Abstract
Purpose
To propose guidelines for the management of patients with wet age-related macular degeneration (wAMD), taking into account the results of large multicenter studies and clinical experience of retina experts.
Method
A team of retina experts developed a consensus paper after three consecutive meetings. The group was focused on guidelines to help clinical decision-making around the definition of successful treatment and the definition of non-response to therapy.
Results
Parameters suggestive of a successful response to treatments included: any gain in best corrected visual acuity (BCVA) or vision loss that is less than 5–10 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, reduction of central retinal thickness, partial or complete absorption of subretinal fluid (SRF), reduction of intraretinal fluid, reduction of pigment epithelial detachment or restoration of the anatomy of outer retinal layers. Non-response to current treatment was considered in the case of loss of BCVA greater than 10 ETDRS letters, increased retinal edema or increase of SRF as evidenced by optical coherence tomography or new bleeding in biomicroscopy.
Conclusion
The introduction of anti-VEGF agents revolutionized the treatment of wAMD. Given the complexity of the disease, the emerging new agents and the difference of cases recruited in clinical trials compared to those appearing in every-day practice, it is essential to individualize treatment options taking into account the results of clinical trials.
Keywords: Age-related macular degeneration, Consensus, Ophthalmology, Recommendations
Introduction
According to recent epidemiological data, 2.3% of the population aged over 65 years in Europe is suffering from neovascular (wet) age-related macular degeneration (wAMD), with the disease posing a significant public health problem [1–3]. wAMD is diagnosed less often than dry AMD, yet it accounts for 90% of severe vision loss cases [1, 2].
During the past decade, new targeted treatments against vascular endothelial growth factor (anti-VEGF agents) have been introduced in the management of wAMD patients changing the visual prognosis in these patients [4]. A large benefit in vision of patients treated with ranibizumab was initially highlighted in large multicenter trials, followed by the use of bevacizumab and recently aflibercept [5–9]. Moreover, diagnostic technologies, and especially optical coherence tomography (OCT) have revolutionized the AMD diagnosis and treatment algorithm [10]. However, despite positive developments in disease management, there are no commonly accepted therapeutic response evaluation criteria. This is particularly true when the discussion is not limited to patients that are selected based on criteria similar to those in large clinical trials, but when referring to the individual patient, treated by an ophthalmologist in everyday clinical practice. In addition, there is ongoing research for parameters predictive or suggestive of no response to anti-VEGF therapy since the characteristics of patients not responding to treatment are not clearly defined. Thus, deriving the maximum possible benefit for each individual patient in the clinic becomes more difficult.
The purpose of this article is to present guidelines, compiled by a panel of specialized ophthalmologists in the area of wAMD, and propose commonly accepted therapeutic response criteria and define treatment discontinuation criteria for patients suffering from wAMD treated with anti-VEGF agents.
Methods
A team of Greek retina experts developed a consensus paper after three consecutive meetings. The expert panel reviewed the peer-reviewed literature with the goal to establish practical guidelines for the practicing ophthalmologist. The group was focused on guidelines to help clinical decision-making around the definition of successful treatment and the definition of non-response to therapy. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Initial Evaluation
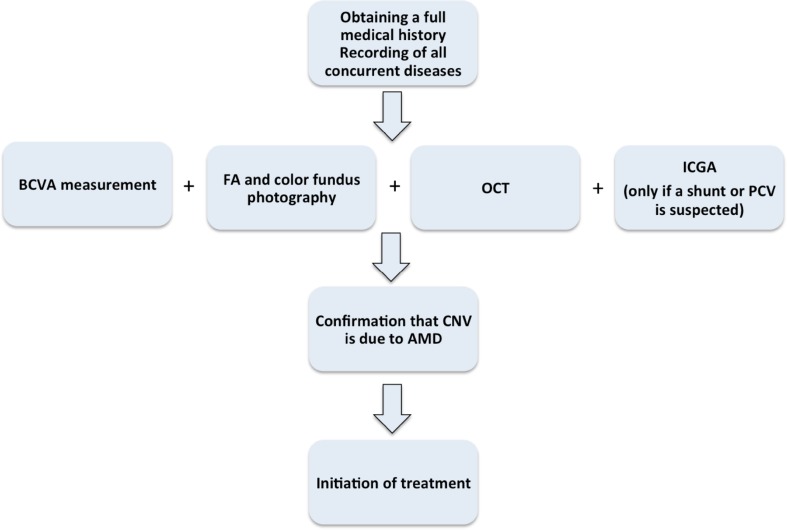
Prior to treatment initiation, compliance with the examination algorithm depicted in Fig. 1 is suggested. It is important to establish an initial diagnosis of wAMD before initiating treatment. Naturally, signs of the disease include central vision loss and metamorphopsia. Family history of AMD as well as social habits like smoking is important and should be recorded. Concomitant disease, e.g., arterial hypertension or diabetes mellitus should also be recorded. At baseline, it is expected that best corrected visual acuity (BCVA), dilated fundus examination, fluorescein angiography and optical coherence tomography (OCT) should be performed in order to confirm the diagnosis of wAMD and establish baseline criteria for evaluation of the treatment effect. Indocyanine green angiography (ICG) is not performed routinely. Ιt is, however, indicated in those patients where choroidal neovascularization (CNV) due to other etiologies is suspected and needs to be ruled out. The differential diagnosis from chronic central serous chorioretinopathy, retinal angiomatous proliferation (RAP) and polypoidal choroidal vasculopathy (PCV) is important also due to their different prognosis and treatment strategy [11–14].
Fig. 1.
Patient management prior to treatment initiation. AMD age-related macular degeneration, CNV choroidal neovascularization, FA fluorescein angiography, OCT optical coherence tomography, PCV polypoidal choroidal vasculopathy, BCVA best-corrected visual acuity measurement, ICGA indocyanine green angiography
After initiation of treatment, three monthly injections are advised. The frequency of follow-up and criteria for treatment administration thereafter depends on the treatment scheme that is adopted (monthly, pro re nata or treat and extend strategy, etc.). Follow-up is based on the evaluation of functional and morphological parameters to assess disease activity.
Various dosage schemes have been offered to patients given the real-life limitations that a costly and intensive treatment scheme of fixed monthly injections can encounter in practice. Results from the use of less intensive dosing schemes of ranibizumab, bevacizumab and aflibercept, coming from PIER (Clinicaltrials.gov identifier, NCT00090623), PrONTO (NCT00344227), SECURE (NCT00504959), HARBOR (NCT00891735), AURA (NCT01447043), CATT (NCT00593450), IVAN (ISRCTN92166560) and CLEAR-IT2 (NCT00320788) have been generally variable and inferior to monthly dosing; however, the pro re nata dosing regimen is commonly used in practice [7–9, 15–19].
Functional Parameters
Visual acuity constitutes the main functional parameter for quantitative evaluation of functional response in the treatment of wAMD and the main criterion that influences clinical decision-making. Tests to assess function are of primary importance, since they try to measure the loss that the patient experiences in life. Additional psychophysical methods such as contrast sensitivity and microperimetry may be used as ancillary methods, but are not usually part of the clinic routine (Table 1). Near visual acuity is also important. Standardized testing of BCVA with Early Treatment Diabetic Retinopathy Study (ETDRS) charts helps reduce variability that is associated with the measurement of this parameter. Identical procedures should ideally be followed in every visit.
Table 1.
Functional parameters to characterize treatment response
| Functional parameters | Method of measurement | Comments |
|---|---|---|
| Best-corrected visual acuity | Snellen optotype | Most popular. Widely used in clinical practice |
| ETDRS optotype | More precise examination. Optotype of choice in multicenter clinical studies | |
| Contrast sensitivity | Contrast sensitivity optotypes (e.g., Pelli–Robson) | Ancillary examination as per treating physician clinical judgment |
| Microperimetry | Fundus microperimeter | Ancillary examination as per treating physician clinical judgment |
ETDRS Early Treatment Diabetic Retinopathy Study
Therapeutic Response Definition Based on Functional Parameters
Changes in visual symptoms and in patient’s subjective complaints regarding vision should be recorded. Any level of vision improvement in a wAMD patient may be defined in effect as a positive outcome. Vision loss that does not exceed 5–10 EDRTS letters (1–2 Snellen lines) can also be considered an outcome that is better compared to the natural history of the disease. Left untreated, the CNV lesion will grow to involve the fovea and cause more profound vision loss.
In reality, the minimum visual benefit for the patient would be to sustain vision loss that is less compared to that if left untreated, according to the natural history of the disease. The natural history of the disease has been documented in several studies and also supported by findings in untreated eyes in the Macula Photocoagulation Study (Clinicaltrials.gov identifier, NCT00000158) [20–23]. The results from the large scale, carefully conducted, multicenter, randomized controlled clinical trials can also be used to reveal the natural history of the disease, where comparisons are made against the placebo arm. Measurement variability and uncertainty exists in trials and clinical practice as well. Results from the MARINA study (Clinicaltrials.gov identifier, NCT00056836) indicated a 2-year loss of 14.9 ETDRS letters in the sham injection group [24]. In the ANCHOR study (Clinicaltrials.gov identifier, NCT00061594), a loss of 9.5 letters with verterporfin at 12 months was reported [6]. The PIER study found a mean loss of 16.3 ETDRS letters in 1 year [9]. A recent meta-analysis of the literature also supports a mean decrease of 4 lines in visual acuity at 24 months [25]. It is also recognized that the visual gain is dependent on starting visual acuity and there is also a ceiling effect when exploring results in patients with good starting BCVA. There is also a floor effect in cases of low visual acuity, highlighting the limitations in looking only at the change in the number of letters, without taking into consideration the starting BCVA. However, in order to provide a recommendation for use in clinical practice, the panel ascribed any level of visual gain or a loss that is less than 10 ETDRS letters as a response that is better than the natural history of the disease.
Anatomic Parameters
Morphological evidence of the disease status is essential, since it often precedes functional deficit and it can alert the physician as to the disease activity.
In general, structure and function do not correlate very well and are supposed to provide different information on the disease status [26]. This disconnect between structure and function has long been recognized and attempts have been made in order to propose morphological parameters that could predict the functional outcome [27]. In fact, morphological signs of disease activity on OCT, are given primary importance since they correspond to early signs of recurrence, usually observed before BCVA loss [28]. Moreover, it has been recognized that anatomic parameters at baseline may also carry prognostic information, with foveal intraretinal fluid being associated with worse visual outcome and subretinal fluid interestingly, being associated with better visual outcome [29, 30].
Table 2 depicts the main anatomic parameters taken into consideration. Their evaluation is performed based on spectral domain-OCT scans.
Table 2.
Anatomic parameters used to evaluate treatment response
| Anatomic parameters |
|---|
| Central retina thickness |
| Subretinal fluid |
| Intraretinal fluid |
| Anatomy of the outer retinal layers |
| Pigment epithelium detachment |
Techniques, such as angiography and autofluorescence are particularly important in special circumstances (Table 3). Angiography is also useful in case of recurrence. Otherwise, OCT has emerged as the most useful tool to provide evidence of morphological response to treatment or signs of recurrence. Parameters that are important to monitor on OCT are central retina thickness (CRT), presence of subretinal fluid (SRF) or intraretinal fluid, the anatomy of the outer retina layers and the presence and extent of pigment epithelial detachment (PED). Most of these parameters can quantitatively measure the efficacy of the treatment regimen.
Table 3.
Proposed scheme for evaluation of anatomic response
| Evaluation method | Comments |
|---|---|
| Optical coherence tomography (OCT) |
Routine examination during baseline visit Examination of choice at the monthly follow-up |
| Fluorescein angiography (FA) |
Routine examination during baseline visit Lesion size and leak extent/intensity monitoring |
| Indocyanine green angiography |
Not a routine examination during baseline visit Complementary to FA in order to collect information in case of a diagnostic problem or suspicion of latent or other lesion (such as PCV) |
| Autofluorescence (AF) | Useful for the assessment and evaluation of macular atrophy. Ancillary examination as per treating physician clinical judgment |
PCV polypoidal choroidal vasculopathy
Therapeutic Response Definition Based on Anatomic Parameters
Response to treatment is evaluated based on anatomic and functional criteria. Anatomic criteria can provide qualitative and quantitative information. Based on anatomic parameters, a positive outcome of a therapeutic intervention is defined as:
Reduction in CRT compared to baseline. CRT ranging between 250–350 μm is generally considered satisfactory.
Partial or complete resolution of subretinal fluid.
Reduction in subretinal fluid versus baseline.
Reduction in PED extent and height.
Restoration of the outer retinal layers anatomy.
Observations
Lack of relapses constitutes an additional efficacy criterion. The time free of disease activity should be taken into account when evaluating the effect of treatment.
Re-treatment criteria include non-stabilization and fluctuation of visual acuity (continuing improvement or gradual deterioration), as well as anatomic deterioration (e.g., retinal thickness increase by 100 μm, PED or increase in SRF).
In the case of bilateral disease, concomitant and independent treatment administration is recommended for both eyes [31].
Non-response to Treatment with Anti-angiogenic Factors in wAMD
It is a recognized fact that not all patients respond to treatment. Nine percent and 10% of patients treated in the MARINA and ANCHOR trials with monthly ranibizumab experienced a loss in BCVA of more than 15 letters at 2 years [24]. The definition of non-response to treatment is based on the findings described below from functional and anatomic parameters. However, it is understood that ongoing visual acuity loss may in fact result from the development of scarring or geographic atrophy in the center of the fovea, and not only from non-response to the administered anti-VEGF treatment [32]. Therefore, anatomic signs of ongoing wAMD activity should in fact be carefully taken into account to support non-response to anti-VEGF therapy.
Functional Parameters
Vision loss of more than 10 ETDRS letters (or 2 Snellen lines).
Anatomic Parameters
Edema increase (CRT increase >100 μm).
Increase in SRF.
New hemorrhages in dilated fundus exam.
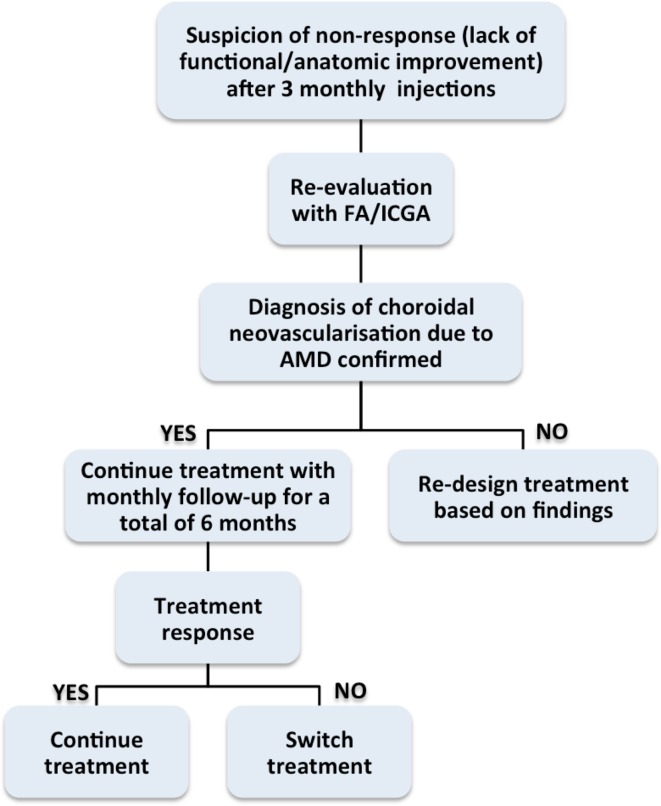
If there is no functional and/or anatomic improvement (i.e., stable low or declining vision and/or persisting fluid in the OCT) after a series of at least three monthly treatment repetitions, non-response to treatment is being suspected. Compliance with the monthly injection scheme is important to evaluate response. In fact the problem of undertreatment is very important and may underlie the lack of observed response to anti-VEGF therapy. Therefore, it should be emphasized that the decision of non-response to treatment may only be made when the strict protocol of 4-weekly treatment intervals has been followed.
In this case, a re-evaluation should be performed using FA/ICGA or any other method considered appropriate by the treating physician to rule out other entities.
If the diagnosis of choroidal neovascularization due to wAMD is confirmed then:
The treatment should be continued on a monthly basis, for up to a total of 6 months. Findings from subgroup analysis from patients enrolled in the MARINA and ANCHOR pivotal trials support a net benefit in vision with continued monthly injections [33]. It has also been suggested that the change in visual acuity with ranibizumab is associated with lesion characteristics [24].
If a benefit is not observed afterwards, the treating physician may choose to switch to a different drug. The evidence to support a change in the administered anti-VEGF agent mainly comes from retrospective case series, without a control group [34–39]. However, analysis of data from the CATT showed in fact that eyes that are considered non-responders, may in fact benefit from continued treatments, with an increase in vision and a better morphological outcome, without switching to another agent [40]. Future studies will provide more evidence to guide clinical practice in this regard.
It has also been proposed that some patients may develop tachyphylaxis after repeated doses of the drug [41]. If this is the case, patients usually respond well initially, but gradually become resistant to further injections. A change in the drug of choice may be indicated in these patients [42, 43].
Chronicity of the disease that has already caused irreversible damage might also be suspected. Presence of permanent structural damage or extensive fibrotic lesion or central atrophy would not allow the vision to recover. Hence, switching to a different drug in these patients is less likely to provide improvement in vision. Presence of polypoidal choroidal vasculopathy might also need combined treatment with anti-angiogenic intravitreal injections and photodynamic therapy. It has also been suggested that some patients may require more frequent dosing than others [44].
The algorithm in Fig. 2 shows patient follow-up upon suspicion of non-response or development of factors able to negatively affect response.
Fig. 2.
Management algorithm in patients when there is suspicion of non-response to anti-VEGF injection. AMD age-related macular degeneration, FA fluorescein angiography, ICGA indocyanine green angiography
Treatment Discontinuation Criteria
In the case of a patient that fulfills the below-mentioned criteria, (s)he is characterized as non-responder and may discontinue anti-angiogenetic factor treatment:
Visual acuity decline at a level less than 1/20 in three sequential visits, due to wAMD.
Lesion morphology deterioration, suggesting ongoing activity, despite appropriate treatment (e.g., progressive increase in the size of the lesion confirmed with fluorescein angiography, features on OCT suggesting disease activity or new hemorrhages or exudates (not related to vascular disease), providing evidence of ongoing activity).
Guidance for Patients that Already Sustained Profound Vision Loss in the Other Eye
Patients that already sustained profound central vision loss in the other eye should probably be treated individually, having a lower threshold for administering treatment. These patients are already at a higher risk of developing wAMD in the fellow eye, therefore close monitoring and timely treatment is indicated [45–47].
Conclusion
The purpose of this paper is to provide a consensus document concerning functional and anatomic criteria used in the evaluation of anti-VEGF treatment response and treatment discontinuation, as a guide in everyday clinical practice regarding the optimal treatment of wAMD. It has already been a decade since the FDA and EMA approval of ranibizumab in the United States and Europe for all wAMD lesion types and recently, a second drug, aflibercept, has been approved [5, 8]. It is true that intravitreal anti-angiogenetic treatment has changed the prognosis for patients with wAMD [48, 49]. Therefore, it is important to determine qualitative and quantitative criteria that can characterize a patient’s response to treatment [31, 50, 51].
Real-world studies have generally been associated with inferior outcomes, compared to those reported in the pivotal trials [52]. It is recognized that the clinical situations and variations that arise in clinical practice are not identical to those met in the well-designed clinical trials that led to the introduction of the drugs. Moreover, patients are generally harder to adhere to the intensive monitoring and treatment scheme in clinical practice. Importantly, early diagnosis and initiation of treatment are essential for a successful outcome. Compliance with treatment is another crucial factor. It is currently believed that initiation of anti-VEGF therapy should not be delayed to ensure that anatomical signs of activity are short lived and that chronic changes have not already damaged the photoreceptor layer [28]. Success has also been variable with different treatment schemes to ensure the same outcome can be achieved with less-than-monthly dosing used in the pivotal ranibizumab trials. Efforts oriented to improve patient compliance with treatment schedule are advocated.
Additionally, knowledge around genetic factors that appear to be important factors controlling response to anti-VEGF treatment is also emerging. Interestingly, low-risk complement factor H phenotypes may in fact predict a favorable response compared to the high-risk group [53]. It remains, however, unknown how this increasing knowledge can translate in clinical practice and whether it will change our treatment approach.
Advances in imaging modalities have definitely revolutionized diagnosis and follow-up of wAMD. OCT has emerged as an essential tool in the care of patients with wAMD nowadays. Fundus autofluorescence imaging and indocyanine green angiography provide useful information in selected cases. It remains to be seen how advances in OCT angiography will change the approach to diagnosis and treatment in those patients.
Morphological evidence of disease activity is crucial, since it provides objective information and can earlier detect activity or recurrence. Fibrosis resulting in permanent disruption of tissue architecture and loss of the outer layers cause irreversible loss of central vision. Therefore, treatment of the lesion before permanent damage has occurred is essential. Finally, progression of atrophy should be carefully observed during prolonged treatment, since it represents an important factor in determining visual outcome [54]. Given the chronic nature of the disease, future studies may further highlight factors that are important for the long-term visual prognosis of the individual wAMD patient.
Acknowledgments
No funding or sponsorship was received for the publication of this article. The consensus discussion was finalized during an Advisory Board Meeting held in Athens, 22.5.2015, sponsored by Novartis Hellas. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
A Dastiridou and C. Symeonidis have nothing to disclose.
S. Androudi, N. Pharmakakis, M. Stefaniotou, C. Kalogeropoulos, A. Charonis and M. Tsilimbaris received honoraria from Novartis.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/02C4F06016FF9EC8.
References
- 1.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Arch Ophthalmol. 2006;124:529–535. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Keenan TD, Kelly SP, Sallam A, et al. Incidence and baseline clinical characteristics of treated neovascular age-related macular degeneration in a well-defined region of the UK. Br J Ophthalmol. 2013;97:1168–1172. doi: 10.1136/bjophthalmol-2013-303233. [DOI] [PubMed] [Google Scholar]
- 4.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, MARINA Study Group et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age related macular degeneration: two year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heier JS, Brown DM, Chong V, VIEW 1 and VIEW 2 Study Groups et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103:1260–1270. doi: 10.1016/S0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 11.Ying GS, Huang J, Maguire MG, Comparison of Age-related Macular Degeneration Treatments Trials Research Group et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharbiya M, Parisi F, Cruciani F, Bozzoni-Pantaleoni F, Pranno F, Abdolrahimzadeh S. Intravitreal anti-vascular endothelial growth factor for retinal angiomatous proliferation in treatment-naive eyes: long-term functional and anatomical results using a modified PrONTO-style regimen. Retina. 2014;34:298–305. doi: 10.1097/IAE.0b013e3182979e62. [DOI] [PubMed] [Google Scholar]
- 13.Kang HM, Koh HJ, Lee CS, Lee SC. Combined photodynamic therapy with intravitreal bevacizumab injections for polypoidal choroidal vasculopathy: long-term visual outcome. Am J Ophthalmol. 2014;157(598–606):e1. doi: 10.1016/j.ajo.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Koh A, LeeWK Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin PDT in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–1464. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 15.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Silva R, Axer-Siegel R, Eldem B, et al. The SECURE study: long-term safety of ranibizumab 0.5 mg in neovascular age-related macular degeneration. Ophthalmology. 2013;120:130–139. doi: 10.1016/j.ophtha.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–1056. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2014;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressler SB, Bressler NM, Fine SL, et al. Natural course of choroidal neovascular membranes within the foveal avascular zone in senile macular degeneration. Am J Ophthalmol. 1982;93:157–163. doi: 10.1016/0002-9394(82)90410-X. [DOI] [PubMed] [Google Scholar]
- 21.Guyer DR, Fine SL, Maguire MG, Hawkins BS, Owens SL, Murphy RP. Subfoveal choroidal neovascular membranes in age-related macular degeneration. Visual prognosis in eyes with relatively good initial visual acuity. Arch Ophthalmol. 1986;104:702–705. doi: 10.1001/archopht.1986.01050170092029. [DOI] [PubMed] [Google Scholar]
- 22.Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol. 1991;109:1220–31. [DOI] [PubMed]
- 23.Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Archives of ophthalmology. 1993;111:1200–9. [DOI] [PubMed]
- 24.Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Roberts P, Mittermueller TJ, Montuoro A, et al. A quantitative approach to identify morphological features relevant for visual function in ranibizumab therapy of neovascular AMD. Invest Ophthalmol Vis Sci. 2014;55:6623–6630. doi: 10.1167/iovs.14-14293. [DOI] [PubMed] [Google Scholar]
- 27.Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Schmidt-Erfurth U. Predictive value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology. 2016;123:60–69. doi: 10.1016/j.ophtha.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Amissah-Arthur KN, Panneerselvam S, Narendran N, et al. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond) 2012;26:394–399. doi: 10.1038/eye.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S, Toth CA, Daniel E, Comparison of Age-related Macular Degeneration Treatments Trials Research Group et al. Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:865–875. doi: 10.1016/j.ophtha.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunwald JE, Daniel E, Huang J, CATT Research Group et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Royal College of Ophthalmologists. Age-related macular degeneration: guidelines for management; 2013.
- 32.Ying GS, Kim BJ, Maguire MG, CATT Research Group, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol. 2014;132:915–21. [DOI] [PMC free article] [PubMed]
- 33.Wolf S, Holz FG, Korobelnik JF, et al. Outcomes following three-line vision loss during treatment of neovascular age-related macular degeneration: subgroup analyses from MARINA and ANCHOR. Br J Ophthalmol. 2011;95:1713–1718. doi: 10.1136/bjophthalmol-2011-300471. [DOI] [PubMed] [Google Scholar]
- 34.Yonekawa Y, Andreoli C, Miller JA, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:29–35. doi: 10.1016/j.ajo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Eadie JA, Gottlieb JL, Ip MS, et al. Response to aflibercept in patients with persistent exudation despite prior treatment with bevacizumab or ranibizumab for age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2014;9:1–4. doi: 10.3928/23258160-20140909-03. [DOI] [PubMed] [Google Scholar]
- 36.Ehlken C, Jungmann S, Bohringer D, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye. 2014;28:538–545. doi: 10.1038/eye.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aslankurt M, Aslan L, Aksoy A, et al. The results of switching between 2 anti-VEGF drugs bevacizumab and ranibizumab in the treatment of neovascular age-related macular degeneration. Eur J Ophthalmol. 2013;23:553–557. doi: 10.5301/ejo.5000268. [DOI] [PubMed] [Google Scholar]
- 38.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97:1032–1035. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 39.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156(15–22):e1. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Ying GS, Maguire MG, Daniel E, Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT) Ophthalmology. 2015;122(2523–31):e1. doi: 10.1016/j.ophtha.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008;115:2199–2205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 42.De Geus SJR, Jager MJ, Luyten GPM, Dijkman G. Shifting exudative age-related macular degeneration patients to ranibizumab after insufficient response to bevacizumab. Acta Ophthalmol. 2013;91:e411–e413. doi: 10.1111/aos.12090. [DOI] [PubMed] [Google Scholar]
- 43.Ho SY, Yeh S, Olsen TW, et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013;156:23–28e2. [DOI] [PMC free article] [PubMed]
- 44.Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye) Retina. 2012;32:434–457. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 45.Maguire MG, Daniel E, Shah AR, Comparison of Age-Related Macular Degeneration Treatments Trials (CATT Research Group) et al. Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:2035–2041. doi: 10.1016/j.ophtha.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis MD, Gangnon RE, Lee LY, Age-Related Eye Disease Study Group et al. The age-related eye disease study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macular Photocoagulation Study Group Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–747. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- 48.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 49.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803–808. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Cruess AF, Berger A, Colleaux K, et al. Canadian expert consensus: optimal treatment of neovascular age-related macular degeneration. Can J Ophthalmol. 2012;47:227–235. doi: 10.1016/j.jcjo.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Erfurth U, Chong V, Loewenstein A, European Society of Retina Specialists et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA) Br J Ophthalmol. 2014;98:1144–1167. doi: 10.1136/bjophthalmol-2014-305702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond). 2016;30:270–286. doi: 10.1038/eye.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah AR, Williams S, Baumal CR, Rosner B, Duker JS, Seddon JM. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016;163(154–166):e8. doi: 10.1016/j.ajo.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, Zhang K. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159(915–24):e2. doi: 10.1016/j.ajo.2015.01.032. [DOI] [PubMed] [Google Scholar]