Abstract
As per incidence, ovarian carcinoma is the second most common gynaecological malignancy in women. In spite of advanced technology, patient awareness and effective screening methods, epithelial ovarian cancer is usually diagnosed at an advanced stage (stage III). Surgical debulking of disease is mainstay of improving the patient survival even in advanced stages. Thus exact delineation of cancer spread in the abdominal cavity guides the surgeon prior to the surgery, help them to decide resectability of lesion and plan for further need of other surgical speciality or need of neoadjuvant chemotherapy. Imaging particularly well-planned contrast-enhanced computed tomography answers most of the queries raised by the treating surgeon. The aim of this article is to review the way ovarian carcinoma spread in the peritoneal cavity and to stress the accurate interpretation of cancer deposits on imaging which can help the treating team to reach optimal management of patients.
Keywords: Ovarian carcinoma, Computed tomography, Peritoneal deposits
Core tip: The extent of ovarian cancer spread in the abdominal cavity determines the treatment options. The exact delineation of cancer spread in the abdominal cavity guides the surgeon prior to the surgery, help them to decide resectability of lesion and plan for further need of other surgical speciality or need of neoadjuvant chemotherapy. Imaging particularly well-planned contrast-enhanced computed tomography answers most of the queries raised by the treating surgeon. This article highlights the role of computed tomography in evaluating the peritoneal spread of ovarian cancer, which help in optimal management of patients.
INTRODUCTION
Ovarian carcinoma is the second most common cause of death from gynaecological malignancies. Epithelial ovarian cancer arises from the surface of the ovary. Serosal lining of the ovary continues with the peritoneal lining of the abdomino-pelvic cavity. The most common route of ovarian tumor spread is direct exfoliation of cells from the ovary into the peritoneal cavity. Other unusual mode of spread is via a lymphatic or hematogenous way. The shed cells in the peritoneal cavity implant in the peritoneal cavity depending upon the gravity directed mobility of peritoneal fluid. This spread in the peritoneal cavity is called peritoneal carcinomatosis and is a typical presentation in advanced carcinoma and in recurrent tumors (Figure 1). Initial debulking followed by chemotherapy and neoadjuvant chemotherapy followed by surgery are two standardized protocols of treatment in advanced ovarian carcinoma. Radiologic imaging helps in proper selection of treatment, determine resectability of lesion and prognosticate the case. Accurate interpretation of imaging findings help surgeons in planning the management[1,2]. Here we attempt to present a comprehensive in-depth review of extraovarian imaging manifestations of advanced ovarian carcinoma.
Figure 1.
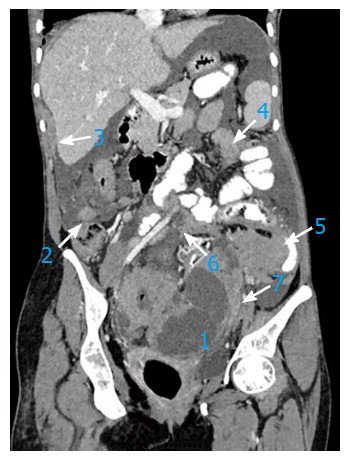
In a 67-year-old patient with advanced stage ovarian carcinoma, the post-contrast computed tomography coronal image depicts preferential spread of peritoneal implant involvement of rectouterine pouch (1), right paracolic gutter (2), right perihepatic space (3), inframesocolon (4), left paracolic gutter (5), mesentery (6) and mesosigmoid (7).
Recent advances in surgical techniques have made resection possible in difficult sites or in initially diagnosed non-resectable disease. Even there is no universally acceptable criteria to define a disease as non-resectable, survival rate in patients undergoing neoadjuvant chemotherapy followed by debulking surgery is equivalent to that among patients who underwent initial surgery followed by chemotherapy[3]. As a result, our role as radiologists is to warn clinicians about critical site involvement, which may preclude optimal debulking or complicate the surgery and not to define a disease as non-resectable.
An early sign of disease spread may be missed if subtle signs of deposits are not sought. Critical site involvement may complicate the surgery and may kill the aim of optimal debulking. Thus accurate interpretation of disease spread on imaging is essential for structured standard reporting of ovarian carcinoma to guide the surgeon for optimal management.
CRITICAL DISEASE SITE
Peritoneal carcinomatosis
Visible implants outside the pelvis (FIGO Stage IIIb) and retroperitoneal lymphadenopathy (IIIc) constitute 60% of epithelial ovarian carcinoma cases[4]. Symptoms of peritoneal progression or advanced disease are often non-specific. Aim of the preoperative diagnostic assessment in patients with epithelial ovarian cancer is to estimate as accurately as possible the extent and anatomic location of disease[5].
Subtle thickening, fine reticulonodular pattern, nodularity, peritoneal enhancement, soft tissue infiltration in omental fat and loculated ascites are subtle signs of peritoneal spread (Figure 2). The nodule may be only a few millimeters in size. Detection of nodules may be enhanced by use of an oral contrast agent. However calcified metastases may be missed due to the presence of intraluminal contrast in bowel loop. So some authors have advocated to use water as a negative oral contrast agent[6]. Coalesced nodules may give an appearance of plaque-like thickening or a single large mass may be the sole manifestation. These findings may be seen in isolation or together in varying proportions. Involvement of the transverse mesocolon must be sought and documented in the radiology report.
Figure 2.
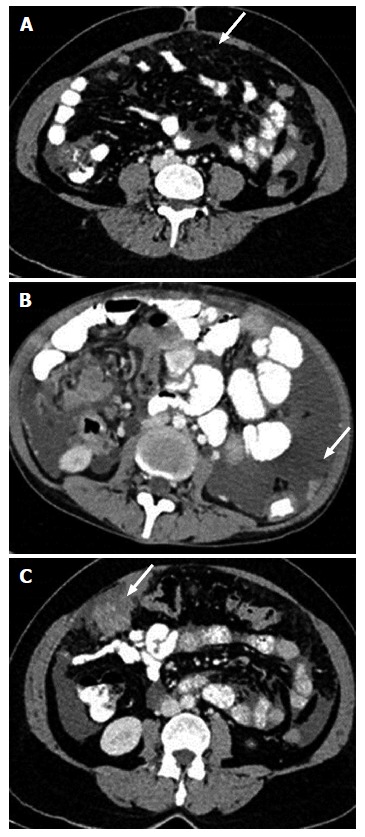
Computed tomography image showing peritoneal and omental deposits in ovarian carcinoma. Subtle stranding (A), nodule (B) and mass like deposit (C) are three forms of peritoneal involvement.
Mesentery: Usually tumor cells get implanted in the small bowel mesentery near terminal ileum[7]. Tiny ill-defined soft tissue, misty mesentery or well-defined mass like deposit are the forms of mesenteric involvement (Figure 3). Retraction of bowel loops may be seen. Infiltration of mesenteric root precludes surgery. Careful assessment is required.
Figure 3.
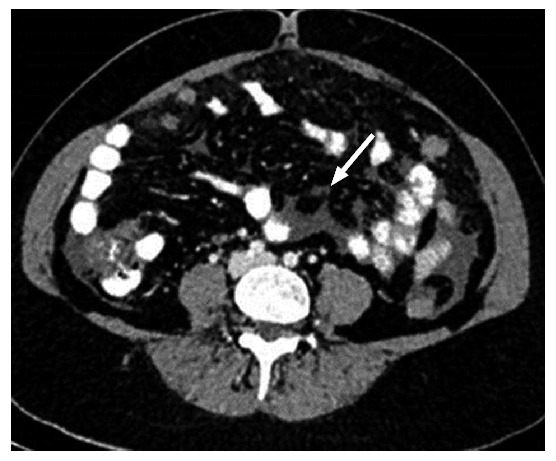
Axial contrast-enhanced computed tomography image shows a small nodule in the mesentery.
Lymph nodes: Involvement of suprarenal lymph nodes including epiphrenic should be documented as it makes the disease prone to suboptimal resection and warrants neoadjuvant chemotherapy[1].
Pelvis: Pelvic side wall invasion should be commented upon in the structured report. Mass extension within 3 mm or encasing more than 90% of iliac vessel circumference confirms the pelvic side wall invasion[1] (Figure 4).
Figure 4.
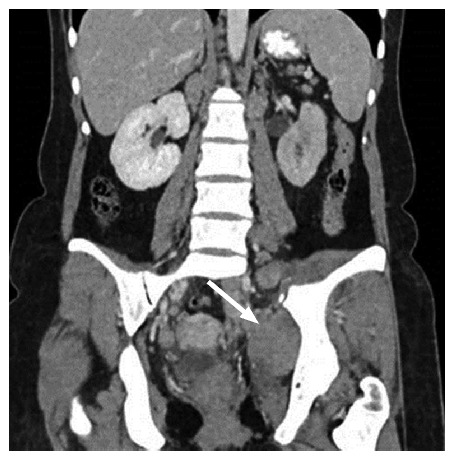
Coronal post-contrast computed tomography image shows pelvic side wall involvement.
Visceral surfaces
Liver: Number, site and size of liver implants have to be mentioned in the report. Deposit in the region of Morrison’s pouch, near the right hepatic vein and inferior vena cava has to be documented as it may lead to excessive bleeding on operative table. Another point which has to be mentioned is secondary invasion of surface implants into liver parenchyma (Figure 5). It is recognized by the absence of well-defined liver - lesion interface[8]. Presence of liver invasion necessitates involvement of hepatobiliary surgeon in the treating team.
Figure 5.
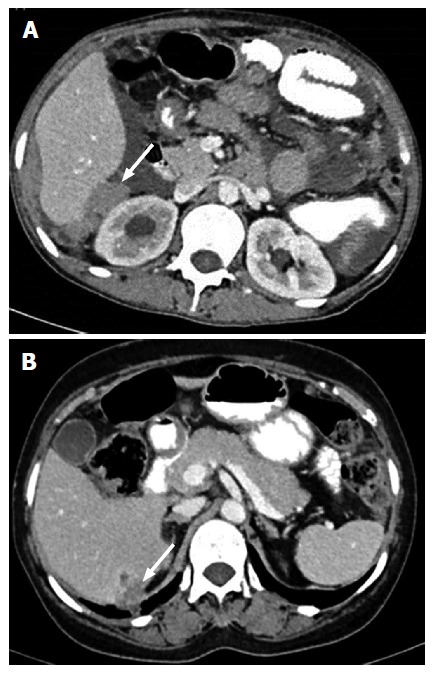
Contrast-enhanced computed tomography in two different patients showing deposits in Morrison’s pouch (A) and liver parenchyma invasion by surface deposit (B).
Spleen: Imaging appearance of spleen surface involvement and secondary parenchymal invasion is the same as that in the liver and helps surgeon to decide between splenectomy and spleen sparing surgery. Splenic hilum has to be documented upon[1].
Subphrenic involvement: Subphrenic involvement is common on the right side due to preferential flow of peritoneal fluid to the right upper quadrant and it warrants upper quadrant peritonectomy. Post-contrast computed tomography or magnetic resonance imaging in coronal and sagittal sections is the best to look for the subphrenic involvement. Apart from nodule, irregular thickening or mass and thin peritoneal enhancement along the diaphragm over the liver surface are the imaging signs of subphrenic involvement (Figure 6)[9-11].
Figure 6.
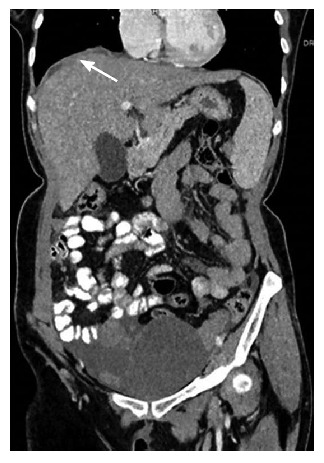
Coronal image showing smooth minimal peritoneal thickening in right subphrenic space.
Bowel: Partial or complete small bowel obstruction is the common presentation of serosal deposit. Nodule, infiltration, bowel wall thickening or definite mass are the imaging forms of bowel involvement (Figure 7). Extensive bowel involvement is considered a contraindication for surgery as per most of the institutional protocols[12-14].
Figure 7.
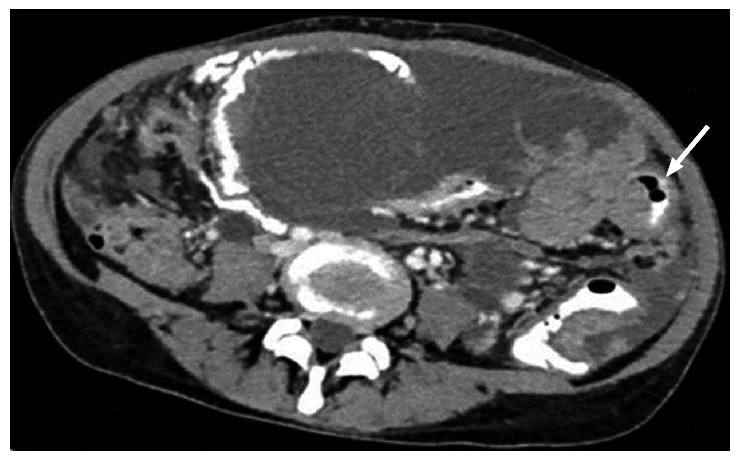
Axial computed tomography showing smooth, diffuse, eccentric bowel wall thickening involving one of the small bowel loops. Mild thickening best seen in oral contrast distended bowel loop.
Ligaments
Lesser omentum and associated ligaments: Gastrohepatic ligament and hepatoduodenal ligament are two parts of the lesser omentum. Gastrohepatic ligament is identified by the left gastric artery and hepatoduodenal ligament by the portal vein, common bile duct and hepatic artery. Size and number of deposits in this region have to be identified and reported[10,11] (Figure 8).
Figure 8.
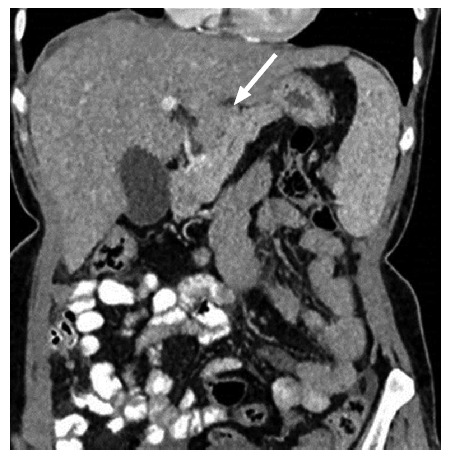
Solid nodular deposit in lesser omentum (gastrohepatic ligament) in a 40-year-old female patient with stage III ovarian carcinoma.
Lesser sac: Posterior to the stomach and anterior to the pancreas, this space involvement by carcinomatosis often precludes resection. Fluid distention of lesser sac is also taken as a sign of implant spread like other signs including thickening, nodule, mass and stranding (Figure 9).
Figure 9.
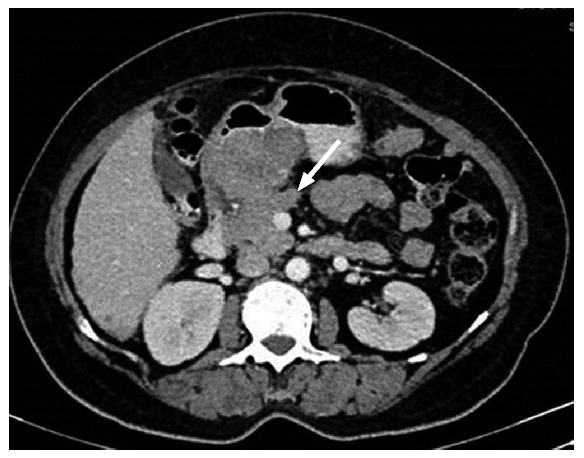
Lobulated mass like deposit in lesser sac in a case of advanced ovarian carcinoma.
Perihepatic peritoneal ligament: Falciform ligament, Gall Bladder fossa and periportal space all communicate with each other. Falciform ligament involvement should be reviewed in multiple planes to distinguish it from liver parenchymal deposit - the two entities widely differing in surgical approach (Figure 10). Extensive involvement of these ligaments are predictors of nonoptimal debulking[15].
Figure 10.
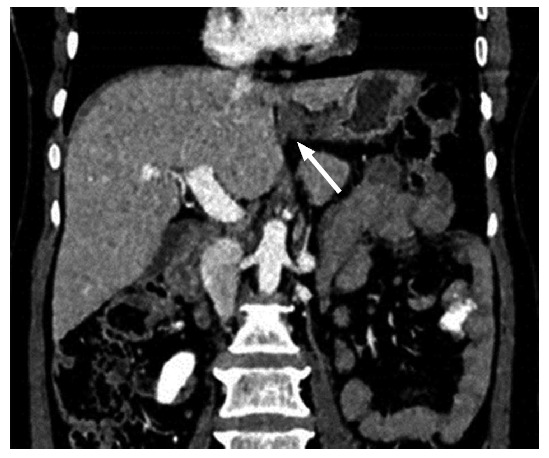
III-defined soft tissue along falciform ligament in a 50-year-old patient with ovarian carcinoma. It has to be differentiated from liver parenchyma lesion.
CONCLUSION
Primary treatment modality for ovarian cancer is surgery. The surgery usually aims at aggressive optimal debulking. Despite treatment, 5-year survival rate in ovarian carcinoma patients is low. The pattern of treatment failure is mostly local-regional, involving only the peritoneum and adjacent intra-abdominal organ usually at the site difficult to evaluate during surgery. Thus preoperative imaging can help in modifying surgical plan by demonstrating surgical hidden site involvement and bulk of disease. This requires knowledge of peritoneal cavity anatomy and acquaintance with tendency of ovarian carcinoma growth and forms of carcinomatosis.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 5, 2015
First decision: November 27, 2015
Article in press: January 29, 2016
P- Reviewer: Shen J S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Li D
References
- 1.Nougaret S, Addley HC, Colombo PE, Fujii S, Al Sharif SS, Tirumani SH, Jardon K, Sala E, Reinhold C. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics. 2012;32:1775–1800; discussion 1800-1803. doi: 10.1148/rg.326125511. [DOI] [PubMed] [Google Scholar]
- 2.Fagotti A, Gallotta V, Romano F, Fanfani F, Rossitto C, Naldini A, Vigliotta M, Scambia G. Peritoneal carcinosis of ovarian origin. World J Gastrointest Oncol. 2010;2:102–108. doi: 10.4251/wjgo.v2.i2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenkop SM, Spirtos NM. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecol Oncol. 2001;82:489–497. doi: 10.1006/gyno.2001.6312. [DOI] [PubMed] [Google Scholar]
- 4.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 5.Halkia E, Spiliotis J, Sugarbaker P. Diagnosis and management of peritoneal metastases from ovarian cancer. Gastroenterol Res Pract. 2012;2012:541842. doi: 10.1155/2012/541842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pannu HK, Bristow RE, Montz FJ, Fishman EK. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics. 2003;23:687–701. doi: 10.1148/rg.233025105. [DOI] [PubMed] [Google Scholar]
- 7.Meyers MA. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med. 1973;119:198–206. doi: 10.2214/ajr.119.1.198. [DOI] [PubMed] [Google Scholar]
- 8.Akin O, Sala E, Moskowitz CS, Ishill N, Soslow RA, Chi DS, Hricak H. Perihepatic metastases from ovarian cancer: sensitivity and specificity of CT for the detection of metastases with and those without liver parenchymal invasion. Radiology. 2008;248:511–517. doi: 10.1148/radiol.2482070371. [DOI] [PubMed] [Google Scholar]
- 9.Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204:513–520. doi: 10.1148/radiology.204.2.9240546. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703–727, xiii. doi: 10.1016/s1055-3207(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J Surg Oncol. 2010;101:713–724. doi: 10.1002/jso.21484. [DOI] [PubMed] [Google Scholar]
- 12.Colombo PE, Mourregot A, Fabbro M, Gutowski M, Saint-Aubert B, Quenet F, Gourgou S, Rouanet P. Aggressive surgical strategies in advanced ovarian cancer: a monocentric study of 203 stage IIIC and IV patients. Eur J Surg Oncol. 2009;35:135–143. doi: 10.1016/j.ejso.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Beecham JB, Bonfiglio TA. Survival time, causes of death, and tumor/treatment-related morbidity in 100 women with ovarian cancer. Hum Pathol. 1988;19:1273–1279. doi: 10.1016/s0046-8177(88)80281-8. [DOI] [PubMed] [Google Scholar]
- 14.Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Stoler MH, Beecham JB, Bonfiglio TA. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19:57–63. doi: 10.1016/s0046-8177(88)80316-2. [DOI] [PubMed] [Google Scholar]
- 15.Forstner R, Sala E, Kinkel K, Spencer JA. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20:2773–2780. doi: 10.1007/s00330-010-1886-4. [DOI] [PubMed] [Google Scholar]


