Abstract
Representing 2%-3% of adult cancers, renal cell carcinoma (RCC) accounts for 90% of renal malignancies and is the most lethal neoplasm of the urologic system. Over the last 65 years, the incidence of RCC has increased at a rate of 2% per year. The increased incidence is at least partly due to improved tumor detection secondary to greater availability of high-resolution cross-sectional imaging modalities over the last few decades. Most RCCs are asymptomatic at discovery and are detected as unexpected findings on imaging performed for unrelated clinical indications. The 2004 World Health Organization Classification of adult renal tumors stratifies RCC into several distinct histologic subtypes of which clear cell, papillary and chromophobe tumors account for 70%, 10%-15%, and 5%, respectively. Knowledge of the RCC subtype is important because the various subtypes are associated with different biologic behavior, prognosis and treatment options. Furthermore, the common RCC subtypes can often be discriminated non-invasively based on gross morphologic imaging appearances, signal intensity on T2-weighted magnetic resonance images, and the degree of tumor enhancement on dynamic contrast-enhanced computed tomography or magnetic resonance imaging examinations. In this article, we review the incidence and survival data, risk factors, clinical and biochemical findings, imaging findings, staging, differential diagnosis, management options and post-treatment follow-up of RCC, with attention focused on the common subtypes.
Keywords: Papillary renal cell carcinoma, Multidetector computed tomography, Clear cell renal cell carcinoma, Magnetic resonance imaging, Chromophobe renal cell carcinoma, Tumor staging, Treatment protocols
Core tip: The common renal cell carcinoma (RCC) subtypes can often be discriminated non-invasively based on characteristic imaging appearances. Clear cell RCC typically shows a heterogeneous consistency (secondary to necrosis, cystic change or hemorrhage), has high signal intensity on T2-weighted magnetic resonance imaging (MRI), and is hypervascular on dynamic contrast-enhanced computed tomography or MRI examinations. Most papillary RCCs are detected while at a low grade and small size, show low signal intensity on T2-weighted MRI, and are hypovascular following contrast administration. Chromophobe RCCs may have a homogeneous solid appearance even when large, and may exhibit a central stellate scar and spoke-wheel enhancement.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 90% of adult renal malignancies and is the most lethal of all urologic cancers[1-3]. RCC is not a single entity but rather a heterogeneous group of neoplasms with varying histological findings, cytogenetic abnormalities, biologic behavior, prognosis and response to therapy[1,4-10]. The 2004 World Health Organization Classification of adult renal tumors stratifies RCC into several distinct subtypes of which clear cell, papillary and chromophobe tumors account for 70%, 10%-15%, and 5%, respectively[1]. Other RCC subtypes are rare and include carcinoma of the collecting ducts of Bellini, renal medullary carcinoma, Xp11.2 translocation carcinoma, multilocular clear cell RCC, carcinoma associated with neuroblastoma, mucinous tubular and spindle cell carcinoma and unclassified RCC[1]. Sarcomatoid or rhabdoid differentiation, a rare finding that can occur in any subtype, is associated with a highly aggressive behavior and poor prognosis[1].
Clear cell RCC has a golden yellow appearance on cut specimen due to rich lipid content while microsco-pically an alveolar, acinar or solid architectural pattern is commonly detected, including a clear or eosinophilic cytoplasm and a delicate vascular network[1]. Chromosome 3p deletions are found in up to 96% of clear cell RCCs including somatic inactivating mutations of the Von Hippel-Lindau (VHL) gene[11,12] Papillary RCC is characterized by malignant epithelial cells that form papillae and tubules on histology[1]. Type 1 tumors show papillae covered by small cells with a scanty cytoplasm arranged in a single layer while type 2 tumors show papillae with pseudostratified nuclei and an eosinophilic cytoplasm, and generally carry a worse prognosis than type 1 tumors due to higher stage and grade[1,11]. Cytogenetic abnormalities associated with the papillary subtype include trisomies of chromosomes 3, 7, 12, 16, 17 and 20, c-MET mutations and loss of the Y chromosome[11,13,14]. Chromophobe RCC has a homogeneous light brown or tan appearance on cut specimen while large polygonal cells with a reticulated cytoplasm and prominent cell membranes are detected histologically[1]. Unlike clear cell RCC, the blood vessels in chromophobe RCC are thick walled and eccentrically hyalinized[1]. Cytogenetic abnormalities associated with chromophobe RCC include loss of multiple chromosomes such as 1, 2, 6, 10, 13, 17 and 21[15].
The clear cell subtype shows a less favorable outcome compared with papillary and chromophobe subtypes, and is more likely to be symptomatic, present at an advanced stage, and show a greater propensity to metastasize[1,4-6,8,9,11,16]. The 5-year survival rate is 44%-69% in clear cell tumors, 82%-92% in papillary tumors and 78%-92% in chromophobe tumors[6-10,17]. Considering metastases from RCC, clear cell tumors account for 94%, papillary tumors 4% and chromophobe tumors 2%[6-8,10,17]. The most common site of organ metastasis varies according to the subtype with the lung being most frequently involved in clear cell tumors and the liver in chromophobe tumors[8]. Surgery is the mainstay of treatment in localized disease, irrespective of subtype. In advanced disease, a tailored management approach is recommended as the effectiveness of systemic therapy including the specific regime used may be influenced by the RCC subtype[5,18-22]. Studies have suggested that clear cell, papillary and chromophobe subtypes can be differentiated non-invasively on imaging[11,15,17-20,23-29]. It is important therefore that radiologists are familiar with the imaging appearances of RCC given that accurate subtyping has therapeutic and prognostic implications for patients. In this article, we review the incidence and survival data, risk factors, clinical and biochemical findings, imaging findings, staging, differential diagnosis, management options and post-treatment follow-up of RCC.
INCIDENCE AND SURVIVAL DATA
Renal cancer represents 2%-3% of adult malignancies[2]. The median age at diagnosis is 65 years with most patients being in the 6th to 8th decade of life[2,23]. Males are 2 to 3 times as affected as females[1,2,23]. Over the last 65 years, the incidence of RCC has increased at a rate of 2% per year[2]. According to data from the National Cancer Institute’s Surveillance, Epidemiology and End Results Program, there are 61500 estimated new cases of kidney cancer in the United States in 2015. Of these, 38270 are males (7th most common male cancer at 5%) and 23290 are females (10th most common female cancer at 3%)[3]. Correspondingly, there are 14080 estimated deaths from kidney cancer in the United States in 2015[3]. Of these, males account for 9070 (9th most fatal cancer in males at 3%) and females 5010 (outside the top 10 most fatal cancers in females)[3]. The overall survival from renal cancer has improved over time - the 5-year relative survival rate in the United States was 50% from 1975 to 1977, 57% from 1987 to 1989 and 74% from 2004 to 2010[3]. In the United States from 2004 to 2010, localized disease was found in 64%, regional disease in 17% and distant disease in 16% of patients[3].
RISK FACTORS FOR RCC
In a meta-analysis involving 26 studies, Hunt et al[30] found a link between cigarette smoking and the development of RCC. The authors found that ever smokers had a relative risk of 1.38 (95%CI: 1.27-1.50) for RCC compared to lifetime non-smokers[30]. The risk was dose-dependent and related to the number of cigarettes smoked per day. The study also suggested that smoking cessation for > 10 years lowered the risk. A meta-analysis of 141 studies by Renehan et al[31] implicated obesity in the development of RCC. The study found that a 5 kg/m2 increase in body mass index conferred a 1.34 relative risk (95%CI: 1.15-1.34) of RCC[31]. In a prospective study involving 296638 subjects from 8 European countries, Weikert et al[32] found that high blood pressure was associated with an increased risk of RCC. A systolic blood pressure ≥ 160 mmHg vs < 120 mmHg was associated with a relative risk of 2.48 (95%CI: 1.53-4.02) and a diastolic blood pressure ≥ 100 mmHg vs < 80 mmHg with a relative risk of 2.34 (95%CI: 1.54-3.55)[32]. A study by Hofmann et al[33] involving 1217 patients with RCCs and 1235 controls found that chronic renal failure and dialysis were independently associated with an increased risk of RCC with an OR of 4.7 (95%CI: 2.2-10.1) and 18.0 (95%CI: 3.6-91), respectively. Studies have also found that RCCs that develop in patients with end-stage renal disease (ESRD) tend to be less aggressive than RCCs that occur in the general population[34-36]. In a long-term comparative study, Breda et al[34] showed that RCCs occurring in ESRD patients were smaller (P = 0.001) and of lower grade and stage (P = 0.001) than RCCs diagnosed in the general population. The study also noted a significantly higher incidence of papillary RCC in ESRD patients (pre-transplant, 17.2% and post-transplant, 27.3%) compared with the general population (11.1%) (P = 0.01)[34]. There were no significant differences in the incidence of clear cell RCC between the groups. Chemicals implicated in the development of RCC include petroleum products, asbestos, cadmium, benzene, vinyl chloride, herbicides and acetaminophen abuse[37,38].
Hereditary RCCs account for 4% and show a predilection towards early-onset, bilaterality and multicentricity[39]. VHL syndrome, an autosomal dominant condition caused by mutations in the VHL gene, predisposes to the development of central nervous syndrome hemangioblastomas, pancreatic neuroendocrine tumors, pheochromocytomas and RCCs (predominantly clear cell subtype)[39]. Around 25% to 60% of VHL patients develop RCC with the risk of metastasis related to tumor size[39-41]. In a study of 181 VHL patients, 27.4% of patients with RCCs > 3 cm had metastases while there were no cases of metastases in patients with RCCs ≤ 3 cm[42]. As such, the standard of care for VHL patients with RCCs ≥ 3 cm is surgical resection. Birt-Hogg-Dube syndrome, an autosomal dominant condition caused by mutations in the folliculin gene, predisposes to cutaneous tumors, oncocytomas and clear cell, papillary and chromophobe RCCs[39,43]. Hereditary leiomyomatosis renal cell cancer, an autosomal dominant condition caused by mutations in the fumarate dehydratase gene, predisposes to uterine and cutaneous leiomyomas, and type 2 papillary RCCs in 25%-30%[39,44]. Hereditary papillary renal carcinoma, an autosomal dominant condition due to mutations in the MET proto-oncogene, is associated with the development of multifocal type 1 papillary RCCs[39]. Recently, it has been discovered that patients with hereditary succinate dehydrogenase mutations are at risk of developing aggressive early-onset RCCs in addition to pheochromocytomas and paragangliomas[39,45].
CLINICAL AND BIOCHEMICAL FINDINGS
Most RCCs are asymptomatic and discovered as unexpected findings on imaging performed for unrelated clinical indications[46-49]. The frequency of these incidental RCCs appears to be rising - these represent 48%-66% of contemporary RCCs compared with 3%-13% in the 1970s[48,49]. The improved detection of RCCs is likely a reflection of the greater availability of high-resolution cross-sectional imaging modalities over the last few decades[49-51]. Furthermore, these incidental RCCs are often detected at a smaller size and lower stage[50,51]. The classic triad of a palpable mass, flank pain and hematuria is found in 6%-10% and portends a more aggressive histology and advanced disease[23,37,52]. About 20%-30% have metastatic disease at presentation - symptoms may include dyspnea (lung metastases) or bone pain (bone metastases)[48]. The development of hypercalcemia, erythrocytosis, gynecomastia, hypertension or fever may be related to a paraneoplastic syndrome. Non-specific complaints include fatigue, loss of appetite and weight loss[37,46].
Biomarker development is a rapidly growing field in oncology given the potential impact of this technology as a diagnostic and prognostic tool. Investigators have studied serum and urinary compounds to determine their suitability as biomarkers for RCC. These include serum compounds such as tumor necrosis factor receptor-associated factor-1, heat shock protein 27 (HSP27), serum amyloid A, osteopontin, pyruvate kinase type M2 and thymidine kinase 1 and urinary compounds such as nuclear matrix proteins-22, neutrophil gelatinase-associated lipocalin, aquaporin-1, kidney injury molecule-1 and perilipin 2[37]. While preliminary results are encouraging, no serum or urinary biomarker has yet received validation for RCC. Imaging remains the mainstay in RCC for diagnosis, screening, follow-up and treatment monitoring.
IMAGING
RCCs may exhibit a variable spectrum of morphologic appearances ranging from small indolent lesions to large aggressive masses associated with local invasion and metastatic disease. Despite the wide range of findings that may be encountered, careful attention to certain imaging characteristics is often helpful in discriminating between the subtypes.
The gross morphologic profile of the tumor can provide an indication of its subtype. Clear cell RCC typically exhibits exophytic growth and has a tendency to be heterogeneous (Figure 1) due to intratumoral necrosis, cystic change or hemorrhage[11,15]. Moreover, Pedrosa et al[53] found that findings such as large size, intralesional necrosis, retroperitoneal vascular collaterals, and renal vein thrombosis predicted a high grade clear cell subtype (P < 0.05). Interruption of the tumor capsule has also been correlated with high tumor grade[54]. Seventy percent of papillary RCCs are confined to the kidney at presentation and are generally small size (≤ 3 cm) and low grade - these commonly manifest as peripherally located tumors which are well-circumscribed and homogeneous (Figure 2)[11,15,53]. Papillary tumors > 4 cm can show internal heterogeneity due to cystic change and necrosis[55]. Cystic papillary RCCs may show hemorrhagic fluid content and internal mural nodules or papillary projections while cystic clear cell RCCs typically show clear fluid content and irregular walls and septations[11,53,55]. Chromophobe RCC tends to appear well-circumscribed and homogeneous (cystic change and necrosis are uncommon) even when large (Figure 3), and perinephric infiltration and vascular involvement (< 4%) are rare[1,11,55,56]. Other features that can help to discriminate chromophobe RCC from other subtypes include the presence of a central stellate scar (Figure 4) and spoke-wheel enhancement, although these may also be seen in oncocytoma[57,58]. The presence of intralesional fat (either macroscopic or microscopic), is a recognized feature of some clear cell RCCs[11,15,53,59]. However, this finding is not subtype specific as rarely papillary RCC and chromophobe RCC may contain fat[1,11,55,60,61]. Karlo et al[61] found that while all 3 subtypes may contain microscopic fat as visualized by signal intensity loss on opposed-phase compared to in-phase T1-weighted magnetic resonance imaging (MRI) (Figure 5), a > 25% signal loss was predictive for clear cell RCC. The use of a simple two-point Dixon fat-water separation technique derived from a dual-echo chemical shift T1 sequence is often helpful in aiding the radiologist in identifying small quantities of microscopic fat in a renal mass. Calcifications were significantly more frequent in papillary RCCs (32%) and chromophobe RCCs (38%) than clear cell RCCs (11%)[25,56]. Bilaterality and multifocality is also more common in papillary RCC (4% and 22.5% respectively) than clear cell RCC (< 5%)[1,15,16]. However, such findings have limited practical value in subtype discrimination.
Figure 1.
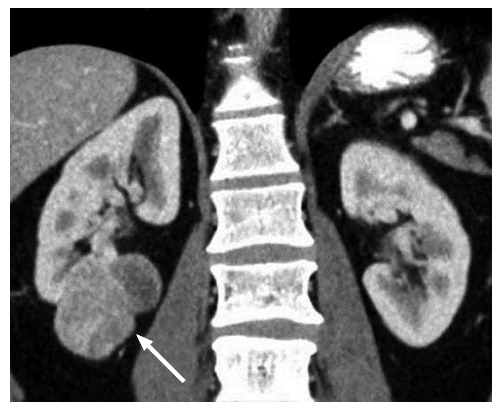
A 45-year-old male with a pathologically proven clear cell renal cell carcinoma in the right kidney on a coronal contrast-enhanced computed tomography image. The exophytic tumor (arrow) has a heterogeneous solid and cystic internal consistency.
Figure 2.

A 47-year-old female with a pathologically proven papillary renal cell carcinoma in the left kidney on an axial contrast-enhanced computed tomography image. The well-circumscribed hypovascular exophytic tumor (arrow) has a homogeneous solid internal consistency.
Figure 3.
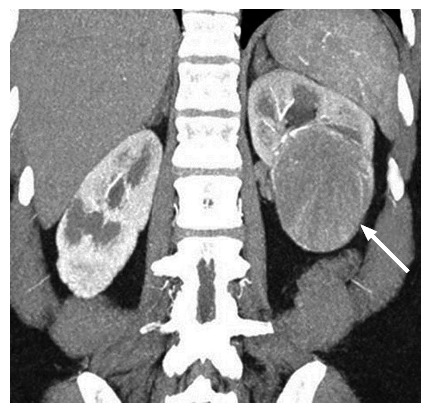
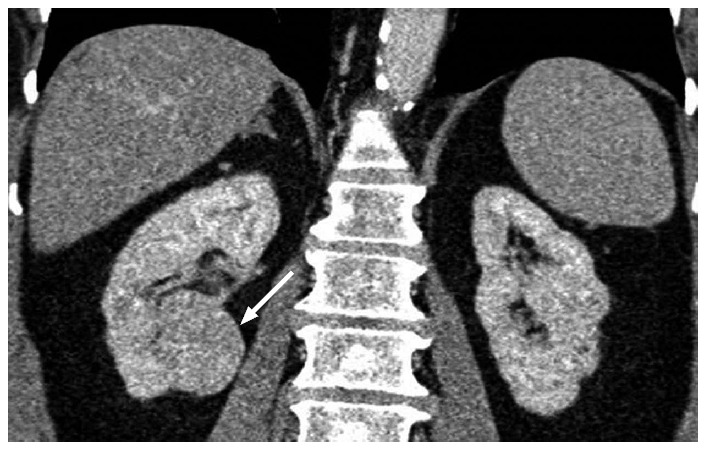
A 53-year-old female with a pathologically proven chromophobe renal cell carcinoma in the left kidney on a coronal contrast-enhanced computed tomography image. The well-circumscribed tumor (arrow) shows a homogeneous solid consistency and peripheral internal tumor vessels.
Figure 4.

A 36-year-old female with a pathologically proven chromophobe renal cell carcinoma in the left kidney on a coronal contrast-enhanced computed tomography image. The large well-circumscribed solid tumor (arrow) shows a hypoattenuating central stellate scar and internal calcification.
Figure 5.
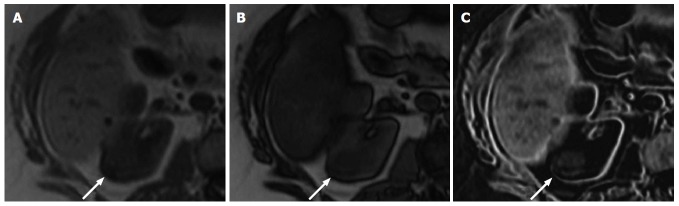
A 61-year-old female with a pathologically proven clear cell renal cell carcinoma in the right kidney. A: Axial in-phase T1-weighted magnetic resonance imaging; B: Axial opposed-phase T1-weighted magnetic resonance image; C: Axial fat-only magnetic resonance image from a two-point Dixon reconstruction which displays the difference between echos from A and B. The tumor (arrow) shows high signal on C due to the presence of microscopic fat. Incidentally, the liver also shows high signal on C due to hepatic steatosis.
An important imaging characteristic is the signal intensity appearance of the tumor on T2 weighted MRI. Most papillary RCCs demonstrate low T2 signal intensity (Figure 6A)[11,20,53,54,62,63]. In contrast, most clear cell RCCs show high T2 signal intensity (Figure 7A)[11,53,54,62,63] while the signal intensity of chromophobe RCCs has yet to be formally profiled in great detail. Oliva et al[63] evaluated the T1 and T2 signal intensity of 49 RCCs (28 clear cell and 21 papillary) and correlated the findings with pathology. The authors found that while the T1 signal intensity of both subtypes was similar, the neoplasms could be discriminated on the basis of the T2 signal intensity with papillary RCC showing an average mean signal intensity ratio of 0.67 ± 0.2, and clear cell RCC showing an average mean signal intensity ratio of 1.41 ± 0.4 (P < 0.05)[63]. The tumor signal intensity ratio was calculated as follows: [Tumor (signal intensity)/renal cortex (signal intensity)]. A tumor T2 signal intensity ratio of ≤ 0.66 was found to have 100% specificity and 54% sensitivity for papillary RCC[63]. The authors also reported that only a papillary architecture on histology correlated with the low T2 signal intensity appearance of papillary RCCs[63]. This was contrary to prior studies that attributed this appearance to the presence of blood degradation products (e.g., hemosiderin or ferritin), fibrosis or a high nucleus-to-cytoplasm ratio[64-66].
Figure 6.

A 42-year-old male with a pathologically proven papillary renal cell carcinoma in the right kidney. A: On an axial T2-weighted magnetic resonance image. The well-circumscribed exophytic solid tumor (arrow) shows relatively homogeneous low T2 signal intensity; B: On an axial contrast-enhanced T1-weighted magnetic resonance image during the corticomedullary phase. The tumor (arrow) is homogeneously hypovascular compared to the adjacent renal cortex, except for mild enhancement of the renal capsule.
Figure 7.
A 61-year-old female with a clear cell renal cell carcinoma in the right kidney. A: On an axial T2-weighted magnetic resonance image. The solid tumor (arrow) shows a heterogeneous high T2 signal intensity; B: On an axial contrast-enhanced T1-weighted magnetic resonance image during the corticomedullary phase. The solid tumor (arrow) shows heterogeneous hypervascularity, of a similar degree to that of the adjacent normal renal cortex.
Several preliminary studies have shown encouraging results in utilizing diffusion-weighted imaging (DWI) for characterizing RCCs into its main subtypes as well as into high grade and low grade tumors[67-70]. In a study of 33 patients with 36 RCCs (clear cell 32 and 4 non-clear cell) of which 23 were grade I or II and 13 were grade III or IV at 1.5-T, Goyal et al[67] found that clear cell RCCs (1.6 x 10-3 mm2/s) had significantly higher mean apparent diffusion coefficient (ADC) values than non-clear RCCs (1.0 x 10-3 mm2/s) (P = 0.005) while lower grade tumors (1.7 x 10-3 mm2/s) had higher mean ADC values than higher grade tumors (1.3 x 10-3 mm2/s) (P = 0.005). In a study of 77 patients with 78 RCCs (59 clear cell tumors, 12 papillary tumors and 7 chromophobe tumors) at 3-T, Choi et al[68] found that papillary RCCs (1.3 x 10-3 mm2/s) and chromophobe RCCs (1.6 x 10-3 mm2/s) had significantly lower mean ADC values than clear cell RCCs (1.8 x 10-3 mm2/s) (P < 0.01). No significant differences were found between papillary and chromophobe tumors (P = 0.26). In addition, high grade clear cell RCCs (1.7 x 10-3 mm2/s) were noted to have significantly lower mean ADC values than low grade clear cell RCCs (2.0 x 10-3 mm2/s) (P = 0.021)[68]. In a study of 83 patients with 85 RCCs (49 clear cell tumors, 22 papillary tumors and 14 chromophobe tumors) at 3-T, Wang et al[69] found that papillary RCCs (1.1 x 10-3 mm2/s) and chromophobe RCCs (1.3 x 10-3 mm2/s) had significantly lower mean ADC values than clear cell RCCs (1.8 x 10-3 mm2/s). No significant differences were found between papillary and chromophobe tumors (P = 0.068). Furthermore, a meta-analysis by Lassel et al[70] of 17 studies with 764 patients found that ADC values on DWI could differentiate RCC (1.6 ± 0.08 x 10-3 mm2/s) from benign renal lesions such as oncocytoma (2.0 ± 0.08 x 10-3 mm2/s) (P < 0.0001).
Several investigators have advocated the adoption of quantitative enhancement metrics as a useful means to discriminate between RCC subtypes on multiphasic cross-sectional imaging modalities[17,26]. Fundamentally, this exploits the principle that papillary RCC shows hypovascularity (Figure 6B), chromophobe RCC intermediate vascularity and clear cell RCC hypervascularity (Figure 7). In a 4-phase (unenhanced phase, corticomedullary phase at 40-55 s, nephrographic phase at 90-120 s and excretory phase at 8 min) computed tomography (CT) study of 298 renal tumors (170 clear cell RCCs, 57 papillary RCCs, 22 chromophobe RCCs and 49 oncocytomas), Young et al[17] found that the mean enhancement of clear cell RCC peaked on the corticomedullary phase compared with that of papillary RCC and chromophobe RCC which peaked on the nephrographic phase. Compared with papillary RCC, clear cell RCC showed greater mean enhancement on all phases - corticomedullary phase (125 HU vs 54 HU, P < 0.01), nephrographic phase (103 HU vs 64 HU, P < 0.001) and excretory phase (80 HU vs 54 HU, P < 0.01). Compared with chromophobe RCC, clear cell RCC showed greater mean enhancement on the corticomedullary phase (125 HU vs 74 HU, P < 0.001) and excretory phase (80 HU vs 60 HU, P = 0.008)[17]. Furthermore, multiphasic enhancement threshold levels enabled clear cell RCC to be discriminated from papillary RCC (threshold of 55 HU on the corticomedullary phase, 65 HU on the nephrographic phase and 55 HU on the excretory phase) and chromophobe RCC (threshold of 75 HU on the corticomedullary phase, 85 HU on the nephrographic phase and 60 HU on the excretory phase) with an accuracy and sensitivity of 85% and 94%, and 84% and 92%, respectively[17]. A multiphasic CT study by Lee-Felker et al[29] of 86 clear cell RCCs, 36 papillary RCCs, 10 chromophobe RCCs, 10 fat-poor angiomyolipomas and 23 oncocytomas found that clear cell RCC had a significantly higher maximum attenuation than papillary RCC on the corticomedullary phase (174.4 HU vs 62.2 HU), nephrographic phase (113.2 HU vs 81.8 HU) and excretory phase (87.9 HU vs 64.5 HU), and significantly higher maximum attenuation than chromophobe RCC on the nephrographic phase (113.2 HU vs 91.4 HU) and excretory phase (87.9 HU vs 71.3 HU). Contrary to Young et al[17] findings, Lee-Felker et al[29] found that chromophobe RCCs showed maximal enhancement on the corticomedullary phase rather than the nephrographic phase - this difference was attributed to the more uniform 4-phase CT protocol adopted in the latter study. Sun et al[26] performed a multiphasic MRI study of 113 renal masses that included 75 clear cell RCCs, 28 papillary RCCs and 10 chromophobe RCCs. The authors found that the tumor signal intensity change was highest for clear cell RCC (205.6% on corticomedullary phase and 247.1% on nephrographic phase), intermediate for chromophobe RCC (109.9% on corticomedullary phase and 192.5% on nephrographic phase) and lowest for papillary RCC (32.1% on corticomedullary phase and 96.6% on nephrographic phase). The percentage signal intensity change of the tumor was calculated as follows: [Signal intensity (post)-signal intensity (pre)/signal intensity (pre)] x 100%. A signal intensity change threshold of 84% on the corticomedullary phase was able to differentiate clear cell RCC from papillary RCC with 93% sensitivity, 96% specificity and an area under the receiver operating curve of 0.99[26]. The study also found that clear cell RCC had a significantly higher tumor to cortex (TCR) ratio than either chromophobe RCC or papillary RCC on the corticomedullary and nephrographic phases (clear cell RCC - TCR of 1.4 and 1.2, chromophobe RCC - TCR of 0.6 and 0.8, and papillary RCC - TCR of 0.2 and 0.4)[26]. In patients with moderate or severe renal impairment, where CT or MR contrast agents may be contraindicated, contrast-enhanced ultrasound (US) may be used as a viable alternative for evaluating renal masses[71]. It can discriminate if a focal lesion is solid or cystic and can differentiate a solid neoplasm from a pseudotumor such as a column of Bertin[71]. In 103 patients with complex cystic renal masses, Xue et al[72] found that contrast-enhanced US was superior to both contrast-enhanced CT and conventional US in evaluating cystic masses including determining the cyst wall thickness, the number of internal septa and the presence of solid components.
CT perfusion is an advanced technique that calculates quantitative parameters that reflect the tumor’s intrinsic microvascular environment such as blood flow, blood volume, capillary permeability and mean transit time[73]. In a study of 85 patients that included a subset of 66 clear cell RCCs, 7 papillary RCCs and 5 chromophobe RCCs, Chen et al[74] found that mean equivalent blood flow and blood volume were significantly higher in clear cell RCCs vs papillary RCCs (P < 0.001), while mean equivalent blood volume was significantly higher in clear cell RCCs vs chromophobe RCCs (P < 0.001). In a CT perfusion study of 15 patients with 15 RCCs, Reiner et al[75] found that parameters such as blood flow and blood volume had a strong correlation with tumor microvascular density on histology with lower blood flow and blood volume noted in poor prognosis RCCs that had lower microvascular density. This suggests that CT perfusion may have a potential role as a prognostic marker as a greater microvascular density is associated with improved prognosis and longer survival for RCC[75,76]. In patients with metastatic RCC, CT perfusion could be used to select patients that would benefit from targeted anti-angiogenic therapy as well to evaluate the post-treatment response[73].
Finally, 18F-fluorodeoxyglucose positron emission tomography (PET)-CT is another modality that has been used to evaluate RCC. In a study of 100 patients with 107 RCCs, Nakajima et al[77] found that clear cell RCCs had significantly higher maximum standardized uptake and tumor-to-normal tissue ratio than non-clear cell RCCs (P < 0.001) when evaluated during the early dynamic phase. During the whole body phase, the authors found that RCCs that were of higher stage, higher grade, and associated with vascular or lymphatic invasion showed higher maximum standardized uptake than less aggressive RCCs[77]. However, PET-CT is limited in primary tumor assessment as physiologic tracer excretion by the kidneys can mask an RCC leading to false negative results. PET-CT has more of a defined role for disease re-staging in advanced RCC and in recurrent RCC[78,79]. Alongi et al[80] suggested that PET-CT was able to predict disease progression and survival in patients with recurrent RCC after surgery and so influence clinical decision making. The study found that patients with a PET positive scan had a worse 5-year survival (19% vs 69%, P < 0.05) and a lower 3-year progression free survival (20% vs 67%, P < 0.05) compared to patients with a PET negative scan[80]. A PET positive scan was also associated with a higher risk of disease progression than a PET negative scan with a HR of 3.8 (P < 0.05)[80].
STAGING OF RCC
The American Joint Committee on Cancer staging system for RCC is as follows[47,81].
Primary tumor (T)
T0: No evidence of a primary tumor
T1: Tumor ≤ 7 cm limited to kidney
T1a: Tumor ≤ 4 cm limited to kidney
T1b: Tumor > 4 cm to 7 cm limited to kidney
T2: Tumor > 7 cm limited to kidney
T2a: Tumor > 7 cm to 10 cm limited to kidney
T2b: Tumor > 10 cm limited to kidney
T3: Tumor extends into major veins or has spread into the perinephric tissues but not beyond Gerota’s fascia
T3a: Tumor extends into the renal vein or the perinephric tissues but not beyond Gerota’s fascia
T3b: Tumor extends into vena cava below the diaphragm (Figure 8)
Figure 8.
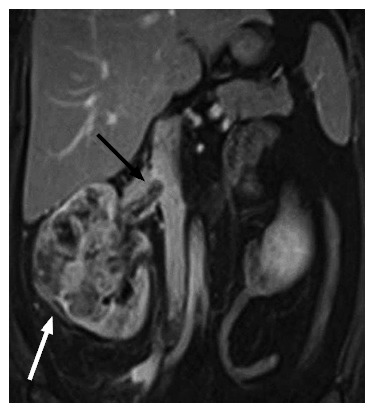
A 42-year-old female with a pathologically proven clear cell renal cell carcinoma in the right kidney on a coronal contrast-enhanced T1-weighted magnetic resonance image. The ill-marginated tumor (white arrow) involves the whole of the kidney and shows extension into the right renal vein (black arrow) and slight protrusion into the inferior vena cava.
T3c: Tumor extends into vena cava above diaphragm or invades the wall of the vena cava
T4: Tumor has spread beyond Gerota’s fascia, which may include the ipsilateral adrenal gland
Regional lymph nodes (N)
N0: Negative for regional lymph node involvement
N1: Positive for regional lymph node involvement
Distant metastasis (M)
M0: Negative for distant metastasis
M1: Positive for distant metastasis
Overall, the most frequent sites of metastases for RCC are the lungs (60%), liver (40%), bone (40%) and brain (5%)[82].
Stages I to IV correspond to the following TNM categories
Stage I: T1, N0, M0
Stage II: T2, N0, M0
Stage III: T1 or T2, N1, M0 or T3, any N, M0
Stage IV: T4, any N, M0 or any T, any N, M1
The 5-year survival for RCC according to stage is 96% for stage I, 82% for stage II, 64% for stage III and 23% for stage IV[2].
DIFFERENTIAL DIAGNOSIS
Tumors that can mimic the appearance of RCC on imaging include lipid-poor angiomyolipoma, oncocytoma, and lymphoma. These are discussed below.
Angiomyolipoma
Angiomyolipoma (AML) - the most common benign renal tumor - is composed of varying amounts of mature adipose tissue, smooth muscle and dysmorphic blood vessels[83]. Most AMLs are sporadic but less frequently may be associated with tuberous sclerosis (< 20%) or lymphangioleiomyomatosis[84]. Seventy-five percent to 80% of patients with tuberous sclerosis develop AMLs - such tumors have a propensity towards multicentricity, bilaterality, larger size and being symptomatic compared with sporadic AMLs[84,85]. AMLs show a female predilection (female to male ratio of 4:1) and are commonly detected in middle-age[86]. Most are asymptomatic although tumors ≥ 4 cm have an increased risk of hemorrhage[85]. The diagnosis of AML on imaging is based on the detection of macroscopic fat (Figure 9). However, 5% of AMLs have an insufficient amount of lipid (equivalent to a fat content of ≤ 25% per high power field on histopathologic examination[87]) to be perceived on cross-sectional imaging modalities[86]. Included are lipid-poor AMLs and AMLs that completely lack fat, and these are a radiologic pitfall for misdiagnosis and unnecessary surgery. Of the 10%-17% of resected renal masses that are ultimately classified as benign on pathologic analysis, AMLs account for 18%-59%[88-92]. Around 14%-33% of AMLs associated with tuberous sclerosis are lipid-poor[85,93,94].
Figure 9.
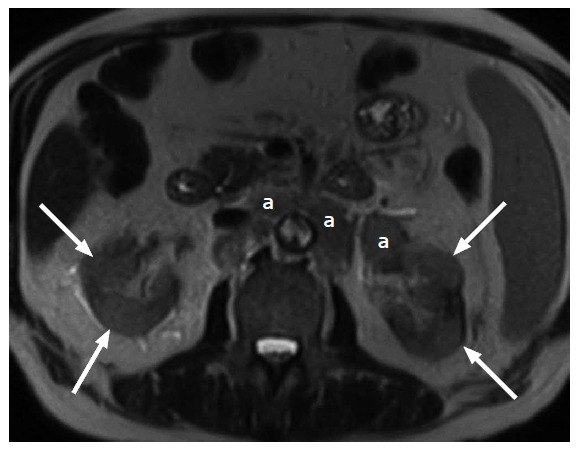
A 81-year-old male with a macroscopic fat containing renal angiomyolipoma. A: On an axial T1-weighted magnetic resonance image. The ovoid lesion (arrow) in the left kidney shows uniform high T1 signal intensity; B: On an axial T2-weighted magnetic resonance image. The ovoid lesion (arrow) in the left kidney shows uniform high T2 signal intensity; C: On an axial fat suppressed T1-weighted magnetic resonance image. The ovoid lesion (arrow) in the left kidney which previously demonstrated uniform high T1 and T2 signal intensities now shows uniform signal loss.
Imaging findings suggestive for a lipid-poor AML include homogeneous isoechogenicity compared with normal renal parenchyma on US[92,95-97], homogeneous hyperdensity compared with normal renal parenchyma on unenhanced CT[92,97-99], rapid homogeneous enhancement followed by rapid washout or persistent enhancement on delayed images[92,97-100], signal loss on opposed-phase compared with in-phase T1-weighted MRI[100,101] and low signal intensity on T2-weighted MRI[96,97,102]. Unfortunately, no single radiologic finding is pathognomonic as the imaging appearances of AML and RCC may overlap. Furthermore, lipid-poor AMLs should be differentiating from AMLs that completely lacks fat. The former displays signal loss on opposed-phase compared with in-phase T1-weighted MRI, at least in some areas, while the latter does not. Yang et al[99] suggested 4 CT parameters for differentiating lipid-poor AML from RCC - an angular tumor interface with the normal parenchyma, an unenhanced density > 38.5 HU, a hypodense rim due to subtle marginal fat and homogeneous enhancement. Lee-Felker et al[29] found that lipid-poor AML could be differentiated from clear cell RCC with 95% accuracy, 70% sensitivity, 98% specificity, 78% positive predictive value and 97% negative predictive value based on the combination of an unenhanced CT density > 45 HU and a relative corticomedullary attenuation {[lesion (region of interest)-cortex (region of interest)/cortex (region of interest)] x 100%} of < 10%. On MRI, Hindman et al[96] found that in-phase and opposed-phase T1-weighted MRI had poor ability to discriminate between lipid-poor AML and clear cell RCC as both may show microscopic fat. While low T2 signal intensity is a common feature of lipid-poor AML and papillary RCC, the tumors may be differentiated on the basis of vascularity as AMLs are hypervascular while papillary RCCs are hypovascular[96,102]. Features such as larger tumor size (> 3 cm), intratumoral necrosis and calcifications favor a diagnosis of RCC[83,96].
Renal oncocytoma
Oncocytoma is the second most common benign tumor after AML and accounts for 3%-7% of renal neoplasms[103,104]. A study of 138 pathologically proven oncocytomas reported a mean patient age of 68 years (24-86 years), male to female ratio of 2.6 and median tumor size of 3.2 cm (0.3-14.5 cm)[105]. Oncocytomas were found to be unilateral in 95%, bilateral in 5%, multiple in 6% while a co-existing RCC was found in 10%[105]. Oncocytomas and chromophobe RCCs share some common imaging and histological findings[58,106-108]. Both arise from the intercalated cells - of the collecting duct in oncocytomas and the cortex in chromophobe RCC[58,106-109]. Imaging findings regarded as suggestive of oncocytoma such as a well-defined margin, homogeneous consistency, central stellate scar, spoke-wheel enhancement and segmental enhancement inversion may also be seen in chromophobe RCC[58,107,110-115] (Figure 10). First described by Kim et al[112] segmental enhancement inversion refers to a renal mass that shows 2 distinct regions of enhancement on the corticomedullary phase which then exhibits enhancement reversal on the nephrographic phase. In a systemic review of 4 studies and 307 patients, segmental enhancement inversion was found to have 87%-100% specificity but 0%-80% sensitivity for oncocytoma[116]. Woo et al[111] suggested that non-uniform CT technique and interpretation errors accounted for the variable sensitivity. Wu et al[107] evaluated the CT findings of pathologically proven oncocytomas (n = 56) and chromophobe RCCs (n = 54). Homogeneous enhancement was found in 64.3% of oncocytomas vs 38.9% of chromophobe RCCs (P = 0.008), a central stellate scar in 46.4% of oncocytomas vs 25.9% of chromophobe RCCs (P = 0.025), spoke-wheel enhancement in 73.2% of oncocytomas vs 20.4% of chromophobe RCCs (P < 0.001), and segmental enhancement inversion in 69.6% of oncocytomas vs 16.7% of chromophobe RCCs (P < 0.001)[107]. The combination of a central stellate scar, spoke-wheel enhancement, and segmental enhancement inversion had 99.1% sensitivity, 100% specificity, 100% positive predictive value and 75% negative predictive value for oncocytoma[107]. The authors also noted that oncocytoma had significantly higher unenhanced CT density than chromophobe RCC and normal renal cortex, and significantly greater enhancement than chromophobe RCC on corticomedullary, nephrographic and excretory phases[107]. In differentiating oncocytoma from clear cell RCC, Ren et al[57] found that a corticomedullary phase TCR < 1 had 93% sensitivity, 84% specificity and 87% accuracy while a nephrographic phase TCR > corticomedullary phase TCR had 71% sensitivity, 97% specificity and 89% accuracy for oncocytoma. Lee-Felker et al[29] found that CT de-enhancement [region of interest (corticomedullary) - region of interest (nephrographic) ] > 50 HU or a relative corticomedullary attenuation > 0% was able to differentiate clear cell RCC from oncocytoma with 74% accuracy, 76% sensitivity, 70% specificity, 90% positive predictive value and 43% negative predictive value. Young et al[17] found that a multiphasic CT threshold level of 106 HU on the corticomedullary phase, 92 HU on the nephrographic phase and 68 HU on the excretory phase was able to differentiate clear cell RCC from oncocytoma with 77% accuracy, 86% sensitivity and 85% positive predictive value. Despite these promising preliminary reports, there remains a strong clinical body of opinion that oncocytoma cannot be reliably differentiated from RCC based on imaging features alone.
Figure 10.
A 59-year-old female with a pathologically proven oncocytoma in the lower pole of the right kidney on a coronal contrast-enhanced computed tomography image. The well-circumscribed tumor (arrow) shows a homogeneous solid consistency.
Renal lymphoma
Renal lymphoma may be primary or secondary. Secondary renal lymphoma is relatively common (> 30% post-mortem incidence) and generally develops in the context of widespread lymphoma as a consequence of hematogenous dissemination or contiguous extension from retroperitoneal adenopathy[117-119] (Figure 11). Primary lymphoma is rare and accounts for < 1% of extranodal lymphomas[117]. El-Sharkawy et al[120] found that renal lymphoma has 5 morphologic patterns on CT: Enlarged lobular non-enhancing kidneys, bilateral multiple renal masses, focal single non-enhancing renal mass, perirenal infiltrations from retroperitoneal extension and bilateral diffuse areas of non-enhancing hypodensities. Multifocal lesions are the most frequent presentation followed secondly by contiguous extension from retroperitoneal adenopathy[117,121]. Renal lymphoma appears homogeneously hypoechoic on US, hypodense on CT and low to intermediate signal intensity on T1- and T2-weighted MRI[121-123]. Due to high cellularity, renal lymphoma generally show restricted diffusion and low DWI values although further analysis is required to determine if DWI can be used to differentiate renal lymphoma from other renal tumors[124].
Figure 11.
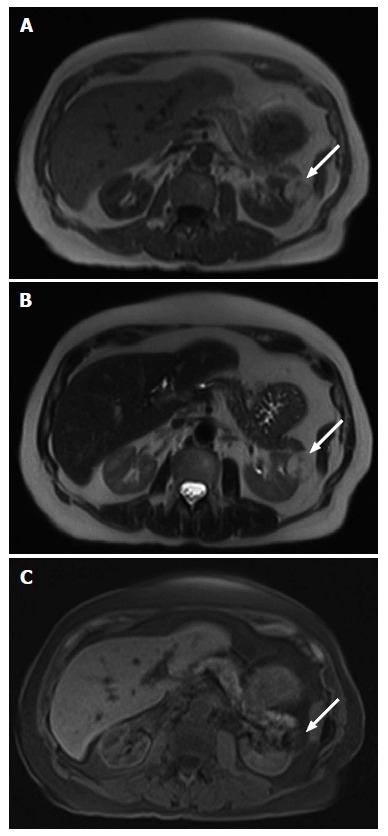
A 62-year-old male with pathologically proven B cell lymphoma on an axial T2-weighted image. Multifocal bilateral poorly-defined masses (arrows) of intermediate to high T2 signal intensity in the kidneys are due to secondary renal lymphoma. In addition, there is lymphomatous involvement of enlarged retroperitoneal lymph nodes (a) in the para-aortic region.
Lymphomatous lesions may show negligible mass effect - deformation of the renal contour, collecting system and ureter (hydronephrosis is a late finding) and displacement of surrounding structures are relatively uncommon findings[23,120]. Renal lymphoma is hypovascular and shows lower enhancement than the renal parenchyma on CT or MRI[121]. This can make differentiation from a hypovascular RCC such as a papillary tumor challenging[125]. Conversely, type 2 papillary RCC may show extensive para-aortic adenopathy which can mimic secondary renal lymphoma[125]. Cystic tumors, calcifications and vascular extension into the renal vein and/or inferior vena cava are atypical findings for lymphoma that should raise the suspicion for an alternative etiology[117,120,121]. Renal biopsy may be required to establish the diagnosis in equivocal cases. Such patients can be spared surgery as lymphoma generally responds well to chemotherapy.
MANAGEMENT OPTIONS AND IMAGING FOLLOW-UP
A variety of management strategies have been formulated for RCC. Accurate radiological staging is essential as therapeutic options are stage dependent. The National Comprehensive Cancer Network (NCCN) multidisciplinary recommendations for the clinical management and imaging follow-up of patients with RCC are discussed below[2,22,126].
Stage Ia
Nephron-sparing partial nephrectomy - with the objective being the complete surgical extirpation of the tumor while retaining sufficient healthy tissue for adequate renal function - is the preferred treatment option for stage Ia[2,47,127]. The technique was originally intended for the selective treatment of: (1) small RCCs; (2) patients at increased risk of post-surgical renal insufficiency due to inadequate renal reserve such as subjects with a solitary kidney, pre-existing borderline renal function, or those with multiple or bilateral tumors; and (3) patients at increased risk for additional RCCs that may require repeat surgeries such as those with a genetic syndrome. Over the last decade, the clinical indications for partial nephrectomy have been expanded to include most patients with low stage tumors as studies have demonstrated that partial nephrectomy is as effective a therapeutic option as radical nephrectomy with comparable rates of tumor-free survival and overall survival[128-131]. Recurrence rates following partial nephrectomy for stage Ia tumors is low at 0%-3%[128]. Radical nephrectomy - first described by Robson et al[132] in 1969 to encompass the en-bloc excision of the diseased kidney with the perirenal fat, ipsilateral adrenal gland and regional lymph nodes - is reserved for stage Ia cases ineligible for partial nephrectomy such as RCCs situated at the renal hilum[2]. In addition to being a more extensive procedure, radical nephrectomy increases the risk of renal impairment which adversely affects quality of life and cardiovascular specific- and overall- survival[127,133,134]. A study by Huang et al[135] of 662 patients that underwent either radical nephrectomy or partial nephrectomy for a stage Ia renal tumor found that the 3-year probability of freedom from new onset glomerular filtration rate (GFR) < 60 mL/min was 80% for partial nephrectomy compared with 35% for radical nephrectomy. For a new onset GFR < 45 mL/min, the 3-year probability of freedom was 95% for partial nephrectomy compared with 64% for radical nephrectomy. For poor surgical candidates such as elderly patients with significant co-morbidities, minimally invasive thermal ablation techniques such as radiofrequency ablation (RFA) or cryoablation represent effective alternatives for stage Ia tumors[2,136-138]. Performed with US or CT-guidance as an elective procedure, these techniques have the advantage of improved patient tolerance and recovery, preservation of renal function and a lower complication rate compared with surgery[138,139]. Ablative therapies may be inferior to surgery for oncologic control with a higher risk of recurrence, but this is often an acceptable compromise in non-surgical candidates[138]. The recurrence rate for stage Ia tumors post-RFA is 2.5%-9%[140,141]. In a study of 200 renal tumors, Wah et al[141] found that a tumor size < 3 cm and an exophytic location were independent predictors of successful RFA. In contrast, a central or lower pole tumor location was an independent predictor of ureteric injury. A systemic review by Klatte et al[139] comparing cryoablation vs partial nephrectomy for the treatment of small renal masses found that cryoablation conferred an 8.5% risk of tumor progression and partial nephrectomy a 1.9% risk. On CT or MRI images, recurrent disease may be detected as a focus of new enhancement ± an increase in size of the viable portion of the ablated tumor. The NCCN recommends that abdominal CT or MRI at 3 and 6 mo be performed to evaluate treatment response followed by annual CT or MRI for 5 years[22]. Active imaging surveillance may be an appropriate strategy in frail patients with small RCCs as these tumors generally have a slow growth rate and low metastatic potential[2,142-148]. Analyzing the results of 6 studies with a total of 937 renal tumors (mean size of 2.4 cm at diagnosis) and a follow-up period of 28-36 mo showed that tumors grew at an average rate of 0.24 cm/year[143-148]. The NCCN recommends that abdominal CT or MRI be performed within 6 mo of surveillance initiation followed by abdominal CT, MRI or US at least annually[22]. A chest X-ray or CT chest yearly is also suggested to evaluate for pulmonary metastases.
Stage Ib
The NCCN recommends that either partial nephrectomy or radical nephrectomy be performed for stage Ib tumors[2]. Both techniques show comparable oncologic control[128,131,134,149,150]. The follow-up schedule suggested by the NCCN for surgically treated stage Ia/b tumors is as follows[22]: (1) a baseline abdominal CT, MRI or US within 3-12 mo of surgery; (2) if the initial postoperative scan following a partial nephrectomy is negative, then abdominal CT, MRI or US should be performed annually for 3 years based on individual risk factors; (3) if the initial postoperative scan following a radical nephrectomy is negative, then abdominal imaging beyond 12 mo may be performed at the discretion of the physician, and (4) chest X-ray annually for 3 years, and beyond that as deemed appropriate.
Stage II and III
The NCCN recommends that radical nephrectomy be performed for stage II and III tumors[2]. Routine adrenalectomy and lymphadenectomy is not advocated in the absence of radiologic disease at these sites as it does not improve survival[151,152]. A laparoscopic approach is favored for stage II tumors while stage III tumors are usually treated by an open approach[47,137]. Comparing laparoscopic vs open radical nephrectomy, Hemel et al[153] found that laparoscopic nephrectomy had the advantage over the open procedure of reduced blood loss and analgesia requirements, reduced hospital stay and improved recovery times. The follow-up schedule suggested by the NCCN for stage II and III patients treated by radical nephrectomy is as follows[22]: (1) baseline abdominal CT or MRI within 3-6 mo, then CT, MRI or US every 3-6 mo for at least 3 years and then annually up to 5 years; (2) baseline chest CT within 3-6 mo after surgery with continued imaging (CT or chest X-ray) every 3-6 mo for at least 3 years and then annually up to 5 years; and (3) site-specific imaging depending on symptoms.
Stage IV
Targeted molecular therapies using vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) (e.g., sunitinib, sorafenib, pazopanib and axitinib) or mammalian target of rapamycin inhibitors (e.g., temsirolimus and everolimus) have largely replaced immunotherapy agents (e.g., interferon-α) for systemic therapy. A randomized trial by Motzer et al[154] involving 750 patients with metastatic clear cell RCC showed that patients treated with sunitinib had longer progression free survival and overall survival compared with patients treated with interferon-α. Several studies have suggested that VEGF-TKIs may be less effective in treating papillary and chromophobe RCCs compared with clear cell RCCs[18,26,155-157]. At present, there is no established first-line therapy for metastatic non-clear cell RCC. As such, the NCCN suggests that the preferred option in these patients is enrollment in a clinical trial[126]. Potential agents include temsirolimus, sorafenib, sunitinib, pazopanib, axitinib, everolimus, bevacizumab or erlotinib[126]. Preliminary studies have suggested that temsirolimus has efficacy in treating papillary RCC[158-161]. Two randomized controlled studies found that cytoreductive nephrectomy followed by immunotherapy improved survival in patients with metastatic RCC compared to immunotherapy alone[162,163]. Similarly, a study of 314 patients with metastatic RCC found that cytoreductive nephrectomy followed by VEGF-TKI therapy improved survival compared with VEGF-TKI therapy alone (19.8 mo vs 9.4 mo, P < 0.01)[164].
The NCCN guidelines for stage 4 patients are as follows[2]: (1) Cases that involve a potentially resectable solitary metastatic site should undergo nephrectomy and surgical metastasectomy; (2) cases that involve a potentially resectable RCC with multiple metastatic sites should undergo cytoreductive nephrectomy in appropriate patients prior to systemic therapy; and (3) cases with medically or surgically unresectable disease should undergo systemic therapy.
The NCCN suggests that stage IV patients should undergo baseline chest, abdominal and pelvic imaging by CT or MRI pre-treatment or prior to observation, followed by repeat imaging every 6-16 wk as per physician discretion and per patient clinical status[22]. The imaging frequency may be modified depending on the rate of disease change and the sites of active disease[22].
CONCLUSION
RCC is not a single uniform entity but a group of related neoplasms in which the histologic findings, cytogenetic abnormalities, biologic behavior and imaging appearances of the tumors are subtype dependent. The 3 main subtypes - clear cell, papillary and chromophobe - can often be differentiated non-invasively based on characteristic radiologic appearances. This knowledge is useful for radiologists as it has an impact on prognosis, clinical management and treatment options.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 21, 2015
First decision: January 15, 2016
Article in press: March 9, 2016
P- Reviewer: Cerwenka HR, Chow J, El-Ghar MA, Nouh MR, Razek AA, Shen J, Siracusano S, Tarazov PG S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, Carducci MA, Chang SS, Choueiri TK, Hancock SL, Hudes GR, et al. Kidney cancer. J Natl Compr Canc Netw. 2011;9:960–977. doi: 10.6004/jnccn.2011.0082. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16:432–443. doi: 10.1111/j.1442-2042.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000;89:604–614. [PubMed] [Google Scholar]
- 8.Hoffmann NE, Gillett MD, Cheville JC, Lohse CM, Leibovich BC, Blute ML. Differences in organ system of distant metastasis by renal cell carcinoma subtype. J Urol. 2008;179:474–477. doi: 10.1016/j.juro.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, Reuter VE, Russo P. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–77. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 10.Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol. 1997;21:621–635. doi: 10.1097/00000478-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Gurel S, Narra V, Elsayes KM, Siegel CL, Chen ZE, Brown JJ. Subtypes of renal cell carcinoma: MRI and pathological features. Diagn Interv Radiol. 2013;19:304–311. doi: 10.5152/dir.2013.147. [DOI] [PubMed] [Google Scholar]
- 12.Sükösd F, Kuroda N, Beothe T, Kaur AP, Kovacs G. Deletion of chromosome 3p14.2-p25 involving the VHL and FHIT genes in conventional renal cell carcinoma. Cancer Res. 2003;63:455–457. [PubMed] [Google Scholar]
- 13.Lubensky IA, Schmidt L, Zhuang Z, Weirich G, Pack S, Zambrano N, Walther MM, Choyke P, Linehan WM, Zbar B. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol. 1999;155:517–526. doi: 10.1016/S0002-9440(10)65147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TD, Eble JN, Cheng L. Application of molecular diagnostic techniques to renal epithelial neoplasms. Clin Lab Med. 2005;25:279–303. doi: 10.1016/j.cll.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, Dalrymple NC, Chintapalli KN. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26:1795–1806; discussion 1806-1810. doi: 10.1148/rg.266065010. [DOI] [PubMed] [Google Scholar]
- 16.Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS. Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology. 2013;267:444–453. doi: 10.1148/radiol.13112617. [DOI] [PubMed] [Google Scholar]
- 18.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 19.Tannir NM, Thall PF, Ng CS, Wang X, Wooten L, Siefker-Radtke A, Mathew P, Pagliaro L, Wood C, Jonasch E. A phase II trial of gemcitabine plus capecitabine for metastatic renal cell cancer previously treated with immunotherapy and targeted agents. J Urol. 2008;180:867–872; discussion 872. doi: 10.1016/j.juro.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vikram R, Ng CS, Tamboli P, Tannir NM, Jonasch E, Matin SF, Wood CG, Sandler CM. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. Radiographics. 2009;29:741–754; discussion 755-757. doi: 10.1148/rg.293085190. [DOI] [PubMed] [Google Scholar]
- 21.Upton MP, Parker RA, Youmans A, McDermott DF, Atkins MB. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH, et al. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151–159. doi: 10.6004/jnccn.2015.0022. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Lefkowitz RA, Bach A. Imaging of kidney cancer. Radiol Clin North Am. 2007;45:119–147. doi: 10.1016/j.rcl.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Prando A, Prando D, Prando P. Renal cell carcinoma: unusual imaging manifestations. Radiographics. 2006;26:233–244. doi: 10.1148/rg.261055060. [DOI] [PubMed] [Google Scholar]
- 25.Park SB, Cho KS, Lee JH, Jeong YK, Choi SH, Kang BS, Kim JK. Unusual manifestations of renal cell carcinoma. Acta Radiol. 2008;49:839–847. doi: 10.1080/02841850802065018. [DOI] [PubMed] [Google Scholar]
- 26.Sun MR, Ngo L, Genega EM, Atkins MB, Finn ME, Rofsky NM, Pedrosa I. Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes--correlation with pathologic findings. Radiology. 2009;250:793–802. doi: 10.1148/radiol.2503080995. [DOI] [PubMed] [Google Scholar]
- 27.Campbell N, Rosenkrantz AB, Pedrosa I. MRI phenotype in renal cancer: is it clinically relevant? Top Magn Reson Imaging. 2014;23:95–115. doi: 10.1097/RMR.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamurthy NK, Moosavi B, McInnes MD, Flood TA, Schieda N. Multiparametric MRI of solid renal masses: pearls and pitfalls. Clin Radiol. 2015;70:304–316. doi: 10.1016/j.crad.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee-Felker SA, Felker ER, Tan N, Margolis DJ, Young JR, Sayre J, Raman SS. Qualitative and quantitative MDCT features for differentiating clear cell renal cell carcinoma from other solid renal cortical masses. AJR Am J Roentgenol. 2014;203:W516–W524. doi: 10.2214/AJR.14.12460. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 31.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 32.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Trichopoulou A, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167:438–446. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann JN, Schwartz K, Chow WH, Ruterbusch JJ, Shuch BM, Karami S, Rothman N, Wacholder S, Graubard BI, Colt JS, et al. The association between chronic renal failure and renal cell carcinoma may differ between black and white Americans. Cancer Causes Control. 2013;24:167–174. doi: 10.1007/s10552-012-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breda A, Lucarelli G, Rodriguez-Faba O, Guirado L, Facundo C, Bettocchi C, Gesualdo L, Castellano G, Grandaliano G, Battaglia M, et al. Clinical and pathological outcomes of renal cell carcinoma (RCC) in native kidneys of patients with end-stage renal disease: a long-term comparative retrospective study with RCC diagnosed in the general population. World J Urol. 2015;33:1–7. doi: 10.1007/s00345-014-1248-y. [DOI] [PubMed] [Google Scholar]
- 35.Shrewsberry AB, Osunkoya AO, Jiang K, Westby R, Canter D, Pattaras J, Turgeon N, Master VA, Ogan K. Renal cell carcinoma in patients with end-stage renal disease has favorable overall prognosis. Clin Transplant. 2014;28:211–216. doi: 10.1111/ctr.12299. [DOI] [PubMed] [Google Scholar]
- 36.Neuzillet Y, Tillou X, Mathieu R, Long JA, Gigante M, Paparel P, Poissonnier L, Baumert H, Escudier B, Lang H, et al. Renal cell carcinoma (RCC) in patients with end-stage renal disease exhibits many favourable clinical, pathologic, and outcome features compared with RCC in the general population. Eur Urol. 2011;60:366–373. doi: 10.1016/j.eururo.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 37.Pastore AL, Palleschi G, Silvestri L, Moschese D, Ricci S, Petrozza V, Carbone A, Di Carlo A. Serum and urine biomarkers for human renal cell carcinoma. Dis Markers. 2015;2015:251403. doi: 10.1155/2015/251403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Mao Y, White K. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond) 2002;52:157–164. doi: 10.1093/occmed/52.3.157. [DOI] [PubMed] [Google Scholar]
- 39.Byler TK, Bratslavsky G. Hereditary renal cell carcinoma: genetics, clinical features, and surgical considerations. World J Urol. 2014;32:623–630. doi: 10.1007/s00345-014-1287-4. [DOI] [PubMed] [Google Scholar]
- 40.Rosner I, Bratslavsky G, Pinto PA, Linehan WM. The clinical implications of the genetics of renal cell carcinoma. Urol Oncol. 2009;27:131–136. doi: 10.1016/j.urolonc.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 42.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, Walther MM. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172:63–65. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 43.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 44.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricketts CJ, Shuch B, Vocke CD, Metwalli AR, Bratslavsky G, Middelton L, Yang Y, Wei MH, Pautler SE, Peterson J, et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J Urol. 2012;188:2063–2071. doi: 10.1016/j.juro.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii65–vii71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 47.Bellmunt J, Puente J, Garcia de Muro J, Lainez N, Rodríguez C, Duran I. SEOM clinical guidelines for the treatment of renal cell carcinoma. Clin Transl Oncol. 2014;16:1043–1050. doi: 10.1007/s12094-014-1219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krabbe LM, Bagrodia A, Margulis V, Wood CG. Surgical management of renal cell carcinoma. Semin Intervent Radiol. 2014;31:27–32. doi: 10.1055/s-0033-1363840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–745. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 50.Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med. 2010;362:624–634. doi: 10.1056/NEJMcp0910041. [DOI] [PubMed] [Google Scholar]
- 51.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 52.Lee CT, Katz J, Fearn PA, Russo P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol. 2002;7:135–140. doi: 10.1016/s1078-1439(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 53.Pedrosa I, Chou MT, Ngo L, H Baroni R, Genega EM, Galaburda L, DeWolf WC, Rofsky NM. MR classification of renal masses with pathologic correlation. Eur Radiol. 2008;18:365–375. doi: 10.1007/s00330-007-0757-0. [DOI] [PubMed] [Google Scholar]
- 54.Pedrosa I, Alsop DC, Rofsky NM. Magnetic resonance imaging as a biomarker in renal cell carcinoma. Cancer. 2009;115:2334–2345. doi: 10.1002/cncr.24237. [DOI] [PubMed] [Google Scholar]
- 55.Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48:166–174. doi: 10.1590/0100-3984.2013.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol. 2002;178:1499–1506. doi: 10.2214/ajr.178.6.1781499. [DOI] [PubMed] [Google Scholar]
- 57.Ren A, Cai F, Shang YN, Ma ES, Huang ZG, Wang W, Lu Y, Zhang XZ. Differentiation of renal oncocytoma and renal clear cell carcinoma using relative CT enhancement ratio. Chin Med J (Engl) 2015;128:175–179. doi: 10.4103/0366-6999.149190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS. MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol. 2010;195:W421–W427. doi: 10.2214/AJR.10.4718. [DOI] [PubMed] [Google Scholar]
- 59.Outwater EK, Bhatia M, Siegelman ES, Burke MA, Mitchell DG. Lipid in renal clear cell carcinoma: detection on opposed-phase gradient-echo MR images. Radiology. 1997;205:103–107. doi: 10.1148/radiology.205.1.9314970. [DOI] [PubMed] [Google Scholar]
- 60.Lesavre A, Correas JM, Merran S, Grenier N, Vieillefond A, Hélénon O. CT of papillary renal cell carcinomas with cholesterol necrosis mimicking angiomyolipomas. AJR Am J Roentgenol. 2003;181:143–145. doi: 10.2214/ajr.181.1.1810143. [DOI] [PubMed] [Google Scholar]
- 61.Karlo CA, Donati OF, Burger IA, Zheng J, Moskowitz CS, Hricak H, Akin O. MR imaging of renal cortical tumours: qualitative and quantitative chemical shift imaging parameters. Eur Radiol. 2013;23:1738–1744. doi: 10.1007/s00330-012-2758-x. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimitsu K, Irie H, Tajima T, Nishie A, Asayama Y, Hirakawa M, Nakayama T, Kakihara D, Honda H. MR imaging of renal cell carcinoma: its role in determining cell type. Radiat Med. 2004;22:371–376. [PubMed] [Google Scholar]
- 63.Oliva MR, Glickman JN, Zou KH, Teo SY, Mortelé KJ, Rocha MS, Silverman SG. Renal cell carcinoma: t1 and t2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am J Roentgenol. 2009;192:1524–1530. doi: 10.2214/AJR.08.1727. [DOI] [PubMed] [Google Scholar]
- 64.Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, Yacoub M, Bouzgarrou M, Ravaud A, Grenier N. Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol. 2015;25:24–31. doi: 10.1007/s00330-014-3380-x. [DOI] [PubMed] [Google Scholar]
- 65.Shinmoto H, Yuasa Y, Tanimoto A, Narimatsu Y, Jinzaki M, Hiramatsu K, Mukai M. Small renal cell carcinoma: MRI with pathologic correlation. J Magn Reson Imaging. 1998;8:690–694. doi: 10.1002/jmri.1880080327. [DOI] [PubMed] [Google Scholar]
- 66.Mancilla-Jimenez R, Stanley RJ, Blath RA. Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer. 1976;38:2469–2480. doi: 10.1002/1097-0142(197612)38:6<2469::aid-cncr2820380636>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 67.Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A, Iyer VK, Das P. Diffusion-weighted MRI in renal cell carcinoma: a surrogate marker for predicting nuclear grade and histological subtype. Acta Radiol. 2012;53:349–358. doi: 10.1258/ar.2011.110415. [DOI] [PubMed] [Google Scholar]
- 68.Choi YA, Kim CK, Park SY, Cho SW, Park BK. Subtype differentiation of renal cell carcinoma using diffusion-weighted and blood oxygenation level-dependent MRI. AJR Am J Roentgenol. 2014;203:W78–W84. doi: 10.2214/AJR.13.11551. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Cheng L, Zhang X, Wang D, Guo A, Gao Y, Ye H. Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology. 2010;257:135–143. doi: 10.1148/radiol.10092396. [DOI] [PubMed] [Google Scholar]
- 70.Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ. Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol. 2014;24:241–249. doi: 10.1007/s00330-013-3004-x. [DOI] [PubMed] [Google Scholar]
- 71.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 72.Xue LY, Lu Q, Huang BJ, Ma JJ, Yan LX, Wen JX, Wang WP. Contrast-enhanced ultrasonography for evaluation of cystic renal mass: in comparison to contrast-enhanced CT and conventional ultrasound. Abdom Imaging. 2014;39:1274–1283. doi: 10.1007/s00261-014-0171-4. [DOI] [PubMed] [Google Scholar]
- 73.Das CJ, Thingujam U, Panda A, Sharma S, Gupta AK. Perfusion computed tomography in renal cell carcinoma. World J Radiol. 2015;7:170–179. doi: 10.4329/wjr.v7.i7.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C, Liu Q, Hao Q, Xu B, Ma C, Zhang H, Shen Q, Lu J. Study of 320-slice dynamic volume CT perfusion in different pathologic types of kidney tumor: preliminary results. PLoS One. 2014;9:e85522. doi: 10.1371/journal.pone.0085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiner CS, Roessle M, Thiesler T, Eberli D, Klotz E, Frauenfelder T, Sulser T, Moch H, Alkadhi H. Computed tomography perfusion imaging of renal cell carcinoma: systematic comparison with histopathological angiogenic and prognostic markers. Invest Radiol. 2013;48:183–191. doi: 10.1097/RLI.0b013e31827c63a3. [DOI] [PubMed] [Google Scholar]
- 76.Yildiz E, Ayan S, Goze F, Gokce G, Gultekin EY. Relation of microvessel density with microvascular invasion, metastasis and prognosis in renal cell carcinoma. BJU Int. 2008;101:758–764. doi: 10.1111/j.1464-410X.2007.07318.x. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima R, Abe K, Kondo T, Tanabe K, Sakai S. Clinical role of early dynamic FDG-PET/CT for the evaluation of renal cell carcinoma. Eur Radiol. 2015 doi: 10.1007/s00330-015-4026-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 78.Safaei A, Figlin R, Hoh CK, Silverman DH, Seltzer M, Phelps ME, Czernin J. The usefulness of F-18 deoxyglucose whole-body positron emission tomography (PET) for re-staging of renal cell cancer. Clin Nephrol. 2002;57:56–62. doi: 10.5414/cnp57056. [DOI] [PubMed] [Google Scholar]
- 79.Nakatani K, Nakamoto Y, Saga T, Higashi T, Togashi K. The potential clinical value of FDG-PET for recurrent renal cell carcinoma. Eur J Radiol. 2011;79:29–35. doi: 10.1016/j.ejrad.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Alongi P, Picchio M, Zattoni F, Spallino M, Gianolli L, Saladini G, Evangelista L. Recurrent renal cell carcinoma: clinical and prognostic value of FDG PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:464–473. doi: 10.1007/s00259-015-3159-6. [DOI] [PubMed] [Google Scholar]
- 81.Novara G, Ficarra V, Antonelli A, Artibani W, Bertini R, Carini M, Cosciani Cunico S, Imbimbo C, Longo N, Martignoni G, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol. 2010;58:588–595. doi: 10.1016/j.eururo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39:588–604. doi: 10.1007/s00261-014-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Logue LG, Acker RE, Sienko AE. Best cases from the AFIP: angiomyolipomas in tuberous sclerosis. Radiographics. 2003;23:241–246. doi: 10.1148/rg.231025109. [DOI] [PubMed] [Google Scholar]
- 85.Schieda N, Kielar AZ, Al Dandan O, McInnes MD, Flood TA. Ten uncommon and unusual variants of renal angiomyolipoma (AML): radiologic-pathologic correlation. Clin Radiol. 2015;70:206–220. doi: 10.1016/j.crad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525–1540. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 87.Milner J, McNeil B, Alioto J, Proud K, Rubinas T, Picken M, Demos T, Turk T, Perry KT. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J Urol. 2006;176:905–909. doi: 10.1016/j.juro.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 88.Yan L, Liu Z, Wang G, Huang Y, Liu Y, Yu Y, Liang C. Angiomyolipoma with minimal fat: differentiation from clear cell renal cell carcinoma and papillary renal cell carcinoma by texture analysis on CT images. Acad Radiol. 2015;22:1115–1121. doi: 10.1016/j.acra.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP, Van Arsdalen KN, Wein AJ, Malkowicz SB. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737–740. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Fujii Y, Komai Y, Saito K, Iimura Y, Yonese J, Kawakami S, Ishikawa Y, Kumagai J, Kihara K, Fukui I. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology. 2008;72:598–602. doi: 10.1016/j.urology.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 91.Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (& lt; 4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2012;263:160–168. doi: 10.1148/radiol.12111205. [DOI] [PubMed] [Google Scholar]
- 92.Farrell C, Noyes SL, Tourojman M, Lane BR. Renal angiomyolipoma: preoperative identification of atypical fat-poor AML. Curr Urol Rep. 2015;16:12. doi: 10.1007/s11934-015-0484-z. [DOI] [PubMed] [Google Scholar]
- 93.Lemaitre L, Claudon M, Dubrulle F, Mazeman E. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997;18:100–114. doi: 10.1016/s0887-2171(97)90054-8. [DOI] [PubMed] [Google Scholar]
- 94.Bret PM, Bretagnolle M, Gaillard D, Plauchu H, Labadie M, Lapray JF, Roullaud Y, Cooperberg P. Small, asymptomatic angiomyolipomas of the kidney. Radiology. 1985;154:7–10. doi: 10.1148/radiology.154.1.3880613. [DOI] [PubMed] [Google Scholar]
- 95.Silverman SG, Mortele KJ, Tuncali K, Jinzaki M, Cibas ES. Hyperattenuating renal masses: etiologies, pathogenesis, and imaging evaluation. Radiographics. 2007;27:1131–1143. doi: 10.1148/rg.274065147. [DOI] [PubMed] [Google Scholar]
- 96.Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, Rofsky NM, Pedrosa I. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012;265:468–477. doi: 10.1148/radiol.12112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, Hiramatsu K, Mukai M, Murai M. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997;205:497–502. doi: 10.1148/radiology.205.2.9356635. [DOI] [PubMed] [Google Scholar]
- 98.Zhang YY, Luo S, Liu Y, Xu RT. Angiomyolipoma with minimal fat: differentiation from papillary renal cell carcinoma by helical CT. Clin Radiol. 2013;68:365–370. doi: 10.1016/j.crad.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 99.Yang CW, Shen SH, Chang YH, Chung HJ, Wang JH, Lin AT, Chen KK. Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma? AJR Am J Roentgenol. 2013;201:1017–1028. doi: 10.2214/AJR.12.10204. [DOI] [PubMed] [Google Scholar]
- 100.Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, Lee JW, Kim S, Cho KS. Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006;239:174–180. doi: 10.1148/radiol.2391050102. [DOI] [PubMed] [Google Scholar]
- 101.Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol. 2005;184:1868–1872. doi: 10.2214/ajr.184.6.01841868. [DOI] [PubMed] [Google Scholar]
- 102.Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, Yacoub M, Bouzgarrou M, Ravaud A, Grenier N. Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol. 2014;24:1068–1080. doi: 10.1007/s00330-014-3107-z. [DOI] [PubMed] [Google Scholar]
- 103.Perez-Ordonez B, Hamed G, Campbell S, Erlandson RA, Russo P, Gaudin PB, Reuter VE. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. 1997;21:871–883. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Lieber MM. Renal oncocytoma. Urol Clin North Am. 1993;20:355–359. [PubMed] [Google Scholar]
- 105.Dechet CB, Bostwick DG, Blute ML, Bryant SC, Zincke H. Renal oncocytoma: multifocality, bilateralism, metachronous tumor development and coexistent renal cell carcinoma. J Urol. 1999;162:40–42. doi: 10.1097/00005392-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 106.Choudhary S, Rajesh A, Mayer NJ, Mulcahy KA, Haroon A. Renal oncocytoma: CT features cannot reliably distinguish oncocytoma from other renal neoplasms. Clin Radiol. 2009;64:517–522. doi: 10.1016/j.crad.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 107.Wu J, Zhu Q, Zhu W, Chen W, Wang S. Comparative study of CT appearances in renal oncocytoma and chromophobe renal cell carcinoma. Acta Radiol. 2016;57:500–506. doi: 10.1177/0284185115585035. [DOI] [PubMed] [Google Scholar]
- 108.Li G, Barthelemy A, Feng G, Gentil-Perret A, Peoc’h M, Genin C, Tostain J. S100A1: a powerful marker to differentiate chromophobe renal cell carcinoma from renal oncocytoma. Histopathology. 2007;50:642–647. doi: 10.1111/j.1365-2559.2007.02655.x. [DOI] [PubMed] [Google Scholar]
- 109.Davidson AJ, Hayes WS, Hartman DS, McCarthy WF, Davis CJ. Renal oncocytoma and carcinoma: failure of differentiation with CT. Radiology. 1993;186:693–696. doi: 10.1148/radiology.186.3.8430176. [DOI] [PubMed] [Google Scholar]
- 110.McGahan JP, Lamba R, Fisher J, Starshak P, Ramsamooj R, Fitzgerald E, Yen P. Is segmental enhancement inversion on enhanced biphasic MDCT a reliable sign for the noninvasive diagnosis of renal oncocytomas? AJR Am J Roentgenol. 2011;197:W674–W679. doi: 10.2214/AJR.11.6463. [DOI] [PubMed] [Google Scholar]
- 111.Woo S, Cho JY, Kim SH, Kim SY, Lee HJ, Hwang SI, Moon MH, Sung CK. Segmental enhancement inversion of small renal oncocytoma: differences in prevalence according to tumor size. AJR Am J Roentgenol. 2013;200:1054–1059. doi: 10.2214/AJR.12.9300. [DOI] [PubMed] [Google Scholar]
- 112.Kim JI, Cho JY, Moon KC, Lee HJ, Kim SH. Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology. 2009;252:441–448. doi: 10.1148/radiol.2522081180. [DOI] [PubMed] [Google Scholar]
- 113.Jasinski RW, Amendola MA, Glazer GM, Bree RL, Gikas PW. Computed tomography of renal oncocytomas. Comput Radiol. 1985;9:307–314. doi: 10.1016/0730-4862(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 114.Ambos MA, Bosniak MA, Valensi QJ, Madayag MA, Lefleur RS. Angiographic patterns in renal oncocytomas. Radiology. 1978;129:615–622. doi: 10.1148/129.3.615. [DOI] [PubMed] [Google Scholar]
- 115.Quinn MJ, Hartman DS, Friedman AC, Sherman JL, Lautin EM, Pyatt RS, Ho CK, Csere R, Fromowitz FB. Renal oncocytoma: new observations. Radiology. 1984;153:49–53. doi: 10.1148/radiology.153.1.6473802. [DOI] [PubMed] [Google Scholar]
- 116.Schieda N, McInnes MD, Cao L. Diagnostic accuracy of segmental enhancement inversion for diagnosis of renal oncocytoma at biphasic contrast enhanced CT: systematic review. Eur Radiol. 2014;24:1421–1429. doi: 10.1007/s00330-014-3147-4. [DOI] [PubMed] [Google Scholar]
- 117.Ganeshan D, Iyer R, Devine C, Bhosale P, Paulson E. Imaging of primary and secondary renal lymphoma. AJR Am J Roentgenol. 2013;201:W712–W719. doi: 10.2214/AJR.13.10669. [DOI] [PubMed] [Google Scholar]
- 118.Richmond J, Sherman RS, Diamond HD, Craver LF. Renal lesions associated with malignant lymphomas. Am J Med. 1962;32:184–207. doi: 10.1016/0002-9343(62)90289-9. [DOI] [PubMed] [Google Scholar]
- 119.Miyake O, Namiki M, Sonoda T, Kitamura H. Secondary involvement of genitourinary organs in malignant lymphoma. Urol Int. 1987;42:360–362. doi: 10.1159/000281993. [DOI] [PubMed] [Google Scholar]
- 120.El-Sharkawy MS, Siddiqui N, Aleem A, Diab AA. Renal involvement in lymphoma: prevalence and various patterns of involvement on abdominal CT. Int Urol Nephrol. 2007;39:929–933. doi: 10.1007/s11255-007-9224-8. [DOI] [PubMed] [Google Scholar]
- 121.Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics. 2006;26:1151–1168. doi: 10.1148/rg.264055125. [DOI] [PubMed] [Google Scholar]
- 122.Semelka RC, Kelekis NL, Burdeny DA, Mitchell DG, Brown JJ, Siegelman ES. Renal lymphoma: demonstration by MR imaging. AJR Am J Roentgenol. 1996;166:823–827. doi: 10.2214/ajr.166.4.8610558. [DOI] [PubMed] [Google Scholar]
- 123.Hauser M, Krestin GP, Hagspiel KD. Bilateral solid multifocal intrarenal and perirenal lesions: differentiation with ultrasonography, computed tomography and magnetic resonance imaging. Clin Radiol. 1995;50:288–294. doi: 10.1016/s0009-9260(05)83418-x. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen DD, Rakita D. Renal lymphoma: MR appearance with diffusion-weighted imaging. J Comput Assist Tomogr. 2013;37:840–842. doi: 10.1097/RCT.0b013e3182a55d0a. [DOI] [PubMed] [Google Scholar]
- 125.Mitsufuji T, Fujimitsu R, Ida M, Urakawa H, Kora S, Takeshita M, Miyajima S, Yoshimitsu K. Papillary renal cell carcinoma with extensive paraaortic nodal metastasis mimicking malignant lymphoma. Magn Reson Med Sci. 2011;10:201–204. doi: 10.2463/mrms.10.201. [DOI] [PubMed] [Google Scholar]
- 126.Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Derweesh IH, Gupta S, et al. Kidney cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:175–182. doi: 10.6004/jnccn.2014.0018. [DOI] [PubMed] [Google Scholar]
- 127.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471; discussion 472-473. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 128.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 129.Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000;163:730–736. [PubMed] [Google Scholar]
- 130.Delakas D, Karyotis I, Daskalopoulos G, Terhorst B, Lymberopoulos S, Cranidis A. Nephron-sparing surgery for localized renal cell carcinoma with a normal contralateral kidney: a European three-center experience. Urology. 2002;60:998–1002. doi: 10.1016/s0090-4295(02)01993-3. [DOI] [PubMed] [Google Scholar]
- 131.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, Cindolo L, Han KR, De La Taille A, Tostain J, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181–2185, quiz 2435. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 132.Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- 133.Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, Kaouk JH, Klein EA, Novick AC. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 134.Milonas D, Skulčius G, Baltrimavičius R, Auškalnis S, Kinčius M, Matjošaitis A, Gudinavičienė I, Smailytė G, Jievaltas M. Comparison of long-term results after nephron-sparing surgery and radical nephrectomy in treating 4- to 7-cm renal cell carcinoma. Medicina (Kaunas) 2013;49:223–228. [PubMed] [Google Scholar]
- 135.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Poppel H, Becker F, Cadeddu JA, Gill IS, Janetschek G, Jewett MA, Laguna MP, Marberger M, Montorsi F, Polascik TJ, et al. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60:662–672. doi: 10.1016/j.eururo.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 137.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 138.Klatte T, Kroeger N, Zimmermann U, Burchardt M, Belldegrun AS, Pantuck AJ. The contemporary role of ablative treatment approaches in the management of renal cell carcinoma (RCC): focus on radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), and cryoablation. World J Urol. 2014;32:597–605. doi: 10.1007/s00345-014-1284-7. [DOI] [PubMed] [Google Scholar]
- 139.Klatte T, Grubmüller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol. 2011;60:435–443. doi: 10.1016/j.eururo.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 140.Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486–492. doi: 10.1016/j.eururo.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 141.Wah TM, Irving HC, Gregory W, Cartledge J, Joyce AD, Selby PJ. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113:416–428. doi: 10.1111/bju.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nguyen MM, Gill IS. Effect of renal cancer size on the prevalence of metastasis at diagnosis and mortality. J Urol. 2009;181:1020–1027; discussion 1027. doi: 10.1016/j.juro.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 143.Bahouth Z, Halachmi S, Meyer G, Avitan O, Moskovitz B, Nativ O. The natural history and predictors for intervention in patients with small renal mass undergoing active surveillance. Adv Urol. 2015;2015:692014. doi: 10.1155/2015/692014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mason RJ, Abdolell M, Trottier G, Pringle C, Lawen JG, Bell DG, Jewett MA, Klotz L, Rendon RA. Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol. 2011;59:863–867. doi: 10.1016/j.eururo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 145.Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177:849–853; discussion 853-854. doi: 10.1016/j.juro.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 146.Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, Tanguay S, Rendon RA, Gleave ME, Drachenberg DE, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 147.Smaldone MC, Kutikov A, Egleston BL, Canter DJ, Viterbo R, Chen DY, Jewett MA, Greenberg RE, Uzzo RG. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 149.Crépel M, Jeldres C, Perrotte P, Capitanio U, Isbarn H, Shariat SF, Liberman D, Sun M, Lughezzani G, Arjane P, et al. Nephron-sparing surgery is equally effective to radical nephrectomy for T1BN0M0 renal cell carcinoma: a population-based assessment. Urology. 2010;75:271–275. doi: 10.1016/j.urology.2009.04.098. [DOI] [PubMed] [Google Scholar]
- 150.Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors & gt; 4 cm: intermediate-term oncologic and functional outcomes. Urology. 2009;73:1077–1082. doi: 10.1016/j.urology.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 151.Lane BR, Tiong HY, Campbell SC, Fergany AF, Weight CJ, Larson BT, Novick AC, Flechner SM. Management of the adrenal gland during partial nephrectomy. J Urol. 2009;181:2430–2436; discussion 2436-2437. doi: 10.1016/j.juro.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 152.Blom JH, van Poppel H, Maréchal JM, Jacqmin D, Schröder FH, de Prijck L, Sylvester R. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 153.Hemal AK, Kumar A, Kumar R, Wadhwa P, Seth A, Gupta NP. Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. J Urol. 2007;177:862–866. doi: 10.1016/j.juro.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 154.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 156.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 157.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 158.Schrader AJ, Olbert PJ, Hegele A, Varga Z, Hofmann R. Metastatic non-clear cell renal cell carcinoma: current therapeutic options. BJU Int. 2008;101:1343–1345. doi: 10.1111/j.1464-410X.2008.07462.x. [DOI] [PubMed] [Google Scholar]
- 159.Dutcher JP SC, Tannir N, Benedetto P, Ruff P, Hsu A, Berkenblit A, Thiele A, Strahs A, Feingold J. Correlation of survival with tumor histology, age, and prognostic risk group for previously untreated patients with advanced renal cell carcinoma (adv RCC) receiving temsirolimus (TEMSR) or interferon-alpha (IFN) J Clin Oncol. 2007;25:5033. [Google Scholar]
- 160.Dutcher JP, de Souza P, McDermott D, Figlin RA, Berkenblit A, Thiele A, Krygowski M, Strahs A, Feingold J, Hudes G. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 161.Bergmann L, Maute L, Guschmann M. Temsirolimus for advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2014;14:9–21. doi: 10.1586/14737140.2014.864562. [DOI] [PubMed] [Google Scholar]
- 162.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 163.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 164.Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, McDermott DF, Rini BI, Heng DY. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–66. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]