that should be considered when designing wound healing research protocols. The goal of any investigator selecting a model should be to find a situation that simplifies (yet mimics) the wound process as it occurs in humans to test potential therapeutic approaches. In many cases, humans might be the only appropriate model.
Three factors potentially limit the rate of epithelial coverage: infection (length and intensity of the inflammatory phase), keratinocyte cornification (contact with air), and the presence of a non-optimal migration base (wound bed). The first two factors are nowadays fairly well controlled with therapy: the use of antibiotics in the mid-20th century provided the greatest advance in wound care since the development of antiseptics, and animal models developed at the end of the 20th century have greatly facilitated improvements of wound dressing materials and techniques. Much remains to be learned to successfully develop therapies aimed at optimizing the keratinocyte migration base. Hopefully, the 21st century will facilitate great discoveries in this area.
Acknowledgments
The author acknowledges all the published work that was not cited because of space limitations. She also thanks the librarians at the University of Michigan for their tremendous help with retrieving the older (and newer) documents cited herein.
References
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Arao H, Shimada T, Hagisawa S, Ferguson-Pell M. Morphological characteristics of the human skin over posterior aspect of heel in the context of pressure ulcer development. J Tissue Viability. 2013;22:42–51. doi: 10.1016/j.jtv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Arey LB. Certain basic principles of wound healing. Anat Rec. 1932;51:299–313. doi: 10.1002/ar.1090510309. [DOI] [Google Scholar]
- Argenbright LW, Forbes PD. Erythema and skin blood content. Br J Dermatol. 1982;106:569–574. doi: 10.1111/j.1365-2133.1982.tb04560.x. [DOI] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg. 1998;102:124–131. doi: 10.1097/00006534-199807000-00019. [DOI] [PubMed] [Google Scholar]
- Bigelman J, Mertz PM. Human and swine models of epidermal wound healing. In: Rovee DT, Maibach HI, editors. The epidermis in wound healing. Boca Raton: CRC Press; 2004. pp. 113–123. [Google Scholar]
- Billingham RE, Russell PS. Studies on wound healing, with special reference to the phenomenon of contracture in experimental wounds in rabbits’ skin. Ann Surg. 1956;144:961–981. doi: 10.1097/00000658-195612000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GH. Regeneration after experimental removal of skin in man. Am J Anat. 1945;76:153–181. doi: 10.1002/aja.1000760202. [DOI] [Google Scholar]
- Blanpain C, Fuchs E (2014) Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281 [DOI] [PMC free article] [PubMed]
- Bramble DM, Lieberman DE. Endurance running and the evolution of homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Butterworth RJ (1992) The histology of human granulating wounds. M.D. Thesis # U053163. University of Leicester, UK
- Carrel A, Hartmann A. Cicatrization of wounds : I. The relation between the size of a wound and the rate of its cicatrization. J Exp Med. 1916;24:429–450. doi: 10.1084/jem.24.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella LF, Buscemi L, Godbout C, Meister JJ, Hinz B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci. 2010;123:1751–1760. doi: 10.1242/jcs.066795. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Cutaneous tissue repair: basic biologic considerations. I. J Am Acad Dermatol. 1985;13:701–725. doi: 10.1016/S0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Overview and general considerations of wound repair. In: Clark RAF, Henson PM, Henson PM, editors. The molecular and cellular biology of wound repair. New York: Plenum Press; 1988. pp. 3–33. [Google Scholar]
- Clark RAF. Wound repair: overview and general considerations. In: Clark RAF, editor. The molecular and cellular biology of wound repair. 2. New York: Plenum Press; 1996. pp. 3–50. [Google Scholar]
- Clark RA, Winn HJ, Dvorak HF, Colvin RB. Fibronectin beneath reepithelializing epidermis in vivo: sources and significance. J Investig Dermatol. 1983;80(Suppl 1):26s–30s. doi: 10.1038/jid.1983.7. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644–650. doi: 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G, Damstetter E, Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care. 2016;5:32–41. doi: 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Dimick AR. Delayed wound closure: indications and techniques. Ann Emerg Med. 1988;17:1303–1304. doi: 10.1016/S0196-0644(88)80355-X. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Donati G, Watt FM. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16:465–476. doi: 10.1016/j.stem.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Du Noüy PL. Cicatrization of wounds : Iii. The relation between the age of the patient, the area of the wound, and the index of cicatrization. J Exp Med. 1916;24:461–470. doi: 10.1084/jem.24.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Noüy PL. Cicatrization of wounds : Ii. Mathematical expression of the curve representing cicatrization. J Exp Med. 1916;24:451–460. doi: 10.1084/jem.24.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62:216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434–439. doi: 10.1111/1523-1747.ep12476467. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH, Davis SC, Mehle AL, Mertz PM. Optimal use of an occlusive dressing to enhance healing. Effect of delayed application and early removal on wound healing. Arch Dermatol. 1988;124:392–395. doi: 10.1001/archderm.1988.01670030058022. [DOI] [PubMed] [Google Scholar]
- Elias PM, Goerke J, Friend DS. Mammalian epidermal barrier layer lipids: composition and influence on structure. J Investig Dermatol. 1977;69:535–546. doi: 10.1111/1523-1747.ep12687968. [DOI] [PubMed] [Google Scholar]
- Ennis WJ, Sui A, Bartholomew A. Stem cells and healing: impact on inflammation. Adv Wound Care. 2013;2:369–378. doi: 10.1089/wound.2013.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagrell B. Microcirculation of the skin. In: Mortillaro NA, editor. The physiology and pharmacology of the microcirculation. New York: Academic; 1984. pp. 133–180. [Google Scholar]
- Ferry LL, Argentieri G, Lochner DH. The comparative histology of porcine and guinea pig skin with respect to iontophoretic drug delivery. Pharm Acta Helv. 1995;70:43–56. doi: 10.1016/0031-6865(94)00050-6. [DOI] [PubMed] [Google Scholar]
- Forage AV. The effects of removing the epidermis from burnt skin. Lancet. 1962;2:690–693. doi: 10.1016/S0140-6736(62)90504-4. [DOI] [PubMed] [Google Scholar]
- Forbes PD (1967) Radiation effects in swine. I. Vascular supply of the skin and hair. Usnrdl-tr-67-141. Res Dev Tech Rep: 1–18 [PubMed]
- Forbes PD, Urbach F. Vascular and neoplastic changes in mice following ultraviolet radiation. In: Urbach F, editor. The biologic effects of ultraviolet radiation (with emphasis on the skin) Oxford: Pergamon Press; 1969. pp. 279–289. [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Investig Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Chaponnier C, Huttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76:561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Gibbins JR. Migration of stratified squamous epithelium in vivo. The development of phagocytic ability. Am J Pathol. 1968;53:929–951. [PMC free article] [PubMed] [Google Scholar]
- Gillman T, Penn J, Bronks D, Roux M. A re-examination of certain aspects of the histogenesis of the healing of cutaneous wounds; a preliminary report. Br J Surg. 1955;43:141–153. doi: 10.1002/bjs.18004317804. [DOI] [PubMed] [Google Scholar]
- Gordillo GM, Bernatchez SF, Diegelmann R, Di Pietro LA, Eriksson E, Hinz B, Hopf HW, Kirsner R, Liu P, Parnell LK, Sandusky GE, Sen CK, Tomic-Canic M, Volk SW, Baird A. Preclinical models of wound healing: is man the model? Proceedings of the wound healing society symposium. Adv Wound Care. 2013;2:1–4. doi: 10.1089/wound.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JE. Function of the panniculus carnosus—a hypothesis. Vet Rec. 2010;167:760. doi: 10.1136/vr.c6210. [DOI] [PubMed] [Google Scholar]
- Grillo HC, Gross J. Collagenolytic activity during mammalian wound repair. Dev Biol. 1967;15:300–317. doi: 10.1016/0012-1606(67)90029-2. [DOI] [PubMed] [Google Scholar]
- Gross J, Farinelli W, Sadow P, Anderson R, Bruns R. On the mechanism of skin wound “contraction”: a granulation tissue “knockout” with a normal phenotype. Proc Natl Acad Sci U S A. 1995;92:5982–5986. doi: 10.1073/pnas.92.13.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark RA, Uitto VJ, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Investig Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- Hadfield G. The tissue of origin of the fibroblasts of granulation tissue. Br J Surg. 1963;50:870–881. doi: 10.1002/bjs.18005022627. [DOI] [PubMed] [Google Scholar]
- Hartwell SW., Sr Surgical wounds in human beings: a histologic study of healing with practical applications: I. Epithelial healing. Arch Surg. 1929;19:835–847. doi: 10.1001/archsurg.1929.01150050066007. [DOI] [Google Scholar]
- Hartwell SW. Surgical wounds in human beings: a Histologic study of healing with practical applications: Ii. Fibrous healing. Arch Surg. 1930;21:76–96. doi: 10.1001/archsurg.1930.01150130079004. [DOI] [Google Scholar]
- Hartwell SW, Sr. (1955) The mechanisms of healing in human wounds; a correlation of the clinical and tissue factors involved in the healing of human surgical wounds, burns, ulcers, and donor sites. Springfield, Ill.: Thomas. 166 p
- Havran WL, Jameson JM. Epidermal t cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higton DI, James DW. The force of contraction of full-thickness wounds of rabbit skin. Br J Surg. 1964;51:462–466. doi: 10.1002/bjs.1800510616. [DOI] [PubMed] [Google Scholar]
- Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–378. doi: 10.1038/200377a0. [DOI] [PubMed] [Google Scholar]
- Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoke JB, Lobitz WC., Jr Histologic variations in the structure of human eccrine sweat glands. J Investig Dermatol. 1952;18:147–167. doi: 10.1038/jid.1952.18. [DOI] [PubMed] [Google Scholar]
- Hwang K, Baik SH. Distribution of hairs and sweat glands on the bodies of Korean adults: a morphometric study. Acta Anat (Basel) 1997;158:112–120. doi: 10.1159/000147920. [DOI] [PubMed] [Google Scholar]
- Inoue M, Kratz G, Haegerstrand A, Stahle-Backdahl M. Collagenase expression is rapidly induced in wound-edge keratinocytes after acute injury in human skin, persists during healing, and stops at re-epithelialization. J Investig Dermatol. 1995;104:479–483. doi: 10.1111/1523-1747.ep12605917. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358:1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci U S A. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman MF. Introduction, survey of the literature and aim of this study. In: Jonkman MF, editor. Epidermal wound healing between moist and dry: the enhancing effects of a new poly(ether urethane) wound covering on the reepithelialization of partial-thickness wounds. Groningen: Rijksuniversiteit Groningen; 1989. pp. 13–30. [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qiao L, Huang X, Yuan K, Yang H. Clinical evaluation of polyurethane foam dressing on wound healing of skin graft donor site. Journal of Shanghai Jiaotong University. Med Sci. 2013;33:663–666. [Google Scholar]
- Lobitz WC, Jr, Holyoke JB, Montagna W. Responses of the human eccrine sweat duct to controlled injury: growth center of the epidermal sweat duct unit. J Investig Dermatol. 1954;23:329–344. doi: 10.1038/jid.1954.116. [DOI] [PubMed] [Google Scholar]
- Lu C, Fuchs E (2014) Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med 4: [DOI] [PMC free article] [PubMed]
- Lu S, Xiang J, Qing C, Jin S, Liao Z, Shi J. Effect of necrotic tissue on progressive injury in deep partial thickness burn wounds. Chin Med J. 2002;115:323–325. [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen S-C, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf S, Vergou T, Sterry W, Lademann J, Patzelt A. Comparative study of hair follicle morphology in eight mammalian species and humans. Skin Res Technol. 2014;20:147–154. doi: 10.1111/srt.12098. [DOI] [PubMed] [Google Scholar]
- Marples MJ. The ecology of human skin. Springfield: Charles C. Thomas; 1965. Cutaneous appendages: the sweat gland; pp. 22–45. [Google Scholar]
- Martin CW, Muir IF. The role of lymphocytes in wound healing. Br J Plast Surg. 1990;43:655–662. doi: 10.1016/0007-1226(90)90185-3. [DOI] [PubMed] [Google Scholar]
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ecm traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, Nishimura EK (2016) Hair follicle aging is driven by transepidermal elimination of stem cells via col17a1 proteolysis. Science 351: aad4395 [DOI] [PubMed]
- Mehendale F, Martin P. The molecular and cellular events of wound healing. In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz Ltd; 2001. pp. 15–38. [Google Scholar]
- Meyer W. Hair follicles in domesticated mammals with comparison to laboratory animals and humans. In: Mecklenburg L, Linek M, Tobin DJ, editors. Hair loss disorders in domestic animals. Ames: Wiley-Blackwell; 2009. pp. 43–64. [Google Scholar]
- Miller SJ, Burke EM, Rader MD, Coulombe PA, Lavker RM. Re-epithelialization of porcine skin by the sweat apparatus. J Investig Dermatol. 1998;110:13–19. doi: 10.1046/j.1523-1747.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- Montagna W. Comparative anatomy and physiology of the skin. Arch Dermatol. 1967;96:357–363. doi: 10.1001/archderm.1967.01610040007003. [DOI] [PubMed] [Google Scholar]
- Montagna W. The skin of nonhuman primates. Am Zool. 1972;12:109–124. doi: 10.1093/icb/12.1.109. [DOI] [Google Scholar]
- Montagna W. Morphology of cutaneous sensory receptors. J Investig Dermatol. 1977;69:4–7. doi: 10.1111/1523-1747.ep12497855. [DOI] [PubMed] [Google Scholar]
- Montagna W. Embryology and anatomy of the cutaneous adnexa. J Cutan Pathol. 1984;11:350–351. doi: 10.1111/j.1600-0560.1984.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Montagna W. Some particularities of human skin and the skin of nonhuman primates. G Ital Dermatol Venereol. 1984;119:1–4. [PubMed] [Google Scholar]
- Montagna W, Yun JS. The skin of the domestic pig. J Investig Dermatol. 1964;42:11–21. doi: 10.1038/jid.1964.110. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Saga K. Proliferating cells in human eccrine and apocrine sweat glands. J Histochem Cytochem. 1995;43:1217–1221. doi: 10.1177/43.12.8537637. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland G, Ross R. Human wound repair. I Epidermal regeneration. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortonne JP, Loning T, Schmitt D, Thivolet J. Immunomorphological and ultrastructural aspects of keratinocyte migration in epidermal wound healing. Virchows Arch A Pathol Anat Histopathol. 1981;392:217–230. doi: 10.1007/BF00430822. [DOI] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailler-Mattei C, Debret R, Vargiolu R, Sommer P, Zahouani H. In vivo skin biophysical behaviour and surface topography as a function of ageing. J Mech Behav Biomed Mater. 2013;28:474–483. doi: 10.1016/j.jmbbm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Poblet E, Jimenez-Acosta F, Rocamora A. Qbend/10 (anti-cd34 antibody) in external root sheath cells and follicular tumors. J Cutan Pathol. 1994;21:224–228. doi: 10.1111/j.1600-0560.1994.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Rea TH., Jr The anatomic site of vascular injury in mouse skin exposed to ultraviolet light. J Investig Dermatol. 1968;51:100–107. doi: 10.1038/jid.1968.98. [DOI] [PubMed] [Google Scholar]
- Rendell MS, McIntyre SF, Terando JV, Kelly ST, Finney DA. Skin blood flow in the wistar-kyoto rat and the spontaneously hypertensive rat. Comp Biochem Physiol A. 1993;106:349–354. doi: 10.1016/0300-9629(93)90524-8. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Arnott EC, Whitehouse CJ, Lawrence CM, Reynolds AJ, Hole N, Jahoda CA. Plasticity of rodent and human hair follicle dermal cells: implications for cell therapy and tissue engineering. J Investig Dermatol Symp Proc. 2005;10:180–183. doi: 10.1111/j.1087-0024.2005.10101.x. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT (2015) Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151 [DOI] [PMC free article] [PubMed]
- Rittié L. Another dimension to the importance of the extracellular matrix in fibrosis. J Cell Commun Signal. 2015;9:99–100. doi: 10.1007/s12079-015-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370. doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Stoll SW, Kang S, Voorhees JJ, Fisher GJ. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738–751. doi: 10.1111/j.1474-9726.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- Rittié L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;182:163–171. doi: 10.1016/j.ajpath.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Orringer JS, Sachs DL, Voorhees JJ, Fisher GJ. Eccrine sweat gland-derived keratinocytes rapidly express epidermal differentiation markers during repair of human wounds. J Investig Dermatol. 2013;133:S251. [Google Scholar]
- Rittié L, Farr EA, Orringer JS, Voorhees JJ, Fisher GJ (2016) Reduced cell cohesiveness of outgrowths from eccrine sweat glands delays wound closure in elderly skin. Aging Cell accepted: [DOI] [PMC free article] [PubMed]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GB, Cliff WJ, Gabbiani G, Irle C, Montandon D, Statkov PR, Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974;5:55–67. doi: 10.1016/S0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92:2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga K. Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem. 2002;37:323–386. doi: 10.1016/S0079-6336(02)80005-5. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol. 1983;245:R203–R208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. Ii Disorders of sweat gland function. J Am Acad Dermatol. 1989;20:713–726. doi: 10.1016/S0190-9622(89)70081-5. [DOI] [PubMed] [Google Scholar]
- Semer NB, Adler-Lavan M. Skin grafts. In: NSemer B, Adler-Lavan M, editors. Practical plastic surgery for nonsurgeons. Philadelphia: Hanley & Belfus; 2001. pp. 97–109. [Google Scholar]
- Sinclair R, Chapman A, Magee J. The lack of significant changes in scalp hair follicle density with advancing age. Br J Dermatol. 2005;152:646–649. doi: 10.1111/j.1365-2133.2005.06310.x. [DOI] [PubMed] [Google Scholar]
- Siver A, Montagna W, Karacan I. Age and sex differences in spontaneous, adrenergic and cholinergic human sweating. J Investig Dermatol. 1964;43:255–265. doi: 10.1038/jid.1964.153. [DOI] [PubMed] [Google Scholar]
- Snowden JM, Kennedy DF, Cliff WJ. Wound contraction. The effects of scab formation and the nature of the wound bed. Aust J Exp Biol Med Sci. 1982;60:73–82. doi: 10.1038/icb.1982.5. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Alvarez OM, Bere EW, Jr, Eaglstein WH, Katz SI. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J Investig Dermatol. 1981;77:240–243. doi: 10.1111/1523-1747.ep12480082. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Su Y, Richmond A. Chemokine regulation of neutrophil infiltration of skin wounds. Adv Wound Care. 2015;4:631–640. doi: 10.1089/wound.2014.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Rennert RC, Rodrigues M, Sorkin M, Glotzbach JP, Januszyk M, Fujiwara T, Longaker MT, Gurtner GC. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells. 2014;32:1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philos Trans R Soc Lond B. 1967;252:447–485. doi: 10.1098/rstb.1967.0029. [DOI] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/S0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tiwari VK. Burn wound: How it differs from other wounds? Indian J Plast Surg. 2012;45:364–373. doi: 10.4103/0970-0358.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Turcan I, Jonkman MF. Blistering disease: insight from the hemidesmosome and other components of the dermal-epidermal junction. Cell Tissue Res. 2015;360:545–569. doi: 10.1007/s00441-014-2021-7. [DOI] [PubMed] [Google Scholar]
- Van Winkle W., Jr The epithelium in wound healing. Surg Gynecol Obstet. 1968;127:1089–1115. [PubMed] [Google Scholar]
- Vardaxis NJ, Brans TA, Boon ME, Kreis RW, Marres LM. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat. 1997;190(Pt 4):601–611. doi: 10.1046/j.1469-7580.1997.19040601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viziam CB, Matoltsy AG, Mescon H. Epithelialization of small wounds. J Investig Dermatol. 1964;43:499–507. doi: 10.1038/jid.1964.192. [DOI] [PubMed] [Google Scholar]
- Wagner KJ. Epithelial regeneration in anesthetic areas after spinal cord injuries. Plast Reconstr Surg. 1964;34:268–274. doi: 10.1097/00006534-196409000-00008. [DOI] [PubMed] [Google Scholar]
- Watts GT, Grillo HC, Gross J. Studies in wound healing: Ii. The role of granulation tissue in contraction. Ann Surg. 1958;148:153–160. doi: 10.1097/00000658-195808000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. The biological foundations of wound repair. Harvey Lect. 1961;55:13–42. [PubMed] [Google Scholar]
- Welch MP, Odland GF, Clark RAF. Temporal relationships of f-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MG, Hunter R. Studies on epidermal regeneration by means of the strip method. J Investig Dermatol. 1957;29:407–413. doi: 10.1038/jid.1957.116. [DOI] [PubMed] [Google Scholar]
- Winstanley EW. The epithelial reaction in the healing of excised cutaneous wounds in the dog. J Comp Pathol. 1975;85:61–75. doi: 10.1016/0021-9975(75)90085-7. [DOI] [PubMed] [Google Scholar]
- Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- Winter GD. Healing of skin wounds and the influence of dressings on the repair process. In: Harkiss KJ, editor. Surgical dressings and wound healing: proceedings of a symposium held on 7–8 July 1970 at the university of Bradford. London: Crosby Lockwood [for] Bradford University Press; 1971. pp. 46–60. [Google Scholar]
- Winter GD. Epidermal regeneration studied in the domestic pig. In: Maibach HI, Rovee DT, editors. Epidermal wound healing. Chicago: Year Book Medical Publishers; 1972. pp. 71–112. [Google Scholar]
- Woodley DT, Briggaman RA. Re-formation of the epidermal-dermal junction during wound healing. In: Clark RAF, Henson PM, editors. The molecular and cellular biology of wound repair. New York: Plenum Press; 1988. pp. 559–586. [Google Scholar]
- Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15:S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Zahir M. Formation of scabs on skin wounds. Br J Surg. 1965;52:376–380. doi: 10.1002/bjs.1800520516. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Forrester JV, McCaig CD. Direct visualization of a stratified epithelium reveals that wounds heal by unified sliding of cell sheets. FASEB J. 2003;17:397–406. doi: 10.1096/fj.02-0610com. [DOI] [PMC free article] [PubMed] [Google Scholar]