Abstract
Unresectable primary and secondary liver malignancies present a major problem in the treatment of solid tumors. Transarterial radioembolization (TARE) is an increasingly used technique for treating various types of malignant liver tumors. This approach is appealing, as the mechanism of action is independent from other loco-regional treatments and potentially complementary to systemic therapies. There are two commercially available products in use for TARE: 90Y-resin and 90Y-glass microspheres. Currently available data indicates TARE so be safe and effective in hepatocellular carcinoma (HCC) and metastatic liver disease. In HCC the results compare well with chemoembolization, while the role of TARE in combination with kinase inhibitors has yet to be established. Current data on TARE in metastatic liver disease is promising, but there is a strong need for prospective randomized trials comparing TARE and modern chemotherapeutic regimen to support the growing role of TARE in metastatic liver disease.
Keywords: Hepatocellular carcinoma, Selective internal radiation therapy, Radioembolization, Liver, Neoplasm, Metastasis
Core tip: Transarterial radioembolization (TARE) with 90Y microspheres is a targeted therapy indicated for unresectable primary and secondary liver malignancies. Current data proves its safety and effectiveness, but its definitive role in the treatment of hepatocellular carcinoma and metastatic liver disease within interdisciplinary treatment algorithms is still to be established. There is a strong need for randomized controlled trials comparing TARE to transarterial chemoembolization in primary liver cancer and to modern chemotherapeutic regimen in metastatic liver disease.
INTRODUCTION
Transarterial radioembolization (TARE) describes a group of treatment options currently in use for the treatment of primary and secondary liver tumors. Primary and secondary malignant liver neoplasms are common. Hepatocellular carcinoma (HCC) is the most common primary malignant hepatic neoplasm with about 782000 new cases per year, with a particularly high incidence in the Western Pacific Region[1]. In addition, the liver is the most common site for metastases from different solid tumors, most importantly colorectal cancer (CRC). Patients with colorectal liver metastases (CRLM) present a particularly relevant group of patients. With 1361000 estimated new cases of CRC per year and a frequency of 15%-25% of all CRC patients presenting synchronous metastatic disease and one third of patients eventually developing metachronous metastatic liver disease it also is the largest group of potential patients[2-4]. About 20%-30% of patients with metastatic liver disease are thought to be candidates for resection. However, reported resection rates of liver metastases are only around 5%-15%[5,6]. Thus there is a large number of patients in need of alternative therapies.
There are substantial differences in the treatment strategies for primary and secondary malignancies of the liver. While loco-regional treatments are a mainstay in primary liver cancers[7], transcatheter techniques such as conventional or drug-eluting beads transarterial chemoembolization (cTACE/DEB-TACE) are not commonly used in metastatic liver disease. With 90Y-based TARE there is a new and increasingly accepted treatment option for both primary and secondary malignancies to the liver.
The goal of this review is to provide an overview on the current status of TARE. As this is not a systematic review it may contain personal biases of the author.
CONCEPT AND HISTORY OF RADIOEMBOLIZATION
Radiation based tumor treatment is long known and has a clear rationale, as radiation is: (1) known to be cytocidal; and (2) independent from chemical or other energy based ablation techniques. Practically there are some limitations for the use of radiation for the treatment of liver tumors: Most importantly > 70 Gy are needed for the destruction of solid liver tumors[8], whereas the tolerance of normal liver tissue is only about 30 Gy[9]. Thus a selective delivery of radiation is the key for a safe and successful radiation therapy in hepatic malignancies. As all transcatheter techniques aim on a selective delivery of the anticancer treatment, it was an obvious choice to combine transcatheter delivery techniques with radiation based cancer treatments. Consequently the concept of TARE was introduced several decades ago and over time a variety of radioactive substances were used for treatment, particularly 131I-lipiodol[10]. While the term TARE is currently associated with the application of 90Y-microspheres, other radioactive microspheres based on 166 Ho and 188 Re and are under investigation[11,12].
Initial reports on TARE date back to the 1960s, when 90Y microspheres have first been reported for embolizing the prostate gland in dogs. Clinical data from the early days of radioembolization reported its use in inoperable pancreas, liver, lung and bone tumors[13,14]. While initial intravenous applications showed poor outcomes[15], early clinical series with intra-arterial administration of 90Y microspheres via the proper hepatic artery reported promising results. It was observed that hypervascularized tumors benefitted most from this type of therapy[16]. Several dose-escalation studies in animals and humans followed these early reports, indicating doses of up to 150 Gy to be safe, if pre-procedural work-up included a pre-treatment angiogram with occlusion of arteries with hepatofugal flow[17,18]. Although early applications of 90Y-TARE were first reported in the mid-1960s it took until the 1990s to establish this technique as a tool in clinical routine.
COMMERCIALLY AVAILABLE DEVICES
Two distinctively different types of 90Y-microspheres are commercially available: (1) SIR-Spheres® (Sirtex Medical Europe, Bonn, G); and (2) TheraSphere® (BTG International, London, United Kingdom) (Table 1). TheraSphere® were approved in 1999 in the United States for the treatment of unresectable HCC, while SIR-Spheres® were approved in 2002 in the United States for treating CRLM. In many countries both products are commercially available, labeled for treating hepatic neoplasms in general. All other products suited for TARE are either investigational or not in clinically relevant use.
Table 1.
Characteristics of commercially available 90Y-particles
| Feature | SIR-Spheres® | TheraSphere® |
| Isotope | 90Y | 90Y |
| Half life (h) | 64.2 | 64.2 |
| Material | Resin | Glass |
| Diameter (μm) | 20-60 | 20-30 |
| Activity per particle (Bq) | 50 | 2500 |
| Spheres per 3 GBq | 40-80 × 106 | 1.2 × 106 |
| Specific Gravity (g/mL) | 1.6 | 3.2 |
| Embolic effect | Mild | Negligible |
| Contrast injection | During infusion | No |
| FDA approved indication | CRC liver metastases with intrahepatic floxuridine | HCC |
FDA: Food and Drug Administration; CRC: Colorectal cancer; HCC: Hepatocellular carcinoma.
INDICATIONS AND CONTRAINDICATIONS
TARE may be considered for the treatment of unresectable primary or secondary liver malignancies or in patients unfit for surgery. There is an increasing amount of data necessitating more differentiated indications.
In general appropriateness of TARE needs to be determined in a multidisciplinary tumor board. Independent from the underlying disease candidates for TARE should have a life expectancy greater than 3 mo, with an Eastern Cooperative Oncology Group (ECOG) status ≤ 2.
In metastatic liver disease TARE is most commonly used as a salvage therapy in almost any kind of primary tumors. Based on early clinical trials TARE is accepted in CRLM either alone after failure of first-line chemotherapy, as salvage option in combination with 5-fluoruracil (5-FU), leucoverin, oxaliplation or irinotecan. It may also be applied as an adjuvant treatment to first- or second-line chemotherapy ideally within a clinical trial[19-23]. Several ongoing studies are likely to broaden accepted indications for TARE. Only recently the results of the SIRFLOX trial were published, indicating a potential use of TARE in a first line setting[24]. A neoadjuvant indication before resection may also be considered[25].
So far TARE is not yet named in the current treatment recommendations derived from the Barcelona Clinic Liver Cancer (BCLC) staging system. Despite the amount of data on TARE in HCC there is a lack of prospective randomized trials comparing TARE with other accepted treatment options such as TACE or sorafenib. Consequently in many institutions TARE is limited to patients who failed TACE. However, TARE may be considered instead of TACE in patients fulfilling the criteria for TACE according to the BCLC staging system[26]. Moreover, TARE should be considered an option in patients with portal vein thrombosis (PVT)[27].
The use of TARE is limited by only few absolute contraindications. These include inadequate functional liver reserve with an elevated total bilirubin > 2.0 mg/dL and reduced albumin < 3 g/dL, pathological lung shunting fraction potentially causing a lung dose of ≥ 30 Gy in a single application and foreseen non-target embolization that cannot be avoided by adequate transarterial embolization[28]. From an early trial with SIR-spheres® treatment with capecitabine within 3 mo prior to TARE is deemed an absolute contraindication for the use of resin spheres.
Patient preparation and procedural details are described in several practice guidelines[29-31]. These aspects include vascular anatomy of the liver, pre-procedural imaging as well as dosimetry. The latter is of particular interest as it varies depending on the type of spheres used for treatment. Moreover, dose has to be taken into account when comparing outcome and complications.
CURRENT RESULTS IN PRIMARY LIVER CANCER
There is a general consensus to accept 90Y-TARE as a safe and effective treatment. In fact TARE results in a significantly longer survival when compared with a control group without loco-regional treatment[32]. However, there is a substantial variation in response rates and survival. Recent data indicate any response rates [partial response (PR), complete response (CR), stable disease (SD)] according to EASL in the range of 79%-94% with an overall survival of 15-16.4 mo[33-35]. Liver function as determined by Child-Pugh score was shown to be a strong predictor for outcome with CHILD. A patients having a markedly better prognosis, when compared with CHILD B patients with a median survival of 17.2-17.4 mo vs 6-7.7 mo[33,34]. The presence of PVT is another predictor of outcome with significantly reduced time-to-progression (TTP), while evidence regarding overall survival is contradictory[33,34]. Although most HCC patients die of liver failure due to intrahepatic tumor, extensive extrahepatic disease negatively impacts prognosis with 5.4-7.4 mo overall survival in current series from Europe and the United States[33,36].
According to the BCLC staging system and treatment recommendations TACE is the first-line treatment of choice. To assess the role of TARE it therefore is important to compare outcome of TACE and TARE. Unfortunately there is only a single randomized controlled clinical trial (RCT) addressing this issue. This very small RCT comparing TARE and DEB-TACE in only 24 patients failed to show a difference in progression free survival, TTP and overall survival[37]. Typical candidates for TARE often come with more advanced stages of disease and are often considered poor candidates for TACE. Comparison of a large case series on TACE analyzed by BCLC stage[38] and corresponding data on TARE[39] showed median overall survivals of 17.4 mo (95%CI: 13.9-18.8) and 16.9 mo (95%CI: 12.8-22.8) in intermediate BCLC stage B patients. From these data one may assume TARE to be more or less equivalent with TACE. However, a coarse comparison of both methods is problematic as results vary and strongly depend on the stage of disease (Tables 2 and 3).
Table 2.
Summary of studies on 90Y-transarterial radioembolization in hepatocellular carcinoma with more than 50 patients
| Ref. | Patients (n) | Particle type | Stage | Design |
Response (%)
|
Median survival (mo) | ||||
| CR | PR | SD | AR | PD | ||||||
| Lau et al[73] | 71 | Resin | CHILD A/B | Retrospective | 0 | 27 | 65 | 92 | 8 | 9.4 |
| Carr[74] | 65 | Glass | Okuda I/II | Prospective | 3 | 28 | 40 | 71 | 29 | Okuda I = 21.6; Okuda II = 10 |
| Geschwind et al[75] | 80 | Glass | CHILD A/B | Retrospective | NA | NA | NA | NA | NA | CHILD A = 18.9; CHILD B = 8.2 |
| Hilgard et al[34] | 108 | Glass | CHILD A/B | Retrospective | 3 | 37 | 53 | 94 | 6 | 16.4 (CHILD A = 17.4; CHILD B = 6) |
| Salem et al[33] | 291 | Glass | CHILD A/B | Prospective | 23 | 34 | NA | NA | NA | CHILD A = 17.2; CHILD B = 7.7 |
| Sangro et al[39] | 325 | Resin | BCLC A-D | Retrospective | 12.8 (BCLC A = 24.4; BCLC B = 16.9; BCLC C = 10) | |||||
| Mazzaferro et al[35] | 52 | Glass | CHILD A/B | Prospective | 9.6 | 30.8 | 38.4 | 78.8 | 21.2 | 15 |
CR: Complete response; PR: Partial response; SD: Stable disease; AR: Any response; PD: Progressive disease; NA: Not available; BCLC: Barcelona Clinic Liver Cancer.
Table 3.
Summary of studies comparing different treatments to 90Y-transarterial radioembolization in hepatocellular carcinoma
| Ref. | Patients (n) | Particle type | Stage | Design |
Response (%)
|
Median survival (mo) | ||||
| CR | PR | SD | AR | PD | ||||||
| D'Avola et al[32] | 35 | Resin | CHILD A/B | Retrospective | NA | NA | NA | NA | NA | 16 |
| 43 | Control | NA | NA | NA | NA | NA | 8 | |||
| Lewandowski et al[43] | 43 | Glass | UNOS T3 | Retrospective | 47 | 39 | 14 | 100 | 0 | 18.7 |
| 43 | TACE | 17 | 54 | 26 | 97 | 3 | 35.7 | |||
| Kooby et al[76] | 27 | Resin | Okuda I-III | Retrospective | 0 | 11 | 56 | 87 | 33 | 6 |
| 44 | TACE | 0 | 4 | 60 | 64 | 36 | 6 | |||
| Salem et al[41] | 123 | Glass | BCLC A-D | Retrospective | NA | NA | NA | 72 | NA | 20.5 |
| 122 | TACE | NA | NA | NA | 69 | NA | 17.4 | |||
| Lance et al[77] | 38 | Glass | CHILD A/B | Retrospective | NA | NA | NA | NA | NA | 8 |
| 35 | TACE | NA | NA | NA | NA | NA | 10.3 | |||
| Moreno-Luna et al[26] | 55 | Glass | CHILD A/B | Retrospective | 12 | 39 | 39 | 91 | 9 | 15 |
| 61 | TACE | 4 | 47 | 34 | 85 | 15 | 14.4 | |||
| Gramenzi et al[47] | 63 | Resin | BCLC B/C | Retrospective | 14 | 54 | 14 | 72 | 28 | 13.2 |
| 74 | Sorafenib | 0 | 10 | 42 | 52 | 48 | 14.4 | |||
CR: Complete response; PR: Partial response; SD: Stable disease; AR: Any response; PD: Progressive disease; NA: Not available; TACE: Transarterial chemoembolization; BCLC: Barcelona Clinic Liver Cancer; UNOS: United Network for Organ Sharing.
A recent meta-analysis even concluded that microsphere embolization in patients with unresectable HCC provides better response to therapy and improved survival when compared with TACE[40]. As this meta-analysis mixes TARE with other techniques, data has to be analyzed in more detail and forest plots from the same meta-analysis prove TARE to be more effective than TACE in terms of overall survival [HR = 0.73 (0.60-0.88)] and TTP [HR = 0.61 (0.41-0.89)]. However, a more recent case control series comparing TARE vs TACE failed to show significant differences[26]. While CR rate was higher in the TARE groups, there were no differences in objective response rates and most importantly survival, with an overall survival of 15 mo after TARE and 14.4 mo after TACE. A subgroup analysis according to BCLC stage favored TARE over TACE in stage BCLC A/B, while in BCLC C patients TACE resulted in a slightly better survival. However, none of these trends was statistically significant. A more detailed analysis of two substantial patient series using either cTACE[38] or 90Y-glass microspheres[33] revealed median overall survivals of: 40 (15-46) mo vs 26.9 (17-30.2) mo in BCLC A, 17.4 (13.9-18.8) mo vs 17.2 (13.5-29.6) mo in BCLC B and 6.6 (4-9.3) mo vs 7.3 (6.5-10.1) mo in BCLC C. Therefore a prospective randomized controlled trial is needed, which according to Salem et al[41] would require more than 1000 patients as difference in outcome between TACE and TARE is expected to be relatively small.
In terms of quality of life, TARE might be somewhat better than TACE, particularly in terms of embolotherapy specific quality of life scores[42]. However, there was no significant difference in overall quality of life, likely due to the small number of patients included.
A different topic is the choice of loco-regional therapy for downstaging or bridging to transplant. In fact there are several studies assessing the effectiveness of TARE for these indications. In a comparative data analysis comparing TARE and TACE downstaging to UNOS T2 was achieved in 31% of TACE and 58% of 90Y-TARE patients. In this particular analysis TARE was also beneficial in terms of survival[43]. Two case series showed TARE to be effective as a bridging treatment while on the waiting list for transplantation[44,45]. Both of the latter case series also indicated the potential of TARE to downstage patients to meet the transplant criteria. Other case series confirmed the potential of TARE to downstage HCC patients to become eligible for other treatments such as resection, ablation or transplantation[46]. This, however, has to be considered anecdotal and prospective trials addressing this topic are missing.
Only recently Gramenzi et al[47] questioned the use of TARE in HCC by comparing TARE and sorafenib in a retrospective single center analysis. Their key finding is a comparable overall survival of both groups with 14.4 (4.3-24.5) mo in sorafenib and 13.2 (6.1-20.2) mo in TARE patients, with 1-, 2- and 3-year overall survival rates of 52.1%, 29.3% and 14.7% vs 51.8%, 27.8% and 21.6% respectively. Interestingly TARE showed better response rates and was the only technique providing a sufficient downstaging that allowed for liver transplantation in some patients. These data are highly relevant, but require further confirmation.
In view of currently available data TACE has still to be considered the first line method in HCC patients eligible for transarterial therapies. The lack of RCTs proving TARE to be more effective than TACE is a key drawback. The costs of treatment also need to be considered. The only cost effectiveness study on TARE in HCC concludes that the costs of TARE may be justified in BCLC C patients, while TARE appears not to be cost effective in BCLC A patients. Unfortunately there is no recommendation for BCLC B patients, who represent the majority of patients eligible for transarterial therapies[48].
In view of the poor outcome after systemic chemotherapy, TARE is also used for treating intrahepatic cholangiocaracinoma (ICC). A recent systematic review on the use of TARE in ICC treatment identified 12 studies covering 73 patients. PR and SD at 3 mo were reported in 28% and 54% of patients, respectively. In a pooled analysis the overall weighted median survival was 15.5 mo. In seven patients downstaging to surgery was achieved[49]. The combination of TARE and chemotherapy as a strategy for downstaging ICC to achieve resectability has recently been identified and initial data are encouraging[50]. However, when comparing different loco-regional treatments for ICC, TARE may not be the most effective approach. In a comparative analysis TARE was second to hepatic artery infusion chemotherapy (HAI), but more effective than cTACE or DEB TACE in terms of response to treatment as well as overall survival, which was best for HAI (22.8 mo) followed by 90Y-TARE (13.9 mo), cTACE (12.4 mo) and DEB-TACE (12.3 mo). While HAI provided best survival, but also had the highest grade III/IV toxicity[51]. Despite the lack of randomized controlled trials, loco-regional treatment appears to be somewhat more effective when compared with the current standard chemotherapy with oxaliplatin and gemcitabine[52]. In ICC TARE seems to be best suited for patients who are not eligible for HAI.
CURRENT RESULTS IN LIVER METASTASES
There is a vast amount of data on TARE in metastatic liver disease. With CRLM being the most common type of metastatic liver disease a large amount of data is focused on this entity. Currently the integration of TARE in multidisciplinary treatment algorithms is subject of discussion[53].
An early RCT compared early treatment with radioembolization combined with HAI with floxuridine (FUDR) to HAI with FUDR alone. In these patients with unresectable CRLM objective response rate and TTP were significantly longer in the HAI plus TARE group when compared to HAI alone, with 44% and 15.9 mo compared to 17.6% and 9.7 mo respectively[19].
Several prospective trials have examined TARE in combination with systemic chemotherapy vs systemic chemotherapy alone (Table 4). In an early study, a first line setting with TARE combined with systemic 5-FU proved superior to 5-FU alone in terms of objective response rate (73% vs 0%), TTP (18.6 mo vs 3.6 mo) and overall survival (29.4 mo vs 12.8 mo)[21]. As 5-FU alone is an outdated chemotherapeutic regimen, prospective studies assessed TARE with more recent chemotherapeutic regimen. In a first line setting TARE combined with FOLFOX4 achieved a 90% PR rate[23], while TARE with irinotecan in a second line setting after failure of previous chemotherapy reported 87% any response with 48% PR and 39% SD[22]. Only recently the SIRFLOX study, an RCT in 530 patients, reported the results of mFOLFOX 6 with or without bevacizumab compared with TARE + mFOLFOX 6 with or without bevacizumab. While there was no difference in progression free survival, there was a significant difference in progression free survival in the liver, favoring the combination with TARE (20.5 mo) over chemotherapy alone (12.6 mo; P = 0.002). Objective response rates were somewhat better in the combination therapy when compared to chemotherapy alone (68.1% vs 76.4%; P = 0.113)[24].
Table 4.
Summary of randomized controlled clinical trials comparing different treatments to 90Y-transarterial radioembolization using resin spheres in colorectal liver metastases
| Ref. | Patients (n) | Protocol | Design | Setting |
Response (%)
|
Progression free survival (mo) | Median survival (mo) | ||||
| CR | PR | SD | AR | PD | |||||||
| Gray et al[19] | 36 | TARE + HAI FUDR | RCT - Phase III | Early line | 6 | 44 | 28 | 78 | 14 | 15.9 (liver) | 17 |
| 34 | TARE - HAI FUDR | 0 | 22 | 48 | 70 | 30 | 9.7 (liver) | 15.9 | |||
| Van Hazel et al[21] | 11 | TARE + 5-FU/LV | RCT - Phase II | 1. line | 0 | 91 | 9 | 100 | 0 | 18.6 | 29.4 |
| 10 | 5-FU/LV | 0 | 0 | 60 | 60 | 40 | 3.6 | 12.8 | |||
| Hendlisz et al[20] | 21 | TARE + 5-FU | RCT - Phase III | Salvage | 0 | 10 | 80 | 90 | 10 | 4.5 | 10 |
| 23 | 5-FU | 0 | 0 | 36 | 36 | 64 | 2.1 | 7.3 | |||
| Gibbs et al[24] | 267 | TARE + FOLFOX ± Bevacizumab | RCT - Phase III | 1. line | 4.5 | 71.9 | NA | NA | NA | 10.7/20.5 (liver) | NA |
| 263 | FOLFOX ± Bevacizumab | 1.5 | 66.5 | NA | NA | NA | 10.2/12.6 (liver) | NA | |||
CR: Complete response; PR: Partial response; SD: Stable disease; AR: Any response; PD: Progressive disease; NA: Not available; RCT: Randomized controlled clinical trial; HAI: Hepatic artery infusion; FUDR: Floxuridine; TARE: Transarterial radioembolization; 5-FU: 5-fluoruracil; LV: Leucoverin.
Clinically TARE is most often used in a salvage setting. In a phase II study on 50 patients with isolated or predominant liver disease with progression after at least three lines of systemic chemotherapy TARE achieved disease control in 24% of patients with a progression-free-survival of 3.7 mo and an overall survival of 12.6 mo[54]. An RCT on 46 chemorefractory patients comparing systemic 5-FU to 5-FU plus TARE showed an significantly improved time to progression of liver disease (5.5 mo vs 2.1 mo; P = 0.003), but failed to show an significant improvement in overall survival (10.0 mo vs 7.3 mo; P = 0.80)[20]. A recent systematic review on TARE in unresectable, chemorefractory CRLM included 979 patients from 20 studies. After failure of 2 to 5 (median: 3) lines of chemotherapy TARE achieved CR, PR and SD in 0% (range: 0%-6%), 31% (range: 0%-73 %) and 40.5% (range: 17%-76 %) of patients, respectively. The median time to intra-hepatic progression was 9 mo (range: 6-16) and median overall survival was 12 mo (range: 8.3-36)[55]. A large multicenter data analysis proved overall survival being strongly dependent on previous treatment with median survivals (95%CI) receiving 90Y-TARE as a 2nd-, 3rd-, and ≥ 4th line of treatment after chemotherapy of 13.0 mo (95%CI: 10.5-14.6), 9.0 mo (95%CI: 7.8-11.0), and 8.1 mo (95%CI: 6.4-9.3), respectively (P < 0.001)[56]. A recent cost-effectiveness study on TARE using 90Y-resin microspheres compared to best supportive care reported a cost per QALY gained of £28216. The authors concluded that TARE using 90Y-resin microspheres offers a clinically effective and cost-effective treatment option[57].
While aforementioned data was obtained from 90Y resin spheres, there is only little data on 90Y-TARE with glass sphere. In 72 patients with unresectable CRLM after failure of at least one line of chemotherapy time to intrahepatic progression was 15.4 mo with a median survival of 14.5 mo after first 90Y treatment. ECOG stage 0, tumor replacement < 25% of liver volume, lack of extrahepatic tumor and response to therapy were identified as positive prognostic markers[58]. A recent phase II multicenter trial reported slightly worse results for treating liver metastases were, with an 8.8 mo median overall survival in CRLM[59].
There also is encouraging data on TARE in liver metastases from various other tumor entities such as metastatic breast cancer, uveal melanoma or neuroendocrine tumors (NET) (Table 5 and Figure 1). Among these, NET take a special role as these tumors are well arterialized and thus an ideal target for transarterial therapies similarly to HCC. Treatment goral in these patients is control of symptoms as well as survival. The biggest series so far comprises data from 148 patients from ten institutions. This series reported very high response rates with any response in 95.1% of patients and progressive disease in only 4.9% of the patients. The median OS of 70 mo after initial TARE was higher than other studies (Table 5)[60]. This may be due to the variable biology of NET, with pancreatic NET being associated with a markedly poorer prognosis when compared to non-pancreatic NET. However, there are no RCTs on any these entities and further data is needed to confirm these encouraging results.
Table 5.
Summary of studies on 90Y-transarterial radioembolization in liver metastases from various tumor types with more than 10 patients published within the last 5 years
| Ref. | Patients (n) | Particle type | Entity | Setting | Design |
Response (%)
|
Median survival (mo) | ||||
| CR | PR | SD | AR | PD | |||||||
| Saxena et al[78] | 48 | Resin | NET | Salvage | Retrospective | 15 | 40 | 23 | 78 | 22 | 35 |
| Cao et al[79] | 58 | Resin | NET | Mixed | Retrospective | 11.7 | 27.5 | 27.5 | 66.7 | 33.3 | 36 |
| Paprottka et al[80] | 42 | Resin | NET | Mixed | Retrospective | 0 | 22.5 | 75 | 97.5 | 2.5 | NA |
| Memon et al[81] | 40 | Glass | NET | Mixed | Retrospective | 1.2 | 62.7 | 32.5 | 96.4 | 3.6 | 34.4 |
| Peker et al[82] | 30 | Resin | NET | Mixed | Retrospective | 3 | 37 | 43 | 83 | 17 | 39 |
| Haug et al[83] | 58 | Resin | Breast | Salvage | Retrospective | 0 | 25 | 63 | 88 | 12 | 10.8 |
| Cianni et al[84] | 52 | Resin | Breast | Salvage | Retrospective | 0 | 56 | 35 | 91 | 9 | 11.5 |
| Saxena et al[85] | 40 | Resin | Breast | ≥ 1.line CTX | Retrospective | 5 | 26 | 39 | 70 | 30 | 13.6 |
| Gonsalves et al[86] | 32 | Resin | Uveal Melanoma | Salvage | Retrospective | 3 | 3 | 56 | 62 | 38 | 10 |
| Michl et al[87] | 19 | Resin | Pancreas | mixed | Retrospective | 0 | 64.3 | 0 | 64.3 | 45.7 | 9 |
CR: Complete response; PR: Partial response; SD: Stable disease; AR: Any response; PD: Progressive disease; NA: Not available; CTX: Chemotherapy; NET: Neuroendocrine tumor.
Figure 1.
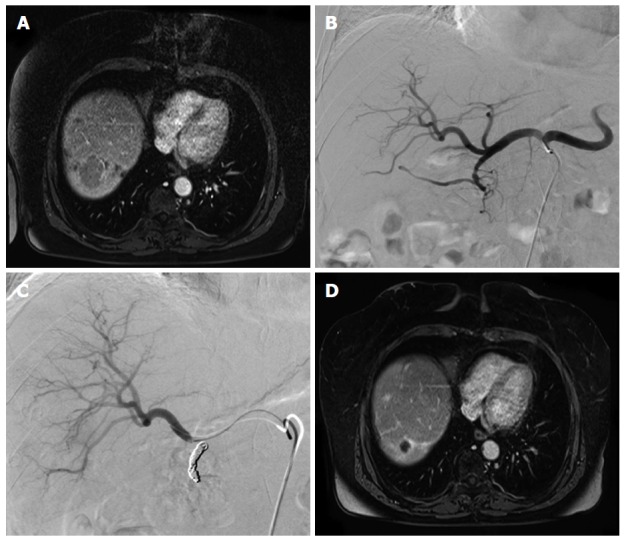
Case study of a 64-year-old female patient suffering from liver metastases from a midgut neuroendocrine tumor. A: Contrast enhanced MRI shows a large liver metastasis in the right hemiliver; B: Prior to TARE an angiogram of the hepatic arteries was obtained; C: The gastroduodenal artery was occluded with multiple microcoils; D: Contrast enhanced MRI obtained 24 mo after therapy shows a maintained partial response of the liver metastasis. MRI: Magnetic resonance imaging; TARE: Transarterial radioembolization.
SIDE EFFECTS AND ADVERSE EVENTS
The so called post-(radio)embolization syndrome with fatigue, nausea, vomiting, anorexia, fever and abdominal discomfort is the most frequent side effect of TARE. It may occur in up to 55% of patients and is self-limiting, lasting no longer than two weeks[61]. A passing elevation of liver enzymes, namely in alkaline phosphatase, alanine transferase and bilirubin are normal side effects of this treatment.
The most common relevant complication of TARE is gastrointestinal (GI) ulceration, caused by non-target embolization of 90Y-microspheres into the GI tract. Thorough pre-interventional imaging work-up and coiling of vessels with hepatofugal flow are key to minimize the risk of GI complications to less than 4%[62]. Proton pump inhibitors are the treatment of choice in GI ulcers. In case of treatment failure surgery may be required.
Eventually radiation leads to fibrosis presenting with imaging signs of portal hypertension. Fortunately, these findings hardly ever have clinical consequences[63]. In patients with HCC signs of portal hypertension are commonly seen on pre-interventional imaging as most of these patients suffer from underlying cirrhosis. However, liver dysfunction potentially progresses to radiation induced liver disease (RILD), which may occur in up to 20% of patients[59,64]. RILD is defined as icteric or anicteric, non-malignant ascites combined with an increase in alkaline phosphatase to at least twice the upper normal level within four months after treatment. So far there is no reliable treatment. Only recently administration of defibrotide (Gentium, Como, Italy) which is used for the treatment of veno-occlusive disease has been suggested[65]. Thus preventing RILD is most important. Consequently selection of patients by liver function is crucial as deranged baseline hepatic function, presence of liver cirrhosis and administered radiation dose are the most important risk factors for developing RILD. The routine administration of ursodeoxycholic acid and low-dose steroids has been shown to significantly reduce the risk of RILD[64]. In addition sequential lobar treatment seems to be safer than single session whole liver treatment[66].
Biliary toxicity with biloma, abscess and radiation induced cholecystitis occurs in ≤ 2% of patients[67]. Fortunately, many imaging findings indicative of biliary complications do not manifest clinically.
Finally, radiation pneumonitis, a restrictive lung dysfunction, is a relevant, but very rare adverse event[68]. It can reliably be avoided if dosimetry and pre-interventional work-up are performed properly with computation of lung shunting fraction from 99mTc-MAA imaging[69]. Lung doses need to be below 30 Gy for a single treatment and less than 50 Gy for repeated TARE. Radiation induced pneumonitis is usually managed with a steroid based therapy.
FUTURE PERSPECTIVES
A steadily growing amount of data shows TARE to be an effective monotherapy in the treatment of HCC. The obvious next step is the adjuvant or neoadjuvant combination of systemic and loco-regional therapies, specifically sorafenib and TARE. From theory both techniques run complementary ways of action. So far there data on the combination of sorafenib and TARE is scarce. In the only prospective study on this type of combination therapy, 39% of patients could not complete the prescribed dose of sorafenib due to side effects. Moreover, an objective response rate of 25% does not support the use of this type of combination therapy[70]. Initial data from a RCT comparing TARE with 90Y-resin microspheres followed by sorafenib with sorafenib only so far only reported safety data from the first 40 patients. These preliminary results indicate a similar tolerance for both treatment arms[71]. Outcome data from ongoing RCTs such as SORAMIC (NCT01126645), SARAH (NCT01482442) or SIRveNIB (NCT01135056) using SIR-spheres® or STOP-HCC using TheraSphere® (NCT01556490) have not yet been published.
While TARE is considered a bridging technique to transplantion, the combination of sorafenib and TARE seems problematic. A RCT in 20 patients undergoing TARE with or without sorafenib prior to transplantation indicated more acute rejections and peri-transplant complications in the treatment arm receiving TARE plus sorafenib. In addition, none of the patients could tolerate the prescribed dose of sorafenib. Half of the patients discontinued sorafenib completely because of side effects[72]. Thus caution on this type of combination therapy appears to be prudent until more data is available.
For metastatic disease RCTs comparing TARE and modern chemotherapeutic regimen are needed, as currently available data compared SIR-spheres® with outdated chemotherapeutic regimen or lacking survival data (Table 4), while there are no comparative data at all for TheraSphere®. The latter is currently addressed in an ongoing phase III trial evaluating treatment with 90Y-glass spheres and second-line chemotherapy after failure of first-line chemotherapy in comparison to second-line chemotherapy alone for CRLM (EPOCH; NCT01483027). The FOXFIRE global trial is an ongoing phase III study assessing the value of additional 90Y-resin spheres in a first line setting with FOLFOX6m (NCT01721954). There are further trials evaluating the role of TARE in uveal melanoma (SIRUM NCT01473004) or the combination of TARE and pasireotide and everolimus in neuroendocrine tumors (NCT01469572).
The use of TARE beyond the liver has been described sporadically for the lung and the spleen and is currently evaluated in a pilot trial for renal cancer (RESIRT, ACTRN 12610000690055).
CONCLUSION
In conclusion, TARE represents a potent technique for treating liver malignancies. The current data justifies its clinical use in HCC and CRLM, while its role outside a salvage setting needs to be identified for liver metastases from other tumor entities. Considering ongoing trials and the increasing clinical experience, a rapid increase in TARE procedures has to be expected.
Footnotes
Conflict-of-interest statement: There is no conflict of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 18, 2015
First decision: September 28, 2015
Article in press: March 14, 2016
P- Reviewer: Facciorusso A, Lau WY, Mao YL S- Editor: Gong XM L- Editor: A E- Editor: Li D
References
- 1.International Agency for Research on Cancer. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. [accessed 2015 Jul 28] Available from: http://globocan.iarc.fr/
- 2.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P, European Colorectal Metastases Treatment Group Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Hsu YN, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, Chang SC, Yen CC, Tzeng CH, Teng HW. A new classification scheme for recurrent or metastatic colon cancer after liver metastasectomy. J Chin Med Assoc. 2011;74:493–499. doi: 10.1016/j.jcma.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 4.van Steenbergen LN, Elferink MA, Krijnen P, Lemmens VE, Siesling S, Rutten HJ, Richel DJ, Karim-Kos HE, Coebergh JW; Working Group Output of The Netherlands Cancer Registry. Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989-2006. Ann Oncol. 2010;21:2206–2212. doi: 10.1093/annonc/mdq227. [DOI] [PubMed] [Google Scholar]
- 5.Hackl C, Gerken M, Loss M, Klinkhammer-Schalke M, Piso P, Schlitt HJ. A population-based analysis on the rate and surgical management of colorectal liver metastases in Southern Germany. Int J Colorectal Dis. 2011;26:1475–1481. doi: 10.1007/s00384-011-1278-5. [DOI] [PubMed] [Google Scholar]
- 6.van der Pool AE, Damhuis RA, Ijzermans JN, de Wilt JH, Eggermont AM, Kranse R, Verhoef C. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14:56–61. doi: 10.1111/j.1463-1318.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, Lawrence TS. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 9.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 10.Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, Bekhechi D, Deugnier YM, Gosselin M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 11.Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Huijbregts JE, van het Schip AD, Elschot M, Bult W, de Jong HW, et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: design of the phase I HEPAR trial. J Exp Clin Cancer Res. 2010;29:70. doi: 10.1186/1756-9966-29-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowicki ML, Cwikla JB, Sankowski AJ, Shcherbinin S, Grimmes J, Celler A, Buscombe JR, Bator A, Pech M, Mikołajczak R, et al. Initial study of radiological and clinical efficacy radioembolization using 188Re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers. Med Sci Monit. 2014;20:1353–1362. doi: 10.12659/MSM.890480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariel IM, Pack GT. The treatment of cancer metastases in the lung by means of radiating microspheres. Thoraxchir Vask Chir. 1966;14:286–307. doi: 10.1055/s-0028-1101262. [DOI] [PubMed] [Google Scholar]
- 14.Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres) Ann Surg. 1965;162:267–278. doi: 10.1097/00000658-196508000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard RJ, Lafave jw, kim ys, frye cs, ritchie wp, perry jf. treatment of patients with advanced cancer utilizing Y90 microspheres. Cancer. 1965;18:375–380. doi: 10.1002/1097-0142(196503)18:3<375::aid-cncr2820180314>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Mantravadi RV, Spigos DG, Tan WS, Felix EL. Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142:783–786. doi: 10.1148/radiology.142.3.7063703. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd FA, Rotstein LE, Houle S, Yip TC, Paul K, Sniderman KW. A phase I dose escalation trial of yttrium-90 microspheres in the treatment of primary hepatocellular carcinoma. Cancer. 1992;70:2250–2254. doi: 10.1002/1097-0142(19921101)70:9<2250::aid-cncr2820700906>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–1644. [PubMed] [Google Scholar]
- 19.Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, Gebski V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 20.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 21.Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, Cardaci G, Gray B. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 22.van Hazel GA, Pavlakis N, Goldstein D, Olver IN, Tapner MJ, Price D, Bower GD, Briggs GM, Rossleigh MA, Taylor DJ, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089–4095. doi: 10.1200/JCO.2008.20.8116. [DOI] [PubMed] [Google Scholar]
- 23.Sharma RA, Van Hazel GA, Morgan B, Berry DP, Blanshard K, Price D, Bower G, Shannon JA, Gibbs P, Steward WP. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs P, Heinemann V, Sharma NK, Findlay MPN, Ricke J, Gebski V, Van Buskirk M, Van Hazel GA; SIRFLOX Study Group. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab (bev) versus mFOLFOX6 + selective internal radiation therapy (SIRT) ± bev in patients (pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2015;33 Suppl:3502. [Google Scholar]
- 25.Van den Eynde M, Flamen P, El Nakadi I, Liberale G, Delatte P, Larsimont D, Hendlisz A. Inducing resectability of chemotherapy refractory colorectal liver metastasis by radioembolization with yttrium-90 microspheres. Clin Nucl Med. 2008;33:697–699. doi: 10.1097/RLU.0b013e318184b9a0. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokabi N, Camacho JC, Xing M, El-Rayes BF, Spivey JR, Knechtle SJ, Kim HS. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164–2174. doi: 10.1002/cncr.29275. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, Soulen MC, Geschwind JF, Kulik L, Kim YH, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giammarile F, Bodei L, Chiesa C, Flux G, Forrer F, Kraeber-Bodere F, Brans B, Lambert B, Konijnenberg M, Borson-Chazot F, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011;38:1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 31.Mahnken AH, Spreafico C, Maleux G, Helmberger T, Jakobs TF. Standards of practice in transarterial radioembolization. Cardiovasc Intervent Radiol. 2013;36:613–622. doi: 10.1007/s00270-013-0600-8. [DOI] [PubMed] [Google Scholar]
- 32.D’Avola D, Lñarrairaegui M, Bilbao JI, Martinez-Cuesta A, Alegre F, Herrero JI, Quiroga J, Prieto J, Sangro B. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1683–1688. [PubMed] [Google Scholar]
- 33.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 35.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 36.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, Weinmann A, Schreckenberger M, Galle PR, Otto G, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38:352–360. doi: 10.1007/s00270-014-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, Ibrahim SM, Abecassis MI, Miller FH, Sato KT, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 40.Xie F, Zang J, Guo X, Xu F, Shen R, Yan L, Yang J, He J. Comparison of transcatheter arterial chemoembolization and microsphere embolization for treatment of unresectable hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:455–462. doi: 10.1007/s00432-011-1117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358–1365.e1. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 44.Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, Geller DA, Marsh JW, Tsung A. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24:1632–1638. doi: 10.1016/j.jvir.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Abdelfattah MR, Al-Sebayel M, Broering D, Alsuhaibani H. Radioembolization using yttrium-90 microspheres as bridging and downstaging treatment for unresectable hepatocellular carcinoma before liver transplantation: initial single-center experience. Transplant Proc. 2015;47:408–411. doi: 10.1016/j.transproceed.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim SM, Kulik L, Baker T, Ryu RK, Mulcahy MF, Abecassis M, Salem R, Lewandowski RJ. Treating and downstaging hepatocellular carcinoma in the caudate lobe with yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2012;35:1094–1101. doi: 10.1007/s00270-011-0292-x. [DOI] [PubMed] [Google Scholar]
- 47.Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036–1047. doi: 10.1111/liv.12574. [DOI] [PubMed] [Google Scholar]
- 48.Rostambeigi N, Dekarske AS, Austin EE, Golzarian J, Cressman EN. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25:1075–1084. doi: 10.1016/j.jvir.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, Boucher E, Boudjema K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 51.Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, Gamblin TC. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213–220. doi: 10.1002/jso.23781. [DOI] [PubMed] [Google Scholar]
- 52.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 53.Wasan H, Kennedy A, Coldwell D, Sangro B, Salem R. Integrating radioembolization with chemotherapy in the treatment paradigm for unresectable colorectal liver metastases. Am J Clin Oncol. 2012;35:293–301. doi: 10.1097/COC.0b013e3182005747. [DOI] [PubMed] [Google Scholar]
- 54.Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saxena A, Bester L, Shan L, Perera M, Gibbs P, Meteling B, Morris DL. A systematic review on the safety and efficacy of yttrium-90 radioembolization for unresectable, chemorefractory colorectal cancer liver metastases. J Cancer Res Clin Oncol. 2014;140:537–547. doi: 10.1007/s00432-013-1564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy AS, Ball D, Cohen SJ, Cohn M, Coldwell DM, Drooz A, Ehrenwald E, Kanani S, Rose SC, Nutting CW, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol. 2015;6:134–142. doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pennington B, Akehurst R, Wasan H, Sangro B, Kennedy AS, Sennfält K, Bester L. Cost-effectiveness of selective internal radiation therapy using yttrium-90 resin microspheres in treating patients with inoperable colorectal liver metastases in the UK. J Med Econ. 2015;18:797–804. doi: 10.3111/13696998.2015.1047779. [DOI] [PubMed] [Google Scholar]
- 58.Mulcahy MF, Lewandowski RJ, Ibrahim SM, Sato KT, Ryu RK, Atassi B, Newman S, Talamonti M, Omary RA, Benson A, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115:1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 59.Benson AB, Geschwind JF, Mulcahy MF, Rilling W, Siskin G, Wiseman G, Cunningham J, Houghton B, Ross M, Memon K, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional phase II study. Eur J Cancer. 2013;49:3122–3130. doi: 10.1016/j.ejca.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 61.Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, Omary RA, Salem R. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–1130; quiz 1131. doi: 10.1016/j.jvir.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 62.Lam MG, Banerjee S, Louie JD, Abdelmaksoud MH, Iagaru AH, Ennen RE, Sze DY. Root cause analysis of gastroduodenal ulceration after yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36:1536–1547. doi: 10.1007/s00270-013-0579-1. [DOI] [PubMed] [Google Scholar]
- 63.Jakobs TF, Saleem S, Atassi B, Reda E, Lewandowski RJ, Yaghmai V, Miller F, Ryu RK, Ibrahim S, Sato KT, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci. 2008;53:2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 64.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, Benito A, Dominguez I, D’Avola D, Herrero JI, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57:1078–1087. doi: 10.1002/hep.26191. [DOI] [PubMed] [Google Scholar]
- 65.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seidensticker R, Seidensticker M, Damm R, Mohnike K, Schütte K, Malfertheiner P, Van Buskirk M, Pech M, Amthauer H, Ricke J. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–1118. doi: 10.1007/s00270-011-0295-7. [DOI] [PubMed] [Google Scholar]
- 67.Atassi B, Bangash AK, Lewandowski RJ, Ibrahim S, Kulik L, Mulcahy MF, Murthy R, Ryu RK, Sato KT, Miller FH, et al. Biliary sequelae following radioembolization with Yttrium-90 microspheres. J Vasc Interv Radiol. 2008;19:691–697. doi: 10.1016/j.jvir.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Wright CL, Werner JD, Tran JM, Gates VL, Rikabi AA, Shah MH, Salem R. Radiation pneumonitis following yttrium-90 radioembolization: case report and literature review. J Vasc Interv Radiol. 2012;23:669–674. doi: 10.1016/j.jvir.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 69.Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Ibrahim S, Mulcahy MF, Kulik L, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol. 2008;31:431–438. doi: 10.1097/COC.0b013e318168ef65. [DOI] [PubMed] [Google Scholar]
- 70.Chow PK, Poon DY, Khin MW, Singh H, Han HS, Goh AS, Choo SP, Lai HK, Lo RH, Tay KH, et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One. 2014;9:e90909. doi: 10.1371/journal.pone.0090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K, Verslype C, Walecki J, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015;35:620–626. doi: 10.1111/liv.12622. [DOI] [PubMed] [Google Scholar]
- 72.Kulik L, Vouche M, Koppe S, Lewandowski RJ, Mulcahy MF, Ganger D, Habib A, Karp J, Al-Saden P, Lacouture M, et al. Prospective randomized pilot study of Y90+/-sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol. 2014;61:309–317. doi: 10.1016/j.jhep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Lau WY, Ho S, Leung TW, Chan M, Ho R, Johnson PJ, Li AK. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–592. doi: 10.1016/s0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- 74.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 75.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 76.Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1697–1705. doi: 10.1016/j.jvir.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251:910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 79.Cao CQ, Yan TD, Bester L, Liauw W, Morris DL. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg. 2010;97:537–543. doi: 10.1002/bjs.6931. [DOI] [PubMed] [Google Scholar]
- 80.Paprottka PM, Hoffmann RT, Haug A, Sommer WH, Raessler F, Trumm CG, Schmidt GP, Ashoori N, Reiser MF, Jakobs TF. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol. 2012;35:334–342. doi: 10.1007/s00270-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 81.Memon K, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83:887–894. doi: 10.1016/j.ijrobp.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peker A, Çiçek O, Soydal Ç, Küçük NÖ, Bilgiç S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol. 2015;21:54–59. doi: 10.5152/dir.2014.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP, Uebleis C, Bartenstein P, Heinemann V, Hacker M. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 84.Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O, Filippi L, Cortesi E. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013;23:182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 85.Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21:1296–1303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 86.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196:468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 87.Michl M, Haug AR, Jakobs TF, Paprottka P, Hoffmann RT, Bartenstein P, Boeck S, Haas M, Laubender RP, Heinemann V. Radioembolization with Yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology. 2014;86:24–32. doi: 10.1159/000355821. [DOI] [PubMed] [Google Scholar]


